Abstract
The rat is one of the most commonly used experimental animal species in biomedical research. The availability of new research tools in rats could therefore provide considerable advances in the areas where this mammal is extensively used. We report the development of a new Green Fluorescent Protein (GFP) rat strain suitable for organ transplantation and the birth of GFP rats following orthotopic transplantation of neonatal ovaries from this newly developed GFP rat strain to a wild-type Fischer 344 (F344) strain. A new GFP rat strain was developed by backcrossing eGFP Sprague-Dawley (SD-Tg(CAG-EGFP)Cz-004Osb) to wild-type F344 for eight generations. Whole ovaries from postnatal day 8 GFP rats were transplanted orthotopically to bilaterally ovariectomized wild-type adult females (n = 6). All recipients were mated, and three of the five resulting litters contained GFP pups. In the PND 8 group, all recipients cycled regularly and the ovarian morphology appeared normal when collected at 9 months post-transplantation. In the PND 21 group, 60% of the recipients displayed regular estrous cycles at 9 months post-transplantation, but showed reduced ovarian size. This new strain and neonatal orthotopic transplantation could be useful for many biomedical fields including transplantation, development, and reproductive toxicology.
Keywords: inbred GFP rat, organ transplantation, ovary, environment, endocrine disruptors, toxicology
INTRODUCTION
The rat is one of the most studied mammalian species in biomedical research, as over 1.2 million publications describe research with this mammal. Its size, fecundity, and ease of care have made the rat a preferred animal model in many areas of experimental medicine, including surgery, physiology, pharmacology, and toxicology [1, 2]. Therefore, the availability of experimental tools, such as traceable tissues that are suitable for transplantation and do not require immunosuppression would be of great interest to those scientists who use rats for their studies. For tissue tracing, green fluorescent protein (GFP) has been the preferred genetic marker because it can be directly observed under UV illumination without staining. Although various alternatives are available (e.g., beta-galactosidase), their detection is more cumbersome [3]. Inbred GFP rat strains are available, but not all the strains can be used successfully in tissue transplantation, due to immunogenicity problems. For example, it has been reported that the skin grafts from transgenic GFP inbred Lewis (CAG/GFP/LEW tg) rats to wild-type Lewis rats are rejected within 6–9 days after transplantation [4].
Ovary transplantation has been previously reported in many species, ranging from rats [5] to humans [6]. Ovaries can be transplanted either heterotopically (i.e., in any location other than the normal location of the host’s ovary, such as under the skin [7]) or orthotopically (i.e., in the location normally occupied by the host ovary [8]). Both types of ovary transplantation are used for studying ovarian biology and the direct (intraovarian) effects of environmental factors on the ovary [9, 10]. However, only orthotopic transplantation allows the study of all functions of the ovary, including generation of offspring.
One of the challenges of orthotopic ovary transplantation is keeping the reproductive tract fully functional while completely removing the ovary. On the one hand, if the reproductive tract becomes dysfunctional during complete removal of the ovary, this will defeat the original purpose of orthotopic transplantation. On the other hand, if the host ovary is not completely removed, it will not be possible to distinguish whether the offspring originated from the donor or the host ovary. Several groups addressed this issue by using ovary donors with distinct genetic markers [5, 11, 12].
