Abstract
Dishevelled (Dvl) is a positive regulator of the canonical Wnt signaling pathway, which regulates the levels of β-catenin. The β-catenin oncoprotein depends upon the association of Dvl and Axin proteins through their DIX domains, and its accumulation directs the expression of specific developmental-related genes at the nucleus. Here, the 1H, 13C, and 15N resonances of the human Dishevelled 2 DIX domain are assigned using heteronuclear nuclear magnetic resonance (NMR) spectroscopy. In addition, helical and extended elements are identified based on the NMR data. The results establish a structural context for characterizing the actin and phospholipid interactions and binding sites of this novel domain, and provide insights into its role in protein localization to stress fibers and cytoplasmic vesicles during Wnt signaling.
Keywords: Dishevelled, DIX domain, Resonance assignments
Introduction
The Wnt signaling pathway is crucial in cell differentiation and proliferation of embryonic and adult tissues, and its disruption leads to human tumors including colorectal cancer (Nelson and Nusse, 2004). Signaling is initiated by secreted Wnt glycoproteins, which bind to seven transmembrane Frizzled receptors and the low-density receptor-related lipoproteins at the cell surface. Downstream events include positive and negative regulation of b-catenin levels by two DIX domain-containing proteins, Dishevelled (Dvl) and Axin, respectively. In unstimulated cells, β-catenin is targeted for destruction in the ubiquitin-proteosome pathway by a protein complex that includes the Axin protein, a putative tumor suppressor (Satoh et al., 2000). Under specific Wnt stimulation, Dvl binds to Axin and disassembles the β-catenin destruction complex. Thus, Dvl boosts the intracellular levels of β-catenin, which translocates to the nucleus where it binds to transcription factors leading to changes in gene expression.
The DIX domain is a 85-residue conserved module found in a family of seven human signaling proteins. Dvl and Axin represent the two major subtypes of DIX domain-containing proteins, and their homo and hetero oligomerization is mediated by their DIX domains (Kishida et al., 1999). A third subtype of a DIX-related protein, the coiled-coil-DIX1, has been recently shown to positively regulate Wnt signaling by hetero oligomerization with Dvl and Axin (Shiomi et al., 2003). The DIX domain interacts with actin stress fibers and vesicular membranes, partitioning Dvl between these two pools and segregating the proteins to distinct pathways (Capelluto et al., 2002). In addition to the N-terminal DIX domain, the three mammalian Dvl proteins (Dvl1, Dvl2, and Dvl3) contain DEP and PDZ domains in their sequences of approximately 700 residues, and these modules are generally thought to mediate membrane and protein interactions, respectively (Wharton, 2003). The involvement of Dvl in the Wnt pathway requires the integrity of its DIX and PDZ domains while its DEP domain may coordinate planar cell polarity through the Jun N-terminal kinase cascade (Wharton, 2003).
In order to obtain further insights into structure-function relationships of Dvl, we carried out NMR studies of its conserved DIX domain. Here we report the NMR characterization of the homodimeric DIX domain of the Dishevelled 2 (Dvl2) protein.
Materials and Methods
The DIX domain of human Dvl2 (residues 10-94) was overexpressed in E. coli BL21(DE3)pLysS strain (Novagen, Madison, USA) as a glutathione S-transferase (GST) fusion protein. Unlabeled protein was purified from cells grown at 30°C in Luria-Bertani broth. Uniformly 15N and 13C/15N labeled DIX was produced in M9 minimal media supplemented with 15NH4Cl and 13C6-glucose (Cambridge Isotope Laboratories). Harvested cells were disrupted by sonication. The GST- fusion protein was immobilized and purified on Glutathione Sepharose 4B beads (Amersham, Arlington Heights, USA). Dvl2 DIX was eluted by cleavage with thrombin (Sigma Chemical Co, St. Louis, USA), concentrated in the presence of 7 mM perdeuterated dodecylphosphocholine (DPC-d38) (Cambridge Isotope Laboratories), and its purity was verified by SDS-PAGE. The dimeric state of the DIX domain was evidenced from its diffusion coefficient determined by the pulse field gradient experiments and from SDS PAGE and bis(sulfosuccinimidyl) suberate crosslinking experiments (Capelluto et al., 2002).
