Abstract
Macrophage death in advanced atherosclerosis causes plaque necrosis, which promotes plaque rupture and acute atherothrombotic vascular events. Of interest, plaque necrosis and atherothrombotic disease are markedly increased in diabetes and metabolic syndrome. We discovered a novel "multi-hit" macrophage apoptosis pathway that appears to be highly relevant to advanced atherosclerosis. The elements of the pathway include: (a) activation of the unfolded protein response (UPR) by cholesterol overloading of the endoplasmic reticulum or by other UPR activators known to exist in atheromata; and (b) pro-apoptotic signaling involving the type A scavenger receptor (SRA). The downstream apoptosis effectors include CHOP (GADD153) for the UPR and JNK for SRA signaling. Remarkably, components of this pathway are enhanced in macrophages with defective insulin signaling, including UPR activation and SRA expression. As a result, insulin-resistant macrophages show increased susceptibility to apoptosis when exposed to UPR activators and SRA ligands. Moreover, the advanced lesions of atherosclerosis-prone mice reconstituted with insulin-resistant macrophages show increased macrophage apoptosis and plaque necrosis. Based on these findings, we propose that one mechanism of increased plaque necrosis and atherothrombotic vascular disease in insulin resistant syndromes is up-regulation of a two-hit signal transduction pathway involved in advanced lesional macrophage death.
Keywords: macrophage, apoptosis, atherosclerosis, insulin resistance, diabetes, unfolded protein response, necrotic core, scavenger receptor, cholesterol
Introduction
Diet and life style habits of industrialized societies have led to a situation where almost all adult individuals in these societies have variable numbers of subendothelial deposits of cholesterol, inflammatory cells, and extracellular matrix in focal areas of the arterial tree (Braunwald 1997). The vast majority of these focal subendothelial deposits, called atherosclerotic lesions or plaques, are relatively small, stable, and asymptomatic and will remain so during the lifespan of the individual. However, in approximately 50% of this population, a small number of these atherosclerotic lesions will undergo a gradual transformation that can prove deadly. This transformation is characterized by changes in the morphology of the lesions that render them susceptible to erosion or rupture (Aikawa & Libby 2004, Kolodgie et al 2004, Libby 2000). If erosion or rupture does eventually occur in these susceptible, or "vulnerable," plaques, the exposure of thrombogenic plaque material to the blood stream can lead to acute thrombotic vascular occlusion and infarction of the tissue supplied by the affected artery. In the heart, this scenario results in sudden cardiac death and/or myocardial infarction, and in the brain, this process can lead to certain types of stroke (Aikawa & Libby 2004, Kolodgie et al 2004, Libby 2000).
Understanding the mechanisms of stable-to-vulnerable plaque transformation at a cellular and molecular level will almost certainly suggest new therapeutic strategies to combat atherothrombotic vascular disease. Because both atherogenesis itself and the transformation to plaque vulnerability are complex processes, a reductionist approach is necessary to elucidate molecular-cellular mechanisms. In this context, we have focused on one of the more prominent cellular events associated with plaque vulnerability, namely, advanced lesional macrophage death. In early life, the arterial subendothelium is largely devoid of cells and extracellular lipids. However, by the early teen years, areas of the arterial tree characterized by disturbed blood flow begin to accumulate circulating lipoproteins in the subendothelial space (Williams & Tabas 1995, Williams & Tabas 1998). The most important of these lipoproteins are LDL, derived from hepatic secretion of VLDL, and so-called remnant lipoproteins, derived from partial catabolism of intestinally derived chylomicrons and hepatic-derived VLDL (Williams & Tabas 1995, Williams & Tabas 1998). The key initiating event of subendothelial lipoprotein retention, which is influenced by the plasma level of these "atherogenic" lipoproteins and poorly understood genetic factors at the level of the arterial wall, triggers a series of biological responses (Williams & Tabas 1995, Williams & Tabas 1998). The most prominent response to subendothelial lipoprotein retention is monocyte infiltration. The monocytes differentiate into macrophages in the subendothelial space, and the macrophages then ingest the retained lipoproteins by a variety of processes including endocytosis, pinocytosis, and phagocytosis (Tabas 2002). Lipoprotein uptake by macrophages results in a large delivery of lipoprotein-derived lipids, particularly cholesterol. Although excess cholesterol can be toxic to cells, early lesional macrophages remain healthy by effluxing the cholesterol and by storing it in a relatively harmless form called cholesteryl fatty acid esters (Glass & Witztum 2001). This latter process, which gives rise to cytoplasmic lipid droplets and thus a "foamy" appearance to the macrophages, is catalyzed by an endoplasmic reticulum (ER) enzyme called acyl-CoA:cholesterol acyltransferase (ACAT) (Chang et al 2001). Early foam cell lesions tend be non-occlusive and stable against erosion or rupture, which is in part due to a "protective" collagenous "cap" that separates the lesion from the blood stream (Aikawa & Libby 2004, Kolodgie et al 2004).
