Abstract
18F-Fluorodeoxyglucose (FDG) PET has become an important tool in the management of non-Hodgkin’s lymphoma (NHL), but its role in the evaluation of primary CNS lymphoma (PCNSL) has not been established. We investigated the ability of body FDG PET to detect systemic disease in the staging and restaging of PCNSL. The records of 166 PCNSL patients seen at Memorial Sloan-Kettering Cancer Center were examined. Forty-nine patients who underwent body FDG PET for staging of PCNSL were identified. Clinical data were reviewed to determine FDG PET results and their influence on therapy. Body FDG PET disclosed a systemic site of malignancy in 15% of patients. NHL was found in 11% of all patients, 7% of patients at diagnosis, and 27% of patients at CNS relapse. Four percent had a second systemic neoplasm. Workup with conventional staging did not reveal systemic disease, and in 8% of patients, body FDG PET was the only abnormal diagnostic exam suggestive of lymphoma. FDG PET findings altered patient treatment and resulted in additional chemotherapy, surgery, or radiotherapy. Our findings suggest that FDG PET may be more sensitive than conventional body staging and may disclose higher rates of concomitant systemic disease at PCNSL diagnosis. Body FDG PET may be an important noninvasive adjunct to conventional PCNSL staging, and its utility should be evaluated prospectively.
Keywords: FDG PET, primary CNS lymphoma
The imaging of tumor metabolism with PET has emerged as an important technique in the diagnostic and therapeutic assessment of cancer.1 18F-Fluorodeoxyglucose (FDG), used to assay cellular glucose uptake and utilization, is the most commonly employed and studied radiotracer in the management of non-Hodgkin’s lymphoma (NHL). A substantial body of evidence reinforces the value of metabolic imaging in NHL; studies have demonstrated the ability of FDG PET to assess postchemotherapy residual disease and to differentiate indolent from aggressive histologies.2–4 The use of FDG PET in NHL has recently been standardized and incorporated into treatment and assessment guidelines.5
Primary CNS lymphoma (PCNSL) is, by definition, an NHL restricted to the CNS. FDG PET has not been evaluated specifically in this rare subset of NHL. However, FDG PET’s role in systemic NHL implies several key potential uses in the staging evaluation of PCNSL,6 and it may have other important applications in the prognostication and treatment of PCNSL.7
The management of PCNSL consists of establishing diagnosis with tissue biopsy or cerebrospinal fluid cytology, staging for extent of disease, and treatment with chemoradiotherapy.8,9 Standard staging for PCNSL requires contrast-enhanced CT of the chest, abdomen, and pelvis (CAP) and bone marrow biopsy to exclude systemic lymphoma.10 Conventional staging evaluation in PCNSL identifies a systemic site of disease in about 4% of patients.11 However, recent studies suggest that subclinical systemic disease at diagnosis may be more frequent than previously recognized.12 The clinical significance of identifiable systemic disease is uncertain, but at least 5% of PCNSL patients relapse outside the CNS.13 The detection of systemic lymphoma at PCNSL diagnosis may provide important clues regarding the origin of this disease and the pathophysiology of relapse.
FDG PET has the potential of identifying and visualizing systemic involvement in patients with apparent PCNSL better than conventional anatomic imaging. This study sought to determine the utility of FDG PET in disclosing systemic foci of disease and to consider whether this test should be incorporated into the routine staging of PCNSL.
Materials and Methods
Patients
The records of 166 immunocompetent PCNSL patients evaluated in the Department of Neurology at Memorial Sloan-Kettering Cancer Center between January 2000 and August 2005 were reviewed in a study approved by the institutional review board. Forty-nine patients with a median age of 65 years (range, 35–80 years) who underwent 57 body FDG PET scans were identified using the electronic medical record. Clinical documents were reviewed to determine the reason for the study, results of FDG PET, and impact on management. CT scans, bone marrow biopsy results, MRI, and brain and tissue biopsy results were also reviewed.
