Abstract
Cisplatin chemotherapy frequently causes severe vomiting in two temporally-separated clusters of bouts dubbed the acute and delayed phases. Cannabinoids can inhibit the acute phase, albeit through a poorly understood mechanism. We examined the substrates of cannabinoid-mediated inhibition of both emetic phases via immunolabeling for serotonin, Substance P, cannabinoid receptors 1 and 2 (CB1, CB2), and the neuronal activation marker Fos in the least shrew (Cryptotis parva). Shrews were injected with cisplatin (10 mg/kg ip), and one of vehicle, Δ9-THC, or both Δ9-THC and the CB1 receptor antagonist SR141716A (2 mg/kg ip), and monitored for vomiting. Δ9-THC-pretreatment caused concurrent decreases in the number of shrews expressing vomiting and Fos-immunoreactivity (Fos-IR), effects which were blocked by SR141716A-pretreatment. Acute phase vomiting induced Fos-IR in the solitary tract nucleus (NTS), dorsal motor nucleus of the vagus (DMNX), and area postrema (AP), whereas in the delayed phase Fos-IR was not induced in the AP at all, and was induced at lower levels in the other nuclei when compared to the acute phase. CB1 receptor-IR in the NTS was dense, punctate labeling indicative of presynaptic elements, which surrounded Fos-expressing NTS neurons. CB2 receptor-IR was not found in neuronal elements, but in vascular-appearing structures. All areas correlated with serotonin- and Substance P-IR. These results support published acute phase data in other species, and are the first describing Fos-IR following delayed phase emesis. The data suggest overlapping but separate mechanisms are invoked for each phase, which are sensitive to antiemetic effects of Δ9-THC mediated by CB1 receptors.
Keywords: Emesis, Chemotherapy, Cannabinoid, Serotonin, Substance P, Vagus, Solitary Tract Nucleus
1. Introduction
Chemotherapy-induced vomiting (CIV) is among the most common and debilitating side effects of antitumor therapy [38]. Despite extensive research, the origin of CIV remains elusive. Cisplatin (CIS) and related chemotherapeutics produce vomiting in cancer patients biphasically, beginning with emetic bouts up to 24 hours post-CIS infusion (the acute or immediate phase), then a quiescent period of one to several days, and finally a second set of bouts occurring 2–7 days post-CIS (the delayed phase) [19].
Mechanistically, CIV involves a substantial release of serotonin (5-HT) by CIS in the gastrointestinal tract (GIT), and probably in the brain as well, which initiates a reflex arc via vagal 5-HT3 receptors activating the dorsal vagal complex (DVC) of the central nervous system (CNS) [15,19,20]. The DVC is a cluster of medullary nuclei which performs several emetic functions: The area postrema (AP), a circumventricular organ, allows bloodborne emetogenic chemicals access to the brain. The dorsal motor nucleus of the vagus (DMNX) may receive vagal afferents from the nodose ganglion [27], but is primarily innervated by the medial solitary tract nucleus (NTS). It sends efferents to the GIT and to a central pattern generator which modulates emetic retroperistaltic activity. The medial solitary tract nucleus (NTS) is a key site for integrating diverse emesis-related afferents from the vagus and a wide range of brain areas (for review see [22]).
5-HT3 receptor antagonists (e.g. ondansetron) are effective antiemetics against the acute phase of CIV [5,26], but not against the delayed phase [3]. Research has implicated neurokinin NK1 receptor activation by Substance P (SP) as one putative mediator of the delayed phase [3,39]. In fact, SP itself is emetic, and both NK1 antagonists [13] and Δ9-tetrahydrocannabinol (THC; [10]) can block SP-induced vomiting. Whereas animal studies demonstrate potent antiemetic activity of NK1 receptor antagonists against both phases of CIV [35,39], clinical studies have been disappointing. NK1 antagonists alone are ineffective against either phase of CIV [19,34], but potentiate antiemetic efficacy when combined with standard antiemetics [18].
Cannabinoid neurotransmission, mediated by two G-protein coupled receptors (CB1 or CB2), can also modulate vomiting. In animals, THC, synthetic CB1 receptor agonists, and related ligands are potent, broad-spectrum antiemetics [11,37,41] whose activity may also include interactions with 5-HT3 [26] or TRPV1 vanilloid [36] receptors, while the CB2 receptor does not appear emetically relevant [8,37]. In limited clinical use, cannabinoids appeared antiemetic against both phases of CIV [1,7]. CB1 antagonists reverse the antiemetic effects of THC [11,41], but require high doses to be emetogenic themselves [11,37]. The antivomiting effect of exogenous cannabinoids appears improved when combined with standard antiemetic drugs [7,38].
Mechanistic aspects of vomiting and the antiemetic action of cannabinoids remain unclear. Emesis-related functional studies have been limited by only examining the acute phase [28,33,41] or using non-vomiting species [21,42]. CNS cannabinoid receptors are widespread and multifunctional [17,42], complicating study of the anatomical substrates of their antiemetic activity. Furthermore, the exact locus (or loci) of effect of antiemetic cannabinoids has not been clearly identified, although CB1 receptors on vagal afferents to the DVC are implicated [41]. Finally, data are lacking on the relationship of cannabinoid receptors to components of other emetogenic neurotransmitter systems (e.g. 5-HT or SP).
This study sought to elucidate the neural substrates mediating CIV and THC-related antiemesis in both phases. We used immunohistochemistry (IHC) for Fos protein (Fos) to indicate neuronal activation, alone and in combination with IHC for CB1 and CB2 receptors, SP, and 5-HT, to study the DVC anatomically and neurochemically during both phases of CIV in the least shrew, Cryptotis parva. This mammal is emesis-competent, with well-defined acute (0–3 hours post-CIS exposure, peaking at 2 h) and delayed (28–35 hours post-CIS, peaking at 33 h) emetic phases, and emetic responses similar to those of humans [5,7,12,31].
2. Methods
2.1. Animals
Adult female least shrews (C. parva, N = 44) from the Western University Animal Facilities colony were housed in groups on a 14:10 light:dark cycle and fed and watered ad libitum. The shrews were 45–60 days old and weighed 3.7 – 5.4g. All experiments were performed between 11:00 and 17:30 hours, and in accordance with Western University IACUC standards. On the day of experimentation, shrews were brought from the animal facility and separated to individual cages, and allowed to adapt to the new conditions for 2–3 hours minimum to reduce potential novelty-induced Fos immunoreactivity.
