Abstract
Pyrazole can induce CYP2E1 and 2A5, which produce reactive oxygen species (ROS). Nuclear factor erythroid 2-related factor 2 (Nrf2) regulates important antioxidant enzymes to remove ROS. In this study, we applied Nrf2 knockout mice to test the hypothesis that pyrazole will cause hepatotoxicity and elevate oxidative stress to a greater extent in Nrf2 knockout compared to wild type mice. Pyrazole induced severe oxidative liver damage in Nrf2 knockout mice but not in wild type mice. Activities and levels of CYP2E1 and 2A5 were elevated by pyrazole in the wild type mice but not in the Nrf2 knockout mice. However, expression or activity of Nrf2-regulated antioxidant enzymes, such as γ-glutamylcysteine synthetase (GCS), heme oxygenase-1(HO-1) and glutathione S-transferase (GST), were upregulated in the pyrazole-treated wild type mice, but to a lesser extent or not at all in the pyrazole-treated Nrf2 knockout mice. Treatment with antioxidants such as vitamin C or S-Adenosyl-L-methionine (SAM) or an inhibitor of iNOS prevented the pyrazole-induced oxidative liver damage, thus validating the role of oxidative/nitrosative stress in the pyrazole-induced liver injury to the Nrf2 knockout mice. In summary, even though ROS-producing CYP2E1/2A5 were not elevated by pyrazole, impaired antioxidant capacity resulting from Nrf2 deficiency appear to be sufficient to promote pyrazole-induced oxidative liver injury.
Keywords: Nuclear factor-erythroid 2-related factor 2 (Nrf2), pyrazole, CYP2E1, CYP2A5, oxidative/nitrosative stress, free radicals
Pyrazole has been used as an inducer of cytochrome P450 2E1 (CYP2E1) to study CYP2E1-mediated oxidative stress and liver injury (Yang and Cederbaum, 1996; Wu and Cederbaum, 2000; Guan et al, 1992.). Microsomes isolated from rats treated with pyrazole for 2–3 days showed an approximately two- to four-fold increase in CYP2E1 (Palakodety et al., 1988; Winters and Cederbaum, 1992). After administration of pyrazole to rats, mRNA levels did not increase, suggesting that the mechanism of induction was at the level of protein stabilization (Song et al., 1989; Winters and Cederbaum, 1992). Due to being poorly coupled with NADPH-cytochrome P450 reductase, CYP2E1 exhibits enhanced NADPH oxidase activity and elevated rates of production of reactive oxygen species (ROS) such as superoxide anion radical and hydrogen peroxide (H2O2) and, in the presence of iron catalysts, produces powerful oxidants such as the hydroxyl radical (Boveris et al., 1983; Ekstrom and Ingelman-Sundberg, 1989; Rashba-Step et al., 1993). Usually, pyrazole-treated animals with higher levels of CYP2E1 and 2A5 do not show liver injury, but they are more sensitive to other hepatotoxins such as LPS (Lu and Cederbaum, 2006a). In addition to CYP2E1 induction, pyrazole also induces CYP2A5 (Juvonen et al., 1985; Gilmore et al., 2003). Pyrazole induction of CYP2A5 is also believed to be related to oxidative stress and liver damage. Vitamin E attenuates CYP2A5 induction by pyrazole, and GSH depletion by BSO induces CYP2A5 (Gilmore et al., 2003). Induction of CYP2A5 by pyrazole is a direct consequence of endoplasmic reticulum damage, dysfunction, and stress, which is believed to be related to pyrazole-induced oxidative stress (Gilmore and Kirby, 2004). In a previous study (Lu and Cederbaum, 2006a), we showed that, while either pyrazole or LPS alone did not induce liver injury, combination of pyrazole plus LPS induced severe liver injury, and the liver injury involves oxidative stress and induction of CYP2E1 and 2A5 by pyrazole.
Oxidative stress reflects an unbalance between production of ROS and antioxidant capacity to remove ROS. Nuclear factor erythroid 2-related factor 2 (Nrf2) plays an important role in antioxidant response element (ARE)-mediated antioxidant gene expression (Alam et al., 1999; Kang et al., 2005). Under normal physiological conditions, Nrf2 is bound to Kelch-like ECH-associated protein-1 (Keap1) and thereby sequestered in the cytoplasm, but upon oxidation of cysteine residues, Nrf2 is dissociated and released from Keap1 and translocates to the nucleus, where it binds to ARE sequences leading to transcriptional activation of antioxidant and phase II detoxifying genes (Zhang, 2006). In previous studies (Gong and Cederbaum, 2006 a and b), we showed that Nrf2 is increased in cells over-expressing CYP2E1, and the increased Nrf2 activates two Nrf2-regulated antioxidant enzymes gamma-glutamylcysteine synthetase (GCS) and heme oxygenase 1 (HO-1) expression, which protect against CYP2E1-dependent cytotoxicity. Nrf2 is also increased in livers from mice and rats treated with pyrazole (Gong and Cederbaum, 2006 a), but the toxicological or functional significance of this increase is not known. In the present study, we found that, pyrazole did not cause liver injury in wild type mice, due to compensative increases in Nrf2-regulated antioxidant capacity, although ROS producing CYP2E1 and 2A5 were induced. However, in Nrf2 knockout mice, due to failed or impaired upregulation of antioxidant capacity, pyrazole induced severe oxidative liver injury, even though CYP2E1 and 2A5 were not elevated.
Materials and Methods
Reagents
Pyrazole, lipopolyssachride (LPS), N(omega)-Nitro-L-arginine methyl ester (L-NAME), S-adenosyl-methionine (SAM), 1-chloro-2,4-dinitrobenzene (CDNB), p-nitrophenol (PNP), H2O2, 7-ethoxycoumarin, Coumarin, Ac-DEVD-AMC, were purchased from Sigma (St. Louis, MO); thiobarbituric acid (TBA), o-phthalaldehyde, and vitamin C were from Fisher (Pittsburgh, PA).
Antibodies
Anti-3-nitrotyrosine (3-NT) adducts Ig G was from Upstate (Lake Placid, NY); Ig G for Nrf2 was from Santa Cruz Biotechnology (Santa Cruz, CA); Ig G for heme oxygenase 1 (HO-1) and iNOS were from Stressgen Biotechnologies (Victoria, Canada); Ig G for β-actin was from Sigma; Ig G for γ-glutamylcysteine synthetase (GCS) was from Lab Vision Corp. (Fremont, CA). Antibodies against CYP2E1 and CYP2A5 were generous gifts from Drs Jerome Lasker (Hackensack Biomedical Research Institute, Hackensack, NJ) and Risto Juvonen (Department of Pharmacology and Toxicology, University of Kuopio, Kuopio, Finland).
Animals and treatments
C57BL/6 background Nrf2-knockout mice were kindly provided by Dr. Masayuki Yamamoto (Tsukuba University, Japan), and the offspring’s of these mating pairs were used in this study. C57BL/6 wild type mice were from Charles River laboratory. All mice were housed in temperature-controlled animal facilities with 12-hour light/dark cycles and permitted consumption of tap water and Purina standard chow ad libitum. The mice received humane care and experiments were carried out according to the criteria outlined in the Guide for the Care and Use of Laboratory Animals and with approval of the Mount Sinai Animal Care and Use Committee.