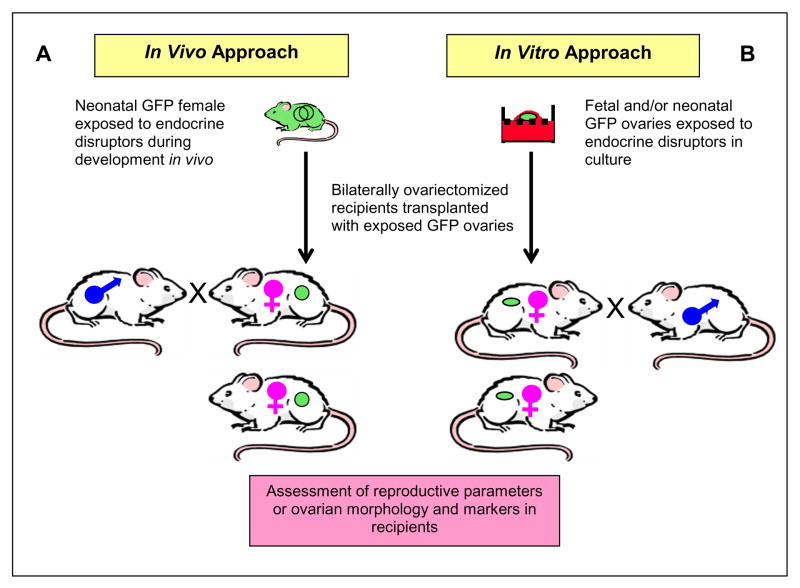
Live birth following orthotopic transplantation of adult rat ovaries has previously been documented [5] but not following transplantation of neonatal rat ovaries. The creation of an orthotopic neonatal rat ovary transplantation model is important for studying ovarian biology and the effects of environmental factors (e.g., estrogenic xenobiotics) on the ovary because major developmental events in the ovary take place during late gestational and early postnatal life [13–15] and are affected by estrogens [16–18]. Thus, neonatal orthotopic GFP ovary transplantation, especially prior to establishment of the hypothalamic-pituitary-gonadal axis [19], provides a powerful tool for studying ovarian biology and environmental toxicology (see Discussion and Figure 3).
Fig. 3.
Proposed use of neonatal GFP ovary transplantation to study direct effects of developmental endocrine disruptor exposure. Wild-type Fischer 344 females are mated with newly developed inbred Fisher 344 GFP males to obtain timed-pregnancies (not shown). (A) Resulting GFP females exposed to endocrine disruptors during fetal and early postnatal stages of ovarian development in vivo are used as ovary donors. (B) Alternatively, fetal or neonatal GFP ovaries exposed to endocrine disruptors in vitro are used as donor ovaries. In vivo or in vitro endocrine disruptor-exposed GFP ovaries are transplanted orthotopically to bilaterally ovariectomized adult females. Following post-surgical recovery, the recipient females are bred with wild-type males and are evaluated for their reproductive parameters. Some recipient females, upon establishment of cyclicity, are sacrificed and the ovaries are collected for assessment of ovarian morphology and molecular markers.
The objectives of this study are to assess a new GFP rat strain that allows allografts without a need for immunosuppression and to use this newly generated inbred GFP strain as a donor for orthotopic neonatal ovary transplantation to develop a model to study ovarian development and toxicology.
MATERIALS AND METHODS
Generations of GFP Donor Animals
Transgenic Sprague-Dawley rats [SD-Tg(CAG-EGFP)Cz-004Osb] carrying the enhanced green fluorescent protein (eGFP) transgene were obtained from Japan SLC., Inc. (Hamamatsu, Japan). This transgenic rat line expresses eGFP gene under the control of the cytomegalovirus enhancer and the chicken β-actin promoter [20]. The new GFP rat strain was created by continuous backcrossing of eGFP Sprague-Dawley males to wild-type Fischer 344 (F344) females for eight generations. The new strain is more than 99% congenic to F344 and is denominated F344.SD-Tg(CAG-EGFP)Cz-004Osb(N8), which is referred to “GFP F344” in this study [21]. The offspring from the 8th generation were used as donors that were hemizygotes for the GFP locus. The GFP F344 rats were sacrificed on postnatal day (PND) 8. Both ovaries were asceptically removed from the animals, cleaned of connective tissue, and kept at 4°C until transfer. The ovaries were transferred within 2 hours of sacrificing the donor animals. One whole ovary was transferred into the bursa ovary of each bilaterally ovariectomized 6- to 8-week-old adult female recipient (n = 6).
Orthotopic ovary transplantation from prepubertal (PND 21) GFP F344 female donors to another set of recipients (n = 6) was used as a control because rats of this age are commonly used in superovulation studies, in which ovaries are fully responsive to exogenous gonadotropins, and their ovaries are expected to be functional in adult recipients. Ovaries from PND 21 donors were prepared similar to the ovaries from PND 8 donors, except PND 21 ovaries were divided into approximately equal two pieces, and each piece transferred to one recipient.