Results and Discussion
The importance of macrophage death in advanced atherosclerotic lesions
As alluded to above, the vast majority of these foam cells lesions remain non-occlusive and stable throughout the life of the individual. Indeed, reversal of atherosclerotic risk factors, especially lowering of plasma LDL by drugs and dietary changes, can cause at least partial regression of these lesions (Williams & Tabas 2005). However, a few of the lesions, particularly in the setting of persistent risk factors such as high plasma LDL, cigarette smoking, and diabetes, can progress to the aforementioned vulnerable plaque stage. One of the key features of vulnerable plaques are vast areas of macrophage debris, which result from death of lesional macrophages (Tabas 2005). These areas of dying macrophages and macrophage debris, often referred to as "necrotic cores" or "lipid cores," almost certainly contribute to plaque erosion or rupture by promoting inflammation, physical stress on the fibrous cap, and thrombosis (Libby & Clinton 1993, Tabas 2005). It is likely that these processes promoted by dead macrophages complement those triggered by living macrophages, such as secretion of matrix proteases and inflammatory cytokines, to induce plaque breakdown and acute vascular thrombosis (Libby & Clinton 1993, Tabas 2005).
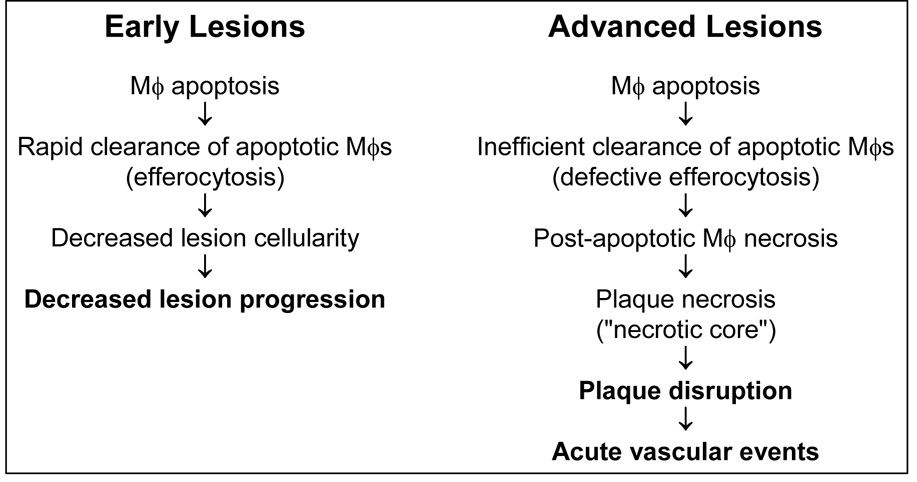
According to the above scenario, advanced lesional macrophage death may be a key cellular event in stable-to-vulnerable plaque transformation. However, simply relating macrophage death to necrotic core formation and worsening of plaques can be misleading (Fig. 1). Macrophages in all stages of atherosclerosis undergo a certain basal rate of turnover by the caspase-dependent death process known as apoptosis (Tabas 2005). In early lesions, these apoptotic macrophages are rarely seen, because they are rapidly and efficiently phagocytosed by neighboring macrophages (Liu et al 2005, Tabas 2005). This process of apoptotic cell clearance via phagocytosis, often referred to as "efferocytosis," is a normal physiologic response to apoptosis and is non-inflammatory and prevents cellular necrosis (Henson, Bratton, & Fadok 2001). Indeed, promoting early lesional macrophage apoptosis by genetic manipulation in mouse models of atherosclerosis results in a decrease in lesion cellularity and size, because the lesional macrophages are safely decreased in number (Liu et al 2005). In advanced lesions, however, there is evidence that efferocytosis of apoptotic macrophages is less than completely efficient (Schrijvers et al 2005, Tabas 2005). Apoptotic cells that do not get rapidly cleared by efferocytosis become leaky and trigger an inflammatory response—a process referred to as post-apoptotic, or secondary, necrosis (Fiers et al 1999, Tabas 2005). By this manner, the necrotic core of advanced atherosclerotic lesions gradually forms.
