Abstract
We tested the herbal extract 2,3,5,6-tetramethylpyrazine (TMP) for possible therapeutic efficacy against a glioma cell line and against gliomas transplanted into rat brains. In the cultured glioma cells, 50 μM TMP significantly inhibited glutamate-induced increase in intracellular calcium. Significant cell damage (30%) and proliferation suppression (10%), however, occurred only at higher concentrations (200–400 μM). Glioma- neuronal co-culturing resulted in significant neuronal damage and higher proliferation of the glioma cells (140%) compared with single cultures. Low concentrations of TMP (⩽200 μM) attenuated the neuronal damage, suppressed glioma migration, and decreased glioma proliferation in the neuronal-glioma co-culture. Gliomas transplanted into the frontal cortical area exhibited high proliferation, with untreated rats dying 10–23 days later. TMP treatment inhibited tumor growth and significantly extended survival time. The results indicate that TMP can suppress glioma activity, including growth, and protect neurons against glioma-induced excitotoxicity, suggesting that TMP may have therapeutic potential in the treatment of malignant gliomas.
Keywords: calcium, excitotoxicity, glioblastoma multiforme, tetramethylpyrazine
Gliomas in their various forms are the most common central nervous system tumors. Glioblastoma multiforme, the most commonly found glioma, is highly malignant and almost uniformly fatal. Although surgery is one treatment option, the results are generally unsatisfactory, and most patients die within 1 year from the time of diagnosis, despite aggressive therapy.1
Previous studies using cell cultures have documented glutamate release by gliomas, giving rise to the hypothesis that excess glutamate, such as that released by gliomas, could be linked to tumor-associated seizures and possibly to neuronal death.2 Takano et al.3 reported that glioma cell lines released glutamate, which when present in excess can cause acute degeneration of neurons, a process commonly referred to as excitotoxicity. They demonstrated that glutamate released by the tumors stimulated tumor growth, which in turn released more glutamate in a vicious positive feedback cycle. A possible underlying mechanism for glutamate-induced tumor proliferation is that glutamate secretion by gliomas might promote tumor expansion by enhancing the inflammatory response in the microenvironment surrounding the tumors. Takano et al.3 have thus described a novel property of experimental tumors that might provide insight into why glioma cells grow so quickly and, more important, provided new clues for possible therapeutic intervention. Ishiuchi et al.4 believe that the Ca2+-permeable glutaminergic α-amino-5-hydroxy-3-methyl-4-isoxazole propionic acid (AMPA) receptor may play crucial roles in the proliferation and migration of glioblastoma, suggesting that a blockade of these Ca2+-permeable receptors may be a useful therapeutic strategy for suppression of glioblastoma invasion. Ideal therapeutic agents for the treatment of gliomas therefore should possess the following properties: (1) inhibition of the Ca2+ influx in glioma cells, (2) protection of neurons against excitotoxicity induced by glioma cells, and (3) good blood-brain barrier penetration.
Chuanxiong is a Chinese herb that is used for the treatment of neurovascular and cardiovascular diseases. 2,3,5,6-tetramethylpyrazine (TMP) is one of the major bioactive principles purified from Chuanxiong. Studies have demonstrated that TMP is effective in the treatment of cardiovascular diseases.5–11 The underlying mechanisms may include relaxation of the aortic, coronary, and pulmonary bronchial arteries. TMP’s vasodilatory effect is mediated by the inhibition of Ca2+ influx as well as by the release of intracellular Ca2+.5–9,11
Ho et al.12 demonstrated that i.p. TMP pretreatment attenuated damage in brain ischemia and significantly increased the survival rate. TMP prevented significant lipid peroxidation and necrosis in neuronal cells around the CA1 region of the hippocampus in rats with hypoxia.13,14 Morphological studies have indicated that kainate can induce apoptosis. It is believed that binding kainate to the kainate receptor would induce an intracellular influx of calcium ions15,16 and initiate calcium-dependent reactions.15,17 Large amounts of oxygen-free radicals18,19 and other free radicals20–22 would be produced, and reactions such as lipid peroxidation would subsequently occur23 and induce excitotoxicity. Our own previous study24 demonstrated that kainate decreased mitochondrial membrane potential and induced reactive oxygen species generation in contributing to neurotoxicity in cultured hippocampal neurons. Mitochondrial stabilization and free radical scavenging may account, at least partially, for the protective effects of TMP.24 Tsai and Liang25 have demonstrated that TMP possesses good blood-brain barrier penetrability.
Using cultures from a C6 glioma cell line and glioma implantation into the rat brain, we sought to determine whether TMP could inhibit the growth of glioma cells under both in vitro and in vivo conditions, and we examined the possible underlying mechanisms, focusing on intracellular calcium concentration, glutamate release, and cell migration.
Materials and Methods
Cell Culture
A C6 glioma cell line was purchased from the Food Industry Research and Development Institute (Hsinchu City, Taiwan) and cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco 12100-046) supplemented with 10% fetal bovine serum. For culturing primary neurons, hippocampi were harvested from postnatal day 7 Sprague-Dawley rats in a laminar flow chamber under sterile conditions, collected in centrifuge tubes containing Ca2+- and Mg2+-free Hank’s buffer (Gibco 14180-061), and centrifuged at 900 rpm for 5 min. The supernatant fraction was discarded. Following the addition to the pellet of DMEM containing 10% fetal bovine serum (FBS), the suspension was thoroughly mixed by titrating 15 times with a sterile glass pipette. The dispersed cells were then cultured in 24-well plates. To inhibit the growth of glial cells in the primary neuronal cultures, 2 μM of cytosine-β-D-arabino-furanoside (Sigma, C-6645) was added to each well, to a final concentration of 2 μM, after 24 h. The fetal serum–containing culture medium was replaced by serum-free DMEM on day 5. TMP (Fluka, F-87915) to final concentrations of 0, 50, 100, 200, and 400 μM was added on day 6 for 24 or 48 h.
Measurement of Intracellular Free Calcium Concentration [Ca2+]i
The glioma cells, cultured on cover slips and treated with TMP for 24 h, were incubated with the calcium indicator Fura-2 acetoxymethyl ester (Fura-2 AM, final concentration 5 μM, Molecular Probes [Invitrogen, Carlsbad, CA, USA], F-1201) at 37°C for 1 h. The glioma cells were then stimulated with 200 μM glutamate and administered by pressure injection for 20 s. The glioma cells were excited at 340 and 380 nm by xenon lamp–based Polychrome II, and the fluorescence emission was captured by timelapse photography using an inverted fluorescence microscope (Olympus IX70) and analyzed using MetaFluor Imaging System software (Molecular Devices, Sunnyvale, CA, USA). Changes in the ratio of 340 nm/380 nm fluorescence were converted to reflect the fluorescence changes within the cells and therefore changes in [Ca2+]i. Quantification, to facilitate statistical comparisons, was based on the means of the peak and trough values (of the fluorescence responses) from 45 cells in three separate experiments.
Assay of Glutamate Concentration in the Medium of Glioma Cell Culture
Prior to TMP treatment, 100 μl of culture medium from 5 × 105 glioma cells was taken out for the measurement of glutamate concentration in the medium. This value was set as the baseline value, or [glutamate]0. Twenty-four hours after TMP treatment, 100 μl of culture medium was again measured for glutamate concentration and the value was assigned [glutamate]T. The difference between [glutamate]T and [glutamate]0 represented the change in glutamate concentrations resulting from TMP treatment for 24 h.
The medium was diluted with an equal volume of distilled water and centrifuged at 500g for 3 min. The supernatant fraction was collected by aspiration and centrifuged at 2,000g for another 3 min. Then 20 μl of supernatant was loaded onto a microdialysis analyzer (CMA/600 [CMA Microdialysis, Stockholm, Sweden]) to determine the glutamate concentration.