Recipient Animals
Adult F344 females (6 to 8 weeks of age) were purchased from Charles River Laboratories (Wilmington, MA). The animals were maintained in a room with controlled illumination (lights on 0700-2100h), temperature (26–28°C), and humidity (30–70%) and given free access to regular rat chow and water. Prior to the transplantation, the recipients’ regular estrous cyclicity was confirmed by daily vaginal cytology. All the procedures were carried out according to guidelines provided by Rutgers University Animal Care and Facilities Committee.
Transplantation of the GFP Ovaries
The transplantation procedure was similar to a previously published protocol [5]. Briefly, recipient females were anesthetized with 45–55 mg/kg sodium pentobarbital (i.p.). Both flanks were shaved and disinfected, and a transverse incision of the skin caudal to the last rib and ventral to the vertebral column was made. A small opening was made bluntly through the musculature and peritoneum to exteriorize each ovary. One ovary was carefully removed through a small incision made in the bursa, excised with microsurgical scissors, and replaced by the donor ovary once hemostasis was obtained. The incision on the bursa was closed with a 9.0 suture (F.S.T., Foster, CA). The other uterine horn was closed with a single ligature using absorbable suture material (Vicryl®-rapid, 4.0, Ethicon, Somerville, NJ), and the ovary was excised with a single cut between the oviduct and the uterine horn. The cuts at the peritoneum and musculature were closed by continuous suture. The skin was closed with Michel clips (F.S.T.), which were removed 10–12 days after surgery.
Assessment of the Fertility of the Recipients
Starting 2 weeks after surgery, the restoration of the reproductive cycle was monitored by vaginal cytology. Females showing regular cycles (2 consecutive proestrus with 4–5 days in between) were mated to wild-type males on the afternoon of proestrus. The animals showing a sperm-positive vaginal smear the next day were followed for another 7 days for a continuous diestrus. Cycles of those animals showing a sperm-negative vaginal smear the next day were followed until the next proestrus day, at which time all the remaining animals mated successfully (i.e., showed a sperm-positive smear). The females were followed daily for the delivery of the litter starting 3 weeks after the sperm-positive day. Females that mated but failed to become pregnant were re-mated two additional times in a similar manner to that described.
Assessment of Cyclicity of the Recipients
In addition to the initial cyclicity, the long-term cyclicity of the recipients was assessed using vaginal cytology after the delivery of the first litter of pregnant animals, or starting in the 3rd month. Post transplantation, the cycles were followed daily for at least 12 days of each month for 9 months. The cycles were classified into normal, persistent estrus, persistent diestrus, or prolonged cycles as previously described (Armenti et al., submitted). One of the females died in the 8th post-transplantation month in both PND 8 and PND 21 groups with no apparent cause. Therefore remaining 5 females were used in analysis thereafter.
Assessment of Remnant of Host Ovary and Ovarian Histology in the Recipients
Nine months post-transplantation, the ovarian tissues were collected, cleaned out of the bursa ovary, oviduct, fats, and connective tissues under a dissection microscope and fixed in 4% paraformaldehyde overnight. Following three rinses with phosphate-buffered saline (PBS), the ovaries were placed in 15% sucrose in PBS overnight and transferred in 30% sucrose in PBS until embedding. The whole fixed ovarian tissue was examined using a Leica MZ FLIII stereo fluorescence microscope (Leica, Deerfield, IL) with GFP filter. Images were acquired with a MagnaFire S99802 CCD camera (Optronics, Goleta, CA) using MagnaFire Software Ver2.1 (Optronics). Images were assembled using Adobe Photoshop CS.
Fixed ovaries were placed in OCT compounds (Tissue Tek) in plastic micro molds and frozen quickly at −80°C. Blocks were sectioned at 8 μm thickness at −20°C in a Leica cryostat. Slides were stored at 4°C until further use. Before mounting, slides were dried at 37°C for 20 minutes and washed in PBS for 15 minutes. After the OCT was removed, sections were stained with ethidium homodimer-2 (EthD-2; 1:200 dilution, Invitrogen, cat# E3599) for 5 minutes and washed with PBS. The sections were then mounted in Prolong Gold Anti-Fade reagent (Invitrogen, cat# 36934), and were observed under a Nikon Eclipse E800 microscope with epifluorescent attachments using red (550 nm) and green (480 nm) filters. Images were acquired with a Nikon DXM1200F camera and ACT1 software (Version 2) and assembled with Adobe Photoshop CS.