FDG PET Imaging
FDG PET scans were performed on a Siemens Biograph PET/CT and a GE Discovery PET/CT. Patients in the fasting state were injected with 12–16.5 mCi of FDG intravenously. Images were obtained 50–75 min afterward and were correlated with the CT examination. Hypermetabolic foci were defined by radiotracer accumulation greater than that seen in normal tissue. Organs with known physiologic uptake of FDG were excluded. Intensity of FDG uptake was quantified by calculating the standardized uptake value (SUV), a semiquantitative ratio of FDG uptake in tumor compared to injected FDG activity per body weight.
Results
FDG-avid foci were identified in 23% of patients (12 of 53) during the course of their illness (Table 1). In eight patients, malignancy was diagnosed. NHL was disclosed in six patients, and in four of these patients, no other abnormal diagnostic tests or imaging suggested systemic sites of lymphoma. Four patients appeared to have false-positive FDG PET scans.
Table 1.
Diagnosis in patients with positive FDG PET
| FDG PET Finding | At Initial Diagnosis (n = 42) | At Relapse (n = 11) | All Patients (n = 53) |
|---|---|---|---|
| Positive FDG PET | 8 (19%) | 4 (36%) | 12 (23%) |
| Cancer (including systemic NHL) | 5 (12%) | 3 (27%) | 8 (15%) |
| Systemic NHL | 3 (7%) | 3 (27%) | 6 (11%) |
| False-positive FDG PET | 3 (7%) | 1 (9%) | 4 (8%) |
Abbreviations: FDG, 18F-fluorodeoxyglucose; NHL, non-Hodgkin’s lymphoma.
Body FDG PET at Initial Diagnosis
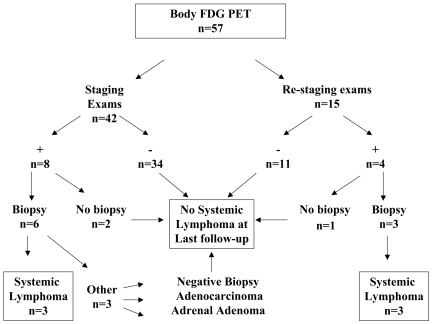
Forty-two exams in 42 patients were performed for the purpose of initial staging (Fig. 1); FDG PET was abnormal in 19% of patients (8 of 42). Biopsies of FDG-avid regions were obtained in six patients, and five revealed malignancy (Table 2). Lymphoma was diagnosed in three patients, one with a focus in the left tibia (SUV 4.1) that required focal radiotherapy (Fig. 2) and one with confirmed lymphoma of mediastinal nodes (highest SUV, 30.5) who then received R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone). The third patient had multiple small foci of abnormal uptake with SUV ranging from 1.8 to 4.5; biopsy of a hepatic lesion demonstrating NHL was obtained months later when a CT CAP demonstrated multiple liver metastases. Two patients had other tumors at biopsy: adrenal adenoma (SUV 3.5) and metastatic duodenal adenocarcinoma (SUV 4.9). One biopsy of a cervical lymph node (SUV 4.0) was nondiagnostic, and no follow-up was available on this patient.
Fig. 1.
Distribution of body 18F-fluorodeoxyglucose (FDG) PET based on reason for exam, positive FDG uptake outside the CNS, biopsy, and biopsy results.
Table 2.
Highest SUV and clinical outcomes in patients with positive FDG PET at initial staging of PCNSL
| Patient | SUV | FDG-Avid Location | BM Bx | CT CAP | Other Abnormal Tests | Biopsy | Change in Therapy |
|---|---|---|---|---|---|---|---|
| 81 F | 30.5 | Mediastinal LN | − | − | Lymphoma | R-CHOP added to PCNSL therapy | |
| 74 F | 8.9 | Supraclavicular LN | ND | − | No biopsy | ||
| 62 M | 4.9 | Abdominal LN | − | + | Adenocarcinoma | Treatment of both malignancies | |
| 76 M | 4.1 | Left tibia | − | − | X-ray: lytic lesion | Lymphoma | RT to left leg |
| 50 M | 4.0 | Cervical LN | − | + | Nondiagnostic biopsy | ||
| 79 F | 4.5 | Multiple | − | − | Biopsy of liver revealed lymphoma | R-CHOP added to PCNSL therapy | |
| 65 M | 3.5 | Adrenal mass | − | − | Adrenal adenoma | ||
| 68 F | 3.0 | Right hilum | − | − | No biopsy |
Abbreviations: SUV, standardized uptake value; FDG, 18F-fluorodeoxyglucose; BM Bx, bone marrow biopsy; CAP, chest/abdomen/pelvis; F, female; LN, lymph node; −, negative; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone; PCNSL, primary CNS lymphoma; ND, not done; M, male; +, positive; RT, radiotherapy.