Assessment of Cell Damage
Propidium iodide (PI) is a fluorescence dye that binds to DNA but does not penetrate intact cell membranes. The permeability of the cell membrane is increased when the cell suffers damage and loses its membrane integrity. PI is then incorporated into the cell and binds to DNA. Positive staining of the nuclei thus indicates loss of membrane integrity and therefore is an index of cytotoxicity. Approximately 5 × 105 glioma cells were treated with the vehicle dimethyl sulfoxide or TMP (0, 50, 100, 200, and 400 μM) for 24 h. After treatment, the cells were washed twice with 0.1 M phosphate buffer for 5 min each time, and then stained with 5 μg/ml PI for 30 min. The cells were then washed thoroughly with Tris buffer (50 mM Tris-HCl, pH 7.3). The staining fluorescence intensity as measured by FACSort (Becton Dickinson, Franklin Lakes, NJ, USA) was used to determine the percentage of damaged cells.
Assay of Cell Cycle
Glioma cells were treated with vehicle or TMP (0, 50, 100, 200, and 400 μM) for 24 h. After treatment, the cells were fixed with 4% paraformaldehyde and 7.5% piric acid in 0.1 M phosphate buffer (pH 7.4). The cells were then further washed twice with 0.1 M phosphate buffer for 5 min each and then stained with 5 μg/ml PI for 30 min. DNA in fixed cells was stained by PI. The staining fluorescence intensity as measured by FACSort was used to determine the G2/M ratio.
Assay of Glioma Cell Proliferation in Glioma Cell Culture Alone or Glioma-Neuronal Co-cultures
To simulate in vivo conditions in which glioma cells may be intermingled with or at least in close proximity to neurons, glioma cells were cultured alone or with neuronal cells in a special transwell system (Corning, Corning, NY, USA) designed to allow delineation of cause and effects. The co-culture system consisted of upper and lower chambers separated by a distance not physically traversable by the cells. The chambers, however, shared the same medium, which covered both cultures, thus allowing access to both cultures by humoral factors. Forming the bottom of the upper chamber was a porous membrane with multiple pores 8 μm in diameter, which allowed movement of cells across the membrane only but no actual mixing of the cells.
Primary hippocampal neurons were cultured in the upper chamber of the transwell co-culture system, with glioma cells (5 × 104 cells/well) cultured in the lower chamber. The cell cultures were treated with TMP (0, 50, 100, 200, and 400 μM) for 24 h. Then the upper Transwell was removed, and the glioma cells in the bottom chamber were counted.
Double Staining of PI and Bisbenzimide to Detect Neuronal Damage and Glioma Migration in the Neuronal and Glioma Cell Co-culture System
Whereas PI penetrates only damaged cells, bisbenzimide (B2883, Sigma), another DNA-binding fluorescence probe, penetrates intact cell membranes. We used this difference in properties to estimate the proportion of damaged cells.
Co-cultures of 5 × 104 glioma cells, this time with glioma cells in the top chamber and neurons (6th day culture of hippocampal neurons) in the bottom chamber, were treated with TMP (0, 50, 100, 200, and 400 μM) for 24 h. Following TMP treatment, the neurons in the bottom chamber were washed twice with 0.1 M phosphate buffer for 5 min each time, stained with 5 μg/ml PI and 1 μg/ml bisbenzimide for 30 min, washed thoroughly with Tris buffer (50 mM Tris-HCl, pH 7.3), and mounted with a mounting medium (Tris-glycerol, 1:1) for observation under a fluorescence microscope.
After the upper surface of the chamber-separating membrane was scraped off, the glioma cells on the lower surface of the membrane were counted to provide an index of glioma cell motility/vitality. The glioma cells that had migrated to the lower surface of the porous membrane were stained with cresyl violet.
Wound heal, another migration assay, was performed to quantify the rate of glioma cell migration. Initial wound quantification was performed on images collected 1 h after wounding, because wound size was unstable before this. Further images were collected randomly in wounded areas 24 h after wounding.
Rat Protein Cytokine Array
Approximately 5 × 104 glioma cells, primary hippocampal neurons, and glioma-neuronal co-culture system were grown in 10% FBS-DMEM. The cells were incubated in serum-free DMEM for 24 h at 37°C, and then the conditioned medium was harvested and centrifuged at 1,500g to remove cell debris. The harvested conditioned medium was used for assay of cytokine proteins using a rat protein cytokine array kit (RayBiotech Rat Cytokine Antibody Array I, R0608001A [RayBiotech, Norcross, GA, USA]). The membranes included in this kit were blocked with a blocking buffer, and then 1 ml of conditioned medium was individually added and incubated at room temperature for 2 h. The membranes were then analyzed according to the manufacturer’s instructions.
Microdialysis Experiments
For the microdialysis experiments, we used a brain microdialysis system consisting of a microinjection pump (CMA/100 [CMA Microdialysis, Stockholm, Sweden]), a fraction collector (CMA/140 [CMA Microdialysis]), and a microdialysis probe. The rats were immobilized in a stereotaxic frame (David Kopf Instruments, Tujunga, CA, USA). The skull was surgically exposed, and a hole was trephined into the skull based on stereotaxic coordinates. Following insertion of the microdialysis probe into the cortex (coordinates: 0.7 mm anterior to bregma, 2.0 mm lateral to midline, 2.0 mm lower to tip), it was perfused with Ringer’s solution (147 mM Na+, 2.2 mM Ca2+, 4 mM K+, pH 7.0) at a flow rate of 2 μl/min. Brain dialysates were collected by the fraction collector at 15-min intervals. Following postsurgical stabilization of the dialysate levels (approximately 2 h), drug-free samples were collected into the fraction collector, and TMP (0.8 mg) was then intraperitoneally administered. The dialysis samples were collected and assayed by liquid chromatography.
Liquid Chromatography
The TMP assay was performed using a liquid chromatography system consisting of a vacuum degasser, a chromatographic pump (model LC-20AT, Shimadzu, Kyoto, Japan), an autosampler (model SIL-20AC, Shimadzu), and a diode-array detector (model SPD-M20A, Shimadzu). Separation was achieved using a reversed-phase C-18 column (250 × 4.6 mm I.D.; particle size 5 μm; Merck, Darmstadt, Germany). The mobile phase consisted of methanol−10 mM NaH2PO4 in water (50:50, v/v, pH 3.0 adjusted with 85% H3PO4) delivered at a flow rate of 1.0 ml/min. The mobile phase was filtered through a Millipore 0.45-μm filter and degassed prior to use. The injection volume was 20 μl for each sample. The analyte was monitored at a wavelength of 295 nm throughout the experiments. Output data from the detector were processed via the workstation software (Class-VP, Shimadzu).
Transplantation of Glioma Cells
Approximately 106 glioma cells were transplanted into the frontal lobe of the left cerebral hemisphere of Sprague-Dawley rats (250–300 g) using a stereotaxic apparatus (A: 0.7 mm, L: 2.0 mm, H: 2.0 mm). Coordinates were set according to the atlas of Paxinos and Watson.26 Seven days after glioma cell implantation, rats in the control group received phosphate-buffered saline (PBS). Rats in the TMP group received TMP delivered by a mini-osmotic pump (Alzet #2ML4 [DURECT, Cupertino, CA, USA]) at a flux of 0.8 mg TMP/day for 56 days (2 × 28 days, the capacity of a mini-osmotic pump).
Histological Examinations
Following terminal anesthesia, the rats were perfused with fixative. Brains were removed, immersed in the fixative solution at 4°C for 24 h, and then switched to PBS containing 30% sucrose prior to cryosectioning. Serial coronal sections (30 μm) were cut from the rostal to the caudal edges of the brain tissues containing the tumors using a cryomicrotome (#CM3050S, Leica, Nussloch, Germany). Tumor size was computed using an Imagepro system (Media Cybernetics Inc., Silver Spring, MD, USA).