NMR samples contained 0.2-1 mM of the DIX domain, 90%H2O/10% 2H2O or 99.9% 2H2O, 20 mM Tris-d11 (pH 6.5) buffer, 600 mM DPC-d38, 1 mM dithiothreitol-d10, 1 mM NaN3 and 50 mM 4-amidinophenylmethane sulfonyl fluoride. NMR experiments were performed at 303 K on Varian INOVA 600 and 500 MHz spectrometers equipped with triple resonance shielded probes with z-axis pulse field gradients. Spin system and sequential assignments were made from 1H,15N-HSQC, CBCA(CO)NNH, HNCACB, HNCO, HNHA, H(CCO)NH TOCSY, C(CO)NH TOCSY, 15N-edited TOCSY, 15N-edited HSQC-NOESY and HSQC-NOESY-HSQC experiments (τmix=50 and 135 ms) (Grzesiek et al., 1993; Kay et al., 1993; Muhandiram and Kay, 1994) (Fig. 1). Asn and Gln side chain 1H and 15N resonances were assigned using 3D 15N-edited NOESY and 3D CBCA(CO)NNH spectra. The secondary structure elements were identified from JHNHα coupling constants derived from HNHA (Vuister and Bax, 1993) and HMQC-J spectra, medium and sequential NOEs patterns, and 1Hα, 13Cα, 13Cβ, and 13CO chemical shifts (Wishart and Sykes, 1994). Titrations of G-actin (Cytoskeleton) into the 15N-labeled DIX domain were analyzed by HSQC experiments. Spectra were processed with NMRPipe (Delaglio et al., 1995) and analyzed using PIPP (Garrett et al., 1991), nmrDraw and in-house software programs (http://biomol.uchsc.edu).
Fig. 1.
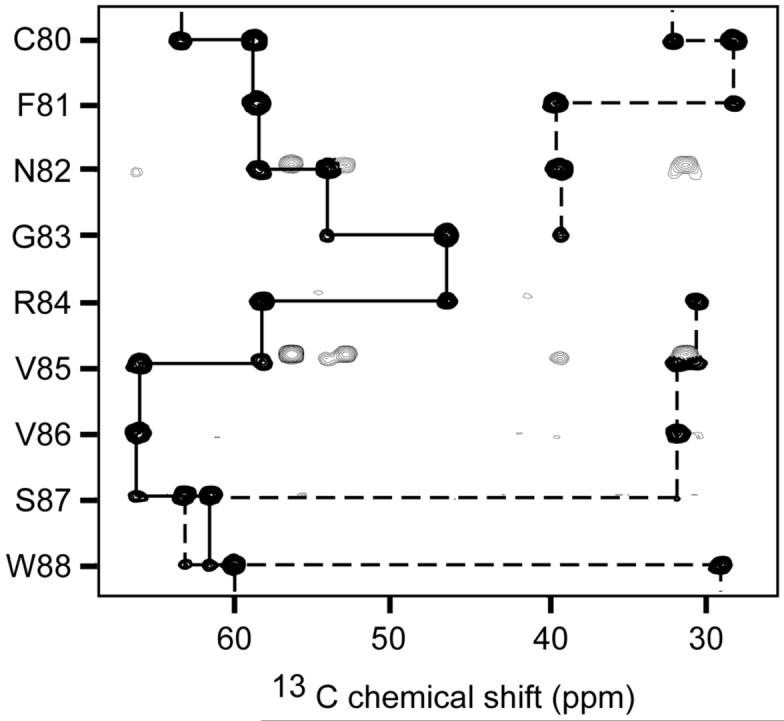
Representative strips from the HNCACB spectrum of Dvl2 DIX domain (1 mM) collected at 303 K showing sequential connectivities for Cα (solid line) and Cβ (dotted line) resonances of residues Cys80-Trp88 of the domain.