2.2. Behavioral Procedures
For the acute phase studies, shrews were placed individually in experiment cages and allowed 2 h to adapt, during which time food was restricted. They were then given four mealworms (Tenebrio sp.) each, and THC (2.5 mg/kg i.p., provided through NIDA, Rockville, MD) or vehicle (ethanol:Emulphor™:saline in a 1:1:18 ratio) injection. Twenty minutes after THC injection, shrews were injected with 10 mg/kg CIS i.p., (Sigma-Aldrich, St. Louis, MO), then observed for vomiting behavior for 30 minutes. For these studies, a shrew was transcardially perfused 75 min after vomiting occurred, typically 20–30 min after cisplatin injection. Thus, vomiting animals were perfused 95–105 min after CIS injection. Animals used in the acute phase studies that did not vomit were perfused 90–100 min after CIS injection.
To begin the delayed phase studies, the shrews were injected with CIS as described above, then placed back in their home cage with food and water ad lib overnight. The next morning the shrews were moved individually to experiment cages and given adaptation time and food restriction as in the acute studies. At 31 h post-CIS injection, shrews were given four mealworms, followed 20 min later by i.p. injection of THC or vehicle, and then monitored for vomiting for 30 min. For the delayed phase studies, shrews were perfused 90–100 min after THC injection if there was no vomiting, or 65–75 min after vomiting (95–105 min post-THC injection). In all cases, injections for a given day were staggered by 45 minutes each, to ensure perfusions would occur during optimal emesis-related Fos expression.
Lastly, two groups of shrews received an i.p. injection of the CB1 receptor antagonist SR141716A (2.0 mg/kg, Sanofi, France) dissolved in the same vehicle as THC, or a vehicle injection, 20 min before receiving a THC injection. Cisplatin injection and monitoring for vomiting behavior, as well as perfusion timing, were then performed as described above.
2.3. Immunohistochemical procedures
After the appropriate time period for acute or delayed phase vomiting, shrews were anesthetized with a lethal dose of pentobarbital (100 mg/kg) and perfused transcardially via blunted needle with a peristaltic pump. The shrew was perfused with ice cold 4% paraformaldehyde and 5% picric acid in pH 7.4, 0.1M phosphate buffer (10 min). Brains were removed and stored in 30% sucrose in 0.1M PB overnight, then embedded in blocks of 12% gelatin in 30% sucrose/PB. The blocks were postfixed for 3 h in 2% paraformaldehyde/PB, then rinsed and immersed in 30% sucrose/PB until they sank (usually 1–2 h). The brain block was cut sagittally on a freezing benchtop microtome (Leica) at 30 μm into 5 series, and stored in PB with 0.03% sodium azide.
Immunolabeling was accomplished by blocking a free-floating series with 10% normal horse serum (NHS) and 3% hydrogen peroxide in PB with 0.3% Triton X-100 (TX) for 30 min. After rinsing in PB, tissue was put in sheep anti-Fos polyclonal antibody (Chemicon/Millipore, Temecula, CA; 1:600), with 5% NHS and 0.3% TX in PB, and incubated for about 42 h at room temperature (RT) with gentle shaking. After rinsing in PB, the tissue was placed in biotinylated donkey anti-sheep IgG secondary antibody (Jackson Immunoresearch, West Grove, PA; 1:600) diluted in the same diluent described for the primary antibody, and the tissue incubated at RT with shaking for 75 min. Tissue was then rinsed and incubated for 60 min in HRP-conjugated avidin-biotin complex (Vector Labs, Burlingame, CA; Vectastain kit diluted 1:2) in PB. Tissue was then rinsed twice in PB, then once in imidazole-acetate buffer (0.1M, pH 7.4), then reacted for 6 min in 0.0006% hydrogen peroxide with 2% nickel ammonium sulfate-enhanced diaminobenzidine (DAB, 0.05% in 0.1M imidazole-acetate buffer). All reagents for DAB processing were purchased from Sigma-Aldrich. Other series were processed for immunofluorescence using one or more primary antibodies including: monoclonal rat anti-Substance P (Chemicon/Millipore, 1:400), affinity-purified polyclonal rabbit anti-CB1 receptor (Chemicon/Millipore, AB5636P, 1:333), affinity-purified polyclonal rabbit anti-CB2 receptor (Chemicon/Millipore, AB5642P, 1:250), and polyclonal rabbit anti-serotonin (Invitrogen/Zymed, 1:400). The cannabinoid receptor antibodies were both affinity-purified stocks with accompanying Western Blot data and characterization references provided by the manufacturer. In addition, several reactions were performed as described, but with normal donkey serum in place of the primary antibody (zero-primary controls). Tissue was blocked, and primary antibodies reacted overnight after rinsing as described for the anti-Fos primary. Secondary antibodies were HRP-conjugated donkey IgG (Jackson Immunoresearch) raised against the appropriate species (rat or rabbit), diluted 1:600 in the same diluent described above. Series were reacted for 90 min, then rinsed 3 times in PB, then reacted in the dark with AlexaFluor-405, 488, or 594 conjugated tyramide produced in lab using reactive AlexaFluor™ dyes from Invitrogen and tyramine from Sigma-Aldrich. Purified tyramide conjugate was reacted for 20 min with 0.006% hydrogen peroxide in PB.
After reacting, tissue was rinsed thoroughly in PB and mounted onto gel-subbed slides out of PB. After air-drying, slides were dehydrated through a series of ascending ethanols (50%–75%–90%–100%), then cleared in xylene. Cleared slides were coverslipped with DEPEX (Electron Microscopy Sciences, Hatfield, PA).
2.4. Analysis
Photomicrographs of regions of interest were taken at 1600×1200 px digital resolution with a SPOT digital camera (Diagnostic Instruments) attached to a PC running version 4.0 of the SPOT software and mounted to a Nikon Eclipse E600 microscope. Images were exported to Adobe Photoshop 7, and passed through a high-pass threshold filter set to 75% black. This filter eliminates potential false positives created by nonspecific background labeling. Relevant structures were identified using an atlas produced in lab [32]. Immunofluorescent series were examined qualitatively for each primary antibody using a Nikon three-laser scanning confocal microscope, noting the relative density of the various markers in the DVC, and noting terminals or other structures which were co-labeled for several different antigens.
2.5. Statistics
The number of shrews vomiting in each treatment group was recorded and the results analyzed using Mann-Whitney U tests for unpaired samples, with a significance level of p ≤ 0.05 being considered statistically significant differences between groups. The number of animals vomiting in the acute phase versus delayed phase was also analyzed via Mann-Whitney U tests. After immunohistochemical processing for Fos-IR, cell counts for each structure were averaged, and two-way analysis of variance and Student’s t-test were used to compare numbers of Fos+ nuclei.
