Abstract
Brain metastases (BM) are among the most devastating and debilitating complications of melanoma. This retrospective study was conducted to gain a better understanding of patient and disease characteristics that have the greatest impact on overall survival in melanoma patients with BM; therapeutic interventions were also assessed. The records of all patients diagnosed with cutaneous melanoma and BM who were seen at Memorial Sloan-Kettering Cancer Center between 1991 and 2001 were retrospectively reviewed. A variety of factors, including age at diagnosis of stage IV disease, gender, race, disease stage at diagnosis, presence of BM at diagnosis of stage IV disease, neurologic symptoms, radiographic findings, number of BM, status and site(s) of extracranial metastasis, and treatment modalities, were analyzed for correlation with overall survival using univariate and multivariate Cox regression models. The records of 355 patients with BM were included in the analysis. On univariate analysis, seven patient and disease characteristics were significantly associated with poorer survival: age > 65 years, extracranial metastases, BM at stage IV diagnosis, neurologic symptoms, four or more BM, hydrocephalus, and leptomeningeal metastases. Of these, age, extracranial metastasis, neurologic symptoms, and number of BM were significantly associated with poorer survival in a multivariate analysis. Multivariate analysis of treatment modalities suggested that patients who had surgery, radiosurgery, or chemotherapy with temozolomide had improved survival outcomes, although this analysis has limitations. The prognostic factors identified in this retrospective study should be considered when making treatment decisions for patients with BM and used as stratification factors in future clinical trials.
Keywords: brain metastases, Cox regression model, melanoma, prognosis, survival
Melanoma is the third most common tumor to metastasize to the brain. The reported incidence of brain metastases (BM) is 10% to 40%, but many patients may have subclinical BM, as evidenced by a higher reported incidence at autopsy (12%–73%) and the frequent discovery of asymptomatic BM on brain imaging studies.1–3 The median survival for patients with untreated BM is only weeks. With treatment, most patients will live less than 1 year from diagnosis of BM, but a few long-term survivors have been reported.1,3,4–12 Historically, the majority of patients with melanoma BM die a neurologic death, although the incidence varies depending on the extent of extracranial metastases.1–3,8,9,12–17 The reason for this dismal outcome is the relative resistance of melanoma to radiation and chemotherapy. Treatment approaches for patients with BM include surgery, whole-brain radiotherapy (WBRT), radiosurgery (RS), and systemic chemotherapy, although systemic chemotherapy has historically had a limited impact on survival. The selection of treatment modality or combined modalities is often based on factors unique to each individual patient and his or her disease. In general, the goal of therapy is to stabilize intracranial disease and improve quality of life.
To better understand the prognostic factors that influence survival in patients with melanoma and BM, we conducted a retrospective analysis of patient- and disease-related factors. Many of these prognostic factors have not been evaluated previously, and their impact on survival is unclear. A better understanding of these factors may allow for more appropriate patient stratification in clinical trials and may improve disease management and treatment outcome.
Materials and Methods
This retrospective study was approved by the Memorial Sloan-Kettering Cancer Center (MSKCC) Institutional Review Board. Data were extracted from the Department of Neurology and the Melanoma Disease Management Team database. All patients were seen at MSKCC between January 1991 and December 2001, but may not have received treatment there. Patients were excluded from the analysis if medical records were incomplete; patients not treated at MSKCC were included only if sufficient information was available about their disease history. Patients with American Joint Committee on Cancer (AJCC) stage IV melanoma (any T, any N, M1a, M1b, M1c: distant skin, lymph node, lung, or visceral metastases)18 and a BM at any time after diagnosis of melanoma and until death were included. Medical records were reviewed for the following: patient age at diagnosis of stage IV disease, gender, and race; date of initial melanoma diagnosis and AJCC stage at diagnosis; date of diagnosis of stage IV melanoma; systemic therapy (chemotherapy, biologic therapy, and/or immunotherapy) before and after stage IV diagnosis; number of treatments after diagnosis of stage IV disease; presence of BM at or after diagnosis of stage IV disease; presence and type of neurologic symptoms at diagnosis of BM; number of BM; evidence of hemorrhage; hydrocephalus; concomitant leptomeningeal metastases (LM); status of extracranial metastases; type of treatment for BM; and overall survival. Radiographic findings were based primarily on MRI and CT reports; films were reviewed if there was a radiographic question and they were available.
These factors were analyzed for correlation with overall survival using univariate and multivariate Cox regression models.19 Survival curves were estimated using the Kaplan-Meier method.20
Results
Patient and Disease Characteristics
We identified 1,114 patients with AJCC stage IV melanoma, of whom 355 had one or more BM. Table 1 summarizes patient and disease characteristics for the 355 patients (217 men and 138 women) who were analyzed. Patients included in this analysis were treated at MSKCC between 1991 and 2001 in an era when brain MRI was the standard imaging modality. Median age at diagnosis of stage IV melanoma was 52.6 years (range, 9–85 years), and 66% of patients had AJCC stage I or II melanoma18 at initial diagnosis. Brain metastases were present at diagnosis of stage IV disease in 49% of patients, and the remaining 51% developed BM at a median of 7 months (range, <1 to 52 months) after stage IV diagnosis. The median time from initial diagnosis of cutaneous melanoma to development of BM was 2.7 years (range, 0–31.5 years). Median survival time from detection of BM was 5.2 months (range, 0.1–155 months). Median follow-up among surviving patients was 18.4 months (range, 0.23–155 months), and 92% of patients were deceased at the time of this analysis.