Immunocytochemistry for Proliferating Cell Nuclear Antigen (PCNA)
Brain sections were fixed with 4% paraformaldehyde in 0.1 M phosphate buffer for 20 min and then washed with 0.1 M phosphate buffer. They were then treated with a blocking solution (0.05% Triton X-100, 5% normal goat serum, 3% bovine serum albumin) for 30 min to prevent nonspecific antibody-antigen binding. The brain sections were then reacted with primary antibodies (mouse anti-PCNA, 1:1000, Sigma P8825) at 4°C for 36 h, washed with 0.1 M PBS, reacted with secondary antibodies at room temperature for 1 h, washed again with 0.1 M PBS, reacted with ABC complex (ABC KIT PK-4000 [Vector Laboratories, Burlingame, CA, USA]) at room temperature for 1 h, washed with 0.1 M PBS, and finally developed with 3,3′-diaminobenzidine (DAB) (5 mg of DAB, 3.5 μl of 30% H2O2 in 10 ml of 50 mM Tris buffer).
Blood Vessel Staining
FITC-dextran (Sigma FD-2000S, 0.1 ml of 50 mg/ml) was administered intravenously through the right femoral vein. After 2 min, the anesthetized animal was killed by decapitation. The brain was rapidly removed from the severed head and placed in 4% paraformaldehyde at 4°C for 24 h. Coronal sections (30 μm) were cut and then observed under a fluorescence microscope.
Effects of TMP on Survival
Survivals (in days) of the rats in the control and TMP groups were compared to evaluate the therapeutic efficacy of TMP in vivo.
Statistical Analyses
One-way or two-way analysis of variance (ANOVA) was used to compare all means, and the least significant difference (LSD) test was used for the posteriori test. In all statistical analyses, p < 0.05 was considered significant. All values are presented as means ± standard error (SE).
Results
TMP Inhibited Glutamate-Induced [Ca2+]i Increase in Glioma Cells in Culture
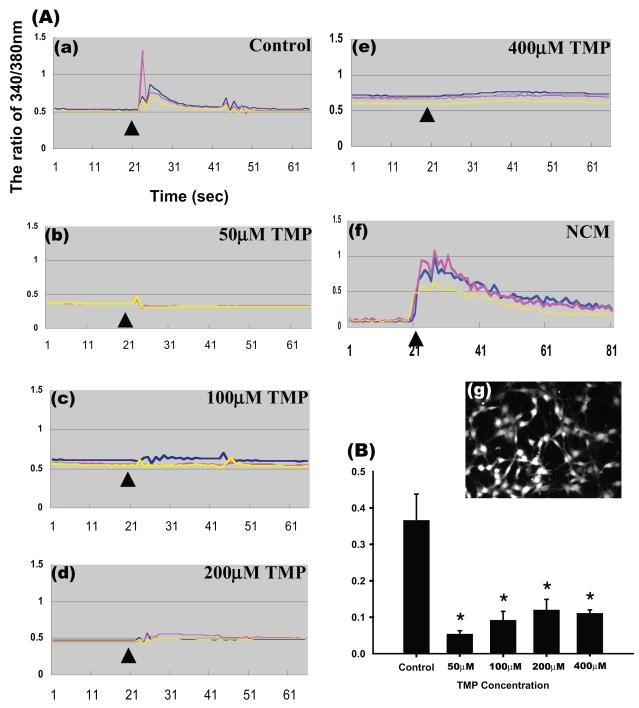
To investigate the effect of TMP on the accumulation of [Ca2+]i in glioma cells, we stimulated Fura-2 AM–loaded glioma cells with 200 μM glutamate. We found that glutamate significantly elevated [Ca2+]i in the glioma cells (Fig. 1A[a]). Treatment with 50 μM TMP significantly attenuated the glutamate-induced [Ca2+]i increase (Fig. 1A[b]); however, no further attenuation was observed with higher concentrations of TMP (100–400 μM) (Fig. 1A[c–e], Fig. 1B, p < 0.01). Fig. 1A(g) shows the formation of cell-to-cell contact by the monolayer of C6 glioma cells. The elevation of [Ca2+]i by 200 μM glutamate was not statistically different from that by 400 or 800 μM glutamate. The accumulation of [Ca2+]i by glutamate was decreased by the glutamate receptor antagonist (10 μM CNQX) (not shown). However, no further attenuation was observed with higher concentrations of TMP (100–400 μM) (Fig. 1A[c–e] and Fig. 1B, p < 0.01). The cultured hippocampal neurons were treated with 2 mM glutamate for 24 h. We used the neuronal-conditioned medium containing glutamate to stimulate the glioma cells in order to elucidate the effect of neuronal excitotoxicity on the accumulation of [Ca2+]i in the glioma cells. The result demonstrated that neuronal-conditioned medium containing glutamate induced a longer [Ca2+ ]i increase (Fig. 1A[f]).
Fig. 1.
Suppression of glutamate-induced [Ca2+]i increase in glioma cells by TMP. (A) Curves representing intracellular Ca concentrations [Ca2+]i as detected by the Fura-2 fluorescence method. In panels (a–e), glioma cells loaded with Fura-2 were treated with various concentrations of TMP ([a] 0 μM, control; [b] 50 μM; [c] 100 μM; [d] 200 μM; [e] 400 μM) prior to the addition of 2 μM of glutamate (arrowhead). In panel (f), glioma cells were stimulated with neuronal-conditioned medium (NCM) in which hippocampal neurons treated with 2 mM glutamate had been cultured for 24 h. Responses of individual cells are represented by different color lines in each panel. (g) Photomicrograph showing density of glioma cells with Fura-2. (B) TMP (50–400 μM) effectively inhibited the rise of [Ca2+]i by glutamate. Results shown are the mean ± SE of 15 randomly selected cells each from three different experiments (n = 3, one-way analysis of variance followed by least significant difference test; *significant difference compared with control group, p < 0.01).
TMP Suppressed Glutamate Release in Cultured Glioma Cells
To explore whether TMP changes the release of glutamate from glioma cells, we assayed the concentration of glutamate in the culture medium of glioma cells. Our results indicated that treatment with 50 μM TMP attenuated the increase in glutamate in the culture medium, from 15.55 ± 3.04 μmol/L without TMP to just 1.97 ± 8.3 μmol/L over a 24-h period. At higher TMP concentrations (100–400 μM), the changes in glutamate concentration in the medium decreased from 10.30 ± 2.58 to 12.84 ± 2.27 μmol/L, suggesting that TMP may modulate the disposition of glutamate, including synthesis, release, or recycling (Table 1, p < 0.01).
Table 1.
TMP changes glutamate releasing and recycling in glioma cells
| [Glu]/group | Pretreatment (μM) | 24 h posttreatment (μM) | Changes of [Glu] in medium (μM) |
|---|---|---|---|
| Control | 29.12 ± 2.41 | 44.66 ± 1.32 | 15.55 ± 3.04 |
| 50 μM TMP | 33.94 ± 4.51 | 35.90 ± 3.79 | 1.97 ± 8.28* |
| 100 μM TMP | 44.76 ± 3.55 | 34.46 ± 6.11 | − 10.30 ± 2.58* |
| 200 μM TMP | 42.10 ± 0.50 | 29.34 ± 0.57 | − 12.76 ± 0.32*,# |
| 400 μM TMP | 45.10 ± 3.03 | 32.27 ± 5.29 | − 12.84 ± 2.27*,# |
Note: Aliquots from the culture medium from the cultured glioma cells pretreated with various concentrations of TMP for 24 h were assayed for glutamate concentration before ([glutamate] 0) and after ([glutamate]T) TMP treatments. The difference between [glutamate]0 and [glutamate]T represents the change in glutamate concentration by 24-h treatment with TMP. TMP treatment reduced the concentration of glutamate in the medium in a dose-dependent manner (n = 4, one-way analysis of variance followed by least significant difference test, p < 0.01,
compared with control,
compared with 50 μM TMP group).