3. Results
3.1. Behavioral effects of THC and SR141716A on cisplatin-induced vomiting
The number of shrews vomiting is described in Table 1. No shrews vomited when injected with vehicle instead of cisplatin. When administered following cisplatin, THC injections blocked vomiting in significantly more shrews than vehicle injections in both the acute (Mann-Whitney U score = 45, 3; p < 0.005) and delayed (U = 43.5, 10.5; p < 0.02) phases. Administration of the CB1 receptor-specific antagonist SR141716A prior to THC injection significantly reversed the antiemetic efficacy of THC in both acute (U = 41, 7; p < 0.01) and delayed (U = 58.5, 22.5; p < 0.05) phases, resulting in vomiting in a number of shrews comparable to the vehicle injected treatment groups in both phases (acute phase, p > 0.24; delayed phase, p > 0.27). No significant differences were found in the numbers of shrews vomiting in the acute phase versus the delayed phase in either the vehicle groups (p > 0.24), the THC groups (p > 0.33), or the SR141716A+THC groups (p > 0.27).
Table 1.
Number of Shrews Vomiting per Experimental Group. All shrews were injected with cisplatin (CIS, 10 mg/kg, ip), then injected at the appropriate time with either vehicle, Δ9-THC (2.5 mg/kg, ip), or Δ9-THC and the CB1 receptor antagonist SR141716A (2 mg/kg, ip). Groups were divided according to pre-treatment condition, treatment condition and emetic phase(acute or delayed). No shrews vomited when vehicle was injected in place of cisplatin. The number of shrews vomiting in response to CIS injection when also treated with Δ9-THC was significantly less than those given CIS followed by either vehicle injection (* p < 0.05; ** p < 0.01), or by both SR141716A and Δ9-THC injection († p < 0.05; †† p < 0.01). This pattern held true for both acute and delayed phases. There were no significant differences in the number of animals vomiting when a given pre-treatment/treatment combination was compared between acute and delayed phase conditions.
| Pre-treatment Condition | Treatment Condition | Vomiting Phase | Number Vomited Per Total in Group |
|---|---|---|---|
| Vehicle | Vehicle | Acute | 0/6 |
| Vehicle | Cisplatin | Acute | 6/6 |
| Δ9-THC | Cisplatin | Acute | 1/8 ** †† |
| Δ9-THC + SR141716A | Cisplatin | Acute | 5/6 |
| Vehicle | Vehicle | Delayed | 0/6 |
| Vehicle | Cisplatin | Delayed | 5/6 |
| Δ9-THC | Cisplatin | Delayed | 2/9 * † |
| Δ9-THC + SR141716A | Cisplatin | Delayed | 6/9 |
3.2. Effects of THC and SR141716A on Fos expression resulting from cisplatin-induced vomiting
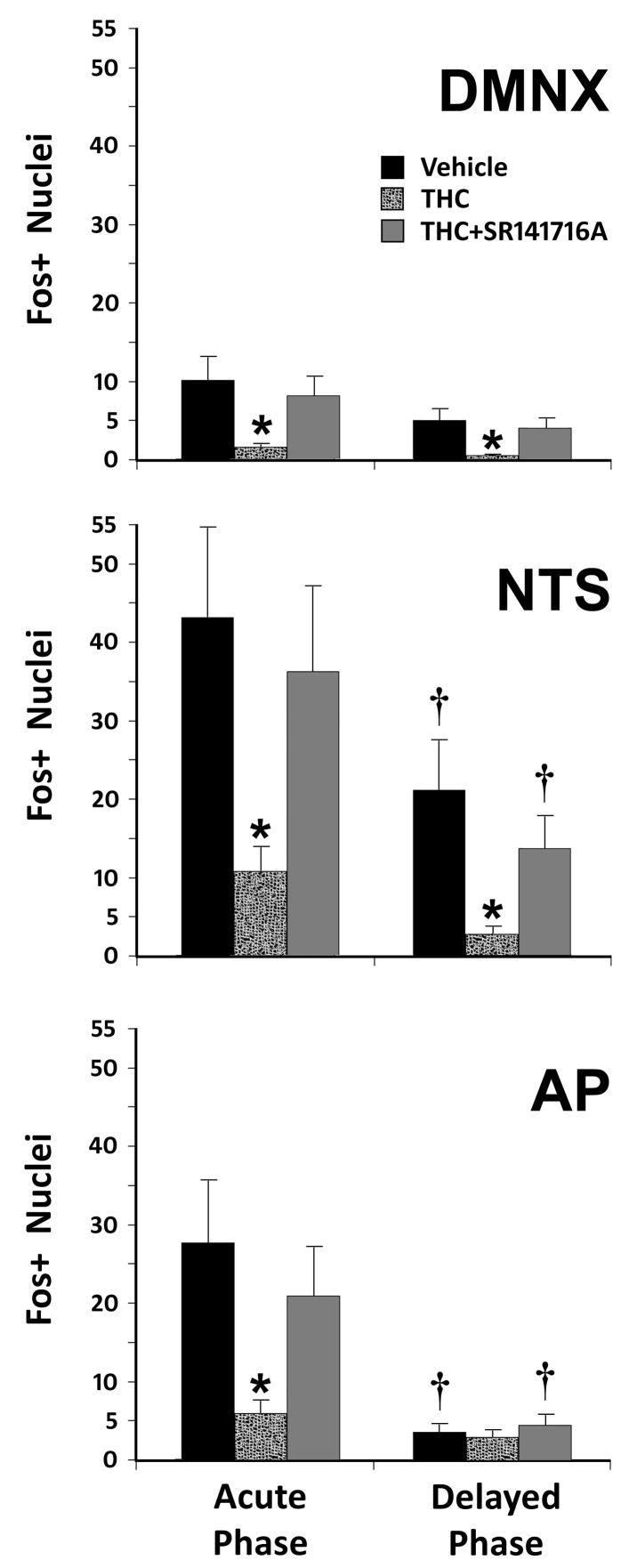
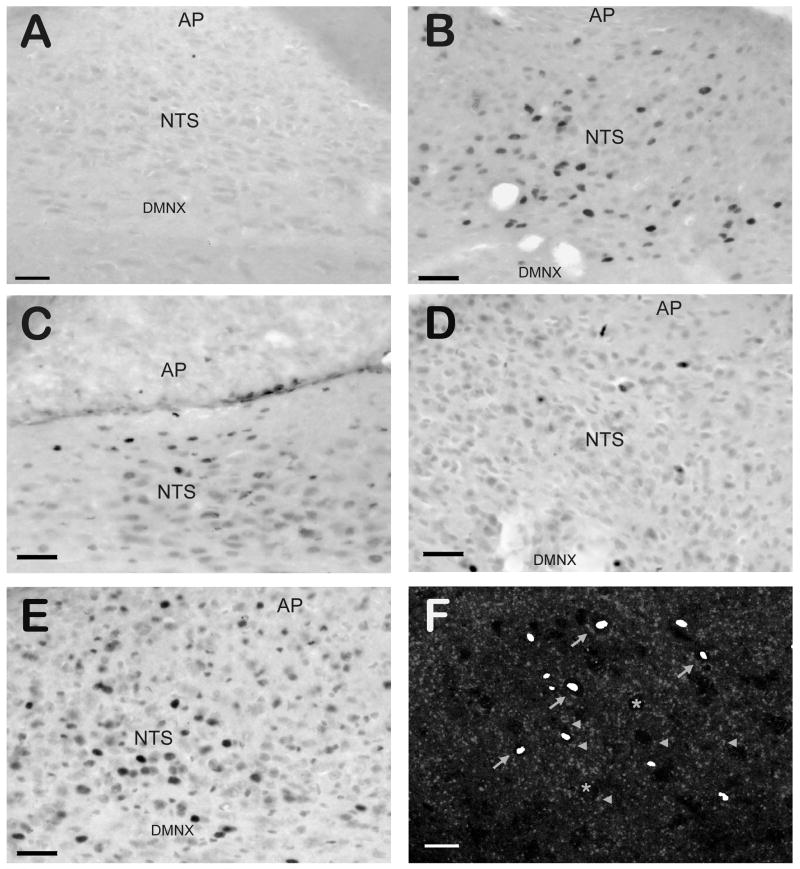
The number of Fos-immunopositive nuclei in the subdivisions of the DVC following cisplatin injections in each group is presented in figure 1, and representative photomicrographs of Fos-IR are presented in figure 2. Vehicle injected in place of CIS (figure 2A) produced almost no Fos-IR in the DVC, while cisplatin-induced vomiting resulted in numerous Fos-IR nuclei (figure 2B). In the acute phase of CIV, THC injections (figure 2D) significantly (p < 0.05) reduced the number of Fos-immunopositive nuclei compared to vehicle injections (figure 2B). This was the case for all three nuclei within the DVC (NTS, AP, and DMNX). When SR141716A was administered prior to THC (figure 2E), the number of Fos+ nuclei was not significantly different (p > 0.2) from vehicle injections, and was significantly higher (p < 0.05) than when THC was administered alone. In the delayed phase (figure 2C), THC-injected animals had significantly fewer (p < 0.05) Fos-immunopositive nuclei than vehicle-injected animals in the NTS and DMNX, but not the AP (p > 0.33). The difference in Fos+ nuclei between vehicle-injected and SR141716A+THC-injected groups was not significant for any part of the DVC (p > 0.18).