Table 1.
Patient and disease characteristics (n = 355)
| n | % | |
|---|---|---|
| Gender | ||
| Male | 217 | 61.1 |
| Female | 138 | 38.9 |
| AJCC stage at melanoma diagnosis | ||
| I–II | 233 | 65.6 |
| III | 64 | 18.0 |
| IV | 58 | 16.3 |
| Race | ||
| Caucasian | 349 | 98.3 |
| Hispanic | 5 | 1.4 |
| African American | 1 | 0.3 |
| Brain metastasis at diagnosis of stage IV | ||
| No | 181 | 51.0 |
| Yes | 174 | 49.0 |
| Neurologic symptoms at diagnosis of brain metastasis | ||
| Asymptomatic | 116 | 32.7 |
| Focal | 107 | 30.1 |
| Nonfocal | 88 | 24.8 |
| Seizures | 39 | 11.0 |
| Unknown | 5 | 1.5 |
| Extracranial metastasis at diagnosis of brain metastasis | ||
| No | 57 | 16.1 |
| Yes | 296 | 83.4 |
| Unknown | 2 | 0.5 |
| Parenchymal metastasis | ||
| No | 7 | 2.0 |
| Yes | 346 | 97.5 |
| Unknown | 2 | 0.5 |
| Number of parenchymal brain metastases | ||
| 0 | 7 | 2.0 |
| 1 | 128 | 36.1 |
| 2–3 | 85 | 23.9 |
| ⩾4 | 130 | 36.6 |
| Unknown | 5 | 1.4 |
| Hemorrhagic brain metastasis | ||
| Yes | 123 | 34.6 |
| No | 219 | 61.7 |
| Unknown | 13 | 3.7 |
| Leptomeningeal metastasis | ||
| Yes | 40 | 11.3 |
| No | 315 | 88.7 |
| Hydrocephalus | ||
| Yes | 26 | 7.3 |
| No | 313 | 88.2 |
| Unknown | 16 | 4.5 |
| Lung metastasis | ||
| Yes | 201 | 56.6 |
| No | 154 | 43.4 |
| Visceral/bone metastasis | ||
| Yes | 135 | 38.0 |
| No | 220 | 62.0 |
| Lymph node/soft tissue metastasis | ||
| Yes | 190 | 53.5 |
| No | 165 | 46.5 |
Abbreviation: AJCC, American Joint Committee on Cancer.
The majority (67%) of patients presented with neurologic symptoms at diagnosis of BM. Radiographic imaging revealed that 88% of patients had parenchymal BM, 2% had LM, 9% had both, and the type of intracranial metastases was not described for 1% of patients (Table 1). At the time of BM diagnosis, 83% of patients had extracranial metastases. Approximately one-third of patients had intratumoral hemorrhage. Of the 107 patients with evidence of intratumoral hemorrhage on MRI at initial diagnosis, 88 (82%) had neurologic symptoms (seizure in 19 and other neurologic symptoms in 69). Among patients with hydrocephalus (n = 26), 10 (38%) had radiographic evidence of LM. Among the 40 patients with LM, 7 (18%) had no parenchymal BM, 3 (8%) had one lesion, 8 (20%) had two or three lesions, and 22 (55%) had four or more lesions.
Treatment
Before the diagnosis of BM, 30% of patients had received systemic therapy for melanoma (Table 2). At some point after the diagnosis of melanoma, 82% of patients had received systemic therapy, and 76% of the 355 patients had received chemotherapy (specifically, 32% of the 355 patients received temozolomide). Among 106 patients treated with temozolomide beginning in 1999, at least 52 (49%) patients received a dose-dense regimen consisting of 75 mg/m2/day continuously for 6 weeks every 8-week cycle, as opposed to the standard 5-day regimen (150–200 mg/m2 × 5 days every 28-day cycle). Temozolomide was not used in conjunction with radiation therapy.
Table 2.