Data Analysis
The experiment used 12 recipient females, half receiving ovaries from PND 8 GFP donors and the other half receiving ovaries from PND 21 GFP donors. Mean ± s.d. of reproductive parameters and litter size of PND 8 and PND 21 groups were compared with student t-test using GraphPad Prism version 4.0a for Macintosh (GraphPad Software, San Diego CA). A p value less than 0.05 was considered significant.
RESULTS
Fertility of the Recipients
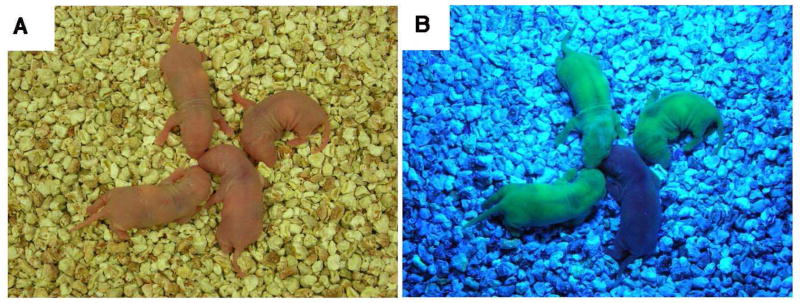
Three recipients of PND 8 GFP ovaries and three recipients of PND 21 GFP ovaries gave births to litters with GFP pups (Table 1). One litter of a recipient of a PND 8 ovary is shown in Figure 1 (see Supplemental Figure 1 for a litter from the PND 21 group).
Table 1.
Reproductive parameters, pregnancy rate, and litter size following GFP ovary transplantation from postnatal day (PND) 8 and 21 rats to wild-type rats.
| Age of donors | Number of recipients | Number of regularly cycling recipients | Days to first proestrus after transplantation (Mean ± s.d.) | Days to first mating after transplantation (Mean ± s.d.) | Days to pregnancy (Mean ± s.d.) | Successful pregnancies | Litters with GFP pups | Number of pups | Number of GFP pups | Litter size(Mean ± s.d.) |
|---|---|---|---|---|---|---|---|---|---|---|
| PND 8 | 6 | 6 | 22.5 ± 4.46 | 26.3 ± 5.27 | 62.6 ± 27.2 | 5 (83%) | 3 (50%) | 16 | 7 | 3.2 ± 1.3 (range: 2–5) |
| PND 21 | 6 | 6 | 18.3 ± 3.14 | 21.0 ± 5.86 | 64.8 ± 23.9 | 5 (83%) | 3 (50%) | 13 | 7 | 2.6 ± 0.55 (range: 2–3) |
Figure 1.
The birth of a green fluorescent protein (GFP) rat following orthotopic PND 8 ovary transplantation to 44-day-old wild-type Fischer rat. A representative litter is shown under regular light (a) and UV light (b), which clearly shows the ubiquitous expression of GFP in the pups. The ovary transplantation was performed as described in Materials and Methods. The reproductive cycle of the recipient was followed starting 2 weeks after transplantation. The recipient gave birth to the shown litter at 95 days after the transplantation. Three out of the six PND 8 ovary recipients gave birth to a GFP-positive litter.
