Abstract
Fifteen articular cartilage-bone specimens from one canine humeral joint were compressed in the strain range of 0% to 50%. The deformation of the extracellular matrices in cartilage was preserved and the same tissue sections were studied using polarized light microscopy (PLM) and Fourier-transform infrared imaging (FTIRI). The PLM results show that the most significant changes in the apparent zone thickness due to ‘re-organization’ of the collagen fibrils based on the birefringence occur between 0% to 20% strain values, where the increase in the superficial zone and decrease in the radial zone thicknesses are approximately linear with the applied strain. The FTIRI anisotropy results show that the two amide components with bond direction perpendicular to the external compression retain anisotropy (amide II in the superficial zone and amide I in the radial zone). In contrast, the measured anisotropy from the two amide components with bond direction parallel to the external compression change their anisotropy significantly (amide I in the superficial zone and amide II in the radial zone). Statistical analysis shows that there is an excellent correlation (r=0.98) between the relative depth of the minimum retardance in PLM and the relative depth of the Amide II anisotropic cross-over. The changes in amide anisotropies in different histological zones are explained by the strain-dependent tipping angle of the amide bonds. These depth-dependent adaptations to static loading in cartilage’s morphological structure and chemical distribution could be useful in the future studies of the early diseased cartilage.
Keywords: compression, cartilage, collagen, polarized light microscopy, Fourier-transform infrared imaging
Introduction
Articular cartilage has an extracellular matrix composed primarily of water, type II collagen fibrils and proteoglycans (Maroudas, 1975; Maroudas et al., 1980; Venn and Maroudas, 1977). Histologically, articular cartilage has unique variations in its morphological structure and molecular composition across its tissue depth, which is commonly considered to comprise three sub-tissue zones based on local fibril orientation (Bayliss et al., 1983; Clarke, 1971; Maroudas et al., 1991; Miosge et al., 1994; Mow and Guo, 2002; Xia, 2000). These three zones are (a) the superficial zone (SZ) where the collagen is oriented parallel to the articular surface, (b) the transitional zone (TZ) where the collagen is oriented rather randomly, and (c) the radial zone (RZ) where the collagen is oriented mainly perpendicular to the articular surface. The critical role of the collagen matrix in cartilage is to preserve the tissue integrity where any alteration of the collagen microstructure due to tissue lesions will inevitably disrupt the molecular environment, consequently modifying the mechanical properties of the tissue.
Several microscopic imaging techniques have been used to study the load-induced deformation of the collagen matrix in articular cartilage. For example, microscopic MRI (µMRI) has been successfully used to image the modification of the tissue morphology in intact tissue blocks based on the proton signals in water molecules (Alhadlaq and Xia, 2004; Alhadlaq and Xia, 2005). Polarized light microscopy (PLM) has been used to study the adaptation of the collagen matrix in compressed cartilage based on optical birefringence (Alhadlaq et al., 2007). Scanning electron microscopy can directly visualize the effect of mechanical loading on the organization of the collagen fibrils in cartilage matrix (Glaser and Putz, 2002; Kaab et al., 2000; Kobayashi et al., 1996). Recently, Fourier-transform infrared imaging (FTIRI) has been used to study the characteristics and distribution of chemical signatures in healthy, uncompressed cartilage (Bi et al., 2005; Camacho et al., 2001; David-Vaudey et al., 2005; Potter et al., 2001; Ramakrishnan et al., 2007a; Rieppo et al., 2004; Saadat et al., 2006; Xia et al., 2007). Of the various infrared vibrational modes of cartilage, the transition moments of amide I and amide II are considered qualitatively perpendicular to each other in the context of the long axis of collagen fibril in cartilage (Bi et al., 2005; Coats et al., 2003; Gadaleta et al., 1996; West et al., 2005). Consequently, one can study the anisotropy of these infrared vibrations in cartilage when the infrared light is polarized (Ramakrishnan et al., 2007a; Ramakrishnan et al., 2007b; Xia et al., 2007).
A compression will inevitably result in the deformation of the collagen matrix in articular cartilage, which will change the molecular anisotropy as measured by PLM and FTIRI. The aim of this study is to correlate the birefringent modification in cartilage using PLM with the anisotropic alterations in cartilage using FTIRI, while the specimen is statically compressed. Although the physical principles of the visible light birefringence and the infrared absorption anisotropy are quite different, we hypothesized that a combined study on the same tissue section has the ability to reveal any correlation between the two imaging techniques. Since PLM is the gold standard in histology and FTIRI can probe tissue’s chemical distribution, changes in which are regarded as the earliest signs in tissue degradation before it becomes a clinical disease, we aim to resolve the depth-dependent consequences of static loading in articular cartilage, providing the background for future study of molecular and morphological modification in the early diseased cartilage.
Materials and Methods
Specimen Preparation
This study used one humeral joint from a healthy and mature dog sacrificed for an unrelated experimental study. This dog (about eighteen month old and mixed-breed) belongs to a group of similar dogs that have been extensively studied in our lab over the last ten years (Xia et al., 2001). The central load-bearing surface of the humeral head was divided into a matrix of 30 tissue blocks, shown in Fig 1, using a tabletop diamond saw. The interface between the soft tissue and the bone was preserved, and the location and orientation of the individual specimen blocks on the joint surface were closely monitored to identify any influence of topographical variation. A number of control specimens were selected from the specimen matrix - each serving as the control for several neighboring blocks. This minimizes the minor but noticeable topographical variation in the tissue thickness over the humeral joint surface (Xia et al., 2002).