Figure 1.
Counts of Fos-immunoreactive Nuclei Following Acute or Delayed Phase Emesis in the Least Shrew. Fos-IR nuclei in the dorsal vagal complex (DVC) were analyzed for each nuclear region in the DVC. Induction of Fos (and vomiting) was through treatment with 10 mg/kg CIS (i.p.). Means and standard errors of Fos-IR nuclei are graphed for each pre-treatment condition, each subnucleus of the DVC, and both phases of CIV. Significantly reduced numbers of Fos-immunopositive nuclei (* p < 0.05) were noted after THC injection when compared to either the vehicle or SR141716A+THC conditions. When regions of the DVC having the same treatment conditions were compared between acute and delayed phases, the AP and NTS had significantly fewer († p < 0.05) Fos+ nuclei in the delayed phase than in the acute phase of emesis when treated with vehicle or SR141716A+THC, but not when treated with THC alone (p > 0.1). Numbers of Fos+ nuclei in the DMNX during the delayed phase were not significantly different from the acute phase (p > 0.12). Abbreviations: AP – area postrema; DMNX – dorsal motor nucleus of the vagus nerve; NTS – nucleus of the solitary tract.
Figure 2.
Examples of Fos-IR in the Dorsal Vagal Complex of the Least Shrew Following Cisplatin-induced Emesis. Sagittal sections through the DVC demonstrate different numbers of Fos-IR nuclei depending on the emetic phase or drug treatment. Vehicle-injected controls (panel A) were effectively devoid of Fos-IR. Cisplatin-injected shrews demonstrated a robust increase in Fos-IR nuclei (black ovals) following the acute phase of emesis (panel B), and a less robust but still significant increase in Fos-IR following the delayed phase (panel C). Injection of THC produces near-control levels of Fos-IR nuclei (panel D), an effect reversible by blockade of CB1 receptors with SR141716A (panel E). When a transmitted light image of Fos-IR is overlaid on a confocal image of CB1 receptor-IR from the same microscopic field of the NTS of shrews following acute phase emesis (panel F), Fos-IR nuclei (pseudocolored white ovals) can be seen within unlabeled somata enmeshed in the CB1 receptor-IR terminal-like structures (grey dots). Asterisks mark the somata of unlabeled (Fos-/CB1-IR negative) neurons, arrowheads mark CB1 receptor-IR terminals apposed to NTS somata, and arrows identify somata with both Fos-IR nuclei and CB1 receptor-IR structures apposed to them. Abbreviations: AP – area postrema; DMNX – dorsal motor nucleus of the vagus; NTS – nucleus of the solitary tract. Scale bars = 50 μm for A–E; 20 μm for F.
When regions of the DVC with the same treatment conditions were compared between acute and delayed phases, there were significantly fewer Fos+ nuclei in the DVC during the delayed phase versus the acute phase. This effect was region and condition-specific, however. The AP and NTS had significantly fewer (p < 0.05) Fos+ nuclei per section in the delayed phase than in the acute phase, but only when treated with vehicle or SR141716A+THC. The number of Fos+ nuclei was not significantly different when treated with THC alone (p > 0.1). In the DMNX, no significant differences (p > 0.12) were found between the delayed phase and the acute phase.