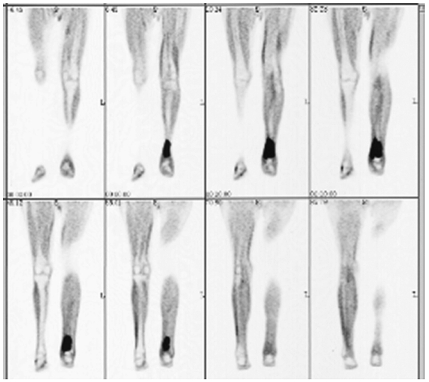
Fig. 2.
A 76-year-old man with 18F-fluorodeoxyglucose-avid focus in left tibia (standardized uptake value 4.1). Plain X-ray corroborated the finding, and biopsy confirmed lymphoma. The patient received radiotherapy to the involved area.
Biopsy was not pursued in two patients: one with abnormal uptake in the right hilum (SUV 3.0) who has not developed evidence of systemic disease after 43 months of follow-up, and one with an FDG-avid supraclavicular lymph node (SUV 8.9) who was not biopsied due to rapid progression of CNS disease.
Body FDG PET at Relapse
A total of 15 body FDG PET scans were performed in 11 patients at the time of CNS relapse (Fig. 1). Seven patients had 11 negative scans and developed no evidence of systemic lymphoma at most recent follow-up. The identification of FDG-avid foci in four patients at relapse led to three biopsies and confirmation of systemic NHL in all three (Table 1). No biopsy was performed in a patient with negative CT CAP and increased uptake in a cervical lymph node (SUV 4.3). The patient has not developed any evidence of systemic disease or other malignancy 19 months after the FDG PET.
The three patients with systemic NHL all had a negative CT CAP, and two had a negative bone marrow biopsy (Table 3). Treatment was modified in all. One patient with a lesion (SUV 22) in his right thigh received focal radiotherapy to the involved area, and follow-up FDG PET demonstrated resolution of the abnormal uptake. A second patient had a right testicular mass on physical examination, and FDG PET revealed a hyper-metabolic lesion (SUV 12.8); he had a right orchiectomy followed by radiotherapy to the left testicle. The third patient had a paraspinal mass (SUV 14.3), and a regimen of cytarabine, rituximab, and etoposide was initiated to treat both CNS and systemic lymphoma (Fig. 3).
Table 3.
Highest SUV and clinical outcomes in patients with positive FDG PET at relapse
| Patient | SUV | FDG-Avid Location | BM Bx | CT CAP | Other Abnormal Tests | Biopsy | Change in Therapy |
|---|---|---|---|---|---|---|---|
| 54 M | 22 | Right thigh | ND | − | Lymphoma | RT to right thigh | |
| 74 M | 14.3 | Paraspinal mass | − | − | Lymphoma | Systemic rituximab and etoposide | |
| 61 M | 12.8 | Right testicle | ND | − | Testicular exam | Lymphoma | Orchiectomy and RT to contralateral testicle |
| 47 F | 4.3 | Cervical LN | − | − | No biopsy |
Abbreviations: SUV, standardized uptake value; FDG, 18F-fluorodeoxyglucose; BM Bx, bone marrow biopsy; CAP, chest/abdomen/pelvis; M, male; ND, not done; −, negative; RT, radiotherapy; F, female; LN, lymph node.