Fig 1.
Schematic of a canine humeral head where the specimens were harvested from the central weight-bearing area, where the black lines marked approximately the locations of the specimens. Gross inspection of the joint revealed no signs of injury or disease.
About 25 tissue blocks were compressed at different strains, by placing each block (about 1.5×2×5 mm) in a Hoffman clamp and compressed by means of screws (Alhadlaq et al., 2007). The compressed specimens remained in the device during the entire histological process (fixation, decalcification, paraffin processing, and embedding) to ensure the preservation of the local environments in the compressed state. The standard histology procedures were used to process the specimens (Xia et al., 2001). The clamp was removed in the paraffin container just before the specimen block was embedded. For each tissue block, multiple unstained 6-µm thick sections were made and placed on the “MirrIR” slides from Tienta Sciences (Indianapolis, IN), which enabled the imaging of the same tissue sections using both PLM and FTIRI. Several tissue blocks were lost during the lengthy histological process. The final data in this report came from a total of fifteen specimens, marked in the matrix in Fig 1. Animal subjects were handled according to the protocols approved by the Institutional Review Board.
PLM Experiments
For each of the fifteen tissue blocks, three to five of the unstained histological sections were imaged in a PLM system, which has a 12-bit CCD camera mounted on a Leica polarized light microscope. Circularly polarized light was used with a liquid crystal compensator, which allows the compensation of birefringent elements of any orientation without rotation of the specimen or mechanical movement of the optical components in the light path (Oldenbourg and Mei, 1995). This method allows the calculation of two quantitative images, representing the optical retardance and the angular orientation of birefringent elements in the tissue (Xia et al., 2001). The pixel resolution in the light microscope was 2.72 µm with a 5x objective. Other experimental details have been documented extensively elsewhere (Xia et al., 2001).
FTIRI Experiments
For each of the fifteen tissue blocks, one representative section, selected from the three to five neighboring sections imaged by PLM, was imaged in a FTIRI system. The FTIRI system consists of a classical FTIR spectrophotometer coupled to an infrared microscope (PerkinElmer Spotlight 300, Wellesley, Massachusetts). The microscope can view/focus the specimen using visible light and has a moving stage on which the specimen can be scanned in a reflection mode. A rectangular region-of-interest (ROI) of 256µm by 981µm was selected from each visible image and the time-domain signals (interferograms) were acquired and subsequently Fourier-transformed into the frequency-domain spectroscopic images.
In order to quantify the infrared anisotropy, each tissue section was scanned nineteen times, each time with the identical experimental parameters and identical tissue orientation except a 10° angle increment of the analyzer (a commercial wire grid infrared polarizer from PerkinElmer, inserted between the tissue section and the detector). The imaging experiment had a spectral step of 8cm−1 with 2 scans per pixel at a pixel resolution of 6.25 µm. It took about 3.25 minutes to acquire one image, which was about 10 MBytes in size. Other experimental details have been described elsewhere (Xia et al., 2007). For both PLM and FTIRI, the same ROI was imaged and used for analysis to obtain better correlation.
Image Analysis
For each tissue section, a single PLM imaging experiment results in two quantitative 2D images, one retardation image (in unit of nm) and one angle image (in unit of degree) (Xia et al., 2001). For articular cartilage, the angle maps in this procedure represent the pixel-averaged orientations of the collagen fibrils in the tissue, where the fibrils are expected to have an approximately 90° difference between the surface and the deep zones; the retardance maps are influenced by several factors in the measurements, including the randomness of the collagen fibrils, the fibril diameter, the packing density of the fibrils, and the thickness of the tissue section. For a single tissue section, a smaller retardance value indicates that the collagen fibrils are less ordered. The 1D profiles from these quantitative 2D PLM images have been used successfully to divide the tissue into three histological zones: the superficial zone (SZ), the transitional zone (TZ), and the radial zone (RZ) (Alhadlaq et al., 2004; Alhadlaq et al., 2007; Xia et al., 2001; Xia et al., 2003; Xia et al., 2002).
For each tissue section, a single FTIRI imaging experiment resulted in one 3D spectroscopic data cube, which has two spatial dimensions in unit of microns and one spectral dimension in unit of wavenumber in cm−1. At any spatial location (a fixed x and y), one can extract a conventional 1D infrared spectrum. At any chemical location (a fixed wavenumber or a range of wavenumbers), one can extract a 2D map of ‘chemical’ distribution (chemi-map). For this cartilage study, the baseline corrected chemi-maps were created by integration of the spectral region 1700 to 1600 cm−1 for amide I and 1600 to 1500 cm−1 for amide II. Because of the desire to seek anisotropy information, each tissue section was imaged nineteen times at different polarization angles. Consequently, each specimen has nineteen 2D amide I images and nineteen 2D amide II images.