3.3. Immunohistochemical labeling of neurochemicals in the dorsal vagal complex
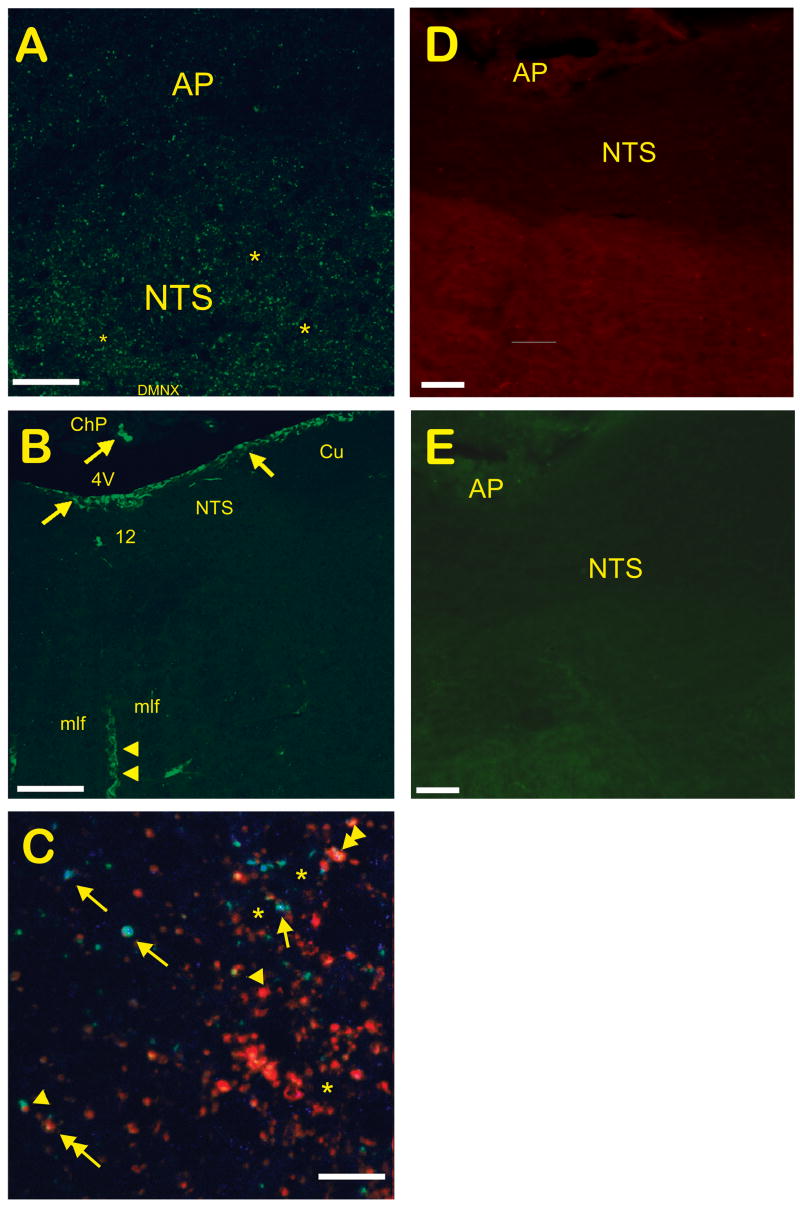
Close examination of overlaid Fos-/CB1-IR images of the NTS demonstrated Fos+ nuclei amidst dense CB1-IR terminal-like structures, including several neurons which appeared apposed to CB1-IR structures (figure 2F). CB1-IR was more prominent in the NTS and DMNX than in the AP (figure 3A). CB2-IR was found on the surface of the brainstem and in the choroid plexus, as well as in ribbon-like structures traversing dorsoventrally through the brainstem (figure 3B). These structures lacked a soma or fine fibers indicative of neurons, suggesting non-neuronal elements such as glia or brain vasculature were being labeled. Additional labeling for 5-HT and SP-IR demonstrated numerous fibers and putative terminals for both neurotransmitters within the DVC. Tissue labeled concurrently for SP, 5-HT, and CB1 receptor-IR showed a wide variety of immunolabeled terminal-like structures, including single-labeled, double-labeled, and triple-labeled structures (figure 3C). Only Fos immunolabeling was quantified, however. When the primary antibodies were left out, no specific labeling was found for either the anti-rat (figure 3D) or anti-rabbit (figure 3E) secondary antibodies. These controls also demonstrated that background fluorescence was much dimmer than specific fluorescence using either Alexa594- or Alexa488-conjugated tyramides.
Figure 3.
Confocal Micrographs of Cannabinoid Receptor, 5-HT, and Substance P Immunolabeling in the Dorsal Vagal Complex of the Least Shrew. A) Sagittal section similar to that in figure 2C, stained for CB1 receptor-IR. CB1-IR was localized mostly to the NTS. B) Coronal section of the DVC demonstrating CB2-IR, which localizes to the brain surface and choroid plexus (arrows), and to non-neuronal elements within the brainstem that appear vascular in nature (arrowheads). Elements suggestive of neuronal origin were absent. C) Multiple-labeling of 5-HT (blue), CB1 receptor (green), and SP (red) in the NTS of the shrew demonstrated a variety of interactions. Asterisks demonstrate unlabeled somata apposed to punctate (terminal-like) immunolabeling for 5-HT, CB1 receptors, and/or SP. While many terminal-like structures were singly-labeled, some showed colocalization of 5-HT/CB1 receptor (arrows), SP/CB1 receptor (arrowheads), or 5-HT/SP (double arrow). A single terminal also demonstrated colocalization of all three antigens (double arrowhead). D) Zero-primary coronal DVC section using the anti-rat secondary antibody and Alexa594-conjugated tyramide amplification. E) Zero-primary coronal DVC section using the anti-rabbit secondary and Alexa488-conjugated tyramide. Abbreviations: 12 – hypoglossal nucleus; 4V – 4th ventricle; AP – area postrema; ChP – choroid plexus; Cu – cuneate nucleus; mlf –medial longitudinal fasciculus; NTS – nucleus of the solitary tract. Scale bars – A, 50 μm; B, 500 μm; C, 10 μm; D and E, 50 μm.
4. Discussion
This is the first study to use functional anatomy to examine the effect of THC on the delayed phase of CIV in a vomiting animal model. It confirms previous work demonstrating the acute-phase antiemetic ability of THC in the least shrew [9,11] and other species [26,41], and also extends the antiemetic ability of THC to delayed-phase CIV. The CB1-specific antagonist SR141716A reversed the antiemetic effect of THC, indicating a CB1 receptor-mediated effect. Furthermore, this effect is mediated at least partially in the DVC, as increased CIV-related Fos-IR in the DVC was attenuated by THC, and restored by prior administration of SR141716A. Both phases of CIV induced DVC activity, but fewer Fos-IR nuclei were found in shrews vomiting during delayed-phase emesis, suggesting that the anatomical substrate for the pharmacological differences between phases could be differential DVC activation. The most obvious difference was the lack of AP activation in the delayed phase, implying that humoral signaling is unnecessary for induction or blockade of delayed phase vomiting. Interestingly, the number of shrews vomiting in the delayed phase was statistically equivalent to the number vomiting in the acute phase. The protocol of short-term food deprivation followed by mealworm presentation is a reliable method of inducing vomiting in CIS-treated shrews. In addition, the intraperitoneal injection of a bolus of CIS produces a shorter and stronger spike of serum CIS when compared to the normal intravenous, longer-term infusion (a 30 minute or longer infusion period) protocol for cancer patients. It is likely that the combined food deprivation protocol and more intense CIS stimulation improve the reliability of inducing vomiting in the delayed phase, to match that of the acute phase.
Van Sickle et al. [41] have used Fos-IR and correlative pharmacological techniques in ferrets to demonstrate increased Fos-IR in the DVC during the acute phase which can be blocked or reduced by THC. Not only do the results in this study in the least shrew support their findings, the magnitudes of the increases in Fos-immunopositive nuclei following vehicle injection, or THC injection preceded by SR141716A, were comparable to the ferret, and the reduction in Fos-IR resulting from THC injection was equally similar [41]. When comparing between phases, these results suggest both phases utilize a similar mechanism, with the important exception that the AP is not activated in the delayed phase. Their work also brings to light one of the drawbacks of using such a tiny animal as the least shrew. The NTS itself is very small, but as in larger animals it appears to subserve several different functions. While van Sickle et al. were able to identify the medial subnucleus of the NTS as the emesis-related subnucleus according to their Fos data [39], in the least shrew it was exceedingly difficult or impossible to define the subnuclei of the NTS. While we believe that as in other animals studied, the majority of emesis-induced Fos-IR changes in the least shrew NTS would be localized to the medial NTS, it will require future studies with better markers for delineating the NTS subnuclei to confirm this in the least shrew.