The data obtained from the recipients of PND 8 and PND 21 ovaries were similar (Table 1). The time (days) to the first estrous cycle (22.5 ± 4.46 and 18.3 ± 3.14), to sperm-positive vaginal smear (26.3 ± 5.27 and 21.0 ± 5.86), and to pregnancy (62.6 ± 27.2 and 64.8 ± 23.9) were not significantly different between the recipients of PND 8 and PND 21 ovaries, respectively (p > 0.05; Table 1). Five of the six recipients of each group (83%) gave birth to a litter. Three GFP-positive litters (50%) were obtained for both PND 8 and PND 21 ovary recipient groups. Average litter size (mean ± s.d.) for PND 8 ovary recipients (3.2 ± 1.3; range was 2–5) was comparable to that of the PND 21 ovary recipients (2.6 ± 0.55; range was 2–3). While 7 of the 16 pups of PND 8 ovary recipients were born GFP-positive (44%), 7 out of the 13 pups of PND 21 ovary recipients were born GFP-positive (54%; Table 1). These results show that the initial performance of the ovaries transplanted from neonatal and prepubertal rats is similar. In addition, the success rate and reproductive parameters in our studies are generally comparable with previous studies using ovaries from adult rats [5, 12] or neonatal mice [11]. Furthermore, some males and females from litters from each PND 8 and PND 21 were allowed to reach adulthood and used in breeding studies. These animals displayed normal general health and fertility (data not shown).
Long-term Cyclicity of the Recipients
In the PND 8 group, all recipients showed normal cycles for 9 months post-transplantation examined (Table 2). In contrast, although all recipients in the PND 21 group showed normal cycle for 8 months post-transplantation, 40% of the females showed irregular cycles in the 9th month. These irregular cycles were classified as persistent estrus.
Table 2.
The long-term cyclicity of bilaterally ovariectomized recipients of post natal day (PND) 8 and PND 21 ovaries.
| Month after ovarian transplantation1 | |||||||
|---|---|---|---|---|---|---|---|
| 1 | 3 | 5 | 6 | 7 | 8 | 9 | |
| PND 8 % Normal2 (n) | 100 (6) | 100 (6) | 100 (6) | 100 (6) | 100 (6) | 100 (53) | 100 (5) |
| PND 21 % Normal2 (n) | 100 (6) | 100 (6) | 100 (6) | 100 (6) | 100 (6) | 100 (53) | 60 (34) |
During the 2nd and 4th months, most recipients were pregnant or pseudo-pregnant. Therefore cyclicity was followed in a limited number of animals and not presented.
Normal = Normal estrus cycles
One of the recipients died at 8th months post-transplantation in PND 8 and PND 21 groups respectively.
Two females showed persistent estrus in PND 21 group.
Assessment of Remnant of Host Ovary and Ovarian Histology of the Recipients
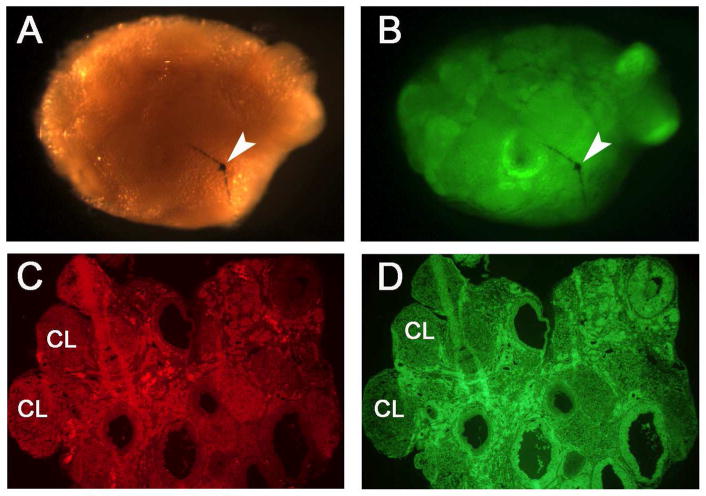
In the PND 8 group, all of the five surviving recipients displayed normal appearing reproductive tracts at 9 months post transplantation (not shown). All of these females had no or negligible non-GFP ovaries in the ovarian tissue (Figure 2 A and B). In the PND 21 groups, three females showed normal reproductive tracts (not shown). Similar to the PND 8 group, these females showed no or negligible non-GFP ovaries (not shown). However, two of the females in the PND 21 group showed hydrosalpinx, and therefore assessment was not performed as the ovaries of these females were mostly degenerated at the time of collection.
Fig. 2.