Treatment (n = 355)
| Modality | n | % |
|---|---|---|
| Systemic treatment before stage IV diagnosis | ||
| Yes | 108 | 30.4 |
| No | 237 | 66.8 |
| Unknown | 10 | 2.8 |
| Surgical resection (i.e., craniotomy) | ||
| Yes | 126 | 35.5 |
| No | 229 | 64.5 |
| Whole-brain radiotherapy | ||
| Yes | 190 | 53.5 |
| No | 154 | 43.4 |
| Unknown | 11 | 3.1 |
| Radiosurgery | ||
| Yes | 78 | 22.0 |
| No | 275 | 77.4 |
| Unknown | 2 | 0.5 |
| Number of systemic therapies for stage IV disease | ||
| 0 | 72 | 20.3 |
| 1 | 130 | 36.6 |
| 2 | 72 | 20.3 |
| 3 | 65 | 18.3 |
| Unknown | 16 | 4.5 |
| Chemotherapy ever | ||
| No | 71 | 20.0 |
| Yes | 268 | 75.5 |
| Unknown | 16 | 4.5 |
| Chemotherapy for stage IV disease | ||
| No | 82 | 23.1 |
| Yes | 256 | 72.1 |
| Unknown | 17 | 4.8 |
| Systemic treatment ever | ||
| No | 50 | 14.1 |
| Yes | 290 | 81.7 |
| Temozolomide treatment ever | ||
| No | 236 | 66.5 |
| Yes | 113 | 31.8 |
| Unknown | 6 | 1.7 |
Nonchemotherapy treatment of BM consisted of surgery, WBRT, or RS. It is important to note that treatment with these modalities was on a continuum; therefore, order and timing were not standardized among patients, thereby limiting the interpretation of the impact of these treatments on survival outcome. Of the 126 (36%) patients who had surgery, 76 (60%) had a single lesion, 30 (24%) had two or three lesions, and 19 (15%) had four or more lesions. Of the 49 patients with one or more BM who underwent surgery, 19 had evidence of hemorrhage on MRI at initial diagnosis, 4 presented with seizure, and 21 presented with neurologic symptoms. The majority of these patients underwent surgery for symptom control. Five other patients developed hemorrhage and/or neurologic symptoms after the diagnosis of BM and underwent surgery at that time. Surgical resection was combined with other treatment modalities in 90 (25%) of 355 patients and was the only treatment in 36 (10%) of 355 patients. WBRT was administered to 190 (54%) patients; it was combined with other modalities in 90 (25%) patients and was the only treatment in 100 (28%) patients. The median and most common dose was 3,000 cGy (range, 400–5,000 cGy). RS was used in 78 (22%) patients; it was combined with other modalities in 52 (15%) patients and was the only treatment in 26 (7%) patients. Of the 78 patients treated with RS, 35 (45%) had one lesion, 35 (45%) had two or three lesions, and 6 (8%) had four or more lesions. The median dose was 1,850 cGy (range, 1,350–3,750 cGy), and the most common doses were 1,800 cGy and 2,100 cGy.
Univariate Analysis
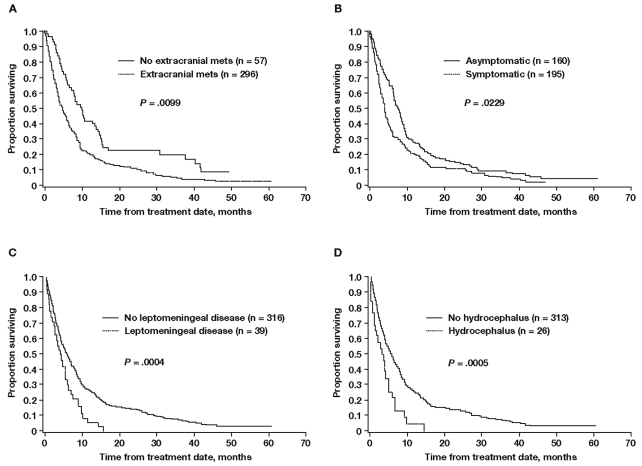
In the univariate analysis, of the 12 patient or disease characteristics assessed, 7 were associated with significantly shorter survival, including age > 65 years, presence of extracranial metastases, presence of BM at stage IV diagnosis, presence of neurologic symptoms at diagnosis of BM, four or more BM, hydrocephalus, and LM (Table 3). Survival curves for patients with or without extracranial metastases, neurologic symptoms, LM, or hydrocephalus are shown in Fig. 1.
Table 3.
Univariate results: overall survival by patient and disease characteristics
| Factor | n | Median | 95% CI | Log-Rank p Value |
|---|---|---|---|---|
| Age, years | ||||
| ⩽65 | 263 | 6.2 | 5.2, 6.9 | 0.0044 |
| >65 | 92 | 3.3 | 2.6, 5.1 | |
| Gender | ||||
| Female | 138 | 5.2 | 4.3, 6.5 | 0.3575 |
| Male | 217 | 5.9 | 4.3, 6.9 | |
| Extracranial disease | ||||
| No | 57 | 10.0 | 7.3, 13.8 | 0.0002 |
| Yes | 296 | 5.0 | 4.1, 6.0 | |
| Brain metastasis at diagnosis of stage IV disease | ||||
| No | 181 | 12.5 | 10.4, 14.4 | 0.0099 |
| Yes | 174 | 8.3 | 6.9, 9.4 | |
| Neurologic symptoms | ||||
| Asymptomatic | 116 | 7.4 | 6.6, 9.4 | 0.0227 |
| Symptomatic | 234 | 4.3 | 4.0, 5.2 | |
| Number of brain metastases | ||||
| 1–3 | 213 | 8.0 | 6.6, 9.5 | <0.0001 |
| ⩾4 | 130 | 3.3 | 2.6, 4.0 | |
| Hydrocephalus | ||||
| No | 313 | 5.9 | 4.8, 6.7 | 0.0005 |
| Yes | 26 | 3.8 | 1.7, 5.1 | |
| Leptomeningeal metastasis | ||||
| No | 315 | 6.0 | 5.0, 6.7 | 0.0004 |
| Yes | 40 | 4.0 | 2.5, 5.2 | |
| Hemorrhagic lesion | ||||
| No | 219 | 5.2 | 4.1, 6.2 | 0.8551 |
| Yes | 123 | 6.0 | 4.8, 8.0 | |
| Parenchymal metastasis | ||||
| No | 7 | 1.3 | 0.8, 6.1 | 0.0055 |
| Yes | 346 | 5.7 | 4.8, 6.6 | |
| AJCC stage at initial diagnosis | ||||
| I–II | 233 | 5.9 | 5.0, 6.7 | 0.7855 |
| III | 64 | 3.2 | 2.7, 6.7 | |
| IV | 58 | 6.1 | 4.6, 8.7 | |
| Brain metastasis only | ||||
| No | 291 | 5.0 | 4.1, 5.9 | 0.0003 |
| Yes | 64 | 9.7 | 6.7, 13.0 | |
| EC metastasis and age, years | ||||
| No EC metastasis, age ⩽ 65 | 40 | 10.2 | 7.9, 14.7 | <0.0001 |
| No EC metastasis, age > 65 | 17 | 8.3 | 3.1, 15.1 | |
| EC metastasis, age ⩽ 65 | 221 | 5.3 | 4.4, 6.5 | |
| EC metastasis, age > 65 | 75 | 2.9 | 1.9, 4.3 | |
Abbreviations: CI, confidence interval; AJCC, American Joint Committee on Cancer; EC, extracranial.