Fig. 1. Model of the opposing roles of macrophage death in atherosclerosis.
In early lesions, apoptotic macrophages are rapidly cleared by efferocytosis, leading to reduced lesion cellularity and decreased lesion progression. In advanced lesions, however, efferocytosis is not as efficient, and so some of the apoptotic macrophages become secondarily necrotic. These necrotic macrophages gradually coalesce, forming the necrotic core. The combination of plaque necrosis, i.e. dead macrophages, and residual living macrophages (not shown) promotes plaque disruption and acute lumenal thrombosis. See text and Ref. (Tabas 2005) for details.
The UPR-SRA model of advanced lesional macrophage apoptosis
Based on the above description of plaque progression, we feel that understanding the cellular-molecular basis of two key processes in advanced lesional macrophages—apoptosis and efferocytosis—is likely to shed new light into how stable atherosclerotic lesions transform into vulnerable plaques. For purposes of focus, this chapter will address the issue of how macrophages might undergo apoptosis in advanced atherosclerotic lesions. Our studies in this area began with a single model based on observations in human vulnerable plaques, but ensuing mechanistic studies of this model uncovered a much broader array of possible triggers that are likely to be relevant to atherosclerosis. The initial model was based on observations that the macrophages in vulnerable human plaques contain more unesterified, or "free," cholesterol (FC) than is typically observed in earlier lesional macrophage foam cells (see above) (Aikawa & Libby 2004, Burke et al 2003, Guyton & Klemp 1994, Kolodgie et al 2004, Kruth 1984, Lundberg 1985, Small 1988). Although the mechanism of FC accumulation is not known, it is likely promoted by dysfunctions of ACAT-mediated cholesterol esterification and cellular cholesterol efflux (above).
Based on this observation and the known cytotoxic effects of excess intracellular FC (Warner et al 1995, Yao & Tabas 2000, Yao & Tabas 2001), we sought to understand how FC accumulation would effect macrophages. The model we chose was one in which primary tissue macrophages (murine peritoneal macrophages) were exposed in culture to atherogenic lipoproteins in the setting of pharmacologic or genetic ACAT dysfunction. We found that FC accumulation was a trigger for caspase-dependent macrophage apoptosis by pathways involving both the Fas death receptor and well-described mitochondrial apoptotic mechanisms (Yao & Tabas 2000, Yao & Tabas 2001). Initially, we imagined that excess FC in the plasma membrane and/or mitochondria might somehow trigger these events. However, our studies revealed that the key organelle was neither of the above but rather the ER (Feng et al 2003b, Feng et al 2003a). In retrospect, the ER would have been a logical candidate, because the ER membrane bilayer normally has a relatively low cholesterol:phospholipid ratio. This property is responsible for the fluid nature of the ER membrane, which is necessary for its proper function (Davis & Poznansky 1987). When the cholesterol:phospholipid ratio of the ER membrane is increased, such as occurs during FC enrichment of macrophages, the membrane undergoes a phase transition to a more ordered state (Li et al 2004). This abnormal state leads to dysfunction of critical ER membrane proteins, including a protein called SERCA, which controls calcium levels in the ER (Li et al 2004).
The macrophage responds to this state of ER "stress" by activating a coordinated signal transduction pathway known as the Unfolded Protein Response (UPR) (Feng et al 2003a). The UPR is triggered by a wide variety of ER stressors, and its major function is to reverse the stress and to keep the ER protected while carrying out this repair function (Ma & Hendershot 2001, Ron 2002, Welihinda, Tirasophon, & Kaufman 1999). Thus, for example, protein translation is suppressed, unfolded proteins are degraded, and protein chaperones are induced (Ma & Hendershot 2001, Ron 2002, Welihinda, Tirasophon, & Kaufman 1999). However, if ER stress is prolonged or unable to be repaired, there is a distal branch of the UPR can promote apoptosis (McCullough et al 2001, Oyadomari et al 2002, Zinszner et al 1998). In many cases, including the situation with FC-enriched macrophages, the apoptotic function of the UPR is effected by the transcription factor called CHOP, or GADD153 (McCullough et al 2001, Oyadomari et al 2002, Zinszner et al 1998). Although the exact mechanism of CHOP-induced apoptosis is still be explored in our and other laboratories, CHOP-deficient macrophages are substantially protected against FC-induced apoptosis (Feng et al 2003a). Moreover, recent unpublished data in our laboratory suggest that advanced lesions of Chop−/− mice on an atherogenic background have less macrophage death and less plaque necrosis than Chop+/+ mice on the same background.