Results and Discussion
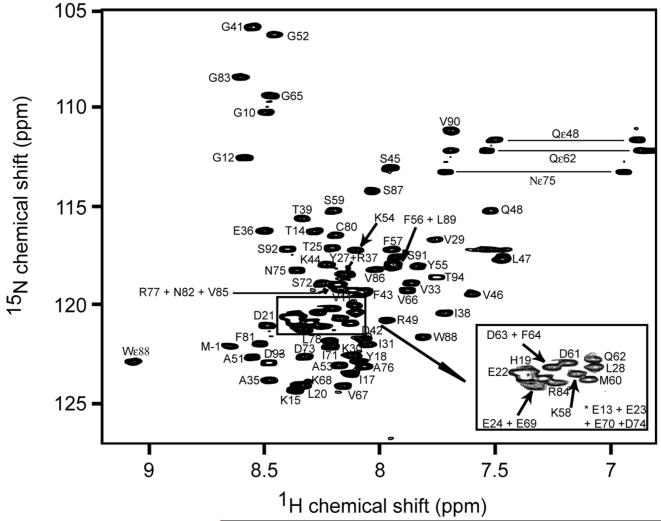
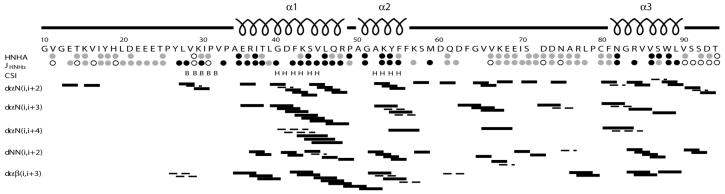
The 1HN and 15N resonances of all 80 backbone amides (excluding the five Pro residues) and 13C resonances of 77 of the 85 backbone carbonyls of the human Dvl2 DIX were assigned. The majority of the spin systems of the Dvl2 DIX domain were identified by the analysis of the H(CCO)NH TOCSY and C(CO)NH TOCSY experiments. In total, 100% of Hα, 99% of Hβ, Hγ, Hδ and Hε, 98% of Cα, 99% of Cβ and 52% of Cγ, Cδ, Cε and Cη resonances were assigned. All the aromatic side chain protons of five Phe, three Tyr, one Trp and three His and the 1H and 15N resonances for the NH2 side chains of 2 Asn and 2 Gln were assigned completely. No assignments were made for the guanidino moiety of Arg and the side chain NH3+ of Lys. The chemical shift values of the 1H, 15N and 13C resonances are represented in Table 1. A typical 1H 15N HSQC spectrum is shown in Fig. 2, where the backbone NHs are labeled for its corresponding amino acid. Chemical shift index analysis of the Hα, 13Cα and 13CO resonance assignments together with NOEs patterns in 3D 15N-edited NOESY and coupling constants estimated from the HNHA experiment indicated the presence of two α-helices (residues Ala35-Gln48 and Ala51-Tyr55), as well as helical and extended elements (Asn82-Leu89 and Val29-Ile31, respectively) (Fig. 3).
Table 1.