Wild type and Nrf2 knockout mice were injected intraperitoneally with pyrazole, 150 mg/kg body wt, once per day for 2 days or 0.9% saline as control, respectively. Twenty four h after the last pyrazole or saline injection, blood was collected from the retro-orbital venous sinus under anesthesia, and then the mice were sacrificed. Livers were removed, washed with cold saline, and excised into fragments; one aliquot of tissue was placed in 10% formalin solution for paraffin blocking, whereas the other aliquots were stored at −80°C for subsequent assays. For intervention study, SAM (50 mg/kg body wt) or vitamin C (125 mg/kg body wt) were injected intraperitoneally every 12 h for 3 days; the mice were treated with pyrazole on days 2 and 3 as above; L-NAME (100 mg/kg body wt) was injected intraperitoneally once a day for 3 days and the mice were treated with pyrazole on days 2 and 3 as above.
Serum ALT and, AST Determination
The collected blood was centrifuged at 3,000 rpm for 5 min, and serum was separated. Alanine aminotransferase (ALT) and asparate aminotransferase (AST) were assayed using diagnostic kits (Thermo Electron, Louisville, CO).
Liver pathology and immunohistochemistry
Liver samples were fixed in 10% formalin solution and embedded in paraffin. Liver sections (5 µm thick) were stained with hematoxylin and eosin for pathological evaluation. Immunohistochemical staining for 3-NT adducts was performed by using anti-3-NT adducts primary antibodies and a rabbit ABC staining kit (Santa Cruz Biotechnology, Santa Cruz, CA).
Western blot analysis
Hepatic proteins (100µg) were separated by SDS-PAGE and transferred to nitrocellulose membranes. Membranes were then incubated for overnight with the indicated antibodies followed by 1 h incubation with correspondent peroxidase secondary antibodies. Chemiluminescence detection was performed by the ECL™ Western blotting method (Amersham Biosciences Corp., Baie d'Urfe, QC). Densitometric analyses were performed by using Molecular Analyst™ software (Version 2.1, Bio-Rad Laboratories).
Terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling (TUNEL) assay and caspase 3 activity
DNA fragmentation was assessed by TUNEL assay using an ApopTag in situ apoptosis detection kit (Chemicon, Temecula, CA). Caspase 3 activity was determined from the rate of AMC formation from 0.2 mM Ac-DEVD-AMC.
Cytochrome P450 2E1 and 2A5, and ECOD Activities
CYP2A5 activity was measured by assessing coumarin 7-hydroxylase activity with 100 µM coumarin as substrate plus 100 µg of microsomal protein and incubation for 10 min at 37°C (Lu and Cederbaum, 2006a). 7-Ethoxycoumarin-O-deethylase (ECOD) activity was measured with the same conditions except for using 100 µM 7-ethoxycoumarin as substrate instead of coumarin (Caro and Cederbaum, 2005). CYP2E1 activity was measured by the rate of oxidation of 1 mM PNP (Reinke and Moyer, 1985). The reaction was performed with 100 µg of microsomal protein for 30 min at 37°C.
Measurement of Catalase Activity
Catalase activity in the homogenate was determined (Lu and Cederbaum, 2006a) by adding liver homogenate to a cuvette containing 100 µl of 20 mM H2O2 in 50 mM potassium phosphate buffer (pH 7.4), and the decrease in absorbance was measured at 240 nm for 1 min.
Measurement of Glutathione S-transferase Activity
Glutathione S-transferase (GST) activity was measured by adding 100 µg of homogenate protein to a cuvette containing 100 µl of 1 mM CDNB and 1 mM GSH in 0.1 M potassium phosphate buffer (pH 6.5) and measuring the increase in absorbance at 340 nm for 1 min.
Measurement of reduced glutathione (GSH) levels
Trichloroacetic acid (TCA) was added to liver homogenate to a final concentration of 5% and the mixture was incubated at 4°C for 30 min to extract GSH. The TCA extract (10 µl) was added to tubes containing 1 mg/ml o-phthalaldehyde (200 µl) in methanol. The tubes were incubated at 37°C in the dark (15 min). Fluorescence was measured (excitation 350 nm, emission 420 nm) using a Perkin-Elmer LC-50B spectrofluorometer. The concentration of GSH was determined from a GSH standard curve (Lu and Cederbaum, 2006 b).
Lipid peroxidation analysis
Hepatic homogenates were incubated with 0.2 ml of TCA [15% (wt/vol)]- TBA [ 0.375% (wt/vol)]-HCl (0.25 N) solution in a boiling water bath for 10 min. After centrifugation at 1,000 rpm for 5 min, the resulting supernatant was used to determine the formation of TBA-reactive substances (TBARS) by evaluating absorbance at 535 nm. MDA treated as above served as a standard (Lu and Cederbaum, 2005).
Statistics
Results are expressed as mean±SD. Statistical evaluation was carried out by analysis of variance (ANOVA) followed by Student-Newman-Keuls post hoc test. Results were considered statistically different if P was less than 0.05.