Fig. 1.
Kaplan-Meier estimate of survival for patients with or without extracranial metastases (A), with or without neurologic symptoms (B), with or without leptomeningeal metastases (C), and with or without hydrocephalus (D). Abbreviation: mets, metastases.
Univariate analysis showed that patients treated with temozolomide (either for systemic melanoma or for BM) and those receiving surgery or RS for the treatment of BM had longer survival (Table 4). Median survival was 8 months for patients treated with temozolomide compared with 4 months if not treated with temozolomide (p = 0.0009) (Table 4). Because temozolomide became commercially available only in 1999, the majority of patients in this cohort were not treated with temozolomide. Among patients who had surgical resection of BM, median survival was 9 months compared with 4 months for those who did not (p < 0.0001) (Table 4). Likewise, patients who received RS had a median survival of 10 months compared with 4 months for patients who did not (p < 0.0001) (Table 4). Patients treated with WBRT had a median survival of 4 months compared with 2 months for patients who received no treatment for BM. Outcomes based on treatment (either single modality or combination) are listed in Table 5.
Table 4.
Univariate analysis: overall survival when one brain-metastasis-directed treatment was part of care
| Treatment | n | Median |
|---|---|---|
| Surgical resection | ||
| No | 229 | 3.9 |
| Yes | 126 | 9.3 |
| Radiosurgery | ||
| No | 275 | 4.3 |
| Yes | 78 | 10.0 |
| Temozolomide | ||
| No | 236 | 4.1 |
| Yes | 113 | 7.9 |
| Whole-brain radiotherapy | ||
| No | 154 | 4.5 |
| Yes | 190 | 6.1 |
| Systemic therapy ever | ||
| No | 50 | 3.6 |
| Yes | 290 | 5.8 |
| Chemotherapy ever | ||
| No | 71 | 4.3 |
| Yes | 268 | 5.9 |
Table 5.
Survival when one or more treatments of brain metastases were part of care (n = 355)
| Treatment | n | % | Median Survival (Months) |
|---|---|---|---|
| None | 83 | 23.3 | 2.04 |
| WBRT alone | 100 | 28.2 | 3.98 |
| RS alone | 26 | 7.3 | 9.87 |
| Surgery alone | 36 | 10.1 | 8.16 |
| WBRT + RS | 20 | 5.6 | 9.44 |
| Surgery + WBRT | 58 | 16.3 | 8.81 |
| Surgery + RS | 20 | 5.6 | 13.75 |
| Surgery + WBRT + RS | 12 | 3.4 | 10.2 |
Abbreviations: WBRT, whole-brain radiotherapy; RS, radiosurgery.
Multivariate Analysis
Multivariate analysis demonstrated that four of the seven patient or disease characteristics were associated with shorter survival, including age > 65 years, presence of extracranial metastases, presence of neurologic symptoms at diagnosis of BM, and having four or more BM (Table 6). In addition, patients treated with surgery, RS, temozolomide, or systemic therapy at any time during the course of disease had the longest survival. As indicated, these treatments may have been used at any point after the diagnosis of BM, so the true benefit cannot be determined by this analysis.
Table 6.
Multivariate analysis: overall survival
| Factor | Risk ratio | 95% CI | p Value |
|---|---|---|---|
| Variable | |||
| Age > 65 years | 1.53 | 1.17, 1.99 | 0.0017 |
| Extracranial metastases | 2.13 | 1.52, 2.98 | <0.0001 |
| Neurologic symptoms | 1.64 | 1.26, 2.12 | 0.0002 |
| Number of brain metastases(1–3 vs.⩾4) | 1.85 | 1.39, 2.46 | <0.0001 |
| Treatment as part of care | |||
| Surgical resection | 0.56 | 0.43, 0.75 | <0.0001 |
| Radiosurgery | 0.69 | 0.50, 0.94 | 0.0191 |
| Temozolomide | 0.69 | 0.53, 0.88 | 0.0034 |
| Systemic treatment ever | 0.70 | 0.49, 1.01 | 0.0538 |
Abbreviation: CI, confidence interval.