Higher Concentrations of TMP Induced Damage in Cultured Glioma Cells
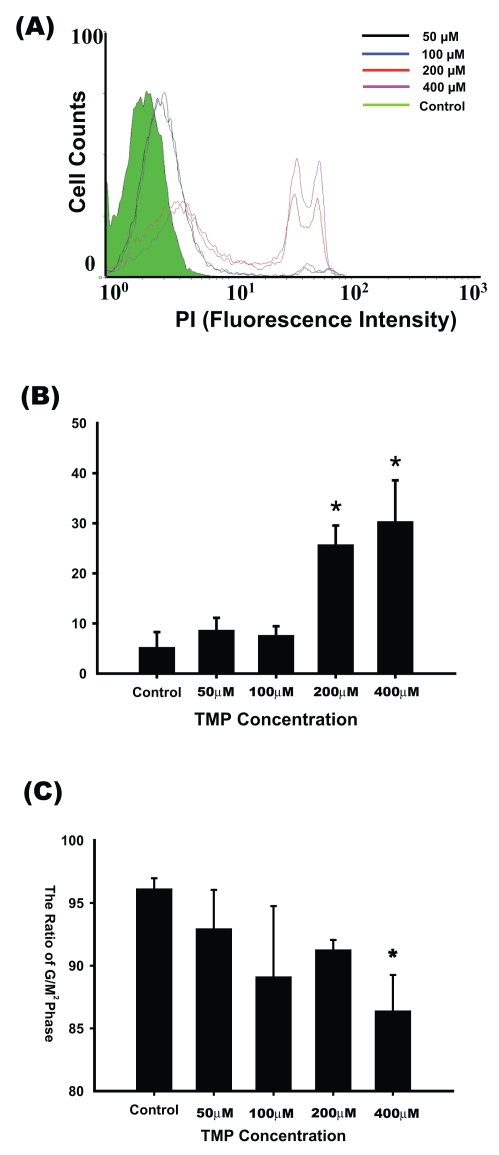
The results of PI staining, used as an index of cell damage under nonfixed cell conditions, showed that treatment with 50 and 100 μM TMP did not cause significant cell damage compared with that in the control group (5.25% ± 3.02%, 8.68% ± 2.44%, and 7.65% ± 1.79% for the control, 50 μM, and 100 μM TMP groups, respectively). Higher percentages of cell damage were induced by 200 and 400 μM TMP (25.75% ± 3.78 % and 30.36% ± 8.21%, respectively, p < 0.01; Fig. 2A, B).
Fig. 2.
Higher concentrations of TMP induced damage in cultured glioma cells. (A) Glioma cells were treated with 0–400 μM TMP for 24 h. Damaged glioma cells were labeled with propidium iodine (PI), and cell counts were confirmed by flow cytometry. Control: green line; 50 μM TMP: black line; 100 μM TMP: blue line; 200 μM TMP: red line; 400 μM TMP: purple line. (B) Histogram showing that higher concentrations of TMP (200–400 μM) caused significant increases in number of PI-labeled glioma cells (n = 6, one-way analysis of variance [ANOVA] followed by least significant difference [LSD] test; *significant difference compared with the control group, p < 0.01). (C) Effects of TMP on the cell cycle in glioma cells. Glioma cells were exposed to 0–400 μM TMP for 24 h and then fixed for PI staining. The G2/M ratio in the 400 μM TMP-treated group was lower than that in the control group (n = 3, one-way ANOVA followed by LSD test; *significant difference compared with the control group, p < 0.05).
Higher Concentrations of TMP Inhibited Proliferation of Cultured Glioma Cells
Cells were fixed, and DNA was labeled by PI. The results showed that the G2/M ratio did not significantly change in glioma cells treated with 50–200 μM TMP (92.97% ± 3.08% to 89.13% ± 5.62% relative to the control, p > 0.05). The G2/M ratio was significantly lower in glioma cells treated with 400 μM TMP (86.41% ± 2.85% relative to the control, p < 0.05) (Fig. 2C).
Co-culturing with Neurons Enhanced Proliferation of Glioma Cells
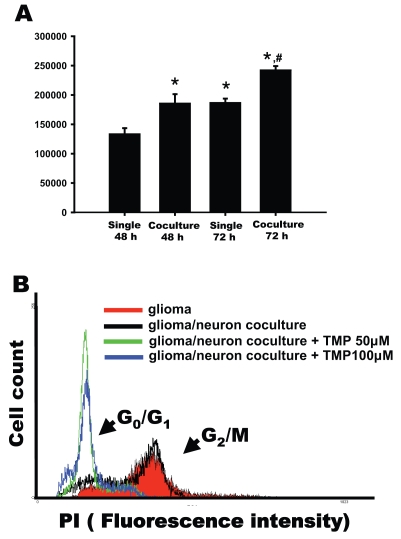
We examined whether glioma-neuronal co-culturing affected the proliferation of glioma cells. For this experiment, the glioma cells were cultured in the top chamber and the neurons in the bottom chamber. Glioma cells (5 × 104) were cultured with neurons separated by porous membranes for 48 and 72 h. The results showed that the number of glioma cells co-cultured with neurons for 48 h was 40% greater than when cultured alone (Fig. 3A, p < 0.01). At 72 h, the number of glioma cells was 130% of those cultured alone for the same period (Fig. 3A, p < 0.01). These results indicated that glioma proliferation was enhanced in the co-culture system.
Fig. 3.
Enhancement of proliferation of glioma cells in glioma and neuronal cells co-culture. (A) Glioma cells were cultured alone or with hippocampal neurons for 48 and 72 h, and then the cells were counted. The growth of glioma cells co-cultured with neurons was enhanced significantly (n = 9, one-way analysis of variance followed by least significant difference test; p < 0.01; *compared with that cultured alone for 48 h, #compared with that co-cultured for 48 h or cultured alone for 72 h). (B) Glioma cells were treated with 50 or 100 μM TMP in co-culture of neuron-glioma cells for 24 h. Cells were fixed, stained with PI, and analyzed for DNA content by FACS.
To further confirm the effect of TMP on the cell cycle in the co-culture system, PI was used to stain the DNA of fixed cells. Values for the G2/M ratios were 67.63% ± 1.43% for the glioma cells alone and 56.54% ± 1.30% for the neuronal-glioma co-culture system (Fig. 3B). Treatment with 50 and 100 μM TMP induced the glioma cells to produce an increase in the G0/G1 ratio and a decrease in the G2/M ratio (Fig. 3B).
TMP Suppressed Migration of Glioma Cells and Protected Neurons against Glioma Cell–Induced Cytotoxicity in Glioma-Neuronal Co-cultures
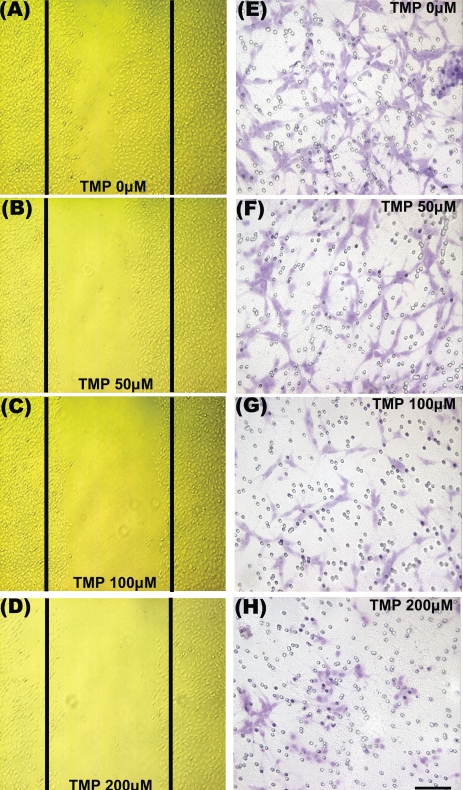
We next examined whether TMP could affect the degree of glioma cell migration in the glioma-neuronal co-culture system. A large number of glioma cells moved across the porous membrane within 24 h in the absence of TMP (Fig. 4E). Treatment with 50 μM TMP led to a slight decrease in the number of migrating cells (Fig. 4F). At the higher TMP concentrations (100 and 200 μM), the number of cells that migrated to the lower surface of the porous membrane was lower (Fig. 4G, H). To study wound-induced migration of glioma cells, we established a cell culture model that permitted direct visualization and measurement of glioma cell movement into a wound. The results indicated that migration was always directed toward the center of the wound. In serum-free medium without added TMP, the glioma cells migrated at a rate of approximately 30 μm/h during the first 24 h (Fig. 4A). Treatment with 50 μM TMP led to a lower migration rate (Fig. 4B). At TMP concentrations of 100 and 200 μM, the number of cells that migrated to the center of the wound was further reduced (Fig. 4C, D).