Results
Pyrazole induces necrotic hepatotoxicity in Nrf2 knockout mice but not in wild type mice
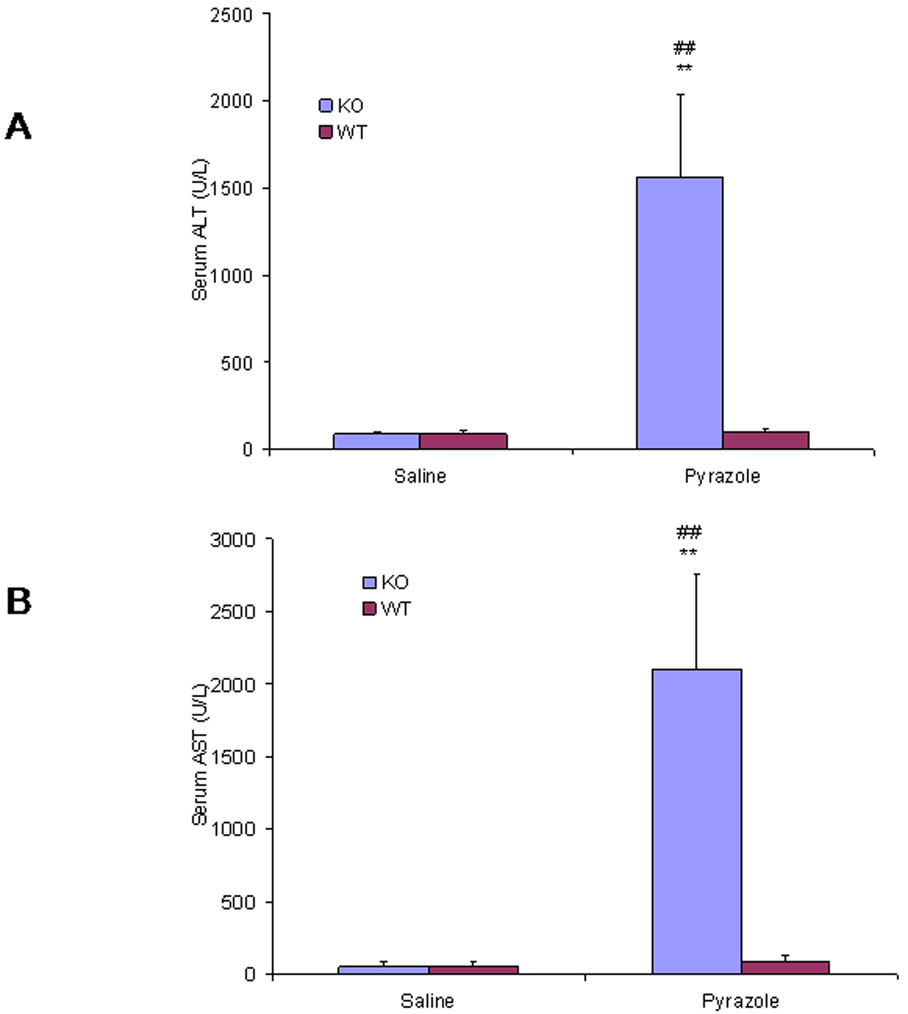
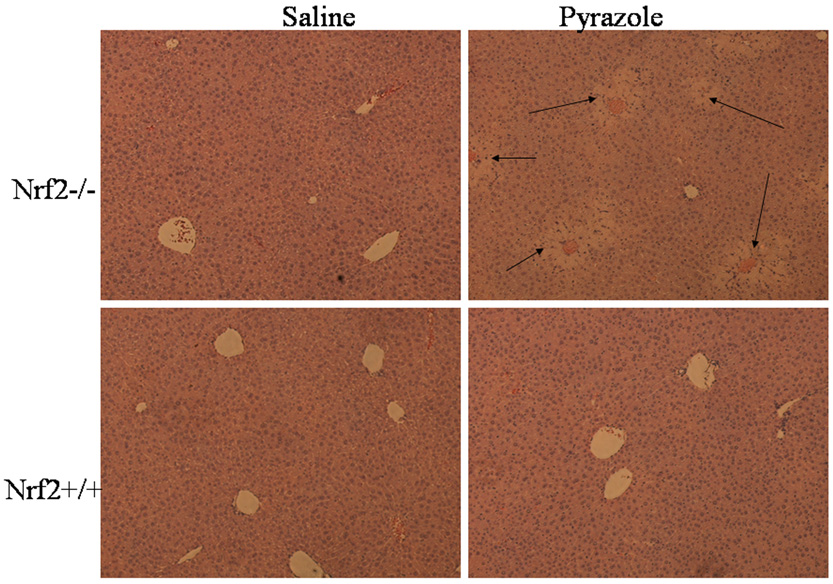
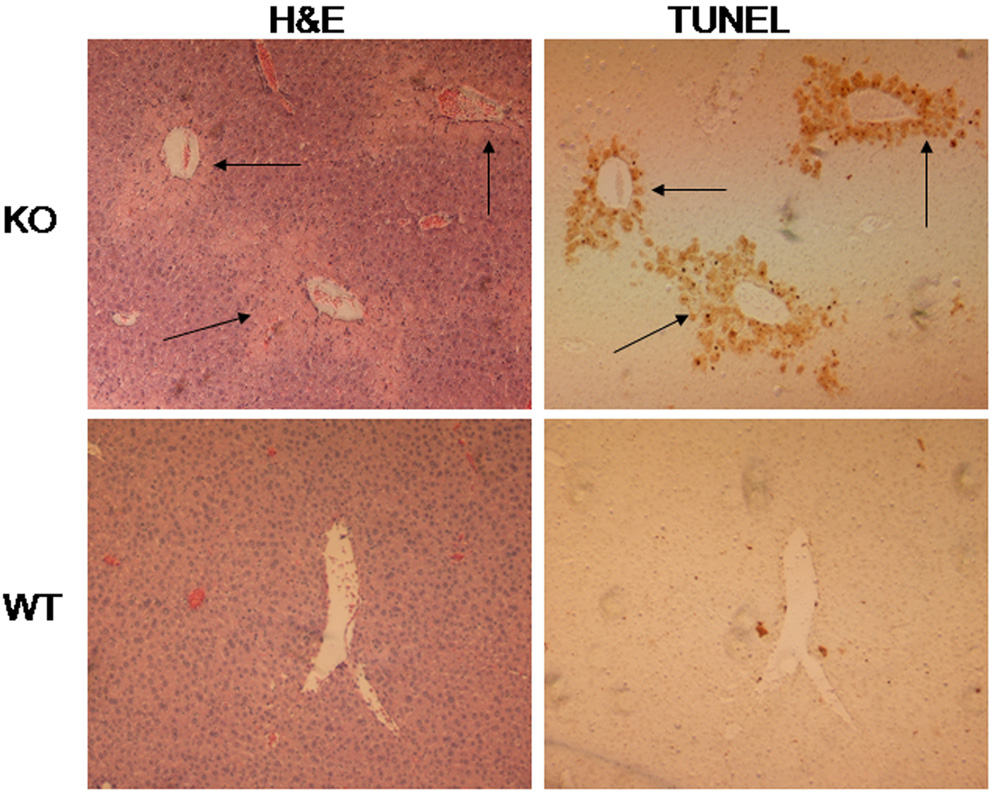
Serum ALT and AST, two markers for hepatic necrosis, increased dramatically after treatment with pyrazole in the Nrf2 knockout mice, but no changes in both serum ALT and AST were observed in the wild type mice (Fig. 1). Pathology evaluation showed that many necrotic areas around the central veins were found in the liver sections from the pyrazole treated Nrf2 knockout mice, but no pathological change was observed in the pyrazole treated wild type mice (Fig. 2). TUNEL staining, an assay for apoptosis, showed that many positive staining cells around the central veins were observed in the liver sections from the pyrazole treated Nrf2 knockout mice, but only casual staining was observed in the pyrazole treated wild type mice (data not shown). However, caspase 3 activity did not significantly increased in the pyrazole-treated Nrf2 knockout mice (data not shown). It is possible that the increase in TUNEL staining reflects necrosis-induced DNA fragmentation rather than apoptosis. H&E staining and TUNEL staining were conducted in successive slices from the pyrazole treated Nrf2 knockout mice or wild type mice; TUNEL positive staining and hepatic necrosis were located in the same areas (Fig. 3). These results suggest that pyrazole induced necrotic but not apoptotic damage in Nrf2 knockout mice.
Fig. 1.
Effect of pyrazole on levels of serum (A) alanine aminotransferase (ALT) and (B) aspartate aminotransferase (AST). Pyrazole (150 mg/kg body wt) or saline was injected ip daily for 2 days in wild type and Nrf2 knockout mice. Blood was collected 24 h after the second injection of pyrazole, and transaminase levels were assayed as described in MATERIALS AND METHODS. **P < 0.01, compared with pyrazole wid type group; ## P<0.01, compared with saline Nrf2 knockout group.