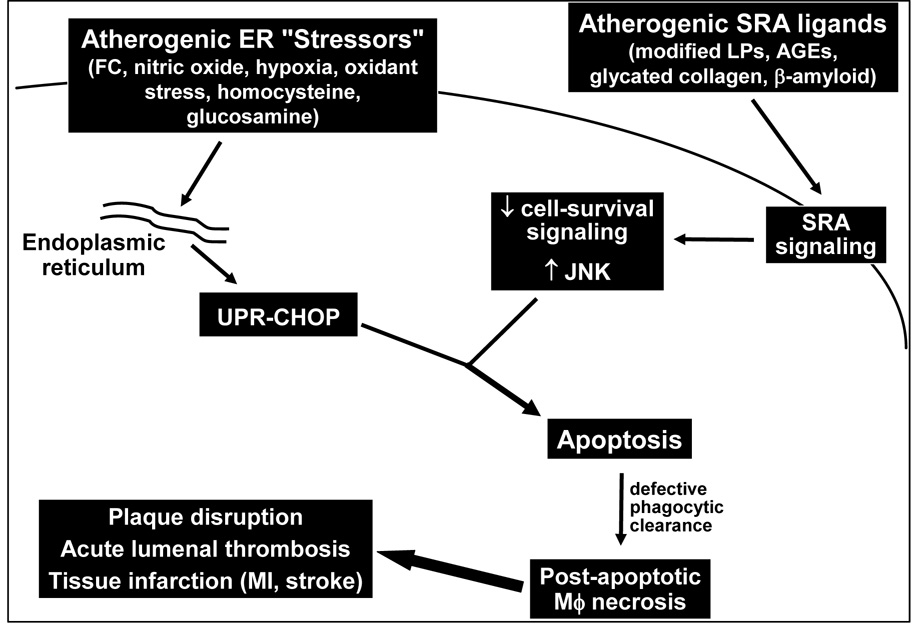
The concept that the UPR is an apoptosis trigger is simplistic. In fact, the UPR normally promotes cell survival to enable repair (above), and apoptosis is induced only when something else goes awry (Ma & Hendershot 2001, Ron 2002, Welihinda, Tirasophon, & Kaufman 1999). Thus, the UPR can, under certain circumstances, be necessary for apoptosis, but it is never sufficient by itself. Rather, the UPR can enable apoptosis in response to another "hit." This two-hit model of UPR-induced apoptosis is critical to understanding how FC enrichment kills macrophages. In this scenario, the UPR-CHOP pathway is necessary but not sufficient for FC-induced apoptosis. We found that the "second hit" in this model involved engagement of a receptor for atherogenic lipoproteins called the type A scavenger receptor (SRA) (DeVries-Seimon et al 2005) (Fig. 2). This second hit was effected by the method we had chosen to deliver cholesterol to the macrophages, namely, because we had used a typical SRA-binding atherogenic lipoprotein to load the cells with cholesterol (DeVries-Seimon et al 2005). Thus, atherogenic lipoproteins induce death by satisfying both hits of the two-hit model: they deliver cholesterol to the ER to activate the UPR-CHOP pathway, and they engage the SRA to supply the second hit. We now know that any combination of UPR activators and SRA ligands, many of which exist in advanced atherosclerotic lesions (Fig. 2), can trigger macrophage apoptosis completely independently of FC enrichment and ACAT dysfunction. These findings are very important, because they extend the array of possible triggers of advanced lesional macrophage death beyond the FC-enrichment/ACAT dysfunction model. How SRA engagement supplies the second apoptotic hit to ER-stressed macrophages is a key area of current focus in the laboratory. Our current data suggest a fascinating mechanism in which SRA engagement triggers activation of the pro-apoptotic mitogen-activated protein kinase JNK while selectively silencing a cell-survival pathway in the macrophages.
Fig. 2. The UPR-SRA two-hit model of macrophage apoptosis.