Discussion
Our goal was to identify prognostic factors for survival in patients with stage IV melanoma and BM. Some prognostic factors have previously been reported for this population.3,6,9 However, the present analysis is based on a more contemporary patient population, and the findings are unique in several respects compared to previous reports. We selected 1991 as the start date for this study because brain MRI scan became the standard imaging modality for BM at about that time, whereas earlier studies used head CT scans or autopsy results. Similar to previous studies, the present study was retrospective, and although patients received a wide variety of treatments and extracranial disease was variable, the outcomes of the regression analysis are similar to those of previous reports. Patients in the present analysis were similar to those included in published studies, although median age was slightly older.1,2,5–7,10–13,15,21–23 In most respects, the survival outcomes in this series are consistent with published studies. The reported median time from diagnosis of melanoma (AJCC stage I or II)18 to BM is 1.4–4 years,1,3,5,8,9,11,12 which is consistent with the median of 2.7 years that we observed. In the present study, median survival from diagnosis of BM was 5.2 months overall and 8.3 months in patients with BM at diagnosis of stage IV disease, similar to results from other series. The reported median survival from diagnosis of stage IV melanoma regardless of disease site is 1–24 months,2,10,24 but patients who develop BM early have a shorter median survival than those who develop BM later.24
Only 16% of patients in the present analysis did not have extracranial metastases, and this factor greatly affected survival in both univariate and multivariate analyses, consistent with other studies.1,3,6,7,9,15 More than half of the patients in the present study had lung, lymph node, or subcutaneous involvement, and about 40% had visceral or bone metastases. In a retrospective analysis of palliative WBRT reported by Morris et al.,23 patients without extracranial disease had a median survival of 3.5 months versus 1.1 months for patients with extracranial metastases, and the number of extracranial metastatic sites correlated inversely with survival. Similarly, in the present study, median survival was 10 months for patients with BM only versus 5 months for patients with concurrent extracranial metastases. These data suggest that extensive extracranial metastases are associated with a decrease in survival. However, the cause of death was not documented in our database, so we are unable to comment on whether death was from a neurologic or systemic cause. We can infer that patients with only BM died a neurologic death, whereas those with BM and systemic disease likely died due to systemic and brain progression.
The results of the present analysis are also consistent with the Radiation Therapy Oncology Group recursive partitioning analysis (RPA) classification for patients with BM based on Karnofsky performance status (< or ⩾ 70%), age (< or ⩾ 65 years), and status of extracranial disease (controlled or uncontrolled).25,26 When the RPA classification was applied to patients with BM from melanoma, median survival was 5–10 months for class I, 2.5–5.9 months for class II, and 0.75–2.5 months for class III.4,7,23 In the present analysis, although information on Karnofsky performance status was not routinely available, outcome based on age and extracranial metastases demonstrated a median survival of 10 months for patients ⩽ 65 years of age with no extracranial metastases (similar to RPA class I) and 3 months for patients > 65 years of age with extracranial metastases (similar to RPA class III) (Table 4).
Our analysis has also confirmed that neurologic symptoms at diagnosis of BM are significant predictors of shorter survival.14,16,27 Interestingly, intratumoral hemorrhage had no significant effect on survival in the present analysis. Although hemorrhage can be fatal, in our series, these patients lived slightly longer than patients without hemorrhage, possibly because of earlier detection of BM. The majority of patients with hemorrhage underwent surgical resection for symptom relief regardless of the total number of BM. Intratumoral hemorrhage has been reported to occur in 29% to 40% of melanoma patients with BM based on imaging studies12,13,16 and in up to 50% of patients by pathologic review.28 In one series, hemorrhage was the cause of death in 20% of patients.2
Hydrocephalus was noted in 26 (7%) patients, and 10 of these patients also had LM on imaging. Patients with hydrocephalus had significantly shorter median survival compared with those who did not (4 vs. 6 months), possibly due to LM not seen on imaging and not accounted for by a posterior fossa BM. Leptomeningeal disease was also a significant negative prognostic factor in the univariate analysis but not in the multivariate analysis. LM was observed in 40 (11%) patients based on MRI findings, which undoubtedly underestimates the true incidence. The combination of LM and BM has been reported to be a significant adverse prognostic factor despite treatment with WBRT.16,23 In our series, LM was correlated with an increased number of parenchymal lesions, which may explain why LM was a significant prognostic factor on univariate analysis.
Number of BM was one of the strongest prognostic factors in both univariate and multivariate analyses. Median survival decreased from 8 months in patients with one to three lesions to only 3 months for patients with four or more lesions. This finding may account for the poor prognosis associated with melanoma BM, because up to 86% of patients have multiple lesions at diagnosis,2,3,6–9,11,13,22 and survival has been shown to decrease inversely with increased number of BM and increased tumor burden.3,4,9,12,22 However, in the present study, the outcome for patients with two or three lesions was not worse than for patients with one lesion. This may reflect recent improvements in surgical and RS techniques.