Fig. 2.
Hematoxylin-eosin-stained liver sections after treatment with pyrazole. Arrows show areas of necrosis around the central veins.
Fig. 3.
Comparison of TUNEL staining with H&E staining in the same field. Arrows show positive TUNEL staining or areas of necrosis around the central veins.
Pyrazole-induced liver injury in the Nrf2 knockout mice involves oxidative/nitrosative stress
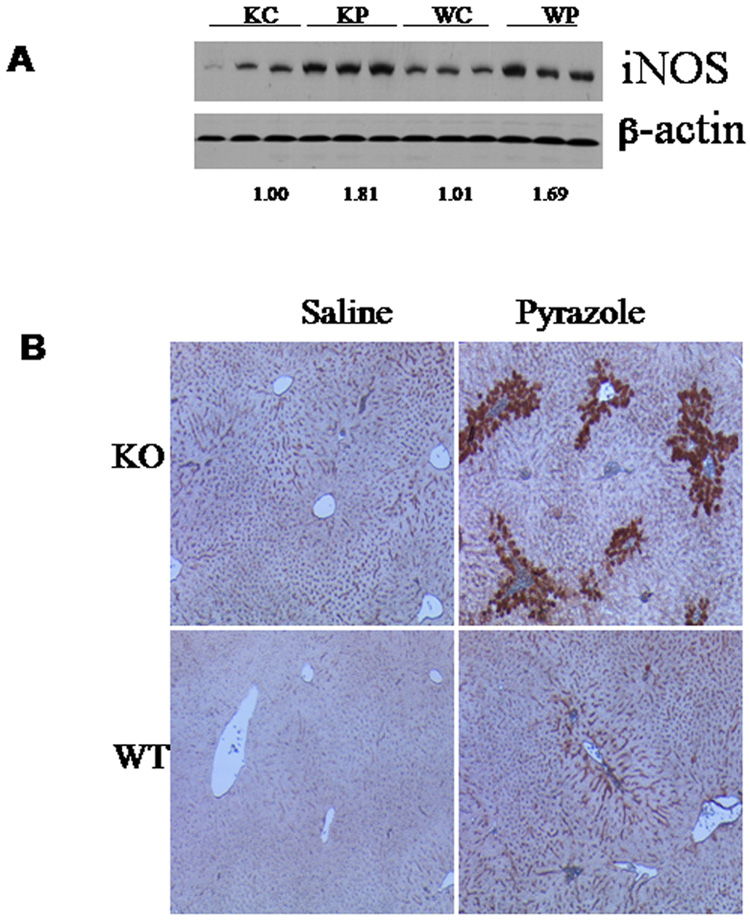
Pyrazole induced iNOS expression both in knockout and wild type mice to comparable extents (Fig.4A). However, after pyrazole treatment positive staining for 3-NT was only observed in the knockout mice, but not in the wild type mice (Fig.4B). The positive staining was around the central veins, the area where the liver injury occurs (Fig.4B). Lipid peroxidation reflected by TBARS levels in liver homogenates was dramatically increased by pyrazole in the knockout mice, but only slightly increased in the wild type mice (Fig.5). Compensative increase in GSH levels by pyrazole was observed in the wild type mice, but not in the knockout mice (Fig.5). Intervention experiments with Vc and SAM, two antioxidants, and L-NAME, an inhibitor of iNOS, were performed to validate whether oxidative/nitrosative stress are important in pyrazole induced liver injury in the Nrf2 knockout mice, since the increases in 3-NT or TBARS (Fig. 4) may just be associated with the liver injury but not be causative. Pyrazole elevation of serum AST and AST was inhibited by Vc, SAM and L-NAME (Fig. 6A); in addition, the increase in TBARS was also inhibited by Vc, SAM and L-NAME (Fig. 6B). These results suggest that oxidative/nitrosative stress induced by pyrazole caused liver injury in the Nrf2 knockout mice.
Fig. 4.
Effects of pyrazole on iNOS expression (A) and 3-NT adduct formation (B) in liver. The expression of iNOS was determined by western blotting analysis. Numbers below the blots refer to the iNOS/β-actin ratio. The formation of 3-NT protein adducts was determined by immunohistochemistry as described in Materials and methods. KC, Nrf2 knockout treated with saline; KP, Nrf2 knockout treated with pyrazole; WC, WP, wild type treated with saline or pyrazole, respectively.
Fig. 5.
Effects of pyrazole on levels of hepatic GSH and TBARS. The levels of GSH and TBARS in liver homogenates were determined as described in Materials and methods.
Fig. 6.
Effects of SAM, vitamin C, and L-NAME on the elevated ALT and AST (A) and TBARS (B) induced by pyrazole in Nrf2 knockout mice. SAM (50 mg/kg body wt) or vitamin C (125 mg/kg body wt) were injected intraperitoneally every 12 h for 3 days; the mice were treated with pyrazole (150 mg/kg body wt, ip) on days 2 and 3; L-NAME (100 mg/kg body wt) was injected intraperitoneally once a day for 3 days; the mice were treated with pyrazole on days 2 and 3 as above. ** P<0.01, compared with the sal group; # P<0.05 and ## P<0.01, compared with the Pyr group. Sal, saline; Pyr, pyrazole; Vc, vitamin C; SAM, S-adenosyl methionine; L-NAME, N(omega)-Nitro-L-arginine methyl ester.
Oxidative liver injury by pyrazole in the Nrf2 knockout mice is independent of induction of cytochrome P450s
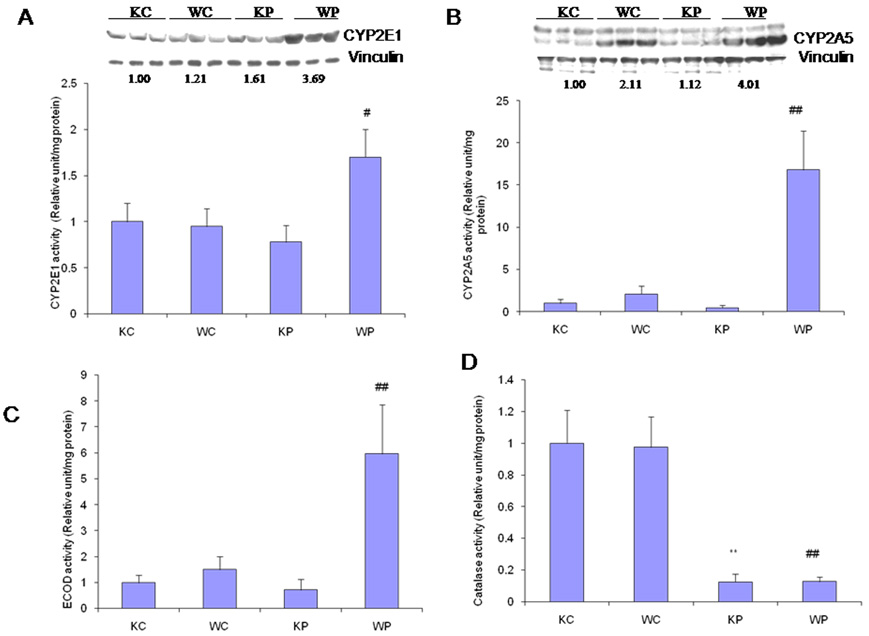
Although as expected, the CYP2E1 catalytic activity and protein expression were induced by pyrazole in the wild type mice, to our surprise, they did not change after pyrazole treatment in the Nrf2 knockout mice (Fig. 7A). Similarly, CYP2A5 activity and protein were elevated by pyrazole in the wild type mice, but did not change in the Nrf2 knockout mice (Fig. 7B). These surprising results were extended to ECOD activity. 7-Ethoxycoumarin is a general P450 substrate, being metabolized by O-deethylation by several enzymes of the CYP1, CYP2 and CYP3 families (Waxman and Chang, 1998). ECOD activity was increased by pyrazole in the wild type mice but no change was observed in the Nrf2 knockout mice (Fig. 7C). The above results suggest that pyrazole-induced hepatotoxicity in Nrf2 knockout mice is not due to the induction of cytochrome P450s. Pyrazole can inhibit the activity of catalase, an important antioxidant enzyme (Lu and Cederbaum, 2006). Catalase activity was inhibited by pyrazole comparably in Nrf2 knockout and wild type mice (Fig.7D).