UPR activation by FC loading or other UPR activators known to be present in advanced atherosclerotic lesions provides the potential for apoptosis through the UPR effector CHOP. However, apoptosis does not occur unless a second hit is present, which in the case of macrophage apoptosis is engagement of the SRA. Ongoing mechanistic suggest that the SRA both triggers a pro-apoptotic JNK pathway and inhibits a cell survival pathway.
The link to insulin resistance
Humans with type II diabetes have a markedly increased risk of atherothrombotic vascular disease (Plutzky, Viberti, & Haffner 2002). Of significant relevance to the topic of this chapter, diabetic lesions are characterized by increased necrotic cores (Burke et al 2004), suggesting an enhancement of advanced lesional macrophage death. The increase in plaque progression in diabetics is likely due to a combination of insulin resistance and hyperglycemia. Certainly, insulin resistance by itself is a strong risk factor, because humans with insulin resistance without hyperglycemia, e.g., in metabolic syndrome, have an increased incidence of coronary artery disease (Grundy 2004).
There are a number of possible mechanisms whereby insulin resistance might promote plaque progression, including enhancement of systemic atherosclerotic risk factors, like low HDL and hypertension, and direct effects on cells of the arterial wall, notably endothelial cells (Plutzky, Viberti, & Haffner 2002). In a recent collaboration with Drs. Alan Tall and Domenico Accili of Columbia University, we have been investigating another possible mechanism, namely, pro-apoptotic effects macrophage insulin resistance in the context of the UPR-SRA model described above. Although macrophages do not have insulin-regulatable glucose transporters, they do have insulin receptors that respond to insulin through the canonical insulin-signaling pathway (Liang et al 2004). Thus, acute treatment of macrophages with physiologic insulin concentrations leads to phosphorylation of the insulin receptor, insulin receptor substrate-2 (IRS-2), and Akt (Liang et al 2004). Most relevant to the topic of this discussion, macrophages isolated from insulin-resistant mouse models, such as the hyperinsulinemic leptin-deficient ob/ob mouse, have down-regulated insulin receptors and depressed insulin signaling (Liang et al 2004). Thus, macrophages, like hepatocytes and skeletal muscle cells, become insulin resistant in the setting of hyperinsulinemia in vivo.
Remarkably, two of the more prominent characteristics of macrophages in the insulin-resistant state are activation of the UPR and up-regulation of the SRA (Han et al 2006, Liang et al 2004). Although the molecular mechanisms behind these two processes are still under investigation, SRA up-regulation appears to be post-transcriptional and causally related to UPR activation (Han et al 2006, Liang et al 2004). These findings directly suggested that insulin-resistant macrophages would be more susceptible to the model of apoptosis described above. Indeed, we found that such macrophages show markedly enhanced apoptosis when exposed to SRA-mediated FC enrichment conditions or when treated with the combination of a UPR activator and an SRA ligand (Han et al 2006). Most importantly, we have data supporting this model in advanced atherosclerotic lesions. Specifically, we found that the advanced lesions of Ldlr−/− mice have increased macrophage apoptosis and lesional necrosis when they are reconstituted with bone marrow from insulin-resistant mice (Han et al 2006).
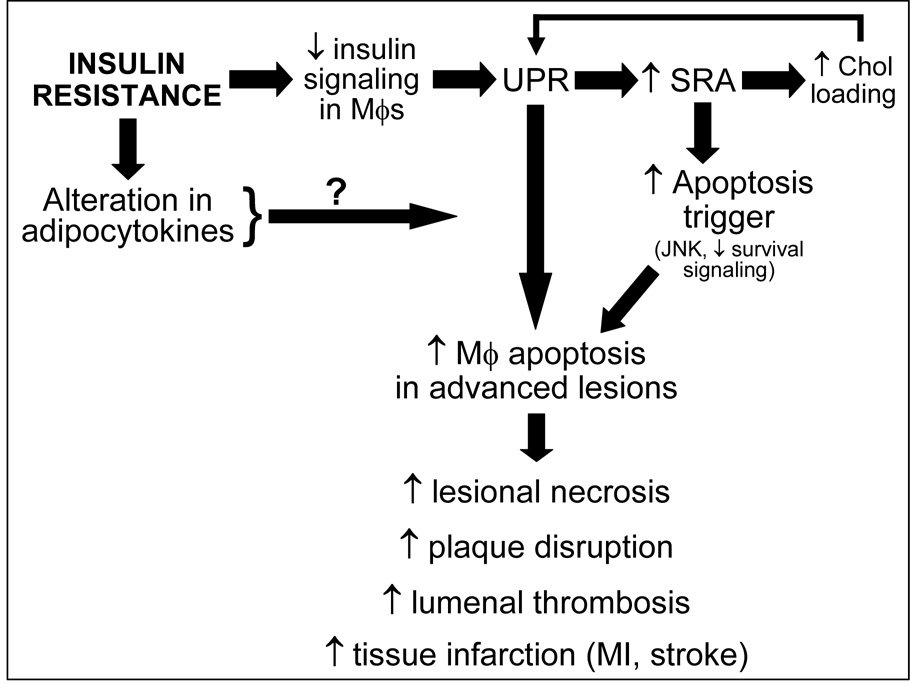
Studies are ongoing to determine whether the increased apoptosis observed in insulin-resistant macrophages represents an enhancement of the same UPR-SRA pro-apoptotic signaling pathway used by insulin-sensitive macrophages (above), as suggested by the UPR activation and SRA up-regulation in these cells (Fig. 3), or whether the insulin-resistant state triggers a second pro-apoptotic pathway that is additive or synergistic with the "basal" pathway. Moreover, the laboratory is investigating molecular links between systemic insulin resistance and advanced lesional macrophage apoptosis, such as those that may be mediated by circulating adipocytokines that are altered in insulin-resistant states.