Chemical shifts of 1HN, 15N, 13CO, 13Ca and 13Cβ of the Dvl2 DIX domain. All chemical shifts were referenced relative to the frequency of the methyl proton resonance of DSS. ND: not determined
| Residue | HN | N | CO | Cα | Cβ |
|---|---|---|---|---|---|
| 10GLY | 8.45 | 109.92 | 173.96 | 45.34 | |
| 11VAL | 8.09 | 119.08 | 176.61 | 62.58 | 32.67 |
| 12GLY | 8.58 | 112.37 | 176.06 | 45.41 | |
| 13GLU | 8.29 | 120.81 | 174.08 | 56.54 | 30.46 |
| 14THR | 8.24 | 116.15 | 174.12 | 62.35 | 69.77 |
| 15LYS | 8.34 | 124.17 | 175.90 | 56.22 | 33.25 |
| 16VAL | 8.18 | 121.42 | 175.42 | 62.37 | 32.83 |
| 17ILE | 8.13 | 123.73 | 175.12 | 60.84 | 38.77 |
| 18TYR | 8.05 | 123.17 | 174.68 | 57.43 | 39.36 |
| 19HIS | 8.31 | 120.34 | 173.80 | 55.28 | 29.75 |
| 20LEU | 8.32 | 123.83 | 176.47 | 55.11 | 42.58 |
| 21ASP | 8.44 | 120.94 | ND | 54.45 | 41.13 |
| 22GLU | 8.35 | 120.91 | 176.42 | 56.86 | 30.35 |
| 23GLU | 8.35 | 121.00 | 176.21 | 56.60 | 30.43 |
| 24GLU | 8.36 | 122.10 | 176.26 | 56.52 | 30.43 |
| 25THR | 8.20 | 117.26 | ND | 60.06 | 69.50 |
| 26PRO | 176.46 | 63.40 | 32.05 | ||
| 27TYR | 8.16 | 118.51 | 175.64 | 58.65 | 38.53 |
| 28LEU | 8.10 | 119.94 | 176.61 | 56.05 | 42.48 |
| 29VAL | 7.74 | 116.73 | 175.06 | 62.02 | 32.99 |
| 30LYS | 8.10 | 122.66 | 175.61 | 55.67 | 33.10 |
| 31ILE | 8.05 | 122.10 | ND | 58.48 | 38.59 |
| 32PRO | 175.13 | 63.07 | 31.31 | ||
| 33VAL | 7.84 | 118.88 | ND | 59.59 | 33.07 |
| 34PRO | 175.42 | 62.94 | 31.73 | ||
| 35ALA | 8.45 | 123.75 | 177.99 | 53.62 | 19.27 |
| 36GLU | 8.46 | 116.36 | 176.20 | 56.89 | 30.11 |
| 37ARG | 8.12 | 118.5 | 175.53 | 55.94 | 30.69 |
| 38ILE | 7.71 | 120.38 | 174.55 | 60.66 | 38.79 |
| 39THR | 8.30 | 115.64 | 175.07 | 60.07 | 71.76 |
| 40LEU | 9.04 | 122.74 | 178.80 | 57.49 | 41.91 |
| 41GLY | 8.51 | 105.80 | 175.86 | 46.75 | |
| 42ASP | 8.03 | 121.59 | 177.77 | 56.43 | 40.91 |
| 43PHE | 8.05 | 119.36 | 176.56 | 60.11 | 39.14 |
| 44LYS | 8.18 | 117.97 | 177.97 | 59.96 | 32.29 |
| 45SER | 7.92 | 113.00 | 176.28 | 60.98 | 62.97 |
| 46VAL | 7.56 | 119.29 | 176.12 | 64.29 | 31.76 |
| 47LEU | 7.44 | 117.68 | 176.35 | 55.68 | 41.85 |
| 48GLN | 7.50 | 115.35 | 175.68 | 55.70 | 29.51 |
| 49ARG | 7.98 | 120.95 | ND | 55.25 | 29.85 |
| 50PRO | 177.02 | 64.07 | 31.82 | ||
| 51ALA | 8.52 | 122.85 | 178.82 | 53.76 | 18.91 |
| 52GLY | 8.43 | 106.33 | 174.49 | 45.93 | |
| 53ALA | 8.15 | 122.97 | 177.99 | 53.77 | 19.06 |
| 54LYS | 8.08 | 117.33 | 176.84 | 58.01 | 32.58 |
| 55TYR | 7.80 | 118.00 | 176.01 | 59.24 | 38.73 |
| 56PHE | 7.92 | 117.78 | 175.39 | 59.29 | 39.69 |
| 57PHE | 7.90 | 116.99 | 175.79 | 58.13 | 39.34 |
| 58LYS | 8.15 | 121.00 | 176.78 | 57.15 | 32.91 |
| 59SER | 8.22 | 115.51 | 174.68 | 58.95 | 63.61 |
| 60MET | 8.16 | 121.12 | 175.75 | 55.88 | 32.79 |