Fig. 7.
Effects of pyrazole on p-nitrophenol (PNP) oxidation activity and CYP2E1 protein expression (A), coumarin 7-hydroxylase activity and CYP2A5 protein expression (B), 7-Ethoxycoumarin-O-deethylase (ECOD) activity (C) and catalase activity (D) in liver. Pyrazole (150 mg/kg body wt) or saline was injected ip daily for 2 days and mice killed 24 h after the second injection of pyrazole. Measurement of enzyme activities was carried out as described in MATERIALS AND METHODS. ** P<0.01, compared with KC group; #P<0.05 and ## P<0.01, compared with WC group.
Impaired upregulation of Nrf2-regulated antioxidant enzymes might contribute to pyrazole-induced oxidative liver injurys
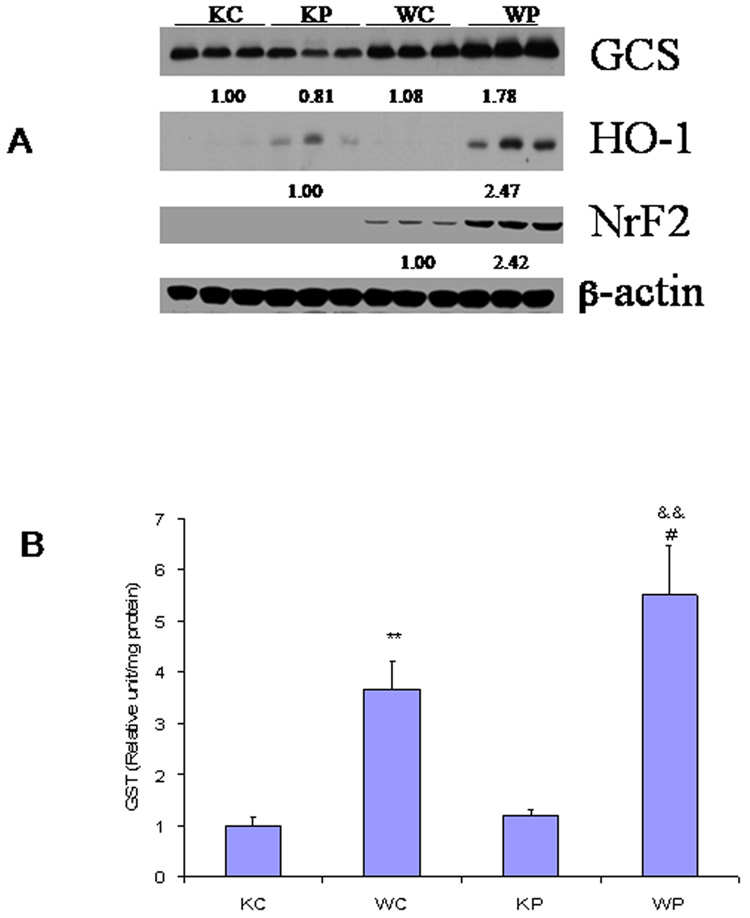
Nrf2-regulated GCS and HO-1 expression was increased in pyrazole-treated wild type mice, compared with saline-treated wild type mice (Fig. 8A). However, in pyrazole-treated Nrf2 knockout mice, GCS expression was not elevated and upregulation of HO-1was lower than that in pyrazole-treated wild type mice (Fig. 8A). The western blots validated the absence of Nrf2 in Nrf2 knockout mice, but in the wild type mice, pyrazole induced upregulation of Nrf2 (Fig. 8A). Glutathione S-transferase (GST) can be regulated by Nrf2 (Kwak et al., 2001). Basal levels of GST activity were about 4-fold lower in the Nrf2 knockout mice than the activity in the wild type mice, and pyrazole produced an increase in GST activity in the wild type mice but not in the Nrf2 knockout mice (Fig. 8B). Thus, treatment with pyrazole caused increase in the activity/expression of several antioxidants (GST, HO-1, GCS) in wild type mice, however, this upregulation was much less effective or did not occur in Nrf2 knockout mice (Fig.8A and B). It appears that oxidative stress resulted from the impairment of Nrf2-regulated upregulation of antioxidant enzymes after pyrazole treatment and this caused liver injury.
Fig. 8.
Effects of pyrazole on hepatic activity or expression of several oxidative/nitrosative stress related enzymes. (A) The indicated enzyme expression was determined by western blotting analysis as described in MATERIALS AND METHODS. Numbers below the blots refer to the enzyme/β-actin ratio. (B) The activity of GST was determined as described in MATERIALS AND METHODS. ** P<0.01, compared with KC group; && P<0.01, compared with KP group; # P<0.05, compared with WC group. KC, Nrf2 knockout treated with saline; KP, Nrf2 knockout treated with pyrazole; WC, WP, wild type treated with saline or pyrazole, respectively.
Discussion
In this study, we found that pyrazole treatment alone induced hepatotoxicity in Nrf2 knockout mice but not in wild type mice. Similarly, elevated oxidative status was detected in pyrazole-treated Nrf2 knockout mice but not in wild type mice. Vc, SAM and L-NAME protected against the pyrazole-induced liver injury in Nrf2 knockout mice. These results suggest that pyrazole-induced liver injury in Nrf2 knockout mice is due to oxidative stress.