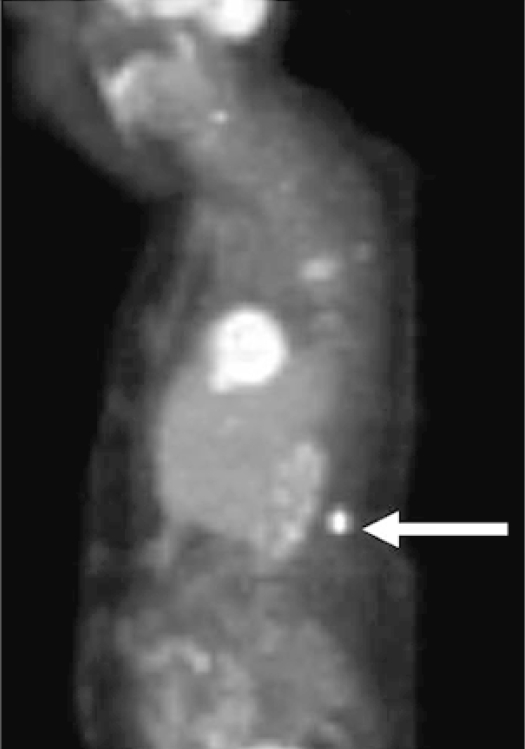
Fig. 3.
18F-Fluorodeoxyglucose PET demonstrated a hypermetabolic paraspinal focus (standardized uptake value 14.3) in a 74-year-old woman during restaging. Subsequent biopsy was consistent with lymphoma, and she began a regimen for both systemic and CNS lymphoma.
Discussion
This study demonstrates the potential utility of PET body imaging in the assessment of patients with a presumptive diagnosis of PCNSL. Seven percent of patients were found to have systemic NHL by staging FDG body PET when body CT scans and bone marrow biopsies were negative. The yield was even higher in the setting of restaging for recurrent disease, where positive body PET imaging led to a confirmed diagnosis of a systemic site of NHL in 27% of patients.
The current recommended staging evaluation for PCNSL includes a CT CAP and bone marrow biopsy; careful testicular examination is recommended in older men.10 Our results suggest that PET may be more sensitive than a conventional staging evaluation in the detection of systemic lymphoma. Three of the FDG-avid foci identified in this study were outside the thoracic, abdominal, and pelvic cavities, underscoring a major limitation of CT CAP: its inability to image outside the torso. This is particularly important considering that PCNSL patients are at higher risk for extranodal NHL.13 FDG PET has been found to add incremental information to conventional CT in various systemic lymphomas,14 and may also be helpful in the assessment of abnormal body CT scans obtained in PCNSL patients. The superior sensitivity of FDG PET compared to bone marrow biopsy has been previously described in aggressive NHL and Hodgkin’s disease;15 further work will be necessary to determine if FDG PET is a reasonable alternative to this invasive test in staging PCNSL. In our own practice, FDG PET is already emerging as an important option in the subset of patients with renal dysfunction, for whom contrast dye used with CT CAP is best avoided. This is critical to prevent nephrotoxicity and allow subsequent safe delivery of high-dose methotrexate. FDG PET may be an important adjunct to our conventional staging evaluation for PCNSL because it can uncover foci not seen on anatomic imaging, it can visualize the entire body, and it is safe and noninvasive.
We found a higher incidence of systemic lymphoma in our PCNSL patients than reported in prior series,11,16,17 suggesting that occult systemic lymphoma may be more common than recognized previously. In a recent study, Jahnke et al.12 described subclinical systemic disease that was not evident on conventional staging by looking for clonally rearranged immunoglobulin in the peripheral blood and bone marrow of PCNSL patients. Their work suggests that the malignant clone may be present systemically at diagnosis but, for reasons yet to be understood, grows preferentially in the CNS. It may be that the systemic clone is controlled by the immune system but grows unchecked in the relatively immune-privileged CNS. One could hypothesize that the uncommon diagnosis of systemic disease may be related to the inadequacy of conventional staging examinations to uncover small foci of disease. A more informative staging evaluation may not only reveal those patients with systemic lymphoma, but also help further our understanding of the biologic origin of PCNSL.