Both 2D images and 1D profiles of the cartilage tissue from PLM and FTIRI were used for analysis and presentation. The 2D images enabled us to examine any topographical variation in the images, whereas the 1D profiles enabled us to examine the depth dependency and wavenumber dependency of the results and to compare the profiles from different imaging experiments. To achieve this, eight data pixels along the direction perpendicular to the tissue depth were averaged to enhance the signal-to-noise ratio of these absorption profiles. Since averaging occurs perpendicular to the tissue depth, the pixel resolution along the tissue depth in the 1D profiles is still 6.25µm for FTIRI and 2.72µm for PLM. For each tissue section, the extraction of infrared profiles was from approximately the same tissue location in all nineteen experiments, and the infrared absorption values shown in the report are the actual experimental values (with no manual scaling).
Using the PLM images, the tissue thickness in the control and compressed specimens was measured. The strain of the compressed tissue was calculated. In order to compare the profiles of several specimens loaded at different strains (hence having different absolute thicknesses). the tissue thickness (depth) was converted into the relative depth (RD), with 0 for the articular surface and 1 for the tidemark line between the radial zone and the calcified tissue.
Statistical Analysis
Data were evaluated for normality and data transformation was considered if data were not normal. Pearson correlation coefficient was calculated between strains (as variable X) and thickness (as variable Y) at each histological zone. The correlation coefficient between variables Y and X, r, has a range of −1 to 1, where |r|=1 means a perfect correlation and 0 means no correlation. A positive correlation coefficient indicates that an increased Y is correlated with an increased X; a negative correlation coefficient, in contrary, indicates an increased Y correlated with a decreased X. A linear regression model was used to study the transformation of PLM to FTIRI on the measurement of tissue depths with the estimation of R2 for modeling goodness-of-fit, where .
Results
Polarized Light Microscopy (PLM) Results
Fig 2 shows the quantitative angle and retardance images (a, b) and angle/retardation profiles (c, d) from a set of four compressed specimens, each at a different strain level. The average thickness of articular cartilage in the control specimens was 625±40µm. For the unloaded tissue (0% strain), the angle map shows the collagen fibrils close to the surface are oriented at 90° to the fibrils deep in the tissue, and while the retardance map shows a minimum value at a relative depth of ~ 0.1 from the articular surface, marking the center of the transitional zone that has the most random fibril orientation (Xia et al., 2001). Under compression, the features of cartilage tissue in both angle and retardance maps changed, as shown clearly in the profiles in Fig 2c and 2d. For this type of canine cartilage from the humeral heads, we have shown in the past that the individual zone thicknesses on the relative scale are about 0.06, 0.1, and 0.84 for SZ, TZ and RZ respectively (Xia et al., 2001). When the strain increases, the superficial zone gets much thicker. For example, the 90° fibril transition for the 50% strain occurs around a relative depth of 0.4 (Fig 2c). It is important to note that the description of the tissue zone’s growth/change in this article is based on the actual fibril arrangement in the tissue, which is being determined via birefringence in PLM (and later in FTIRI). It does not mean that a particular region of the cartilage now belongs to a different histological zone under loading. The description merely means that the orientational changes of the collagen fibrils due to external loading make the local tissue appear to have a different birefringent property in polarization imaging (hence belonging to a different zone).
Fig 2.
The 2D angle maps (a) and retardation maps (b) from four specimens compressed between 0% (control) and 50%. The resolution of these PLM images was 2.72 µm/pixel. The 1D angle profiles (c) and retardation profiles (d) extracted from these images. For the uncompressed tissue (0% strain), three labels (SZ – superficial zone, TZ – transitional zone, RZ – radial zone) mark the approximate location of the three histological zones along the tissue depth. An arrow in (c) marks the shift of the fibril angle transition into the deeper tissue in a compressed tissue, which corresponds to the shifting of the retardation minimum in the same direction in (d).
Using the method established and validated in our lab (Xia et al., 2001), the tissue depth was divided into three histological zones. The zonal thickness is shown in Fig 3 as a function of the strain. It is clear that as the strain increases, the thickness of the SZ increases significantly (correlation coefficient r = 0.725, p = 0.003), the thickness of the RZ decreases significantly (correlation coefficient r = −0.89, p < 0.0001), and no association was observed between the thickness of the TZ and strains (p = 0.05). On the relative thickness scale, the same data shows that the most significant changes in the zone thickness occur from 0% to 20% strain values, where the thickness of the radial zone decreased nearly linearly. Above the 20% strain, the trend in the zone change continues at a slower rate.
Fig 3.
The thickness of the three histological zones (SZ, TZ, RZ) as a function of strain.
Fourier-transform Infrared Imaging (FTIRI) Results
The same tissue sections discussed in PLM results (Fig 2) were studied using the FTIRI method. The visible images from the FTIRI experiments are shown in Fig 4a. A rectangular region of interest in the middle of the tissue section, which contains the full depth of the tissue, was imaged in infrared with no analyzer. The baseline corrected chemi-maps of the two amide components from these specimens, each compressed at a different strain, were extracted from the 3D data and shown in Fig 4b. The infrared absorption patterns change as the tissue is compressed, which suggests the possibility of monitoring the change in the directions of amide bonds due to the reorientation of the collagen fibrils in cartilage when it is being compressed.
Fig 4.