Oxidative stress reflects a balance between production of ROS and antioxidant capacity to remove ROS. In CYP2E1-overexpressing HepG2 cells, total GSH levels, GSH synthetic rate and GCS mRNA were increased (Mari and Cederbaum, 2000). Activity, protein and mRNA levels for other antioxidants such as catalase, alpha- and microsomal GST were also increased (Mari and Cederbaum, 2001). Up-regulation of these antioxidant genes was suggested to reflect an adaptive mechanism to remove CYP2E1-derived oxidants and was dependent on Nrf2 upregulation (Gong and Cederbaum, 2006 b). Induction of HO-1 protects against CYP2E1-dependent toxicity (Gong et al., 2004). The protective effects of Nrf2 against CYP2E1-dependent toxicity can be blocked by L-buthionine-(S,R)-sulfoximine (BSO), a specific inhibitor of GCS, which prevents the synthesis of GSH. Is pyrazole-induced liver injury in Nrf2 knockout mice due to failed or impaired induction of Nrf2-regulated antioxidant capacity? Nrf2 and three of its target genes, GCS, GST and HO-1, were upregulated in wild type mice; but in Nrf2 knockout mice, no upregulation of GST and GCS was observed, and upregulation of HO-1 was lower than that in wild type mice. Pyrazole elevation of GSH levels was observed in pyrazole-treated wild type mice but not in the Nrf2 knockout mice (Fig. 5). Therefore, it is postulated that, in pyrazole-treated Nrf2 knockout mice, the impaired antioxidant capacity causes increased accumulation of ROS, which enhances liver injury. In pyrazole-treated wild type mice, the compensatory increase in antioxidant capacity prevents increased accumulation of ROS, which therefore protects against liver injury. In pyrazole-treated Nrf2 knockout mice, ROS scavengers such as Vc and SAM can take the place of the lowered antioxidant capacity, thereby protecting against pyrazole-induced liver injury. Certainly, other Nrf2-dependent enzymes and cytoprotective factors besides GST, GCS, HO-1 may be important in the protection against the pyrazole toxicity.
Some studies suggest that excessive formation of NO can result in oxidative stress in the liver (Schild et al, 2003), but other reports showed that NO has a protective effect as the production of NO improved the microcirculation and oxygen metabolism (Corso, et al, 1998). In the current study, pyrazole induction of iNOS is comparable in the knockout and wild type mice. However, pyrazole-induced liver damage may be dependent on the balance of the local production of NO and ROS such as O2.−. Peroxynitrite (ONOO−) is formed by the rapid reaction between NO and O2.− and has been shown to nitrate free and protein-associated tyrosine residues and produce nitrotyrosine (Ischiropoulos, 1998). Although the levels of iNOS were the same, higher levels of O2.− due to impaired antioxidant capacity in pyrazole-treated Nrf2 knockout mice elevate ONOO− formation to a greater extent than that found in pyrazole-treated wild type mice. Therefore, the formation of 3-NT was increased and L-NAME, an inhibitor of iNOS, protected against pyrazole liver damage in the Nrf2 knockout mice.
Why is oxidative/nitrosative stress elevated in the pyrazole-treated Nrf2 knockout mice but not in the wild type mice? Pyrazole induces CYP2E1. CYP2E1 exhibits enhanced NADPH oxidase activity and elevated rates of production of O2.− and H2O2 (Ekstrom and Ingelman-Sundberg, 1989). While elevated CYP2E1 alone (pyrazole treatment alone) does not induce significant liver injury, it enhanced liver injury induced by other hepatotoxins such as LPS (Lu et al., 2005; Lu and Cederbaum, 2006 a). In addition to CYP2E1 induction, pyrazole induces CYP2A5 activity. Gilmore et al (2003) reported that vitamin E attenuates CYP2A5 induction by pyrazole and that GSH depletion by BSO induces CYP2A5 suggests a causal association between oxidative stress and CYP2A5 overexpression. Since cytochrome P450 enzymes can produce ROS during their catalytic turnover (White, 1991), pyrazole induction of CYP2E1 and 2A5 should increase P450-generated ROS. However, although pyrazole induced hepatotoxicity in Nrf2 knockout mice, no elevation in CYP2E1 or 2A5 activities or levels were observed, in contrast to the 2-fold increase in CYP2E1 activity and more than 20-fold elevation of CYP2A5 activity in wild type mice. Despite the elevation in CYP2E1 and 2A5, no hepatotoxicity was detected in wild type mice. These results suggest that pyrazole-induced oxidative liver injury in Nrf2 knockout mice is not due to the induction of CYP2E1 and 2A5. Information about pyrazole metabolism might provide an insight into the pathogenesis of pyrazole liver injury; hydroxylation of pyrazole to 4-hydroxypyrazole by CYP2E1 can occur (Clejan and Cederbaum, 1990), however, no other literature is available. Interestingly, 4-hydroxypyrazole can inhibit catalase (MacDonald et al, 1981). While catalase was decreased by pyrazole in both the Nrf2 knockouts and wild type mice, inhibition of this important antioxidant enzyme may have more serious repercussions with respect to the antioxidant status in the pyrazole-treated Nrf2 knockout mice with a failed capacity to upregulate GSH and other antioxidants than in the wild type mice with antioxidant upregulation. How is pyrazole promoting liver injury or elevating oxidative stress in the knockout mice in the absence of increases in CYP2E1 and 2A5? Whether ROS production is increased by other pathways or enzymes cannot be ruled out since pyrazole treatment upregulates and downregulates the expression of many genes in mice as recently shown by microarray analysis (Nichols and Kirby, 2007). Further studies are needed to address this issue.
Cytochrome P450s are generally not considered as Nrf2 target genes. Recent studies have shown that CYP2A5 indeed contains an Nrf2 response element (Abu-Bakar et al., 2004; 2007). Two putative stress response elements (STRE) were localized to positions −2514 to −2386 to −2377 of the CYP2A5 promoter, with the more proximal sequence specifically binding Nrf2 and the authors concluded that Nrf2 activates CYP2A5 transcription by directly binding to the proximal STRE (Abu-Bakar et al., 2007). This would explain why pyrazole is ineffective in inducing CYP2A5 in the Nrf2 knockout mice. More complicated, however, is to understand why pyrazole failed to induce CYP2E1 in the Nrf2 knockout mice. Induction of CYP2E1 by pyrazole is mainly posttranscriptional (Eliasson et al., 1988) as pyrazole can stabilize CYP2E1 against proteasome-mediated degradation (Bardag-Gorce et al., 2002; Roberts, 1997; Yang and Cederbaum, 1997). Why the lack of Nrf2 would block this stabilization of CYP2E1 by pyrazole against proteasome-mediated degradation is not known. Perhaps of importance is the finding that the expression of some subunits of 20S and 19S proteasomes can be transcriptionally induced by dithiolethione in mouse liver in an Nrf2-dependent manner (Kwak et al., 2003). Whether this Nrf2-modulation of the proteasome complex plays a role in the ineffectiveness of pyrazole to elevate CYP2E1 levels will require future studies.
In summary, in wild type mice, although ROS producing CYP2E1 and 2A5 were induced, no liver injury was observed due to compensative increases in Nrf2-regulated antioxidant capacity. However, in Nrf2 knockout mice, due to failed or impaired upregulation of antioxidant capacity, pyrazole induced severe oxidative liver injury, even though CYP2E1 and 2A5 were not elevated. The source of ROS production in pyrazole-treated Nrf2 knockout mice remains unclear.