| 61ASP | 8.17 | 120.32 | 176.15 | 54.65 | 41.13 |
| 62GLN | 8.10 | 119.47 | 176.06 | 56.00 | 29.59 |
| 63ASP | 8.23 | 120.44 | 175.26 | 54.24 | 41.19 |
| 64PHE | 8.24 | 120.50 | 176.17 | 58.37 | 39.24 |
| 65GLY | 8.40 | 109.60 | 173.97 | 45.70 | |
| 66VAL | 7.84 | 119.25 | 176.02 | 62.65 | 32.74 |
| 67VAL | 8.16 | 123.89 | 175.83 | 62.55 | 32.61 |
| 68LYS | 8.36 | 125.37 | 176.13 | 56.43 | 33.19 |
| 69GLU | 8.38 | 122.40 | ND | ND | ND |
| 70GLU | 8.35 | 120.91 | 176.18 | 56.22 | 30.30 |
| 71ILE | 8.19 | 121.90 | 175.96 | 60.97 | 38.60 |
| 72SER | 8.33 | 119.79 | 174.15 | 58.08 | 64.09 |
| 73ASP | 8.35 | 122.91 | 176.21 | 54.35 | 41.31 |
| 74ASP | 8.34 | 121.00 | 176.47 | 54.96 | 40.94 |
| 75ASN | 8.32 | 118.40 | 175.12 | 53.68 | 38.97 |
| 76ALA | 8.05 | 123.17 | 177.24 | 52.67 | 19.18 |
| 77ARG | 8.18 | 119.13 | 175.90 | 56.20 | 31.14 |
| 78LEU | 8.19 | 121.73 | ND | 53.20 | 42.01 |
| 79PRO | 175.82 | 63.07 | 31.87 | ||
| 80CYS | 8.18 | 116.54 | 174.32 | 58.47 | 28.08 |
| 81PHE | 8.51 | 121.87 | 175.03 | 58.18 | 39.34 |
| 82ASN | 8.17 | 118.86 | 175.65 | 53.78 | 39.12 |
| 83GLY | 8.55 | 108.31 | 175.17 | 46.29 | |
| 84ARG | 8.22 | 121.00 | 177.94 | 58.18 | 30.43 |
| 85VAL | 8.16 | 119.14 | 176.96 | 63.43 | 31.94 |
| 86VAL | 8.00 | 118.10 | 177.05 | ND | 31.89 |
| 87SER | 8.00 | 114.03 | 175.86 | 61.32 | 62.93 |
| 88TRP | 7.78 | 121.52 | 177.14 | 59.84 | 28.94 |
| 89LEU | 7.91 | 117.70 | 176.93 | 57.03 | 43.00 |
| 90VAL | 7.65 | 110.88 | 175.71 | 61.50 | 32.49 |
| 91SER | 7.89 | 117.47 | 174.25 | 58.38 | 63.81 |
| 92SER | 8.37 | 117.03 | 174.14 | 58.18 | 64.05 |
| 93ASP | 8.45 | 122.88 | 175.43 | 54.55 | 41.16 |
| 94THR | 7.72 | 118.49 | ND | 63.12 | 70.75 |
Fig. 2.
Two dimensional 1H 15N HSQC spectrum of 200 μM 15N-labeled Dvl2 DIX domain acquired at 600 MHz. Selected peaks are labeled with the corresponding residue numbers.
Fig. 3.
Predicted secondary structure of the Dvl2 DIX domain. The predictions are based on consensus chemical shift indices (CSI’s), coupling constants, and local NOEs. The CSI predictions of helical (H) and extended (B) conformations were based on each residues 1Hα, 13Cα, 13Cβ, and 13C’ chemical shifts. The JHNHα coupling constants under 6 Hz (black circle), over 8 Hz (open circle), and between 6 and 8 Hz (grey circles) are consistent with helical, extended, and indeterminate secondary structure, respectively, and were estimated from HNHA and HMQC-J spectra. The NOE connectivities provide distances (d) between the HN (N), Ha (a), and Hb (b) resonances of residues that are i = 2, 3, or 4 positions apart, and are indicated as solid and dashed lines depending on whether they are resolved or partially overlapped, respectively, in NOESY spectra collected with NOE mixing times of 50 and 135 ms.