Fig. 3. Model of how insulin resistance promotes advanced lesional macrophage apoptosis.
Macrophage insulin resistance directly activates the UPR, which in turn leads to up-regulation of the SRA. Each of these processes contributes to apoptosis by the two-hit model outlined in Fig. 2. In addition, the up-regulation of the SRA might promote enhanced internalization of lipoprotein-derived cholesterol, which might further activate the UPR in the setting of dysfunctional ACAT and suppressed cholesterol efflux. We are also exploring the possibility that alterations in adipocytokines in the setting of systemic insulin resistance might affect advanced lesional macrophage apoptosis.
Summary and Conclusions
Advanced lesional macrophage death is a key event in the transformation of asymptomatic atherosclerotic lesions into plaques that have the potential to rupture and cause acute vascular events. While multiple mechanisms are likely responsible for advanced lesional macrophage death, in-vivo evidence suggests that a two-hit pro-apoptotic pathway involving the unfolded protein response (UPR) and the type A scavenger receptor (SRA) plays an important role. Apoptosis in this two-hit model results from pro-apoptotic CHOP from the UPR branch and pro-apoptotic JNK, plus suppression of cell-survival signaling, from the SRA branch. Remarkably, both of these hits are up-regulated in macrophages that are insulin resistant, and an atherosclerotic mouse models with insulin-resistant macrophages shows evidence of increased advanced lesional macrophage death and plaque necrosis. Further studies are needed to define exactly how defective insulin signaling in macrophages leads to UPR activation and SRA up-regulation; whether these events are responsible for the advanced lesional macrophage death observed in vivo; and whether other events associated with insulin resistance, such as alterations in adipocytokines, might contribute to this process.
Acknowledgements
The work described in this chapter was supported by NIH grants HL75662 and HL54591 and USA Medical Research and Material Command Grant PR054352.
References
- Aikawa M, Libby P. The vulnerable atherosclerotic plaque: pathogenesis and therapeutic approach. Cardiovasc Pathol. 2004;13:125–138. doi: 10.1016/S1054-8807(04)00004-3. [DOI] [PubMed] [Google Scholar]
- Braunwald E. Cardiovascular medicine at the turn of the millennium: triumphs, concerns, and opportunities. N Engl J Med. 1997;337:1360–1369. doi: 10.1056/NEJM199711063371906. [DOI] [PubMed] [Google Scholar]
- Burke AP, Kolodgie FD, Zieske A, et al. Morphologic findings of coronary atherosclerotic plaques in diabetics: a postmortem study. Arterioscler Thromb Vasc Biol. 2004;24:1266–1271. doi: 10.1161/01.ATV.0000131783.74034.97. [DOI] [PubMed] [Google Scholar]
- Burke AP, Virmani R, Galis Z, Haudenschild CC, Muller JE. 34th Bethesda Conference: Task force #2--What is the pathologic basis for new atherosclerosis imaging techniques? J Am Coll Cardiol. 2003;41:1874–1886. doi: 10.1016/s0735-1097(03)00359-0. [DOI] [PubMed] [Google Scholar]
- Chang TY, Chang CC, Lin S, Yu C, Li BL, Miyazaki A. Roles of acyl-coenzyme A:cholesterol acyltransferase-1 and -2. Curr Opin Lipidol. 2001;12:289–296. doi: 10.1097/00041433-200106000-00008. [DOI] [PubMed] [Google Scholar]
- Davis PJ, Poznansky MJ. Modulation of 3-hydroxy-3-methylglutaryl-CoA reductase by changes in microsomal cholesterol content or phospholipid composition. Proc Natl Acad Sci U S A. 1987;84:118–121. doi: 10.1073/pnas.84.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries-Seimon T, Li Y, Yao PM, et al. Cholesterol-induced macrophage apoptosis requires ER stress pathways and engagement of the type A scavenger receptor. J Cell Biol. 2005;171:61–73. doi: 10.1083/jcb.200502078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng B, Yao PM, Li Y, et al. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nat Cell Biol. 2003a;5:781–792. doi: 10.1038/ncb1035. [DOI] [PubMed] [Google Scholar]