(a) Visible images of the same cartilage specimens as used in the PLM illustrations in Fig 2. The rectangular area (256µm by 981µm) was infrared imaged from each tissue section. (b) The chemi-maps of the amide I (A1) and amide II (A2) components in cartilage. These chemi-maps are the integration of the unpolarized infrared absorption spectra. The maximum absorbance settings for the amide images are 0.6 for amide I and 0.4 for amide II.
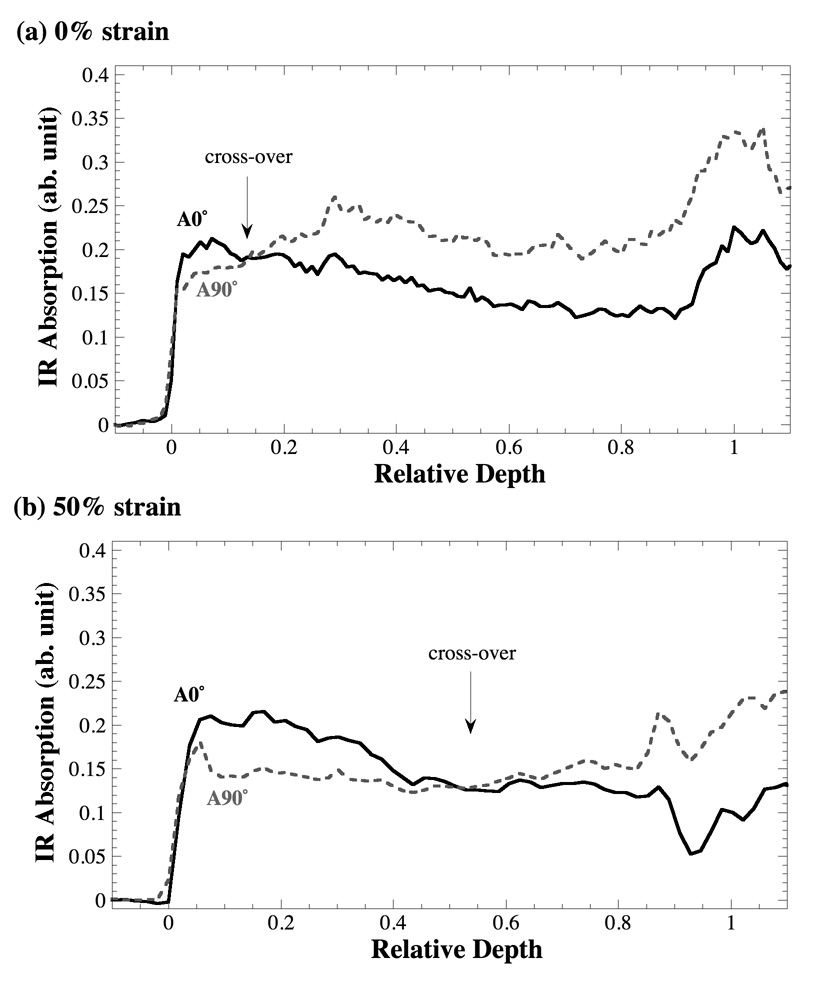
Fig 5a and 5b compare the depth profiles of the amide II component anisotropy obtained at 0° and 90° of the analyzer angle from the strain 0% and 50% respectively. In our previous work, we have identified a crossover point in the amide II profile, occurring at about the relative depth of 0.12 (Ramakrishnan et al., 2007a). We have demonstrated that this crossover point marks the center of the transitional zone and can be used to divide the cartilage thickness into histological zones, based on the tissue’s infrared anisotropy (Ramakrishnan et al., 2007b). In this work, the same crossover phenomenon was observed in all specimens, with one significant difference: the relative depth at which this crossover occurs has been found to be dependent upon the strain, as evident in Fig 5.
Fig 5.
The depth profiles of the amide II anisotropy at two infrared polarizations, 0° (solid line) and 90° (dashed line), from the tissue at the 0% (a) and 50% (b) strains. The delay of the infrared crossover (marked with arrows) due to the applied strain is clear.
Fig 6a and 6b show the anisotropy profiles of amide I and amide II at the superficial zone (RD ~ 0.058, about 37.5µm from the articular surface for the tissue at 0% strain) respectively. When the tissue is not compressed, the anisotropy profiles of amide I and amide II are opposite, which agrees with our previous work (Xia et al., 2007). (Hence the directions of the transition moments of these two amide components can be considered 90° to each other.) When the tissue is compressed, the amide II anisotropy maintains the same profile and becomes much stronger (larger variations between the maximum and minimum), which implies a higher ‘density’ in unit voxel of the amide bonds in compressed tissue. However, the amide I component lost its anisotropy at the strains of 17% and 30%, and acquired the same anisotropy as that of amide II at 50% strain.
Fig 6.
FTIRI anisotropy plots at cartilage’s “superficial zone” (RD ~ 0.058, or 37.5µm from the articular surface when the tissue is not compressed) (a–b) and “radial zone” (RD ~ 0.6, about 360µm from the articular surface when the tissue is not compressed) (c–d). The solid lines are the fitted lines from a theoretic equation that models the anisotropic changes in the amide absorption (Xia et al., 2007).