Fig. 4.
TMP suppressed glioma migration in co-culture of neuron-glioma cells. Representative phase contrast images show that the wound- induced glioma cell migration in the presence of (A) 0, (B) 50, (C) 100, and (D) 200 μM TMP for 24 h. Panels E–H represent cresyl violet staining (purple) of glioma cells found on the lower surface of the porous membrane forming the bottom of the upper chamber in which they were cultured. The glioma cells became more sparse as the TMP was increased from 0 to 200 μM, indicating dose-dependent suppression of motility/vitality of the glioma cells by TMP. The small circles represent the pores of the membrane. Scale bar = 100 μm.
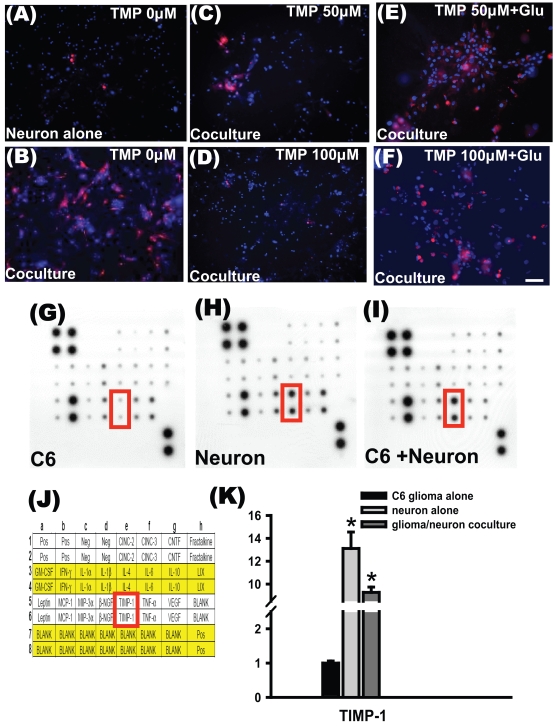
Finally, we estimated whether TMP might change the degree of neuronal damage in the co-culture system. Double staining with PI and bisbenzimide indicated higher neuronal damage in glioma-neuronal co-cultures without TMP treatment than in neurons cultured alone (Fig. 5A, B). Treatment with 50 and 100 μM TMP attenuated neuronal death in the glioma-neuronal co-cultures. It appears that the cytotoxic effects of glioma cells were blocked significantly by treatment with 50 and 100 μM TMP (Fig. 5C, D). The protective effect of 50 and 100 μM TMP on neurons in the glioma-neuronal co-culture was decreased by the addition of glutamate (Fig. 5E, F).
Fig. 5.
TMP attenuated neuronal damage. Double staining with propidium iodide (red), which penetrates only damaged cells, and bisbenzimide (blue), which penetrates both damaged and intact cells, was used to determine the percentage of cytotoxicity. Photomicrographs showing fluorescence staining of cultured hippocampal neurons indicate that co-culturing with glioma cells markedly increased neuronal cytotoxicity, which was attenuated in a dose-dependent manner by TMP. All cultures were treated for 24 h. (A) Relatively few damaged cells were observed in neurons cultured alone. (B) The damage was markedly increased when neurons were co-cultured with glioma cells. (C) TMP at 50 μM markedly reduced the number of damaged neurons; (D) at 100 μM the damage was hardly noticeable. (E and F) The protective effect of TMP on neurons was decreased by additional glutamate (scale bars = 50 μm in A–F). Cytokine antibody array of conditioned medium is shown for glioma cells (G), neuron (H), and glioma-neuronal co-culture (I). Nineteen rat cytokines were blotted onto a membrane; (J) the corresponding position is as shown. The intensities of the relative expression levels of cytokines were quantified by densitometry. (K) The relative density of tissue inhibitor of metalloproteinases-1 (TIMP-1) was higher in the conditioned medium of neurons and glioma-neuronal co-culture than in the conditioned medium of glioma cells alone.
Conditioned media from glioma cells, neurons, and glioma-neuronal co-cultures were then prepared and incubated with membranes containing an array of 19 rat protein cytokine antibodies (Fig. 5J). Autoradiographs were scanned, and the density of each cytokine at the corresponding position was determined. The relative intensities of each cytokine were normalized to control spots on the same membrane. Major increases among cytokines are represented graphically in Fig. 5G, H, and I. Tissue inhibitor of metalloproteinases-1 (TIMP-1) was significantly increased in neuronal-conditioned medium and in glioma-neuronal co-cultured conditioned medium (Fig. 5K).
TMP Could Penetrate the Brain-Blood Barrier
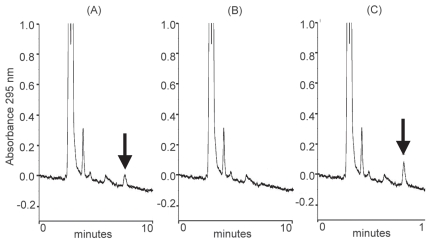
Under the chromatographic conditions, good separation and detection of TMP were achieved in brain dialysates. The retention time of TMP was 7.6 min. Typical chromatograms of TMP in brain dialysates are shown in Fig. 6. No detectable interfering peak was found with a retention time close to that of TMP. Fig. 6A represents a brain dialysate spiked with TMP (0.01 μg/ml), and Fig. 6B shows a blank brain dialysate. Fig. 6C shows a brain dialysate sample containing TMP (0.02 μg/ml) collected 45 min after TMP administration (0.8 mg, i.p.). On the basis of TMP concentrations of 1 and 10 μg/ml, the average in vitro recovery of TMP microdialysate in rat brain was approximately 5.6% ± 0.11%. TMP concentrations were 0.14, 1.39, 0.35, and 0.31 μg/ml (equivalent to 0.67, 6.66, 1.68, and 1.49 μM) at 30, 45, 60, and 75 min after TMP administration (0.8 mg, i.p.).
Fig. 6.
Existence of TMP in the brain tissue. (A) Chromatograms of rat brain dialysate spiked with TMP (0.01 μg/ml), (B) blank brain dialysate, and (C) rat brain dialysate sample containing TMP spike (0.02 μg/ml) collected 45 min after TMP administration (3.2 mg/kg, i.p.). Arrows indicate TMP peak.
The concentration of TMP in the plasma was 2.26 ± 1.0 μg/ml (equivalent to 10.83 μM) at 30 min after TMP administration (0.8 mg/kg, i.p., n = 4).