The identification of a systemic focus of lymphoma or second malignancy is critical, as this alters patient management. Patients with concomitant systemic and CNS lymphoma require treatment with a chemotherapy regimen that addresses both disease compartments, typically one that includes both high-dose methotrexate and doxorubicin. Response assessment also requires careful evaluation of both CNS and systemic disease. Second malignancies may require additional therapy. Because FDG PET lacks the precision necessary to distinguish discrete histologies, the presence of an FDG-avid lesion may be associated with an unexpected malignancy and highlights the importance of confirmation with tissue biopsy.18
SUV in this study was variable and not informative beyond the identification of an abnormality. Values ranged from 3.8 to 30.5 in patients with histologic confirmation of systemic lymphoma. In systemic NHL, lesions with SUV > 2.5 or 3 are often considered to be malignant with good sensitivity and specificity.19,20 However, four of our patients had lesions with a high SUV (3.0, 3.5, 4.0, and 4.3) and an indeterminate biopsy or no clinical evidence of systemic NHL at last follow-up. These foci may represent systemic NHL that responded to chemotherapy administered for the CNS disease. Current recommendations for use of FDG PET in lymphoma depend on visual assessment of PET abnormality rather than on any quantitative measures and are likely the most appropriate guideline to use in PCNSL.5 Further evaluation of PET in PCNSL should incorporate other radiotracers, such as 18F-fluorothymidine, a marker of cellular proliferation that has successfully identified foci of disease and predicted higher tumor grade in preliminary studies of systemic lymphoma.21 This may be particularly useful in identifying aggressive subsets of PCNSL.
This is the first study to evaluate the role of body FDG PET in the staging evaluation of PCNSL. Although it reflects a relatively large collection of patients, it is inherently limited by its retrospective nature. In particular, there may have been clinical suspicions in the patients selected for FDG PET that were not evident by record review. Therefore, our findings need to be confirmed and validated in prospective studies in which FDG PET is employed in the staging of PCNSL patients and compared to the yield of CT CAP and bone marrow biopsy. An emerging role for FDG PET in staging could have important implications for the management of PCNSL as well as our understanding of this disease.
References
- 1.Juweid ME, Cheson BD. Positron emission tomography and assessment of cancer therapy. New Engl J Med. 2006;354:496–507. doi: 10.1056/NEJMra050276. [DOI] [PubMed] [Google Scholar]
- 2.Schöder H, Noy A, Gönen M, et al. Intensity of 18fluorodeoxyglucose uptake in positron emission tomography distinguishes between indolent and aggressive non-Hodgkin’s lymphoma. J Clin Oncol. 2005;23:4643–4651. doi: 10.1200/JCO.2005.12.072. [DOI] [PubMed] [Google Scholar]
- 3.Spaepen K, Stroobants S, Dupont P, et al. Prognostic value of positron emission tomography (PET) with fluorine-18 fluorodeoxyglucose ([18F]FDG) after first-line chemotherapy in non-Hodgkin’s lymphoma: is [18F]FDG-PET a valid alternative to conventional diagnostic methods? J Clin Oncol. 2001;19:414–419. doi: 10.1200/JCO.2001.19.2.414. [DOI] [PubMed] [Google Scholar]
- 4.Zijlstra JM, Lindauer-van der Werf G, Hoekstra OS, Hooft L, Riphagen II, Huijgens PC. 18F-Fluorodeoxyglucose positron emission tomography for post-treatment evaluation of malignant lymphoma: a systematic review. Haematologica. 2006;91:522–529. [PubMed] [Google Scholar]
- 5.Juweid ME, Stroobants S, Hoekstra OS, et al. Use of positron emission tomography for response assessment of lymphoma: consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J Clin Oncol. 2007;25:571–578. doi: 10.1200/JCO.2006.08.2305. [DOI] [PubMed] [Google Scholar]