Fig 6c and 6d show the anisotropy profiles of amide I and amide II approximately in the middle of the radial zone (RD ~ 0.6, about 360µm from the articular surface in the 0% strain tissue). When the tissue is not compressed, the amide I and amide II profiles are opposite to each other, as in the superficial zone. (The anisotropy of the same amide component between the superficial and radial zones is also opposite, since the fibrils at these two zones are about 90° apart.) When the tissue is compressed, however, amide I in the radial zone still retains the same (but weaker) anisotropy at 50% strain, where as amide II in the radial zone becomes nearly isotropic at 50% strain.
Correlation between PLM and FTIRI on measurement of the tissue depths
Fig 2d shows that the relative depth of the minimum retardance in PLM increases as the strain increases, while Fig 5b shows that the amide II cross-over depth increases as the strain increases. Since both parameters represent the location where the fibrils have the most random orientation (via different physical mechanisms) (Ramakrishnan et al., 2007a; Xia et al., 2001), the correlation between the two quantities is assessed using the linear regression. Fig 7 shows the statistical assessment between the two quantities, where the tissue depths using FTIRI can be expressed as the function of PLM in the formula as
with goodness-of-fit 0.96 and the Pearson coefficient r=0.98.
Fig 7.
The linear regression correlation between the depth of the minimum retardation in PLM and the depth of the amide II crossover in FTIRI, as the function of strains. The central shaded band marks the 95% confidence limits. Two outlines mark the 95% prediction limits. The Pearson correlation coefficient between the two variables is 0.9812.
Discussion
Because of its depth-dependent structure and composition, the mechanical properties of articular cartilage vary greatly with the depth away from articular surface (Broom and Myers, 1980; Gore et al., 1983; Kaab et al., 2000; O'Connor et al., 1988). Such depth-dependent variations have been well documented by several reports in literature (Chen et al., 2001; Klein et al., 2007; Schinagl et al., 1997; Wang et al., 2001; Wilson et al., 2007). For example, Klein et al has found that the compressive moduli of fetal and newborn bovine cartilage increased with depth by a factor of 4–5 from the top 0.1 mm to 1 mm depth into the tissue, and that the glycosaminoglycan and collagen content increased with depth, and correlated with the modulus (Klein et al., 2007). These studies demonstrate the complex depth-dependent structure/function in articular cartilage and warrant the necessity of using high-resolution imaging in osteoarthritic cartilage study.
When articular cartilage is compressed, a number of structural changes occur in articular cartilage – a dominant one being the reorganization of the collagen matrix. Since different zones in cartilage have different compressive moduli, the softest one gets compressed the most at a given strain. Since the division of tissue into the histological zones is based on the local fibril orientation, the reorganization of collagen matrix will result inevitably in the reorganization of the individual zone thicknesses. In the PLM results, the thickness of the superficial zone is found to expand at the expense of the radial zone, as a function of strain. Consequently, the minimum retardance location in PLM shifted away from the articular surface, indicating a reorganization of the most randomly oriented collagen fibrils within the tissue matrix.
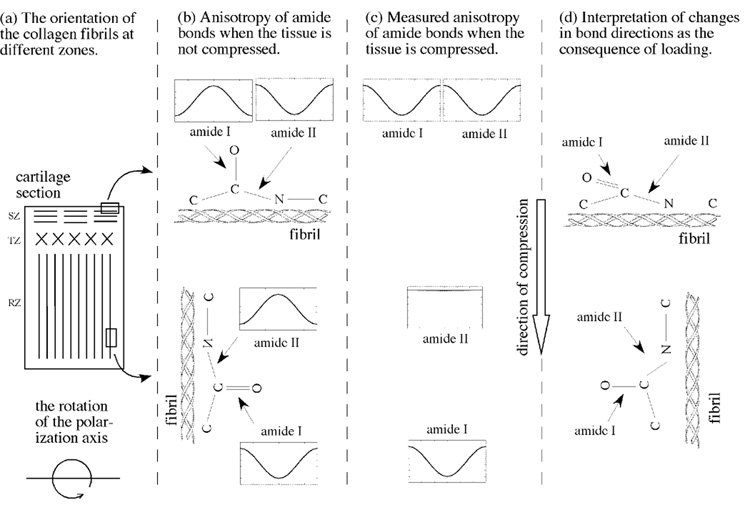
In FTIRI results, a set of complex variations in the infrared anisotropy have been found in cartilage as a function of strain, with the most striking observations being the amide I in the superficial zone reversed its anisotropy and the amide II in the radial zone became isotropic. The origin of the infrared complexity is due to the two perpendicularities in cartilage, illustrated schematically in Fig 8. First, the collagen fibrils in the superficial zone are approximately perpendicular to the fibrils in the radial zone (Fig 8a). Second, the transition moments of amide I and amide II components, each associated with a particular molecular group in a fibril, are considered perpendicular to each other in the context of the long axis of collagen in cartilage (Fig 8b).
Fig 8.
(a) The schematic illustration of the fibril orientation in different histological zones in a cartilage section. In infrared polarization experiments the polarization axis is rotated in the plane of the tissue section. (b) The fibril orientation in the superficial and radial zones and their associated anisotropy of amide bonds (the inserts) when the tissue is not compressed. (The schematic inserts represent the actual infrared measurements, where the horizontal axis is the angle between 0° and 180°, and the vertical axis is the infrared absorbance.) (c) The measured anisotropy of amide bonds when the tissue is compressed. (d) The schematic interpretation of the changes in bond directions as the consequence of the external loading. While the amide I in the surface fibrils can be ‘pressed flat’, the numerous amide II bonds in a mechanically stronger radial zone, each oriented on a helical fibril, produces an isotropic appearance due to its averaging effect.