Acknoledgement
We thank Drs Jerome Lasker (Hackensack Biomedical Research Institute, Hackensack, NJ) and Risto Juvonen (Department of Pharmacology and Toxicology, University of Kuopio, Kuopio, Finland) for generously providing antibodies against CYP2E1 and CYP2A5, respectively.
Grant: These studies were supported by United State Public Health Service Grant AA06610from the National Institute on Alcohol Abuse and Alcoholism.
Abbreviations
- CYP
Cytochrome P450
- GST
glutathione S-transferase
- 3-NT
3-nitrotyrosine
- LPS
lipopolyssachride
- Vc
Vitamin C
- Nrf2
Nuclear factor erythroid 2-related factor 2
- iNOS
inducible nitric oxide synthase
- GCS
γ-glutamylcysteine synthetase
- SAM
S-adenosyl methionine
- HO-1
heme oxygenase-1
- CDNB
1-chloro-2,4-dinitrobenzene
- ARE
antioxidant response element
- L-NAME
N(omega)-Nitro-L-arginine methyl ester
- TBA
thiobarbituric acid
- TBARS
TBA-reactive substances
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- Abu-Bakar A, Satarug S, Marks GC, Lang MA, Moore MR. Acute cadmium chloride administration induces hepatic and renal CYP2A5 mRNA, protein and activity in the mouse: involvement of transcription factor NRF2. Toxicol. Lett. 2004;148:199–210. doi: 10.1016/j.toxlet.2003.10.029. [DOI] [PubMed] [Google Scholar]
- Abu-Bakar A, Lamsa V, Arpiainen S, Moore MR, Lang MA, Hakkola J. Regulation of CYP2A5 gene by the transcription factor nuclear factor (erythroid-derived 2)-like 2. Drug Metab. Dispos. 2007;35:787–794. doi: 10.1124/dmd.106.014423. [DOI] [PubMed] [Google Scholar]
- Alam J, Stewart D, Touchard C, Boinapally S, Choi AM, Cook JL. Nrf2, a Cap'n'Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J. Biol. Chem. 1999;274:26071–26708. doi: 10.1074/jbc.274.37.26071. [DOI] [PubMed] [Google Scholar]
- Bardag-Gorce F, Li J, French BA, French SW. Ethanol withdrawal induced CYP2E1 degradation in vivo, blocked by proteasomal inhibitor PS-341. Free Radic. Biol. Med. 2002;32:17–21. doi: 10.1016/s0891-5849(01)00768-7. [DOI] [PubMed] [Google Scholar]
- Boveris A, Fraga CG, Varsavsky AI, Koch OR. Increased chemiluminescence and superoxide production in the liver of chronically ethanol-treated rats. Arch. Biochem. Biophys. 1983;227:534–541. doi: 10.1016/0003-9861(83)90482-4. [DOI] [PubMed] [Google Scholar]
- Caro AA, Cederbaum AI. Inhibition of CYP2E1 catalytic activity in vitro by S-adenosyl-L-methionine. Biochem. Pharmacol. 2005;69:1081–1093. doi: 10.1016/j.bcp.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Clejan LA, Cederbaum AI. Oxidation of pyrazole by reconstituted systems containing cytochrome P-450 IIE1. Biochim. Biophys. Acta. 1990;1034:233–237. doi: 10.1016/0304-4165(90)90082-8. [DOI] [PubMed] [Google Scholar]
- Corso CO, Gundersen Y, Dorger M, Lilleaasen , Aasen AO, Messmer K. Effects of the nitric oxide synthase inhibitors NG-nitro-L-arginine methyl ester and aminoethyl-isothiourea on the liver microcirculation in rat endotoxemia. J. Hepatol. 1998;28:61–69. doi: 10.1016/s0168-8278(98)80203-1. [DOI] [PubMed] [Google Scholar]
- Ekstrom G, Ingelman-Sundberg M. Rat liver microsomal NADPH-supported oxidase activity and lipid peroxidation dependent on ethanol-inducible cytochrome P-450 (P-450IIE1) Biochem. Pharmacol. 1989;38:1313–1319. doi: 10.1016/0006-2952(89)90338-9. [DOI] [PubMed] [Google Scholar]
- Eliasson E, Johansson I, Ingelman-Sundberg M. Ligand-dependent maintenance of ethanol-inducible cytochrome P-450 in primary rat hepatocyte cell cultures. Biochem. Biophys. Res. Commun. 1988;150:436–443. doi: 10.1016/0006-291x(88)90539-6. [DOI] [PubMed] [Google Scholar]
- Gilmore WJ, Hartmann G, Piquette-Miller M, Marriott J, Kirby GM. Effects of lipopolysaccharide-stimulated inflammation and pyrazole-mediated hepatocellular injury on mouse hepatic Cyp2a5 expression. Toxicology. 2003;184:211–226. doi: 10.1016/s0300-483x(02)00581-4. [DOI] [PubMed] [Google Scholar]
- Gilmore WJ, Kirby GM. Endoplasmic reticulum stress due to altered cellular redox status positively regulates murine hepatic CYP2A5 expression. J. Pharmacol. Exp. Ther. 2004;308:600–608. doi: 10.1124/jpet.103.060111. [DOI] [PubMed] [Google Scholar]
- Gong P, Cederbaum AI, Nieto N. Heme oxygenase-1 protects HepG2 cells against cytochrome P450 2E1-dependent toxicity. Free Radic. Biol. Med. 2004;36:307–318. doi: 10.1016/j.freeradbiomed.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Gong P, Cederbaum AI. Nrf2 is increased by CYP2E1 in rodent liver and HepG2 cells and protects against oxidative stress caused by CYP2E1. Hepatology. 2006a;43:144–153. doi: 10.1002/hep.21004. [DOI] [PubMed] [Google Scholar]
- Gong P, Cederbaum AI. Transcription factor Nrf2 protects HepG2 cells against CYP2E1 plus arachidonic acid-dependent toxicity. J. Biol. Chem. 2006b;281:14573–14579. doi: 10.1074/jbc.M600613200. [DOI] [PubMed] [Google Scholar]
- Ischiropoulos H. Biological tyrosine nitration: a pathophysiological function of nitric oxide and reactive oxygen species. Arch. Biochem. Biophys. 1998;356:1–11. doi: 10.1006/abbi.1998.0755. [DOI] [PubMed] [Google Scholar]
- Juvonen RO, Kaipainen PK, Lang MA. Selective induction of coumarin 7-hydroxylase by pyrazole in D2 mice. Eur. J. Biochem. 1985;152:3–8. doi: 10.1111/j.1432-1033.1985.tb09156.x. [DOI] [PubMed] [Google Scholar]
- Kang KW, Lee SJ, Kim SG. Molecular mechanism of nrf2 activation by oxidative stress. Antioxid. Redox. Signal. 2005;7:1664–1673. doi: 10.1089/ars.2005.7.1664. [DOI] [PubMed] [Google Scholar]