TMP Caused Regression of Malignant Gliomas and Extended Survival of Rats with Glioma Transplantation
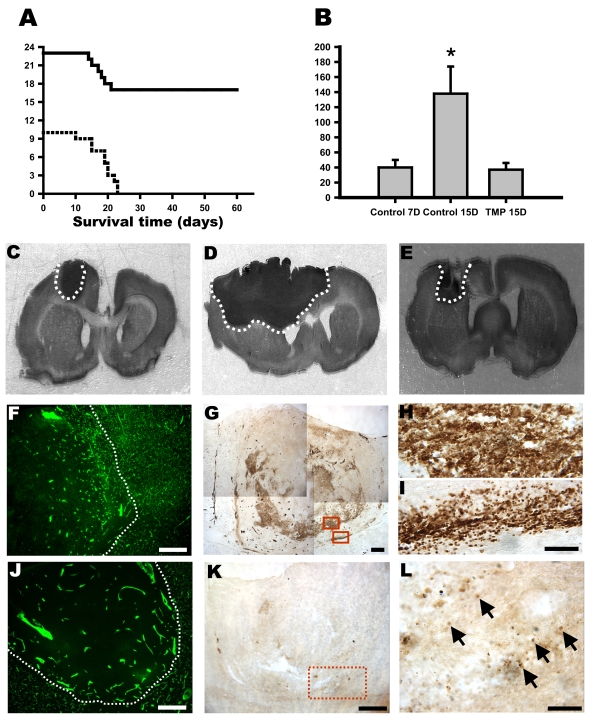
Tumor sizes in the control group were 40 ± 10 mm3 (n = 6) and 138 ± 36 mm3 (n = 10) on days 7 and 15 after implantation, respectively (Fig. 7B, C, D, p < 0.05). Tumor growth was significantly inhibited in rats treated with 0.8 mg TMP/day compared with growth in control rats on day 15 after glioma cell implantation (i.e., TMP treatment for 7 days). The tumor volume in rats treated with TMP for 7 days was 37 ± 9 mm3 (n = 12), similar to the value 7 days after glioma cell implantation (Fig. 7B, C, E). All the control rats (n = 10) died between days 10 and 23. Seventeen of the TMP-treated rats surpassed the survival times of the control rats and survived beyond 60 days (Fig. 7A). To assess peritumor angiogenesis, FITC-dextran was used to perfuse the rats. The results showed that microcirculation around the tumor margin was denser in the control rats than in the TMP-treated rats (Fig. 7F, J). To confirm the proliferation of glioma cells, PCNA immunohistostaining was performed.27 In the TMP-treated rats, PCNA-positive nuclei were sparse and restricted to the tumor margin (Fig. 7K, L). In the control rats, PCNA-positive cells were distributed spaciously and ranged from the center of tumor to a more intense expression (Fig. 7G, H). Some PCNA-positive cells were found to have invaded the boundary of the tumor (Fig. 7G, I).
Fig. 7.
TMP induced regression of gliomas in SD rats. (A) Kaplan-Meier survival curves of rats with brain tumors. Tumors were induced in 33 rats by intracerebral injection of 106 C6 glioma cells (day 0). Ten rats were left untreated (dotted line), and 23 were treated with TMP (solid line) beginning on day 8. Tumor-bearing rats treated with TMP lived significantly longer than did rats in the control group. (B) Tumor size in the control and TMP-treated rats 7 and 15 days after implantation of glioma cells. Each point represents the size of individual tumors (two-way analysis of variance followed by least significant difference test, p < 0.01, *compared with controls on day 7 and TMP treatment on day 15). (C) Photomicrographs showing tumors from the control group on days 7 and 15 (D) and TMP treatment (E) on day 15 after glioma implantation. Photomicrographs showing FITC-dextran (green) perfused microcirculation in the glioma of the control (F) and TMP-treated (J) rats. Immunocytostaining for PCNA-positive cells in glioma (G). Nuclear immunoreactivity was expressed strongly and distributed widely in control rats. Upper and lower boxes show the higher magnification of the cells in (H) and (I), respectively. (K) In the TMP-treated rats, the PCNA-positive cells were few. (L) Higher magnification of the demarked area shown in K. Scale bars = 0.5 mm in F, G, J, and K; 100 μm in H, I, and L.
Discussion
In the present study, we found that lower concentrations of TMP inhibited the increase of intracellular calcium concentration. We suggest that inhibition of calcium influx by TMP may be the crucial factor in suppressing the activities of glioma cells, including cell migration, cell proliferation, and release of glutamate.
It is known that the properties of C6 glioma cells can change under different culture conditions. Armelin et al.28 showed the diverse effect of hydrocortisone on glioma cells in suspension and monolayer cultures. Bignami et al.29 reported that astroglia-specific GFA protein was expressed in aggregating cultures of C6 glioma cells, suggesting that cell-to-cell interaction promoted the production GFA protein. In our study, although glioma cells were cultured in monolayer, the cell density was nevertheless high enough to form cell-to-cell contact (Fig. 1A[g]). In each experiment, under the same culture conditions, about 75% of the glioma cells responded to glutamate. Similar results were reported by Labrakakis et al.,30 who found that 66.7% of glioblastoma cells in coverslip culture and 50% in brain slices responded to glutamate and kainite.
In our study, glutamate triggered an increase of [Ca2+]i m in glioma cells. Pre-incubation of CNQX or TMP (⩽200 μM) reduced but did not abrogate the increase of [Ca2+]i by glutamate. Neuronal-conditioned medium containing glutamate induced a longer duration of Ca2+ influx in the glioma cells, indicating that additional factors in the neuronal-conditioned medium might stimulate glioma cells to produce the delayed Ca2+ influx. Previous studies have confirmed that metabotropic glutamate receptors and voltage-gated Ca2+ channels were expressed in human glioma cells.30,31
In our current study, the TIMP-1 was significantly increased in the media of neurons alone and the neuronal- glioma co-culture. The precise role of TIMP-1 in glioma pathophysiology is unclear. It has been shown that TIMP-1 has erythroid-potentiating activity and stimulates proliferation of various cell types.32,33 TIMP-1 may upregulate VEGF expression in mammary carcinoma cell lines.34 Moreover, Groft et al.35 reported that TIMP-1 was expressed in human gliomas, suggesting TIMP-1 elevation may act as a marker of worse outcome in gliomas. We speculate that, in addition to TIMP-1, some cytokines beyond the 19 cytokines we assessed may be involved in the stimulation of glioma cell activity.
Ishiuchi et al.4 demonstrated that the overexpression of the GluR2 subunit resulted in Ca2+ impermeability, inhibition of cell migration, and induction of apoptosis. In contrast, overexpression of Ca2+-permeating AMPA receptors (GluR1 and/or GluR4) facilitates migration and proliferation of tumor cells.4 Therefore, calcium is the critical factor in the activation of glioma cells. Previous studies have demonstrated that TMP not only blocks the entry of extracellular calcium but also inhibits the release of intracellularly stored calcium in vascular smooth muscle cells5,36 and platelets.37 Furthermore, TMP has been found to interact directly with L-type Ca2+ channels6 and to activate the adenylate cyclase/protein kinase A cascade, subsequent to inhibition of L-type Ca2+ channels.9 Results from our experiments are consistent with such notions and suggest that TMP may have therapeutic potential through blockade of calcium permeability and thus suppression of the activation of glioma cells.
Although TMP at higher concentrations inhibited the proliferation of glioma cells, it also caused damage to neurons. At 400 μM, TMP treatment resulted in considerable neuronal damage, as reflected by increased PI staining (data not shown). Taken together, as the results indicated that lower concentrations of TMP were sufficient to inhibit glioma growth and high concentrations may cause excessive neuronal damage, lower doses might therefore be more desirable if TMP were to be used as a therapeutic agent for the treatment of gliomas.
Co-culturing of glioma cells and neurons indicated that both the proliferation rate of glioma cells and damage to neurons increased. We suggest that the glutamate released by glioma cells led to neuronal damage resulting from neuronal excitotoxicity. The neuronal damage in turn might promote the growth of neighboring glioma cells. In line with this, Takano et al.3 reported that the rat C6 glioma cell line could be subcloned into three cell types, C6 Glu+, C6 Glu−, and C6 WT. C6 Glu+ cells actively release glutamate, causing a significant increase in glutamate concentration in the medium. C6 Glu− cells actively take up glutamate, causing a decrease in glutamate concentration in the medium. Transfer of conditioned medium from C6 Glu+ cells to cultured cortical neurons resulted in decreased neuron survival relative to the transfer of conditioned medium from C6 Glu−cells.3
In the present study, glutamate concentration was less than 50 μM in the medium of cultured glioma cells. We speculate that such a concentration of glutamate may not be enough to stimulate the glioma cells but may nonetheless be enough to induce neuronal depolarization. Subsequently, the number of excitatory neurons may increase and the glutamate concentration may be elevated. Finally, the neuronal damage and glioma cell activity may be triggered.