Examination of remnants (if any) of host ovary and morphology of transplanted ovaries 9 months post-transplantation in PND 8 groups. A representative ovary is shown using visible light (A) and UV light (B). To determine whether any remnant of host ovarian tissue remained, the ovaries were cleaned of surrounding bursa, oviduct, and fat tissues under a dissection microscope. The ovaries were then examined and imaged under visible light (A) and UV light (B) following the fixation as described in Materials and Methods. No or negligible host ovarian tissue remnants were observed in the recipient animals. Arrow indicates the suture used for closing the bursa ovary. Sections (8 μm) of quick frozen ovaries were prepared as described in Materials and Methods and used for determining the histology of the ovaries. The GFP ovaries were stained with EthD-2 and imaged using 550 nm (C; red) and 480 nm (D; green) filters. The ovary sections had different stages of the follicles including the corpus lutea (CL) at the time of collection. Original magnification of panel C and D is 40 x.
The ovarian morphology examined in frozen sections in both groups 9 months post-transplantation. Ovaries transplanted on PND 8 had various stages of the follicles and corpora lutea in the tissue (Figure 2 C and D), supporting functionality of the ovaries. In PND 21 group, ovaries from cycling females also contained various stages of the follicles and corpora lutea (not shown), but they were relatively smaller.
DISCUSSION
These results indicate that the newly developed GFP F344 rats are immune-compatible with wild-type F344 rats at the organ level. The transplanted ovaries were fully functional and survived up to 9 months post-transplantation examined, which indicates that the newly developed rat strain is suitable for organ and tissue transplantation studies. This study also showed for the first time successful neonatal ovary transplantation in rats.
The current study shows that tissues from our congenic GFP F344 rat strain may be transplanted to a F344 rat strain without immunosuppression. This will allow studies of the transplanted allografts in immune-competent rats with and without cyclosporin and other immunosuppressants. For example, such studies would be able to determine whether calcineurin-inhibiting immunosuppressants, such as cyclosporine and FK506, affect reproductive functions of the recipients [22, 23] as well as the host’s immune response to the transplanted tissues.
Orthotopic neonatal GFP ovary transplantation provides a powerful experimental rat model for studying ovarian development and the effects of environmental factors on adult ovarian function (Figure 3). Environmental factors and xenobiotics, such as estrogenic endocrine disruptors, affect organs besides the ovaries. To eliminate the possibility that action on the ovary is mediated through other organs (e.g., hypothalamus and/or pituitary), at least two possible approaches can be followed. In our proposed in vivo approach, fetal and neonatal GFP females are exposed to endocrine disruptors. Then, prior to the establishment of the HPG axis [19] in the treated females, ovaries are orthotopically transplanted to unexposed, bilaterally ovariectomized, wild-type females (Fig. 3A). In our proposed in vitro approach, fetal or neonatal GFP ovaries can be exposed to endocrine disruptors in the ovary organ culture [18, 24] and then transplanted orthotopically (Fig 3B). Reproductive parameters (fertility, cyclicity, and aging) as well as ovarian morphology and gene expression can be evaluated in the recipient females. This in vitro approach has an advantage over the in vivo approach because it completely eliminates any likely indirect effects that may occur during in vivo exposure.
Recipients of both PND 8 and PND 21 GFP ovaries showed long term cyclicity. However the success rate was higher in PND 8 ovaries as compared to PND 21 ovaries. This can be due to at least two possible reasons: (1) approximately half size of the PND 21 ovaries were transplanted to each recipient while entire PND 8 ovaries were transplanted, giving a larger follicular pool to the recipient of PND 8 ovaries, and (2) since the size of the PND 8 ovaries are smaller than the half size PND 21 ovaries, it was easier to place the PND 8 ovaries inside the bursa ovary following removal of the host ovary, possibly causing less damage to the bursa ovary. Our data actually supports the later speculation, since 2 out of 5 PND 21 ovary recipients showed hydrosalpinx when the ovaries were collected 9 months post-transplantation, suggesting that the reproductive tract was likely to be damaged in some of the recipients of PND 21 ovaries. Nevertheless, it is more advantageous to use PND 8 ovaries as donors in our model for studying the direct effects of environmental estrogens because the developing ovaries are more vulnerable to exogenous estrogens than the adult ovaries [18, 24].