Our data on treatment suggest that BM-directed therapies do improve survival outcomes compared with supportive care only, similar to data from other reports.3,6,29 Patients treated with surgery and RS as part of BM-directed therapy had the longest survival. However, a selection bias most certainly contributed to this result, in that patients treated with surgery and/or RS likely had a lower intracranial tumor burden and controlled or absent extracranial disease and were likely healthier overall compared with patients receiving WBRT or supportive care. Patients with better RPA class do better than others, confirming the fact that having fewer “negative” factors leads to better outcomes. Meier et al.22 reported that all treatments for BM, including WBRT, had a significant positive effect on survival. However, the benefit of WBRT continues to be debated. Our data suggest that WBRT alone for melanoma BM provided only a marginal survival benefit over no therapy for BM. Some authors have suggested that WBRT may delay central nervous system progression by up to fourfold3,5,14,21 and may delay development of LM.21 Therefore, in select patients, WBRT may provide some benefit. For example, it has been suggested that patients with limited or no extracranial metastases may have improved survival when treated with WBRT after surgery.3,5,6,14,21 Hagen et al.21 reported that patients who had resection of a single BM had a median survival of 6.4 months, which was increased to 8.3 months in patients who received adjuvant WBRT. In general, patients with a single BM and no extracranial metastases appear to benefit the most from aggressive local treatment of BM. The degree of surgical resection for a given lesion has also been shown to correlate with survival,10,12,14 and a second surgical resection can further improve survival.16
Several reports have also demonstrated the important role of RS in the management of melanoma BM, yielding good disease control rates and median survivals between 7 and 9 months.9,15,17,30,31 Seung et al.31 reported similar median survivals whether a single or multiple lesions were treated (~8 months), and median time to development of new BM was approximately 6 months. The role of WBRT in conjunction with RS is controversial. Some studies have shown that WBRT may decrease development of new BM,9 whereas other studies have not.17,30,32 In patients with a single BM, RS achieves excellent disease control, and median survivals up to 22 months have been reported without use of WBRT.15 The Eastern Oncology Cooperative Group found a median survival of 8.3 months for patients treated with RS alone for radioresistant BM (renal cell cancer, melanoma, and sarcoma), with no difference in outcome based on histology.33 Their survival data are similar to ours, but they did have a 50% failure in the brain at 6 months, suggesting that RS alone be used in select cases.
The data for systemic chemotherapy for the treatment of BM are limited and show that it is largely ineffective, with median survivals of 3–4.5 months.34,35 Recent studies have shown that temozolomide induced objective responses in patients with BM when used as a single agent or in combination with thalidomide or docetaxel.36–40 Meier et al.22 also reported that chemotherapy, including treatment with temozolomide, prolonged survival in patients with melanoma BM, and Paul et al.41 demonstrated that temozolomide significantly decreased the incidence of BM compared with dacarbazine when used as primary therapy for stage IV melanoma. In our analysis, the use of temozolomide appeared to improve survival, but further studies are needed to confirm this finding as well as define the optimal dose, schedule, and combinations for the treatment of BM.
In summary, the present analysis confirmed many of the prognostic factors previously reported by others and defined additional new factors. These data can be used to optimize patient care and to guide selection and stratification of patients in future clinical trials. The retrospective nature of this study limits what can be inferred about individual therapies, as timing of treatment was variable and response rates and time to progression were not consistently documented. Despite these limitations, our survival data are similar to those published in the literature. We recommend that aggressive treatment be used for patients with limited or no extracranial disease and for those with one to three BM. In these patients, surgery or RS, alone or in combination, may have the greatest impact on survival. However, the majority of melanoma patients present with multiple BM and extensive concurrent extracranial disease. For patients with four or more BM, treatment with RS, with or without WBRT, may be an alternative approach, as WBRT alone appears to provide only marginal benefit. Patients with hemorrhage or neurologic symptoms who will likely survive for at least a few months should receive palliative surgery. Temozolomide has been shown to be an active agent in BM from melanoma and should be considered as part of therapy for these patients. Use of the RPA classification should help to determine the appropriate level of aggressiveness, and the prognostic index devised by Morris et al.23 may be helpful for stratifying RPA class II patients. Ultimately, treatment decisions must be tailored to the individual patient, and a clear understanding of patient and disease characteristics that influence survival prognosis can be extremely helpful for determining the best treatment approach.
Acknowledgment
This study was supported by Schering-Plough International.