In the present experiments, TMP decreased the glutamate concentration in the culture medium of the glioma cells. Possible mechanisms may include inhibition of glutamate biosynthesis, inhibition of glutamate secretion, and enhancement of glutamate uptake. This reduction of glutamate in the medium might account for the reduction in neuronal damage. As a consequence, proliferation of the glioma cells, which exhibited a direct relationship to neuronal damage, was attenuated.
In addition to acting on glioma cells, TMP may act on neurons directly. Our previous study demonstrated that decreased mitochondrial membrane potential and increased free radical generation in cultured hippocampal neurons accompanied kainate-induced neurotoxicity.24 Mitochondrial stabilization and free radical scavenging may account, at least partially, for the neuro-protective effects of TMP against excitotoxicity.24 Similar findings have been reported by Zhang et al.,38,39 who demonstrated that TMP scavenged superoxide anion and decreased nitric oxide production in human polymorphonuclear leukocytes and cerebellar granule cells. TMP is believed to stimulate blood circulation, inhibit lymphocyte adhesion to vascular endothelial cells, and suppress platelet aggregation.38–41 Therefore we speculate that TMP not only affects the nervous system but also modulates the immune system, including inhibition of polymorphonuclear leukocyte accumulation and prevention of inflammatory response, resulting in prevention of neuronal damage.
In our in vivo study, gliomas transplanted into the frontal cortical area proliferated highly, with untreated rats dying 10–23 days after implantation, whereas TMP treatment inhibited tumor growth and significantly extended the survival time. In the study of Takano et al.3 with the three subtypes of glutamate receptors, they transplanted the three cell types into the rat brain and found that the sizes of the tumors associated with the three types were in the order C6 Glu+ > C6 WT > C6 Glu− They suggested that glutamate release by glioma cells was central in inducing neuronal death, which in turn enhanced glioma cell growth and invasion in vivo.
Six of the TMP-treated rats in which gliomas had been transplanted died between days 14 and 21. We found the tumor sizes in the six rats similar to those in the control rats, indicating that TMP treatment was ineffective in these rats. In our previous study, following pretreatment with 1 or 5 μM TMP for 10 min, the excitotoxicity effects of cultured hippocampal neurons induced by kainate were significantly attenuated.24 Administration of 10, 20, or 40 mg/kg TMP provides neuroprotection against ischemia-induced brain injury.42,43 Microdialysis analysis showed that TMP concentrations were less than 7 μM after TMP administration (0.8 mg, i.p.; 3.2 mg/kg). We suggest that the six rats’ deaths might be related to individual differences in responding to TMP rather than to the neurotoxicity of TMP.
Our study demonstrated that TMP suppressed the activity of glioma cells, including proliferation rate, migration ability, accumulation of intracellular calcium, and release of glutamate. Consequently, neuronal excitotoxicity in neighboring neurons could be decreased or prevented. Possibly through these mechanisms, TMP induced a considerable regression of the malignant gliomas transplanted in rats. These results suggest that TMP may possess therapeutic potential for the treatment of malignant gliomas.
Acknowledgment
This work was supported by Grant NSC95-2314- B075-071-MY2 from the National Science Council, Grant V95S5-001 from Taipei Veterans General Hospital, and Grant 95002-62-080 from Taipei City Hospital, Taiwan.
References
- 1.Benedetti S, Pirola B, Pollo B, et al. Gene therapy of experimental brain tumors using neural progenitor cells. Nat Med. 2000;6:447–450. doi: 10.1038/74710. [DOI] [PubMed] [Google Scholar]
- 2.Ye ZC, Sontheimer H. Glioma cells release excitotoxic concentrations of glutamate. Cancer Res. 1999;59:4383–4391. [PubMed] [Google Scholar]
- 3.Takano T, Lin JHC, Arcuino G, Gao Q, Yang J, Nedergaard M. Glutamate release promotes growth of malignant gliomas. Nat Med. 2001;7:1010–1015. doi: 10.1038/nm0901-1010. [DOI] [PubMed] [Google Scholar]
- 4.Ishiuchi S, Tsuzuki K, Yoshida Y, et al. Blocking of Ca2+-permeable AMPA receptors suppresses migration and induces apoptosis in human glioblastoma cells. Nat Med. 2002;8:971–978. doi: 10.1038/nm746. [DOI] [PubMed] [Google Scholar]
- 5.Kwan CY, Daniel EE, Chen MC. Inhibition of vasoconstriction by tetramethylpyrazine: does it act by blocking the voltage-dependent Ca channel? J Cardiovasc Pharmacol. 1990;15:157–162. [PubMed] [Google Scholar]
- 6.Kwan CY. Plant-derived drugs acting on cellular Ca2+ mobilization in vascular smooth muscle: tetramethylpyrazine and tetrandrine. Stem Cells. 1994;12:64–67. doi: 10.1002/stem.5530120111. [DOI] [PubMed] [Google Scholar]
- 7.Liu SF, Cai YN, Evans TW, McCormack DG, Barer GR, Barnes PJ. Ligustrazine is a vasodilator of human pulmonary and bronchial arteries. Eur J Pharmacol. 1990;191:345–350. doi: 10.1016/0014-2999(90)94167-v. [DOI] [PubMed] [Google Scholar]
- 8.Pu Z, Zhu W, Jing Z, Zeng Z, Zuo W. Effect of tetramethyl pyrazine on coronary vasoconstriction induced by endothelin-1 in dogs. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 1996;18:133–137. [PubMed] [Google Scholar]
- 9.Au ALS, Kwan YW, Kwok CC, Zhang RZ, He GW. Mechanisms responsible for the in vitro relaxation of ligustrazine on porcine left anterior descending coronary artery. Eur J Pharm. 2003;468:199–207. doi: 10.1016/s0014-2999(03)01691-1. [DOI] [PubMed] [Google Scholar]
- 10.Wang ZL. Effects of tetramethylpyrazine on the cardiovascular system. Sheng Li Ke Xue Jin Zhan. 1992;23:313–317. [PubMed] [Google Scholar]
- 11.Wong KL, Chan P, Huang WC, et al. Effect of tetramethylpyrazine on potassium channels to lower calcium concentration in cultured aortic smooth muscle cells. Clin Exp Pharmacol Physiol. 2003;30:793–798. doi: 10.1046/j.1440-1681.2003.03913.x. [DOI] [PubMed] [Google Scholar]
- 12.Ho WK, Wen HL, Lee CM. Tetramethylpyrazine for treatment of experimentally induced stroke in Mongolian gerbils. Stroke. 1989;20:96–99. doi: 10.1161/01.str.20.1.96. [DOI] [PubMed] [Google Scholar]
- 13.Luo XX, Ogata H, Xu X, Ishitobi F. Protective effect of tetramethylpyrazine on ischemic neuronal damage in the gerbil hippocampus. No to Shinkei. 1994;46:841–846. [PubMed] [Google Scholar]
- 14.Huang JB, Liu XF, Chen SY. Effect of tetramethylpyrazine in inhibiting respiratory burst of polymorphonuclears and scavenging oxygen free radicals. Zhongguo Zhong Xi Yi Jie He Za Zhi. 1994;14:607–609. [PubMed] [Google Scholar]