- 6.Juweid ME, Cheson BD. Role of positron emission tomography in lymphoma. J Clin Oncol. 2005;23:4577–4580. doi: 10.1200/JCO.2005.01.904. [DOI] [PubMed] [Google Scholar]
- 7.Roelcke U, Leenders KL. Positron emission tomography in patients with primary CNS lymphomas. J Neurooncol. 1999;43:231–236. doi: 10.1023/a:1006202402010. [DOI] [PubMed] [Google Scholar]
- 8.Shah GD, DeAngelis LM. Treatment of primary central nervous system lymphoma. Hematol Oncol Clin North Am. 2005;19:611–627. doi: 10.1016/j.hoc.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 9.DeAngelis LM, Gutin PH, Leibel SA, Posner JB. Intracranial Tumors. Diagnosis and Treatment. London: Martin Dunitz; 2002. [Google Scholar]
- 10.Abrey LE, Batchelor TT, Ferreri AJM, et al. Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma. J Clin Oncol. 2005;23:5034–5043. doi: 10.1200/JCO.2005.13.524. [DOI] [PubMed] [Google Scholar]
- 11.O’Neill BP, Dinapoli RP, Kurtin PJ, Habermann TM. Occult systemic non-Hodgkin’s lymphoma (NHL) in patients initially diagnosed as primary central nervous system lymphoma (PCNSL): how much staging is enough? J Neurooncol. 1995;25:67–71. doi: 10.1007/BF01054724. [DOI] [PubMed] [Google Scholar]
- 12.Jahnke K, Hummel M, Korfel A, et al. Detection of subclinical systemic disease in primary CNS lymphoma by polymerase chain reaction of the rearranged immunoglobulin heavy-chain genes. J Clin Oncol. 2006;24:4754–4757. doi: 10.1200/JCO.2006.06.7165. [DOI] [PubMed] [Google Scholar]
- 13.Jahnke K, Thiel E, Martus P, et al. Relapse of primary central nervous system lymphoma: clinical features, outcome and prognostic factors. J Neurooncol. 2006;80:159–165. doi: 10.1007/s11060-006-9165-6. [DOI] [PubMed] [Google Scholar]
- 14.Shah N, Hoskin P, McMillan A, Gibson P, Lowe J, Wong WL. The impact of FDG positron emission tomography imaging on the management of lymphomas. Br J Radiol. 2000;73:482–487. doi: 10.1259/bjr.73.869.10884743. [DOI] [PubMed] [Google Scholar]
- 15.Fuster D, Chiang S, Andreadis C, et al. Can [18F]fluorodeoxyglucose positron emission tomography imaging complement biopsy results from the iliac crest for the detection of bone marrow involvement in patients with malignant lymphoma? Nucl Med Commun. 2006;27:11–15. doi: 10.1097/01.mnm.0000185000.81203.49. [DOI] [PubMed] [Google Scholar]
- 16.DeAngelis LM, Yahalom J, Thaler HT, Kher U. Combined modality therapy for primary CNS lymphoma. J Clin Oncol. 1992;10:635–643. doi: 10.1200/JCO.1992.10.4.635. [DOI] [PubMed] [Google Scholar]
- 17.Pollack IF, Lunsford LD, Flickinger JC, Dameshek HL. Prognostic factors in the diagnosis and treatment of primary central nervous system lymphoma. Cancer. 1989;63:939–947. doi: 10.1002/1097-0142(19890301)63:5<939::aid-cncr2820630526>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 18.Sonet A, Graux C, Nollevaux M-C, Krug B, Bosly A, Vander Borght T. Unsuspected FDG-PET findings in the follow-up of patients with lymphoma. Ann Hematol. 2007;86:9–15. doi: 10.1007/s00277-006-0167-4. [DOI] [PubMed] [Google Scholar]
- 19.Freudenberg LS, Antoch G, Schütt P, et al. FDG-PET/CT in re-staging of patients with lymphoma. Eur J Nucl Med Mol Imaging. 2004;31:325–329. doi: 10.1007/s00259-003-1375-y. [DOI] [PubMed] [Google Scholar]
- 20.Naumann R, Vaic A, Beuthien-Baumann B, et al. Prognostic value of positron emission tomography in the evaluation of post-treatment residual mass in patients with Hodgkin’s disease and non-Hodgkin’s lymphoma. Br J Haematol. 2001;115:793–800. doi: 10.1046/j.1365-2141.2001.03147.x. [DOI] [PubMed] [Google Scholar]
- 21.Buck AK, Bommer M, Stilgenbauer S, et al. Molecular imaging of proliferation in malignant lymphoma. Cancer Res. 2006;66:11055–11061. doi: 10.1158/0008-5472.CAN-06-1955. [DOI] [PubMed] [Google Scholar]