These data raise the possibility that peripheral communication via the vagus plays a role in both phases. In both phases of CIV, the DMNX and NTS are stimulated when assessed for Fos-IR. Vagal afferents to the DVC arise from the nodose ganglion, terminate preferrentially in the NTS (and to a lesser extent the DMNX), and possess CB1 immunoreactivity [24,30,41]. Stimulation of these afferents would produce the pattern of Fos-IR seen following CIV, and presynaptic inhibition of these afferents by THC or other CB1 agonists would reduce postsynaptic activation and lower Fos-IR. As these vagal neurons also innervate the GIT, stimulation at the enteric level could also induce vagal activity in the DVC. Indeed, THC at large doses can block emesis induced by brain impenetrant, 5-HT3 receptor-binding emetogens [11], implying that there is a degree of peripheral involvement in vomiting.
Fos-IR measured activation of the DVC during the delayed phase was less than that during the acute phase, although still above baseline in the DMNX and NTS when compared to vehicle-injected controls. The limitations of utilizing Fos-IR for an activity marker have been described thoroughly elsewhere [4], but one relevant characteristic of Fos-IR is that it occurs primarily through a recent quantitative increase in neuronal activation. Qualitative changes in activity (e.g. burst vs. static firing) do not consistently induce Fos expression, nor do reductions in basal activity of neurons (e.g. neurons being actively inhibited). While CIS stimulates the DVC, and especially the NTS, in the acute phase, no data has been collected studying the electrophysiological activity of DVC neurons in the quiescent or delayed phases. DVC basal activity remaining above pre-injection levels could prevent a subsequent increase large enough to re-induce Fos expression. Alternatively, two subsets of chemically encoded, emesis-activated neurons in the NTS and/or DMNX are present. This is consistent with collected anatomical data. SP and 5-HT have been found colocalized [40] and individually [2,40] within terminals and neuronal varicosities throughout the DVC. Furthermore, confocal microscopy of multilabeled tissue suggests CB1 receptors colocalize on putative 5-HT, SP, or 5-HT/SP terminals in the NTS, consistent with published data regarding serotonergic raphe neurons. Thus, CB1 receptors are in position to modulate both systems either simultaneously or separately, resulting in a pair of overlapping yet neurochemically distinct networks in the DVC. This hypothetical model fits with pharmacological studies demonstrating the antiemetic effects of THC against both serotonergic [11] and tachykininergic [10] emetic stimuli, and with the ineffectiveness of either 5-HT3 or NK1 antagonists given individually to completely block CIV [3,38]. Several studies have found differential neurotransmitter changes (e.g. increased turnover) for both 5-HT and SP in response to CIS in humans, such that serotonergic activity is increased in the acute phase, while tachykininergic activity is increased in both phases, but especially in the delayed phase [19,20]. In both cases, patients treated with the combined antagonists responded significantly better than those treated with either antagonist alone without regard to emetic phase. These data suggest that 5-HT dominates activation of the acute phase of emesis, albeit with input from tachykininergic neurons, and that SP dominates activation of the delayed phase. The Fos and IHC data in this study fit within this hypothetical framework, as CB1 receptor labeling did colocalize with SP-IR and/or 5-HT-IR in terminal-like structures in the NTS (see figure 3C). In control animals, immunolabeling demonstrated that neurons within the NTS activated during emesis (i.e. Fos-IR neurons) were enmeshed within dense CB1 receptor-immunopositive, putative terminals. While not conclusive for synaptic contacts, these data are mirrored pharmacologically [10,26,41]. Thus, during the acute phase, input from serotonergic and combined (5-HT and SP) projections to the DVC cause a larger increase in numbers of Fos-immunopositive nuclei than that seen during the delayed phase, when mainly tachykininergic inputs to the DVC are being stimulated. The result is Fos-IR that is elevated intermediately between baseline and acute phase CIV-related Fos-IR.
The presence of CB2 receptors in the brain is controversial. Recent reports have purported to find CB2 receptors in neurons [29], although numerous other studies have found putative CB2 receptors within the vascular endothelium of the brain and other organs [16]. In the least shrew, CB2-IR did not localize to neuronal structures in the brainstem, but to the surface of the brainstem, the choroid plexus, and to ribbon-like structures within the brainstem which appeared vascular in nature. Our data and previous pharmacological data [6,37,41] support a non-neuronal localization for CB2 receptors in the brain and no effect on the emetic reflex. Sources of divergence between studies could include nonspecific binding due to lack of a shrew- or ferret-specific antibody. Another possibility is glial or endothelial CB2 receptors in brain homogenates used to blot for proteins or to recover mRNA, whose presence essentially “contaminates” the samples studied.
One drawback to Fos-based studies is the broad range of Fos-activating stimuli. This can make interpretation difficult in deciphering between Fos-IR related to induction versus expression (e.g. motor output) of behavior. While not easily resolved without alternative methodologies (e.g. electrophysiological recording), hypotheses can be drawn based on previous functional data. In the case of the DVC, the NTS is a major integrative site [14,30], but does not directly generate motor activity. Thus, increased Fos-IR in the NTS is more likely to be related to induction rather than expression of vomiting. The DMNX, however, has both motor output neurons and local circuit cells [2,23,25], and could be related to either or both induction and/or expression.
In conclusion, this study has provided the first evidence in a freely-behaving, emesis-competent species that THC, via CB1 receptors, effectively inhibits emesis in delayed-phase CIV. Functional anatomy demonstrated that this effect is mediated within the DVC, but without involvement of the AP, and that both emetogenic neurotransmitters, 5-HT and SP, could be inhibited by presynaptic CB1 receptors to produce THC’s antiemetic activity. These data support the limited clinical and anecdotal evidence suggesting that THC or other cannabinomimetics (via stimulation of CB1 receptors) would be a successful therapeutic modality, or co-therapy, for the prevention of acute and delayed phase chemotherapy-induced vomiting.