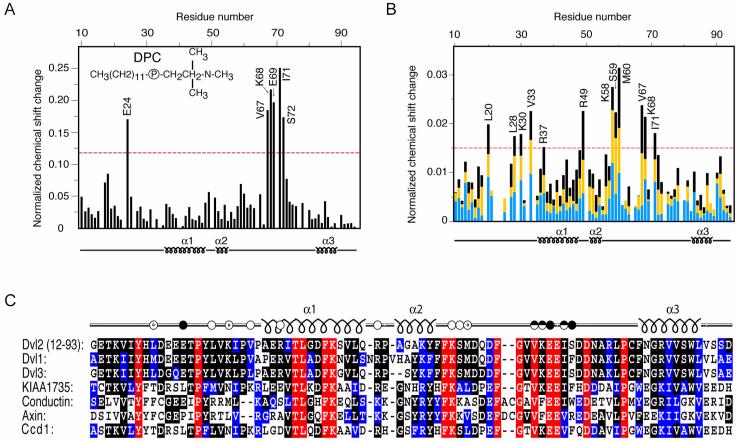
The ability of the DIX domain to interact with membranes was studied using DPC micelles. A number of structural determinations of membrane proteins bound to micelles have indicated that valuable structural information can be obtained from NMR studies of such systems (Henry and Sykes, 1994). The DIX domain mediates Dvl2 localization to vesicular membranes, where activates canonical Wnt signaling by stabilization of β-catenin (Capelluto et al., 2002). HSQC spectra of the DIX domain were collected during stepwise of DPC micelle addition. Several chemical shifts changes in the backbone amides of DIX were detected and plotted as a histogram (Fig. 4A). The data indicates that a short stretch between the last two helical elements in the Dvl2 DIX domain is a putative membrane recognition motif.
Fig. 4.
Identification of ligand-interacting residues of the DIX domain by HSQC experiments. (A) The histogram displays the normalized chemical shift difference for each residues amide group of 15N-labeled Dvl2 DIX domain (100 μM) caused by increasing the d38-DPC concentration from 10 to 400 mM. (B) Histogram showing the progressive changes in chemical shifts of DIX domain (100 μM) residues following actin addition. Blue, yellow and black bars represent reductions after addition of 200, 300, and 400 μM G-actin, respectively. The 1H,15N chemical shifts were normalized as ([(ΔδH)2 + (ΔδN/5)2]/2)0.5, where δ is chemical shift in parts per million (ppm). Labeled residues exhibit changes that exceed the red line, which represents 50% of the largest change. (C) The amino acid sequences of the DIX domains from human Dvl homologs, KIAA1735, Conductin and Axin and zebrafish Ccd1 are aligned. Identical, highly conserved and similar residues are shown in red, black and blue, respectively. The Dvl2 residues that are involved in actin and DPC micelle binding are shown with unfilled and filled dots, respectively.
The DIX domain has also been shown to bind to actin stress fibers, where it may activate non-canonical Wnt signaling (Capelluto et al., 2002). Thus, we mapped the DIX domain actin-binding site using a similar approach as described for DPC binding. The residues interacting with the actin are evident from the chemical shift perturbations (Fig. 4B). This actin-binding site is localized between the second helix and the DPC binding site, as identified by chemical shift changes induced by stepwise addition of G-actin (Fig. 4B). The actin binding motif of DIX is similar to those described for MARCKS and actobindin proteins (Maciver, 1995). As shown in Fig. 4C, the amino acid sequence alignment of DIX domain-containing proteins indicates that both DPC and actin-interacting residues are predominantly conserved among the DIX proteins. This suggests that the actin and micelle interactions may be extended to other members of the family of DIX domain-containing proteins. Thus, the information provided here provides a structural basis for understanding the ligand interactions and molecular function of the DIX domain.
Acknowledgments
We thank D. Jones for discussions, R. Muhandiram and L. E. Kay for NMR pulse sequences. The NMR Center is supported by the University of Colorado Cancer Center Core. This work was supported by the National Institutes of Health, Royal Society and BBSRC (M.O.) and the Cancer League of Colorado (D.G.S.C.).