When the tissue is not compressed (Fig 8b), the perpendicularity of the fibril orientations in the superficial and radial zones has been verified in infrared polarization studies (Ramakrishnan et al., 2007a; Xia et al., 2007) and in this work. The four inserts in Fig 8b show schematically the infrared anisotropy of each amide component (the actual experimental result). When the tissue is compressed (Fig 8c), the measured anisotropy in cartilage shows that two amide components whose bond direction is perpendicular to the external compression retain anisotropic variations (amide II in the superficial zone, amide I in the radial zone). In contrast, the measured anisotropy from the two amide components whose bond direction is parallel with the external compression change their anisotropy significantly (amide I in the superficial zone, amide II in the radial zone).
These infrared anisotropy changes must be related in some way to the changes in the amide bond directions, schematically depicted in Fig 8d. For the superficial fibrils, which are located in the zone that has the lowest compressive modulus (Chen et al., 2001) and hence compressed the most, the amide I bond must have changed its orientation by about 90°. Hence the anisotropy of amide I becomes similar to that of amide II. For the radial fibrils, which are located in the zone that has the highest compressive modulus and hence compressed the least at the same strain, the amide II bond seemed to change its orientation by about 45°. The reason for this 45° assumption is that the illustration in Fig 8d contains only one amide II bond. In practice, any imaging voxel contains numerous amide II bonds, each associated with one molecular location on a fibril. Since the fibrils are triple-helical, these numerous bonds would form a cone. If the bonds in this cone are tipped approximately at 45°, the average of these numerous bonds would behave like isotropic. In fact, one can also notice in Fig 6a that it takes less strains (about 17–30%) to make the amide I anisotropy in the superficial zone to be nearly isotropic (i.e., to be tipped to approximate at 45°). This difference in the stain levels that are needed to change the tipping angles between the superficial and radial zones is entirely consistent with the knowledge that the superficial zone cartilage is softer than the radial zone cartilage; hence the amount of the ‘bond tipping’ is depth-dependent in articular cartilage.
In conclusion, although cartilage in this study comes from one healthy dog, we have in the past sixteen years studied articular cartilage from essentially the same locations of the same type of canine joints using various microscopic imaging techniques (µMRI, PLM, FTIRI, TEM). This current study demonstrates that the combined experimentation using PLM and FTIRI can yield unique information regarding the structural and molecular modifications in articular cartilage due to external loading. To the best of our knowledge, this is the first infrared imaging study of articular cartilage while the tissue is being compressed, and this is also the first combined PLM/FTIRI study of compressed cartilage. Because of their physical natures, the anisotropy information in these two imaging techniques can be used to formulate directly the modifications and adaptations of the collagen fibril network at different compression states in a depth dependent manner. Since the changes in molecular concentration and fibril structures are regarded as the early signs of tissue degradation and since articular cartilage is a load-bearing tissue, these imaging-based methods could be used to determine modification of the molecular organization in the tissue, which often signals the onset of the tissue degradation.
Acknowledgement
Yang Xia thanks the National Institutes of Health (NIH) for the R01 grants (AR045172, AR052353), the National Science Foundation (NSF) for a REU grant (DMR-0552779), and Oakland University for Research Excellence Fund in Biotechnology. The authors thank Mr. Aswin Yerasi for helping in data analysis. The authors are grateful to the labs of Drs. G. Lust and (late) N. Burton-Wurster (James A. Baker Institute for Animal Health, Cornell University, Ithaca, NY) and Dr. C Les (Henry Ford Hospital, Detroit, MI) for providing the canine tissue. Hisham Alhadlaq would like to thank KSU & KACST in Riyadh, Saudi Arabia.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Y Xia, Department of Physics and Center for Biomedical Research, Oakland University, Rochester, MI 48309, USA..
H Alhadlaq, Department of Physics and Astronomy, King Saud University, PO BOX 2455, Riyadh 11451, Saudi Arabia..
N Ramakrishnan, Department of Physics and Center for Biomedical Research, Oakland University, Rochester, MI 48309, USA..
A Bidthanapally, Department of Physics and Center for Biomedical Research, Oakland University, Rochester, MI 48309, USA..
F Badar, Department of Physics and Center for Biomedical Research, Oakland University, Rochester, MI 48309, USA..
M Lu, Department of Biostatistics and Epidemiology, Henry Ford Health System, Detroit, MI 48202, USA..