- Feng B, Zhang D, Kuriakose G, Devlin CM, Kockx M, Tabas I. Niemann-Pick C heterozygosity confers resistance to lesional necrosis and macrophage apoptosis in murine atherosclerosis. Proc Natl Acad Sci U S A. 2003b;100:10423–10428. doi: 10.1073/pnas.1732494100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiers W, Beyaert R, Declercq W, Vandenabeele P. More than one way to die: apoptosis, necrosis and reactive oxygen damage. Oncogene. 1999;18:7719–7730. doi: 10.1038/sj.onc.1203249. [DOI] [PubMed] [Google Scholar]
- Glass CK, Witztum JL. Atherosclerosis. the road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- Grundy SM. Obesity, metabolic syndrome, and cardiovascular disease. J Clin Endocrinol Metab. 2004;89:2595–2600. doi: 10.1210/jc.2004-0372. [DOI] [PubMed] [Google Scholar]
- Guyton JR, Klemp KF. Development of the atherosclerotic core region. Chemical and ultrastructural analysis of microdissected atherosclerotic lesions from human aorta. Arterioscler Thromb. 1994;14:1305–1314. doi: 10.1161/01.atv.14.8.1305. [DOI] [PubMed] [Google Scholar]
- Han S, Liang CP, DeVries-Seimon T, et al. Macrophage insulin receptor deficiency increases ER stress-induced apoptosis and necrotic core formation in advanced atherosclerotic lesions. Cell Metab. 2006;3:257–266. doi: 10.1016/j.cmet.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Henson PM, Bratton DL, Fadok VA. Apoptotic cell removal. Curr Biol. 2001;11:R795–R805. doi: 10.1016/s0960-9822(01)00474-2. [DOI] [PubMed] [Google Scholar]
- Kolodgie FD, Virmani R, Burke AP, et al. Pathologic assessment of the vulnerable human coronary plaque. Heart. 2004;90:1385–1391. doi: 10.1136/hrt.2004.041798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruth HS. Localization of unesterified cholesterol in human atherosclerotic lesions. Demonstration of filipin-positive, oil-red-O-negative particles. Am J Pathol. 1984;114:201–208. [PMC free article] [PubMed] [Google Scholar]
- Li Y, Ge M, Ciani L, et al. Enrichment of endoplasmic reticulum with cholesterol inhibits SERCA2b activity in parallel with increased order of membrane lipids. Implications for depletion of ER calcium stores and apoptosis in cholesterol-loaded macrophages. J Biol Chem. 2004;279:37030–37039. doi: 10.1074/jbc.M405195200. [DOI] [PubMed] [Google Scholar]
- Liang CP, Han S, Okamoto H, et al. Increased CD36 protein as a response to defective insulin signaling in macrophages. J Clin Invest. 2004;113:764–773. doi: 10.1172/JCI19528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P. Changing concepts of atherogenesis. J Intern Med. 2000;247:349–358. doi: 10.1046/j.1365-2796.2000.00654.x. [DOI] [PubMed] [Google Scholar]
- Libby P, Clinton SK. The role of macrophages in atherogenesis. Curr Opin Lipidol. 1993;4:355–363. [Google Scholar]
- Liu J, Thewke DP, Su YR, Linton MF, Fazio S, Sinensky MS. Reduced macrophage apoptosis is associated with accelerated atherosclerosis in low-density lipoprotein receptor-null mice. Arterioscler Thromb Vasc Biol. 2005;25:174–179. doi: 10.1161/01.ATV.0000148548.47755.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg B. Chemical composition and physical state of lipid deposits in atherosclerosis. Atherosclerosis. 1985;56:93–110. doi: 10.1016/0021-9150(85)90087-5. [DOI] [PubMed] [Google Scholar]