References
- 1.Amer MH, Al-Sarraf M, Baker LH, Vaitkevicius VK. Malignant melanoma and central nervous system metastases: incidence, diagnosis, treatment and survival. Cancer. 1978;42:660–668. doi: 10.1002/1097-0142(197808)42:2<660::aid-cncr2820420237>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 2.Patel JK, Didolkar MS, Pickren JW, Moore RH. Metastatic pattern of malignant melanoma. A study of 216 autopsy cases. Am J Surg. 1978;135:807–810. doi: 10.1016/0002-9610(78)90171-x. [DOI] [PubMed] [Google Scholar]
- 3.Sampson JH, Carter JH, Jr, Friedman AH, Seigler HF. Demographics, prognosis, and therapy in 702 patients with brain metastases from malignant melanoma. J Neurosurg. 1998;88:11–20. doi: 10.3171/jns.1998.88.1.0011. [DOI] [PubMed] [Google Scholar]
- 4.Buchsbaum JC, Suh JH, Lee SY, Chidel MA, Greskovich JF, Barnett GH. Survival by Radiation Therapy Oncology Group recursive partitioning analysis class and treatment modality in patients with brain metastases from malignant melanoma: a retrospective study. Cancer. 2002;94:2265–2272. doi: 10.1002/cncr.10426. [DOI] [PubMed] [Google Scholar]
- 5.Ellerhorst J, Strom E, Nardone E, McCutcheon I. Whole brain irradiation for patients with metastatic melanoma: a review of 87 cases. Int J Radiat Oncol Biol Phys. 2001;49:93–97. doi: 10.1016/s0360-3016(00)01355-9. [DOI] [PubMed] [Google Scholar]
- 6.Fife KM, Colman MH, Stevens GN, et al. Determinants of outcome in melanoma patients with cerebral metastases. J Clin Oncol. 2004;22:1293–1300. doi: 10.1200/JCO.2004.08.140. [DOI] [PubMed] [Google Scholar]
- 7.Harrison BE, Johnson JL, Clough RW, Halperin EC. Selection of patients with melanoma brain metastases for aggressive treatment. Am J Clin Oncol. 2003;26:354–357. doi: 10.1097/01.COC.0000020963.71379.FE. [DOI] [PubMed] [Google Scholar]
- 8.Mendez IM, Del Maestro RF. Cerebral metastases from malignant melanoma. Can J Neurol Sci. 1988;15:119–123. doi: 10.1017/s0317167100027463. [DOI] [PubMed] [Google Scholar]
- 9.Mori Y, Kondziolka D, Flickinger JC, Kirkwood JM, Agarwala S, Lunsford LD. Stereotactic radiosurgery for cerebral metastatic melanoma: factors affecting local disease control and survival. Int J Radiat Oncol Biol Phys. 1998;42:581–589. doi: 10.1016/s0360-3016(98)00272-7. [DOI] [PubMed] [Google Scholar]
- 10.Overett TK, Shiu MH. Surgical treatment of distant metastatic melanoma. Indications and results. Cancer. 1985;56:1222–1230. doi: 10.1002/1097-0142(19850901)56:5<1222::aid-cncr2820560544>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 11.Saha S, Meyer M, Krementz ET, et al. Prognostic evaluation of intracranial metastasis in malignant melanoma. Ann Surg Oncol. 1994;1:38–44. doi: 10.1007/BF02303539. [DOI] [PubMed] [Google Scholar]
- 12.Zacest AC, Besser M, Stevens G, Thompson JF, McCarthy WH, Culjak G. Surgical management of cerebral metastases from melanoma: outcome in 147 patients treated at a single institution over two decades. J Neurosurg. 2002;96:552–558. doi: 10.3171/jns.2002.96.3.0552. [DOI] [PubMed] [Google Scholar]
- 13.Byrne TN, Cascino TL, Posner JB. Brain metastasis from melanoma. J Neurooncol. 1983;1:313–317. doi: 10.1007/BF00165714. [DOI] [PubMed] [Google Scholar]
- 14.Choi KN, Withers HR, Rotman M. Intracranial metastases from melanoma. Clinical features and treatment by accelerated fractionation. Cancer. 1985;56:1–9. doi: 10.1002/1097-0142(19850701)56:1<1::aid-cncr2820560102>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 15.Grob JJ, Regis J, Laurans R, et al. Radiosurgery without whole brain radiotherapy in melanoma brain metastases. Club de Cancérologie Cutanée. Eur J Cancer. 1998;34:1187–1192. doi: 10.1016/s0959-8049(98)00026-4. [DOI] [PubMed] [Google Scholar]
- 16.Wronski M, Arbit E. Surgical treatment of brain metastases from melanoma: a retrospective study of 91 patients. J Neurosurg. 2000;93:9–18. doi: 10.3171/jns.2000.93.1.0009. [DOI] [PubMed] [Google Scholar]
- 17.Yu C, Chen JC, Apuzzo ML, et al. Metastatic melanoma to the brain: prognostic factors after Gamma Knife radiosurgery. Int J Radiat Oncol Biol Phys. 2002;52:1277–1287. doi: 10.1016/s0360-3016(01)02772-9. [DOI] [PubMed] [Google Scholar]
- 18.Balch CM, Buzaid AC, Soong S-J, et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol. 2001;19:3635–3648. doi: 10.1200/JCO.2001.19.16.3635. [DOI] [PubMed] [Google Scholar]
- 19.Cox D. Regression models and life tables. J R Stat Soc (B) 1972;34:187–220. [Google Scholar]
- 20.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 21.Hagen NA, Cirrincione C, Thaler HT, DeAngelis LM. The role of radiation therapy following resection of single brain metastasis from melanoma. Neurology. 1990;40:158–160. doi: 10.1212/wnl.40.1.158. [DOI] [PubMed] [Google Scholar]