Acknowledgments
We would like to thank Sanofi-Aventis for generously providing SR141716A. Some of this data has been described in poster form previously (Society for Neuroscience Annual Meeting 2006, Vol. Program #739.16, Atlanta, Georgia, 2006). This study was funded by NIH grant #R01CA115331 through NCI to Dr. Darmani.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abrahamov A, Abrahamov A, Mechoulam R. An efficient new cannabinoid antiemetic in pediatric oncology. Life Sci. 1995;56:2097–2102. doi: 10.1016/0024-3205(95)00194-b. [DOI] [PubMed] [Google Scholar]
- 2.Boissonade FM, Davison JS, Egizii R, Lucier GE, Sharkey KA. The dorsal vagal complex of the ferret: anatomical and immunohistochemical studies. Neurogastroenterol Motil. 1996;8:255–272. doi: 10.1111/j.1365-2982.1996.tb00265.x. [DOI] [PubMed] [Google Scholar]
- 3.Bountra C, Gale JD, Gardner CJ, Jordan CC, Kilpatrick GJ, Twissell DJ, Ward P. Towards understanding the aetiology and pathophysiology of the emetic reflex: novel approaches to antiemetic drugs. Oncology. 1996;53(Suppl 1):102–109. doi: 10.1159/000227649. [DOI] [PubMed] [Google Scholar]
- 4.Dampney RA, Li YW, Hirooka Y, Potts P, Polson JW. Use of c-fos functional mapping to identify the central baroreceptor reflex pathway: advantages and limitations. Clin Exp Hypertens. 1995;17:197–208. doi: 10.3109/10641969509087065. [DOI] [PubMed] [Google Scholar]
- 5.Darmani NA. Serotonin 5-HT3 receptor antagonists prevent cisplatin-induced emesis in Cryptotis parva: a new experimental model of emesis. J Neural Transm. 1998;105:1143–1154. doi: 10.1007/s007020050118. [DOI] [PubMed] [Google Scholar]
- 6.Darmani NA. Delta(9)-tetrahydrocannabinol and synthetic cannabinoids prevent emesis produced by the cannabinoid CB(1) receptor antagonist/inverse agonist SR 141716A. Neuropsychopharmacology. 2001;24:198–203. doi: 10.1016/S0893-133X(00)00197-4. [DOI] [PubMed] [Google Scholar]
- 7.Darmani NA. Endocannabinoids and gastrointestinal function. In: Onaivi ES, Sugiura T, Di Marzo V, editors. The Brain and Body’s Marijuana and Beyond. Boca Raton: CRC; 2005. pp. 393–418. [Google Scholar]
- 8.Darmani NA. Methods evaluating cannabinoid and endocannabinoid effects on gastrointestinal functions. Methods Mol Med. 2006;123:169–189. doi: 10.1385/1-59259-999-0:169. [DOI] [PubMed] [Google Scholar]
- 9.Darmani NA, Crim JL. Delta-9-tetrahydrocannabinol differentially suppresses emesis versus enhanced locomotor activity produced by chemically diverse dopamine D2/D3 receptor agonists in the least shrew (Cryptotis parva) Pharmacol Biochem Behav. 2005;80:35–44. doi: 10.1016/j.pbb.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 10.Darmani NA, Gerdes D, Trinh C. Structurally Diverse Cannabinoids Prevent Substance P-Induced Emesis via Cannabinoid CB1 Receptor in Cryptotis Parva. The 15th Annual Symposium on the Cannabinoids; Clearwater Beach, Florida. 2005. [Google Scholar]
- 11.Darmani NA, Johnson JC. Central and peripheral mechanisms contribute to the antiemetic actions of delta-9-tetrahydrocannabinol against 5-hydroxytryptophan-induced emesis. Eur J Pharmacol. 2004;488:201–212. doi: 10.1016/j.ejphar.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 12.Darmani NA, McClanahan BA, Trinh C, Petrosino S, Valenti M, Di Marzo V. Cisplatin increases brain 2-arachidonoylglycerol (2-AG) and concomitantly reduces intestinal 2-AG and anandamide levels in the least shrew. Neuropharmacology. 2005;49:502–513. doi: 10.1016/j.neuropharm.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 13.Darmani NA, Wang Y, Abad J, Ray AP, Thrush GR, Ramirez J. Utilization of the least shrew as a rapid and selective screening model for the antiemetic potential and brain penetration of substance P NK1 receptor antagonists. Brain Research. 2008;1214:58–72. doi: 10.1016/j.brainres.2008.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis SF, Derbenev AV, Williams KW, Glatzer NR, Smith BN. Excitatory and inhibitory local circuit input to the rat dorsal motor nucleus of the vagus originating from the nucleus tractus solitarius. Brain Res. 2004;1017:208–217. doi: 10.1016/j.brainres.2004.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Endo T, Minami M, Hirafuji M, Ogawa T, Akita K, Nemoto M, Saito H, Yoshioka M, Parvez SH. Neurochemistry and neuropharmacology of emesis - the role of serotonin. Toxicology. 2000;153:189–201. doi: 10.1016/s0300-483x(00)00314-0. [DOI] [PubMed] [Google Scholar]
- 16.Golech SA, McCarron RM, Chen Y, Bembry J, Lenz F, Mechoulam R, Shohami E, Spatz M. Human brain endothelium: coexpression and function of vanilloid and endocannabinoid receptors. Brain Res Mol Brain Res. 2004;132:87–92. doi: 10.1016/j.molbrainres.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 17.Haring M, Marsicano G, Lutz B, Monory K. Identification of the cannabinoid receptor type 1 in serotonergic cells of raphe nuclei in mice. Neuroscience. 2007;146:1212–1219. doi: 10.1016/j.neuroscience.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 18.Hesketh PJ, Grunberg SM, Gralla RJ, Warr DG, Roila F, de Wit R, Chawla SP, Carides AD, Ianus J, Elmer ME, Evans JK, Beck K, Reines S, Horgan KJ. The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin--the Aprepitant Protocol 052 Study Group. J Clin Oncol. 2003;21:4112–4119. doi: 10.1200/JCO.2003.01.095. [DOI] [PubMed] [Google Scholar]
- 19.Hesketh PJ, Van Belle S, Aapro M, Tattersall FD, Naylor RJ, Hargreaves R, Carides AD, Evans JK, Horgan KJ. Differential involvement of neurotransmitters through the time course of cisplatin-induced emesis as revealed by therapy with specific receptor antagonists. European Journal of Cancer. 2003;39:1074–1080. doi: 10.1016/s0959-8049(02)00674-3. [DOI] [PubMed] [Google Scholar]
- 20.Higa GM, Auber ML, Altaha R, Piktel D, Kurian S, Hobbs G, Landreth K. 5-Hydroxyindoleacetic acid and substance P profiles in patients receiving emetogenic chemotherapy. Journal of Oncology Pharmacy Practice. 2006;12:201–209. doi: 10.1177/1078155206072080. [DOI] [PubMed] [Google Scholar]