- Kwak MK, Itoh K, Yamamoto M, Sutter TR, Kensler TW. Role of transcription factor Nrf2 in the induction of hepatic phase 2 and antioxidative enzymes in vivo by the cancer chemoprotective agent, 3H-1, 2-dimethiole-3-thione. Mol. Med. 2001;7:135–145. [PMC free article] [PubMed] [Google Scholar]
- Kwak MK, Wakabayashi N, Greenlaw JL, Yamamoto M, Kensler TW. Antioxidants enhance mammalian proteasome expression through the Keap1-Nrf2 signaling pathway. Mol. Cell Biol. 2003;23:8786–8794. doi: 10.1128/MCB.23.23.8786-8794.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang MA, Juvonen R, Järvinen P, Honkakoski P, Raunio H. Mouse liver P450Coh: genetic regulation of the pyrazole-inducible enzyme and comparison with other P450 isoenzymes. Arch. Biochem. Biophys. 1989;271:139–148. doi: 10.1016/0003-9861(89)90264-6. [DOI] [PubMed] [Google Scholar]
- Lu Y, Wang X, Cederbaum AI. Lipopolysaccharide-induced liver injury in rats treated with the CYP2E1 inducer pyrazole. Am. J. Physiol. Gastrointest. Liver Physiol. 2005;289:G308–G319. doi: 10.1152/ajpgi.00054.2005. [DOI] [PubMed] [Google Scholar]
- Lu Y, Cederbaum AI. Enhancement by pyrazole of lipopolysaccharide-induced liver injury in mice: role of cytochrome P450 2E1 and 2A5. Hepatology. 2006a;44:263–274. doi: 10.1002/hep.21241. [DOI] [PubMed] [Google Scholar]
- Lu Y, Cederbaum AI. Cisplatin-induced hepatotoxicity is enhanced by elevated expression of cytochrome P450 2E1. Toxicol. Sci. 2006b;89:515–523. doi: 10.1093/toxsci/kfj031. [DOI] [PubMed] [Google Scholar]
- MacDonald E, Ihalainen E, Pispa JP. Pharmacological and toxicological properties of 4-hydroxypyrazole, a metabolite of pyrazole. Acta Pharmacol. Toxicol. (Copenh) 1981;48:418–423. doi: 10.1111/j.1600-0773.1981.tb01641.x. [DOI] [PubMed] [Google Scholar]
- Mari M, Cederbaum AI. CYP2E1 overexpression in HepG2 cells induces glutathione synthesis by transcriptional activation of gamma-glutamylcysteine synthetase. J. Biol. Chem. 2000;275:15563–15571. doi: 10.1074/jbc.M907022199. [DOI] [PubMed] [Google Scholar]
- Mari M, Cederbaum AI. Induction of catalase, alpha, and microsomal glutathione S-transferase in CYP2E1 overexpressing HepG2 cells and protection against short-term oxidative stress. Hepatology. 2001;33:652–661. doi: 10.1053/jhep.2001.22521. [DOI] [PubMed] [Google Scholar]
- Nichols KD, Kirby GM. Expression of cytochrome P450 2A5 in a glucose-6-phosphate dehydrogenase-deficient mouse model of oxidative stress. Biochem. Pharmacol. 2007;75:1230–1239. doi: 10.1016/j.bcp.2007.10.032. [DOI] [PubMed] [Google Scholar]
- Palakodety RB, Clejan LA, Krikun G, Feierman DE, Cederbaum AI. Characterization and identification of a pyrazole-inducible form of cytochrome P-450. J. Biol. Chem. 1988;263:878–884. [PubMed] [Google Scholar]
- Quan Z, Khan S, O'Brien PJ. Role of cytochrome P-450IIE1 in N-nitroso-N-methylaniline induced hepatocyte cytotoxicity. Chem. Biol. Interact. 1992;83:221–233. doi: 10.1016/0009-2797(92)90099-7. [DOI] [PubMed] [Google Scholar]
- Rashba-Step J, Turro NJ, Cederbaum AI. Increased NADPH- and NADH-dependent production of superoxide and hydroxyl radical by microsomes after chronic ethanol treatment. Arch. Biochem. Biophys. 1993;300:401–408. doi: 10.1006/abbi.1993.1054. [DOI] [PubMed] [Google Scholar]
- Reinke LA, Moyer MJ. p-Nitrophenol hydroxylation. A microsomal oxidation which is highly inducible by ethanol. Drug Metab. Dispos. 1985;13:548–552. [PubMed] [Google Scholar]
- Roberts BJ. Evidence of proteasome-mediated cytochrome P-450 degradation. J. Biol. Chem. 1997;272:9771–9778. doi: 10.1074/jbc.272.15.9771. [DOI] [PubMed] [Google Scholar]
- Schild L, Reinheckel T, Reiser M, Horn TF, Wolf G, Augustin W. Nitric oxide produced in rat liver mitochondria causes oxidative stress and impairment of respiration after transient hypoxia. FASEB J. 2003;17:2194–2201. doi: 10.1096/fj.02-1170com. [DOI] [PubMed] [Google Scholar]
- Song BJ, Veech RL, Park SS, Gelboin HV, Gonzalez FJ. Induction of rat hepatic N-nitrosodimethylamine demethylase by acetone is due to protein stabilization. J. Biol. Chem. 1989;264:3568–3572. [PubMed] [Google Scholar]
- Wang X, Lu Y, Cederbaum AI. Induction of cytochrome P450 2E1 increases hepatotoxicity caused by Fas agonistic Jo2 antibody in mice. Hepatology. 2005;42:400–410. doi: 10.1002/hep.20792. [DOI] [PubMed] [Google Scholar]
- Waxman DJ, Chang TKH. Use of 7-ethoxycoumarin to monitor multiple enzymes in the human CYP1, CYP2 and CYP3 families. Methods Mol. Biol. 1998;107:175–179. doi: 10.1385/0-89603-519-0:175. [DOI] [PubMed] [Google Scholar]
- White RE. The involvement of free radicals in the mechanisms of monooxygenases. Pharmacol. Ther. 1991;49:21–42. doi: 10.1016/0163-7258(91)90020-m. [DOI] [PubMed] [Google Scholar]
- Winters DK, Cederbaum AI. Time course characterization of the induction of cytochrome P-450 2E1 by pyrazole and 4-methylpyrazole. Biochim. Biophys. Acta. 1992;1117:15–24. doi: 10.1016/0304-4165(92)90156-o. [DOI] [PubMed] [Google Scholar]
- Wu D, Cederbaum A. Ethanol and arachidonic acid produce toxicity in hepatocytes from pyrazole-treated rats with high levels of CYP2E1. Mol. Cell Biochem. 2000;204:157–167. doi: 10.1023/a:1007064706101. [DOI] [PubMed] [Google Scholar]
- Yang MX, Cederbaum AI. Characterization of cytochrome P4502E1 turnover in transfected HepG2 cells expressing human CYP2E1. Arch. Biochem. Biophys. 1997;341:25–33. doi: 10.1006/abbi.1997.9907. [DOI] [PubMed] [Google Scholar]
- Zhang DD. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab. Rev. 2006;38:769–789. doi: 10.1080/03602530600971974. [DOI] [PubMed] [Google Scholar]