References
- Capelluto DG, Kutateladze TG, Habas R, Finkielstein CV, He X, Overduin M. The DIX domain targets dishevelled to actin stress fibres and vesicular membranes. Nature. 2002;419:726–729. doi: 10.1038/nature01056. [DOI] [PubMed] [Google Scholar]
- Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A. NMRpipe-a multidimensional spectral processing system based on Unix pipes. J. Biomol. NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- Garrett DS, Powers R, Gronenborn AM, Clore GM. A common-sense approach to peak picking in 2-dimensional, 3-dimensional, and 4-dimensional spectra using automatic computer-analysis of contour diagrams. J. Magn. Reson. 1991;95:214–220. doi: 10.1016/j.jmr.2011.09.007. [DOI] [PubMed] [Google Scholar]
- Grzesiek S, Anglister J, Bax A. Correlation of backbone amide and aliphatic side-chain resonances in C-13/N-15-enriched proteins by isotropic mixing of C-13 magnetization. J. Magn. Reson. 1993;101:114–119. [Google Scholar]
- Henry GD, Sykes BD. Methods to study membrane protein structure in solution. Methods Enzymol. 1994;239:515–535. doi: 10.1016/s0076-6879(94)39020-7. [DOI] [PubMed] [Google Scholar]
- Kay LE, Xu GY, Singer AU, Muhandiram DR, Formankay JD. A gradient-enhanced HCCH TOCSY experiment for recording side-chain H-1 and C-13 correlations in H2O samples of proteins. J. Magn. Reson. 1993;101:333–337. [Google Scholar]
- Kishida S, Yamamoto H, Hino S, Ikeda S, Kishida M, Kikuchi A. DIX domains of Dvl and Axin are necessary for protein interactions and their ability to regulate beta-catenin stability. Mol. Cell. Biol. 1999;19:4414–4422. doi: 10.1128/mcb.19.6.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciver SK. Microfilament organization and actin binding proteins. In: Hesketh JE, Pryme IF, editors. Treatise on the Cytoskeleton; Structure and Assembly. JAI press; Greenwich, United Kingdom: 1995. pp. 1–45. [Google Scholar]
- Muhandiram DR, Kay LE. Gradient-enhanced triple-resonance 3-dimensional NMR experiments with improved sensitivity. J. Magn. Reson. 1994;103:203–216. [Google Scholar]
- Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh S, Daigo Y, Furukawa Y, Kato T, Miwa N, Nishiwaki T, Kawasoe T, Ishiguro H, Fujita M, Tokino T, Sasaki Y, Imaoka S, Murata M, Shimano T, Yamaoka Y, Nakamura Y. AXIN1 mutations in hepatocellular carcinomas, and growth suppression in cancer cells by virus-mediated transfer of AXIN1. Nat. Genet. 2000;24:245–250. doi: 10.1038/73448. [DOI] [PubMed] [Google Scholar]
- Shiomi K, Uchida H, Keino-Masu K, Masu M. Ccd1, a novel protein with a DIX domain, is a positive regulator in the Wnt signaling during zebrafish neural patterning. Curr. Biol. 2003;13:73–77. doi: 10.1016/s0960-9822(02)01398-2. [DOI] [PubMed] [Google Scholar]
- Vuister GW, Bax A. Quantitative J correlation - a new approach for measuring homonuclear 3-bond J(H(N)H(alpha) coupling-constants in N-15-enriched proteins. J. Am. Chem. Soc. 1993;115:7772–7777. [Google Scholar]
- Wharton KA., Jr. Runnin’ with the Dvl: proteins that associate with Dsh/Dvl and their significance to Wnt signal transduction. Dev. Biol. 2003;253:1–17. doi: 10.1006/dbio.2002.0869. [DOI] [PubMed] [Google Scholar]
- Wishart DS, Sykes BD. The 13C chemical-shift index: a simple method for the identification of protein secondary structure using 13C chemical-shift data. J. Biomol. NMR. 1994;4:171–180. doi: 10.1007/BF00175245. [DOI] [PubMed] [Google Scholar]