The new inbred GFP rat strain and neonatal ovary transplantation model can also be used to advance ovary transplantation studies. The recent report of a live birth following autotransplantation of cryopreserved ovaries has renewed interest in ovary transplantation in humans, which has a major clinical application for women undergoing chemotherapy at a young age [6]. Orthotopic GFP rat ovary transplantation can be used as an experimental model to investigate ovary transplantation from rats of different ages to each other, as well as revascularization and cryopreservation, which appear to be major complicating factors in human ovary transplantation [25].
In summary, we report the production of a new congenic F344 GFP rat strain that is suitable for tissue transplantation, and the birth of GFP rats following orthotopic transplantation of neonatal ovaries from this new GFP rat strain. This new experimental animal model can be used in organ and tissue transplantation in the rat, which is one of the most studied animal models. In addition, the orthotopic neonatal GFP ovary transplantation model can be of use in the study of ovarian biology and environmental reproductive toxicology.
Supplementary Material
Acknowledgments
The authors acknowledge the generous contribution of Sprague-Dawley eGFP transgenic rats from Dr. M. Okabe (Japan SLC, Inc., Japan). The authors wish to thank to Kathy Manger for her help editing the manuscript, Henry John-Alder and Michael Skinner for their critical reading of the manuscript, and Naohide Watanabe from the laboratory of Eric Lam for help with stereomicroscopy of the ovarian tissues. This research was supported by NIH grant ES01385.
Footnotes
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gill TJ, 3rd, Smith GJ, Wissler RW, Kunz HW. The rat as an experimental animal. Science. 1989;245:269–76. doi: 10.1126/science.2665079. [DOI] [PubMed] [Google Scholar]
- 2.Abbott A. Laboratory animals: the Renaissance rat. Nature. 2004;428:464–6. doi: 10.1038/428464a. [DOI] [PubMed] [Google Scholar]
- 3.Okabe M, Ikawa M, Kominami K, Nakanishi T, Nishimune Y. ‘Green mice’ as a source of ubiquitous green cells. FEBS Lett. 1997;407:313–9. doi: 10.1016/s0014-5793(97)00313-x. [DOI] [PubMed] [Google Scholar]
- 4.Inoue H, Ohsawa I, Murakami T, Kimura A, Hakamata Y, Sato Y, et al. Development of new inbred transgenic strains of rats with LacZ or GFP. Biochem Biophys Res Commun. 2005;329:288–95. doi: 10.1016/j.bbrc.2005.01.132. [DOI] [PubMed] [Google Scholar]
- 5.Dorsch M, Wedekind D, Kamino K, Hedrich HJ. Orthotopic transplantation of rat ovaries as a tool for strain rescue. Lab Anim. 2004;38:307–12. doi: 10.1258/002367704323133691. [DOI] [PubMed] [Google Scholar]
- 6.Donnez J, Dolmans MM, Demylle D, Jadoul P, Pirard C, Squifflet J, et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364:1405–10. doi: 10.1016/S0140-6736(04)17222-X. [DOI] [PubMed] [Google Scholar]
- 7.Harris M, Eakin RM. Survival of transplanted ovaries in rats. J Exp Zool. 1949;112:131–63. doi: 10.1002/jez.1401120110. incl 3 pl. [DOI] [PubMed] [Google Scholar]
- 8.Jones EC, Krohn PL. Orthotopic ovarian transplantation in mice. J Endocrinol. 1960;20:135–46. doi: 10.1677/joe.0.0200135. [DOI] [PubMed] [Google Scholar]
- 9.Halling A, Forsberg JG. Ovarian reproductive function after exposure to diethylstilbestrol in neonatal life. Biol Reprod. 1990;43:472–7. doi: 10.1095/biolreprod43.3.472. [DOI] [PubMed] [Google Scholar]