References
- Alhadlaq H, Xia Y. The Structural Adaptations in Compressed Articular Cartilage by Microscopic MRI (µMRI) T2 Anisotropy. Osteoarthritis and Cartilage. 2004;12:887–894. doi: 10.1016/j.joca.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Alhadlaq H, Xia Y, Moody JB, Matyas J. Detecting Structural Changes in Early Experimental Osteoarthritis of Tibial Cartilage by Microscopic MRI and Polarized Light Microscopy. Ann Rheum Dis. 2004;63:709–717. doi: 10.1136/ard.2003.011783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhadlaq HA, Xia Y. Modifications of orientational dependence of microscopic magnetic resonance imaging T(2) anisotropy in compressed articular cartilage. J Magn Reson Imaging. 2005;22:665–673. doi: 10.1002/jmri.20418. [DOI] [PubMed] [Google Scholar]
- Alhadlaq HA, Xia Y, Hansen FM, Les CM, Lust G. Morphological changes in articular cartilage due to static compression: polarized light microscopy study. Connect Tissue Res. 2007;48:76–84. doi: 10.1080/03008200601130950. [DOI] [PubMed] [Google Scholar]
- Bayliss M, Venn M, Maroudas A, Ali SY. Structure of proteoglycans from different layers of human articular cartialge. Biochem J. 1983;209:387–400. doi: 10.1042/bj2090387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi X, Li G, Doty SB, Camacho NP. A novel method for determination of collagen orientation in cartilage by Fourier transform infrared imaging spectroscopy (FTIRIS) Osteoarthritis and Cartilage. 2005;13:1050–1058. doi: 10.1016/j.joca.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Broom ND, Myers DB. A study of the structural response of wet hyaline cartilage to various loading situations. Connect Tissue Res. 1980;7:227–237. doi: 10.3109/03008208009152358. [DOI] [PubMed] [Google Scholar]
- Camacho NP, West P, Torzilli PA, Mendelsohn R. FTIR microscopic imaging of collagen and proteoglycan in bovine cartilage. Biopolymers. 2001;62:1–8. doi: 10.1002/1097-0282(2001)62:1<1::AID-BIP10>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Chen SS, Falcovitz YH, Schneiderman R, Maroudas A, Sah RL. Depth-dependent compressive properties of normal aged human femoral head articular cartilage: relationship to fixed charge density. Osteoarthritis and Cartilage. 2001;9:561–569. doi: 10.1053/joca.2001.0424. [DOI] [PubMed] [Google Scholar]
- Clarke IC. Articular cartilage: A review and scanning electron microscope study. J. Bone Joint Surgery. 1971;53 B:732–750. [PubMed] [Google Scholar]
- Coats AM, Hukins DW, Imrie CT, Aspden RM. Polarization artefacts of an FTIR microscope and the consequences for intensity measurements on anisotropic materials. J Microscopy. 2003;211:63–66. doi: 10.1046/j.1365-2818.2003.01198.x. [DOI] [PubMed] [Google Scholar]
- David-Vaudey E, Burghardt A, Keshari K, Brouchet A, Ries M, Majumdar S. Fourier Transform Infrared Imaging of focal lesions in human osteoarthritic cartilage. Eur Cell Mater. 2005;10:51–60. doi: 10.22203/ecm.v010a06. [DOI] [PubMed] [Google Scholar]
- Gadaleta SJ, Landis WJ, Boskey AL, Mendelsohn R. Polarized FT-IR microscopy of calcified turkey leg tendon. Connect Tissue Res. 1996;34:203–211. doi: 10.3109/03008209609000699. [DOI] [PubMed] [Google Scholar]
- Glaser C, Putz R. Functional anatomy of articular cartilage under compressive loading Quantitative aspects of global, local and zonal reactions of the collagenous network with respect to the surface integrity. Osteoarthritis Cartilage. 2002;10:83–99. doi: 10.1053/joca.2001.0484. [DOI] [PubMed] [Google Scholar]
- Gore DM, Higginson GR, Minns RJ. Compliance of articular cartilage and its variation through the thickness. Phys Med Biol. 1983;28:233–247. doi: 10.1088/0031-9155/28/3/004. [DOI] [PubMed] [Google Scholar]
- Kaab MJ, Ito K, Rahn B, Clark JM, Notzli HP. Effect of mechanical load on articular cartilage collagen structure: A scanning electron-microscopic study. Cells Tissues Organs. 2000;167:106–120. doi: 10.1159/000016774. [DOI] [PubMed] [Google Scholar]
- Klein TJ, Chaudhry M, Bae WC, Sah RL. Depth-dependent biomechanical and biochemical properties of fetal, newborn, and tissue-engineered articular cartilage. J Biomech. 2007;40:182–190. doi: 10.1016/j.jbiomech.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Yonekubo S, Kurogouchi Y. Cryoscanning electron microscopy of loaded articular cartilage with special reference to the surface amorphous layer. J Anat. 1996;188:311–322. [PMC free article] [PubMed] [Google Scholar]
- Maroudas A. Biophysical chemistry of cartilaginous tissues with special reference to solute and fluid transport. Biorheology. 1975;12:233–248. doi: 10.3233/bir-1975-123-416. [DOI] [PubMed] [Google Scholar]
- Maroudas A, Bayliss MT, Venn M. Further studies on the composition of human femoral head cartilage. Ann. Rheum. Dis. 1980;39:514–534. doi: 10.1136/ard.39.5.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroudas A, Wachtel EJ, Grushko G, Katz EP, Weinberg P. The effect of osmotic and mechanical pressures on water partitioning in articular cartialge. Biochim Biophys Acta. 1991;1073:285–294. doi: 10.1016/0304-4165(91)90133-2. [DOI] [PubMed] [Google Scholar]