- 22.Meier S, Baumert BG, Maier T, et al. Survival and prognostic factors in patients with brain metastases from malignant melanoma. Onkologie. 2004;27:145–149. doi: 10.1159/000076903. [DOI] [PubMed] [Google Scholar]
- 23.Morris SL, Low SH, A’Hern RP, et al. A prognostic index that predicts outcome following palliative whole brain radiotherapy for patients with metastatic malignant melanoma. Br J Cancer. 2004;91:829–833. doi: 10.1038/sj.bjc.6602018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brand CU, Ellwanger U, Stroebel W, et al. Prolonged survival of 2 years or longer for patients with disseminated melanoma. An analysis of related prognostic factors. Cancer. 1997;79:2345–2353. [PubMed] [Google Scholar]
- 25.Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37:745–751. doi: 10.1016/s0360-3016(96)00619-0. [DOI] [PubMed] [Google Scholar]
- 26.Gaspar LE, Scott C, Murray K, Curran W. Validation of the RTOG recursive partitioning analysis (RPA) classification for brain metastases. Int J Radiat Oncol Biol Phys. 2000;47:1001–1006. doi: 10.1016/s0360-3016(00)00547-2. [DOI] [PubMed] [Google Scholar]
- 27.Koc M, McGregor J, Grecula J, Bauer CJ, Gupta N, Gahbauer RA. Gamma Knife radiosurgery for intracranial metastatic melanoma: an analysis of survival and prognostic factors. J Neurooncol. 2005;71:307–313. doi: 10.1007/s11060-004-2027-1. [DOI] [PubMed] [Google Scholar]
- 28.Kondziolka D, Bernstein M, Resch L, et al. Significance of hemorrhage into brain tumors: clinicopathological study. J Neurosurg. 1987;67:852–857. doi: 10.3171/jns.1987.67.6.0852. [DOI] [PubMed] [Google Scholar]
- 29.Madajewicz S, Karakousis C, West CR, Caracandas J, Avellanosa AM. Malignant melanoma brain metastases. Review of Roswell Park Memorial Institute experience. Cancer. 1984;53:2550–2552. doi: 10.1002/1097-0142(19840601)53:11<2550::aid-cncr2820531129>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 30.Radbill AE, Fiveash JF, Falkenberg ET, et al. Initial treatment of melanoma brain metastases using Gamma Knife radiosurgery: an evaluation of efficacy and toxicity. Cancer. 2004;101:825–833. doi: 10.1002/cncr.20447. [DOI] [PubMed] [Google Scholar]
- 31.Seung SK, Sneed PK, McDermott MW, et al. Gamma Knife radiosurgery for malignant melanoma brain metastases. Cancer J Sci Am. 1998;4:103–109. [PubMed] [Google Scholar]
- 32.Selek U, Chang EL, Hassenbusch SJ, III, et al. Stereotactic radiosurgical treatment in 103 patients for 153 cerebral melanoma metastases. Int J Radiat Oncol Biol Phys. 2004;59:1097–1106. doi: 10.1016/j.ijrobp.2003.12.037. [DOI] [PubMed] [Google Scholar]
- 33.Manon R, O’Neill A, Knisely J, et al. Eastern Cooperative Oncology Group. Phase II trial of radiosurgery for one to three newly diagnosed brain metastases from renal cell carcinoma, melanoma, sarcoma: an Eastern Cooperative Oncology Group study (E 6397) J Clin Oncol. 2005;23:8870–8876. doi: 10.1200/JCO.2005.01.8747. [DOI] [PubMed] [Google Scholar]
- 34.Franciosi V, Cocconi G, Michiara M, et al. Front-line chemotherapy with cisplatin and etoposide for patients with brain metastases from breast carcinoma, nonsmall cell lung carcinoma, or malignant melanoma: a prospective study. Cancer. 1999;85:1599–1605. [PubMed] [Google Scholar]
- 35.Kaba SE, Kyritsis AP, Hess K, et al. TPDC-FuHu chemotherapy for the treatment of recurrent metastatic brain tumors. J Clin Oncol. 1997;15:1063–1070. doi: 10.1200/JCO.1997.15.3.1063. [DOI] [PubMed] [Google Scholar]
- 36.Agarwala SS, Kirkwood JM, Gore M, et al. Temozolomide for the treatment of brain metastases associated with metastatic melanoma: a phase II study. J Clin Oncol. 2004;22:2101–2107. doi: 10.1200/JCO.2004.11.044. [DOI] [PubMed] [Google Scholar]
- 37.Bafaloukos D, Gogas H, Georgoulias V, et al. Temozolomide in combination with docetaxel in patients with advanced melanoma: a phase II study of the Hellenic Cooperative Oncology Group. J Clin Oncol. 2002;20:420–425. doi: 10.1200/JCO.2002.20.2.420. [DOI] [PubMed] [Google Scholar]
- 38.Bafaloukos D, Tsoutsos D, Fountzilas G, et al. The effect of temozolomide-based chemotherapy in patients with cerebral metastases from melanoma. Melanoma Res. 2004;14:289–294. doi: 10.1097/01.cmr.0000136707.60108.ab. [DOI] [PubMed] [Google Scholar]
- 39.Dvorak J, Melichar B, Zizka J, Hadzi-Nikolov D, Petera J. Complete response of multiple melanoma brain metastases after treatment with temozolomide. Onkologie. 2004;27:171–174. doi: 10.1159/000076908. [DOI] [PubMed] [Google Scholar]
- 40.Hwu WJ, Lis E, Menell JH, et al. Temozolomide plus thalidomide in patients with brain metastases from melanoma: a phase II study. Cancer. 2005;103:2590–2597. doi: 10.1002/cncr.21081. [DOI] [PubMed] [Google Scholar]
- 41.Paul MJ, Summers Y, Calvert AH, et al. Effect of temozolomide on central nervous system relapse in patients with advanced melanoma. Melanoma Res. 2002;12:175–178. doi: 10.1097/00008390-200204000-00011. [DOI] [PubMed] [Google Scholar]