- 10.Iguchi T, Fukazawa Y, Uesugi Y, Takasugi N. Polyovular follicles in mouse ovaries exposed neonatally to diethylstilbestrol in vivo and in vitro. Biol Reprod. 1990;43:478–84. doi: 10.1095/biolreprod43.3.478. [DOI] [PubMed] [Google Scholar]
- 11.Candy CJ, Wood MJ, Whittingham DG. Restoration of a normal reproductive lifespan after grafting of cryopreserved mouse ovaries. Hum Reprod. 2000;15:1300–4. doi: 10.1093/humrep/15.6.1300. [DOI] [PubMed] [Google Scholar]
- 12.Dorsch MM, Wedekind D, Kamino K, Hedrich HJ. Cryopreservation and orthotopic transplantation of rat ovaries as a means of gamete banking. Lab Anim. 2007;41:247–54. doi: 10.1258/002367707780378195. [DOI] [PubMed] [Google Scholar]
- 13.Hirshfield AN. Development of follicles in the mammalian ovary. Int Rev Cytol. 1991;124:43–101. doi: 10.1016/s0074-7696(08)61524-7. [DOI] [PubMed] [Google Scholar]
- 14.Skinner MK. Regulation of primordial follicle assembly and development. Hum Reprod Update. 2005;11:461–71. doi: 10.1093/humupd/dmi020. [DOI] [PubMed] [Google Scholar]
- 15.Obata Y, Kono T. Maternal primary imprinting is established at a specific time for each gene throughout oocyte growth. J Biol Chem. 2002;277:5285–9. doi: 10.1074/jbc.M108586200. [DOI] [PubMed] [Google Scholar]
- 16.Kipp JL, Kilen SM, Bristol-Gould S, Woodruff TK, Mayo KE. Neonatal exposure to estrogens suppresses activin expression and signaling in the mouse ovary. Endocrinology. 2007;148:1968–76. doi: 10.1210/en.2006-1083. [DOI] [PubMed] [Google Scholar]
- 17.Uzumcu M, Zachow R. Developmental exposure to environmental endocrine disruptors: consequences within the ovary and on female reproductive function. Reprod Toxicol. 2007;23:337–52. doi: 10.1016/j.reprotox.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Y, Jefferson WN, Newbold RR, Padilla-Banks E, Pepling ME. Estradiol, progesterone, and genistein inhibit oocyte nest breakdown and primordial follicle assembly in the neonatal mouse ovary in vitro and in vivo. Endocrinology. 2007;148:3580–90. doi: 10.1210/en.2007-0088. [DOI] [PubMed] [Google Scholar]
- 19.Ojeda SR, Ramirez VD. Plasma level of LH and FSH in maturing rats: response to hemigonadectomy. Endocrinology. 1972;90:466–72. doi: 10.1210/endo-90-2-466. [DOI] [PubMed] [Google Scholar]
- 20.Ito T, Suzuki A, Imai E, Okabe M, Hori M. Bone marrow is a reservoir of repopulating mesangial cells during glomerular remodeling. J Am Soc Nephrol. 2001;12:2625–35. doi: 10.1681/ASN.V12122625. [DOI] [PubMed] [Google Scholar]
- 21.Silver L. Mouse Genetics Concepts and Application. Oxford University Press; 1995. [Google Scholar]
- 22.Kantarci G, Sahin S, Uras AR, Ergin H. Effects of different calcineurin inhibitors on sex hormone levels in transplanted male patients. Transplant Proc. 2004;36:178–9. doi: 10.1016/j.transproceed.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 23.Turner HE, Wass JA. Gonadal function in men with chronic illness. Clin Endocrinol (Oxf) 1997;47:379–403. doi: 10.1046/j.1365-2265.1997.2611108.x. [DOI] [PubMed] [Google Scholar]
- 24.Kezele P, Skinner MK. Regulation of ovarian primordial follicle assembly and development by estrogen and progesterone: endocrine model of follicle assembly. Endocrinology. 2003;144:3329–37. doi: 10.1210/en.2002-0131. [DOI] [PubMed] [Google Scholar]
- 25.Donnez J, Martinez-Madrid B, Jadoul P, Van Langendonckt A, Demylle D, Dolmans MM. Ovarian tissue cryopreservation and transplantation: a review. Hum Reprod Update. 2006;12:519–35. doi: 10.1093/humupd/dml032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.