- 15.Choi DW. Glutamate receptors and the induction of excitotoxic neuronal death. Prog Brain Res. 1994;100:47–51. doi: 10.1016/s0079-6123(08)60767-0. [DOI] [PubMed] [Google Scholar]
- 16.Dutrait N, Culcasi M, Cazevieille C, et al. Calcium-dependent free radical generation in cultured retinal neurons injured by kainate. Neurosci Lett. 1985;198:13–16. doi: 10.1016/0304-3940(95)11948-v. [DOI] [PubMed] [Google Scholar]
- 17.Cheng Y, Sun AY. Oxidative mechanisms involved in kainate-induced cytotoxicity in cortical neurons. Neurochem Res. 1994;19:1557–1564. doi: 10.1007/BF00969006. [DOI] [PubMed] [Google Scholar]
- 18.Boldyrev AA, Carpenter DO, Huentelman MJ, Peters CM, Johnson P. Sources of reactive oxygen species production in excitotoxin-stimulated cerebellar granule cells. Biochem Biophys Res Commun. 1999;256:320–324. doi: 10.1006/bbrc.1999.0325. [DOI] [PubMed] [Google Scholar]
- 19.Bondy SC, Lee DK. Oxidative stress induced by glutamate receptor agonists. Brain Res. 1993;610:229–233. doi: 10.1016/0006-8993(93)91405-h. [DOI] [PubMed] [Google Scholar]
- 20.Mikuni N, Babb TL, Chakravarty DN, Christi W. Time course of transient expression of GDNF protein in rat granule cells of the bilateral dentate gyri after unilateral intrahippocampal kainic acid injection. Neurosci Lett. 1999;262:215–218. doi: 10.1016/s0304-3940(99)00074-9. [DOI] [PubMed] [Google Scholar]
- 21.Rauca C, Zerbe R, Jantze H. Formation of free hydroxyl radicals after pentylenetetrazol-induced seizure and kindling. Brain Res. 1999;847:347–351. doi: 10.1016/s0006-8993(99)02084-3. [DOI] [PubMed] [Google Scholar]
- 22.Ueda Y, Yokoyama H, Niwa R, Konaka R, Ohya-Nishiguchi H, Kamada H. Generation of lipid radicals in the hippocampal extracellular space during kainic acid-induced seizures in rats. Epilepsy Res. 1997;26:329–333. doi: 10.1016/s0920-1211(96)00901-1. [DOI] [PubMed] [Google Scholar]
- 23.Puttfarcken PS, Getz RL, Coyle JT. Kainic acid–induced lipid peroxidation: protection with butylated hydroxytoluene and U78517F in primary cultures of cerebellar granule cells. Brain Res. 1993;624:223–232. doi: 10.1016/0006-8993(93)90081-w. [DOI] [PubMed] [Google Scholar]
- 24.Shih YH, Wu SL, Chiou WF, Ku HH, Ko TL, Fu YS. Protective effects of tetramethylpyrazine on kainate-induced excitotoxicity in hippocampal culture. NeuroReport. 2002;13:515–519. doi: 10.1097/00001756-200203250-00032. [DOI] [PubMed] [Google Scholar]
- 25.Tsai TH, Liang C. Pharmcokinetics of tetramethylpyrazine in rat blood and brain using microdialysis. Int J Pharm. 2001;216:61–66. doi: 10.1016/s0378-5173(01)00572-5. [DOI] [PubMed] [Google Scholar]
- 26.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 2nd ed. San Diego: Academic Press; 1986. [Google Scholar]
- 27.Gazitt Y, Erdos GW, Cohen RJ. Ultrastructural localization and fluctuation in the level of the proliferating cell nuclear antigen and myc oncoproteins in synchronized neuroblastoma cells. Cancer Res. 1993;53:1899–1905. [PubMed] [Google Scholar]
- 28.Armelin MCS, Stocco RC, Armelin HA. Control of rat C6 glioma cell proliferation: uncoupling of the inhibitory effects of hydrocortisone hormone in suspension and monolayer cultures. J. Cell Biol. 1983;97:455–458. doi: 10.1083/jcb.97.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bignami A, Swanson J, Dahl D. GFA expression in aggregating cultures of rat C6 glioma. Experientia. 1979;35:1170–1171. doi: 10.1007/BF01963267. [DOI] [PubMed] [Google Scholar]
- 30.Labrakakis C, Patt S, Hartmann J, Kettenmann H. Glutamate receptor activation can trigger electrical activity in human glioma cells. Eur J Neurosci. 1998;10:2153–2162. doi: 10.1046/j.1460-9568.1998.00226.x. [DOI] [PubMed] [Google Scholar]
- 31.Arcella A, Carpinelli G, Battaglia G, et al. Pharmacological blockade of group II metabotropic glutamate receptors reduces the growth of glioma cells in vivo. Neuro-oncology. 2005;7:236–245. doi: 10.1215/S1152851704000961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gasson JC, Golde DW, Kaufman SE, et al. Molecular characterization and expression of the gene encoding human erythroid-potentiating activity. Nature. 1985;315:768–771. doi: 10.1038/315768a0. [DOI] [PubMed] [Google Scholar]
- 33.Bertaux B, Hornebeck W, Eisen AZ, Dubertret L. Growth stimulation of human keratinocytes by tissue inhibitor of metalloproteinases. J Invest Dermatol. 1991;97:679–685. doi: 10.1111/1523-1747.ep12483956. [DOI] [PubMed] [Google Scholar]
- 34.Yoshiji H, Harris SR, Raso E, et al. Mammary carcinoma cells over-expressing tissue inhibitor of metalloproteinases-1 show enhanced vascular endothelial growth factor expression. Int J Cancer. 1998;75:81–87. doi: 10.1002/(sici)1097-0215(19980105)75:1<81::aid-ijc13>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 35.Groft LL, Muzik H, Rewcastle NB, et al. Differential expression and localization of TIMP-1 and TIMP-4 in human gliomas. Br J Cancer. 2001;85:55–63. doi: 10.1054/bjoc.2001.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pang PK, Shan JJ, Chiu KW. Tetramethylpyrazine, a calcium antagonist. Planta Medica. 1996;62:431–435. doi: 10.1055/s-2006-957933. [DOI] [PubMed] [Google Scholar]
- 37.Sheu JR, Kan YC, Hung WC, Lin CH, Yen MH. The antiplatelet activity of tetramethylpyrazine is mediated through activation of NO synthase. Life Sci. 2000;67:937–947. doi: 10.1016/s0024-3205(00)00686-x. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Z, Wei T, Hou J, Li G, Yu S, Xin W. Iron-induced oxidative damage and apoptosis in cerebellar granule cells: attenuation by tetramethylpyrazine and ferulic acid. Eur J Pharmacol. 2003;467:41–47. doi: 10.1016/s0014-2999(03)01597-8. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Z, Wei T, Hou J, Li G, Yu S, Xin W. Tetramethylpyrazine scavenges superoxide anion and decreases nitric oxide production in human polymorphonuclear leukocytes. Life Sci. 2003;72:2465–2472. doi: 10.1016/s0024-3205(03)00139-5. [DOI] [PubMed] [Google Scholar]
- 40.Feng J, Liu R, Wu G, Tang S. Effects of tetramethylpyrazine on the release of PGI2 and TXA2 in the hypoxic isolated rat heart. Mol Cell Biochem. 1997;167:153–158. doi: 10.1023/a:1006837606488. [DOI] [PubMed] [Google Scholar]
- 41.Zhao H, Dong X, Wang X, et al. Studies on single-cell adhesion probability between lymphocytes and endothelial cells with micropipette technique. Microvasc Res. 2002;63:218–226. doi: 10.1006/mvre.2001.2390. [DOI] [PubMed] [Google Scholar]
- 42.Hsiao G, Chen YC, Lin JH, et al. Inhibitory mechanisms of tetramethylpyrazine in middle cerebral artery occlusion (MCAO)-induced focal cerebral ischemia in rats. Planta Medica. 2006;72:411–417. doi: 10.1055/s-2005-917242. [DOI] [PubMed] [Google Scholar]
- 43.Kao TK, Ou YC, Kuo JS, et al. Neuroprotection by tetramethylpyrazine against ischemic brain injury in rats. Neurochem Int. 2006;48:166–176. doi: 10.1016/j.neuint.2005.10.008. [DOI] [PubMed] [Google Scholar]