- 21.Horn CC, Ciucci M, Chaudhury A. Brain Fos expression during 48 h after cisplatin treatment: Neural pathways for acute and delayed visceral sickness. Auton Neurosci. 2006 doi: 10.1016/j.autneu.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hornby PJ. Central neurocircuitry associated with emesis. Am J Med. 2001;111(Suppl 8A):106S–112S. doi: 10.1016/s0002-9343(01)00849-x. [DOI] [PubMed] [Google Scholar]
- 23.Hornby PJ, Abrahams TP. Central control of lower esophageal sphincter relaxation. Am J Med. 2000;108(Suppl 4a):90S–98S. doi: 10.1016/s0002-9343(99)00345-9. [DOI] [PubMed] [Google Scholar]
- 24.Koga T, Fukuda H. Neurons in the nucleus of the solitary tract mediating inputs from emetic vagal afferents and the area postrema to the pattern generator for the emetic act in dogs. Neurosci Res. 1992;14:166–179. doi: 10.1016/0168-0102(92)90078-q. [DOI] [PubMed] [Google Scholar]
- 25.Krowicki ZK, Hornby PJ. Substance P in the Dorsal Motor Nucleus of the Vagus Evokes Gastric Motor Inhibition via Neurokinin 1 Receptor in Rat. J Pharmacol Exp Ther. 2000;293:214–221. [PubMed] [Google Scholar]
- 26.Kwiatkowska M, Parker LA, Burton P, Mechoulam R. A comparative analysis of the potential of cannabinoids and ondansetron to suppress cisplatin-induced emesis in the Suncus murinus (house musk shrew) Psychopharmacology (Berl) 2004;174:254–259. doi: 10.1007/s00213-003-1739-9. [DOI] [PubMed] [Google Scholar]
- 27.Leslie RA, Reynolds DJ, Andrews PL, Grahame-Smith DG, Davis CJ, Harvey JM. Evidence for presynaptic 5-hydroxytryptamine3 recognition sites on vagal afferent terminals in the brainstem of the ferret. Neuroscience. 1990;38:667–673. doi: 10.1016/0306-4522(90)90060-h. [DOI] [PubMed] [Google Scholar]
- 28.Miller AD, Ruggiero DA. Emetic reflex arc revealed by expression of the immediate-early gene c-fos in the cat. J Neurosci. 1994;14:871–888. doi: 10.1523/JNEUROSCI.14-02-00871.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Onaivi ES. Neuropsychobiological evidence for the functional presence and expression of cannabinoid CB2 receptors in the brain. Neuropsychobiology. 2006;54:231–246. doi: 10.1159/000100778. [DOI] [PubMed] [Google Scholar]
- 30.Onishi T, Mori T, Yanagihara M, Furukawa N, Fukuda H. Similarities of the neuronal circuit for the induction of fictive vomiting between ferrets and dogs. Auton Neurosci. 2007;136:20–30. doi: 10.1016/j.autneu.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 31.Ray AP, Darmani NA. A highly restricted pattern of brain fos immunoreactivity following delayed-phase emesis induced by cisplatin administration in the least shrew (Cryptotis parva). Society for Neuroscience Annual Meeting 2006, Vol. Program #739.16; Atlanta, Georgia. 2006. [Google Scholar]
- 32.Ray AP, Darmani NA. A histologically derived stereotaxic atlas and substance P immunohistochemistry in the brain of the least shrew (Cryptotis parva) support its role as a model organism for behavioral and pharmacological research. Brain Res. 2007;1156:99–111. doi: 10.1016/j.brainres.2007.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reynolds DJ, Barber NA, Grahame-Smith DG, Leslie RA. Cisplatin-evoked induction of c-fos protein in the brainstem of the ferret: the effect of cervical vagotomy and the anti-emetic 5-HT3 receptor antagonist granisetron (BRL 43694) Brain Res. 1991;565:231–236. doi: 10.1016/0006-8993(91)91654-j. [DOI] [PubMed] [Google Scholar]
- 34.Rubenstein EB, Slusher BS, Rojas C, Navari RM. New approaches to chemotherapy-induced nausea and vomiting: from neuropharmacology to clinical investigations. Cancer J. 2006;12:341–347. doi: 10.1097/00130404-200609000-00003. [DOI] [PubMed] [Google Scholar]
- 35.Rudd JA, Ngan MP, Wai MK. Inhibition of emesis by tachykinin NK1 receptor antagonists in Suncus murinus (house musk shrew) Eur J Pharmacol. 1999;366:243–252. doi: 10.1016/s0014-2999(98)00920-0. [DOI] [PubMed] [Google Scholar]
- 36.Sharkey KA, Cristino L, Oland LD, Van Sickle MD, Starowicz K, Pittman QJ, Guglielmotti V, Davison JS, Di Marzo V. Arvanil, anandamide and N-arachidonoyl-dopamine (NADA) inhibit emesis through cannabinoid CB1 and vanilloid TRPV1 receptors in the ferret. Eur J Neurosci. 2007;25:2773–2782. doi: 10.1111/j.1460-9568.2007.05521.x. [DOI] [PubMed] [Google Scholar]
- 37.Simoneau II, Hamza MS, Mata HP, Siegel EM, Vanderah TW, Porreca F, Makriyannis A, Malan TP., Jr The cannabinoid agonist WIN55, 212-2 suppresses opioid-induced emesis in ferrets. Anesthesiology. 2001;94:882–887. doi: 10.1097/00000542-200105000-00029. [DOI] [PubMed] [Google Scholar]
- 38.Slatkin NE. Cannabinoids in the treatment of chemotherapy-induced nausea and vomiting: beyond prevention of acute emesis. J Support Oncol. 2007;5:1–9. [PubMed] [Google Scholar]
- 39.Tanihata S, Oda S, Kakuta S, Uchiyama T. Antiemetic effect of a tachykinin NK1 receptor antagonist GR205171 on cisplatin-induced early and delayed emesis in the pigeon. Eur J Pharmacol. 2003;461:197–206. doi: 10.1016/s0014-2999(03)01311-6. [DOI] [PubMed] [Google Scholar]
- 40.Thor KB, Hill KM, Harrod C, Helke CJ. Immunohistochemical and biochemical analysis of serotonin and substance P colocalization in the nucleus tractus solitarii and associated afferent ganglia of the rat. Synapse. 1988;2:225–231. doi: 10.1002/syn.890020309. [DOI] [PubMed] [Google Scholar]
- 41.Van Sickle MD, Oland LD, Mackie K, Davison JS, Sharkey KA. Delta9-tetrahydrocannabinol selectively acts on CB1 receptors in specific regions of dorsal vagal complex to inhibit emesis in ferrets. Am J Physiol Gastrointest Liver Physiol. 2003;285:G566–576. doi: 10.1152/ajpgi.00113.2003. [DOI] [PubMed] [Google Scholar]
- 42.Vera G, Chiarlone A, Martin MI, Abalo R. Altered feeding behaviour induced by long-term cisplatin in rats. Auton Neurosci. 2006;126–127:81–92. doi: 10.1016/j.autneu.2006.02.011. [DOI] [PubMed] [Google Scholar]