- Miosge N, Flachsbart K, Goetz W, Schultz W, Kresse H, Herken R. Light and electron microscopical immunohistochemical localization of the small proteoglycan core proteins decorin and biglycan in human knee joint cartilage. Histochem J. 1994;26:939–945. [PubMed] [Google Scholar]
- Mow VC, Guo XE. Mechano-electrochemical properties of articular cartilage: their inhomogeneities and anisotropies. Ann Rev Biomed Eng. 2002;4:175–209. doi: 10.1146/annurev.bioeng.4.110701.120309. [DOI] [PubMed] [Google Scholar]
- O'Connor P, Orford CR, Gardner DL. Differential response to compressive loads of zones of canine hyaline articular cartilage: micromechanical, light and electron microscopic studies. Ann Rheum Dis. 1988;47:414–420. doi: 10.1136/ard.47.5.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldenbourg R, Mei G. New polarized light microscope with precision universal compensator. J Microscopy. 1995;180:140–147. doi: 10.1111/j.1365-2818.1995.tb03669.x. [DOI] [PubMed] [Google Scholar]
- Potter K, Kidder LH, Levin IW, Lewis EN, Spencer RG. Imaging of collagen and proteoglycan in cartilage sections using Fourier transform infrared spectral imaging. Arthritis Rheum. 2001;44:846–855. doi: 10.1002/1529-0131(200104)44:4<846::AID-ANR141>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan N, Xia Y, Bidthanapally A. Polarized IR microscopic imaging of articular cartilage. Phys Med Biol. 2007a;52:4601–4614. doi: 10.1088/0031-9155/52/15/016. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan N, Xia Y, Bidthanapally A, Lu M. Determination of zonal boundaries in articular cartilage using infrared dichroism. Appl Spectrosc. 2007b;61:1404–1409. doi: 10.1366/000370207783292118. [DOI] [PubMed] [Google Scholar]
- Rieppo J, Hyttinen MM, Jurvelin JS, Helminen HJ. Reference sample method reduces the error caused by variable cryosection thickness in Fourier transform infrared imaging. Appl Spectrosc. 2004;58:137–140. doi: 10.1366/000370204322729577. [DOI] [PubMed] [Google Scholar]
- Saadat E, Lan H, Majumdar S, Rempel DM, King KB. Long-term cyclical in vivo loading increases cartilage proteoglycan content in a spatially specific manner: an infrared microspectroscopic imaging and polarized light microscopy study. Arthritis Res Ther. 2006;8:R147. doi: 10.1186/ar2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinagl RM, Gurskis D, Chen AC, Sah RL. Depth-dependent confined compression modulus of full-thickness bovine articular cartilage. J Orthop Res. 1997;15:499–506. doi: 10.1002/jor.1100150404. [DOI] [PubMed] [Google Scholar]
- Venn M, Maroudas A. Chemical composition and swelling of normal and osteoarthritic femoral head cartilage. Ann Rheum Dis. 1977;36:121–129. doi: 10.1136/ard.36.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CC, Hung CT, Mow VC. An analysis of the effects of depth-dependent aggregate modulus on articular cartilage stress-relaxation behavior in compression. J Biomechanics. 2001;34:75–84. doi: 10.1016/s0021-9290(00)00137-8. [DOI] [PubMed] [Google Scholar]
- West PA, Torzilli PA, Chen C, Lin P, Camacho NP. Fourier transform infrared imaging spectroscopy analysis of collagenase-induced cartilage degradation. J Biomed Opt. 2005;10:14015. doi: 10.1117/1.1854131. [DOI] [PubMed] [Google Scholar]
- Wilson W, Huyghe JM, van Donkelaar CC. Depth-dependent compressive equilibrium properties of articular cartilage explained by its composition. Biomech Model Mechanobiol. 2007;6:43–53. doi: 10.1007/s10237-006-0044-z. [DOI] [PubMed] [Google Scholar]
- Xia Y. Magic Angle Effect in MRI of Articular Cartilage - A Review. Invest Radiol. 2000;35:602–621. doi: 10.1097/00004424-200010000-00007. [DOI] [PubMed] [Google Scholar]
- Xia Y, Ramakrishnan N, Bidthanapally A. The depth-dependent anisotropy of articular cartilage by Fourier-transform infrared imaging (FTIRI) Osteoarthritis Cartilage. 2007;15:780–788. doi: 10.1016/j.joca.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Moody J, Burton-Wurster N, Lust G. Quantitative In Situ Correlation Between Microscopic MRI and Polarized Light Microscopy Studies of Articular Cartilage. Osteoarthritis Cartilage. 2001;9:393–406. doi: 10.1053/joca.2000.0405. [DOI] [PubMed] [Google Scholar]
- Xia Y, Moody J, Alhadlaq H, Hu JN. Imaging the Physical and Morphological Properties of a Multi-Zone Young Articular Cartilage at Microscopic Resolution. J Magn Reson Imaging. 2003;17:365–374. doi: 10.1002/jmri.10269. [DOI] [PubMed] [Google Scholar]
- Xia Y, Moody J, Alhadlaq H, Burton-Wurster N, Lust G. Characteristics of Topographical Heterogeneity of Articular Cartilage over the Joint Surface of a Humeral Head. Osteoarthritis Cartilage. 2002;10:370–380. doi: 10.1053/joca.2002.0523. [DOI] [PubMed] [Google Scholar]