- Ma Y, Hendershot LM. The unfolding tale of the unfolded protein response. Cell. 2001;107:827–830. doi: 10.1016/s0092-8674(01)00623-7. [DOI] [PubMed] [Google Scholar]
- McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol. 2001;21:1249–1259. doi: 10.1128/MCB.21.4.1249-1259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyadomari S, Koizumi A, Takeda K, et al. Targeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetes. J Clin Invest. 2002;109:525–532. doi: 10.1172/JCI14550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plutzky J, Viberti G, Haffner S. Atherosclerosis in type 2 diabetes mellitus and insulin resistance: mechanistic links and therapeutic targets. J Diabetes Complications. 2002;16:401–415. doi: 10.1016/s1056-8727(02)00202-7. [DOI] [PubMed] [Google Scholar]
- Ron D. Translational control in the endoplasmic reticulum stress response. J Clin Invest. 2002;110:1383–1388. doi: 10.1172/JCI16784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrijvers DM, De Meyer GR, Kockx MM, Herman AG, Martinet W. Phagocytosis of Apoptotic Cells by Macrophages Is Impaired in Atherosclerosis. Arterioscler Thromb Vasc Biol. 2005;25:1256–1261. doi: 10.1161/01.ATV.0000166517.18801.a7. [DOI] [PubMed] [Google Scholar]
- Small DM. George Lyman Duff memorial lecture. Progression and regression of atherosclerotic lesions. Insights from lipid physical biochemistry. Arteriosclerosis. 1988;8:103–129. doi: 10.1161/01.atv.8.2.103. [DOI] [PubMed] [Google Scholar]
- Tabas I. Consequences of cellular cholesterol accumulation: basic concepts and physiological implications. J Clin Invest. 2002;110:905–911. doi: 10.1172/JCI16452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabas I. Consequences and therapeutic implications of macrophage apoptosis in atherosclerosis: the importance of lesion stage and phagocytic efficiency. Arterioscler Thromb Vasc Biol. 2005;25:2255–2264. doi: 10.1161/01.ATV.0000184783.04864.9f. [DOI] [PubMed] [Google Scholar]
- Warner GJ, Stoudt G, Bamberger M, Johnson WJ, Rothblat GH. Cell toxicity induced by inhibition of acyl coenzyme A: cholesterol acyltransferase and accumulation of unesterified cholesterol. J Biol Chem. 1995;270:5772–5778. doi: 10.1074/jbc.270.11.5772. [DOI] [PubMed] [Google Scholar]
- Welihinda AA, Tirasophon W, Kaufman RJ. The cellular response to protein misfolding in the endoplasmic reticulum. Gene Expr. 1999;7:293–300. [PMC free article] [PubMed] [Google Scholar]
- Williams KJ, Tabas I. The response-to-retention hypothesis of early atherogenesis. Arterioscler Thromb Vasc Biol. 1995;15:551–561. doi: 10.1161/01.atv.15.5.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KJ, Tabas I. The response-to-retention hypothesis of atherogenesis, reinforced. Curr Opin Lipidol. 1998;9:471–474. doi: 10.1097/00041433-199810000-00012. [DOI] [PubMed] [Google Scholar]
- Williams KJ, Tabas I. Lipoprotein retention--and clues for atheroma regression. Arterioscler Thromb Vasc Biol. 2005;25:1536–1540. doi: 10.1161/01.ATV.0000174795.62387.d3. [DOI] [PubMed] [Google Scholar]
- Yao PM, Tabas I. Free cholesterol loading of macrophages induces apoptosis involving the fas pathway. J Biol Chem. 2000;275:23807–23813. doi: 10.1074/jbc.M002087200. [DOI] [PubMed] [Google Scholar]
- Yao PM, Tabas I. Free cholesterol loading of macrophages is associated with widespread mitochondrial dysfunction and activation of the mitochondrial apoptosis pathway. J Biol Chem. 2001;276:42468–42476. doi: 10.1074/jbc.M101419200. [DOI] [PubMed] [Google Scholar]
- Zinszner H, Kuroda M, Wang X, et al. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 1998;12:982–995. doi: 10.1101/gad.12.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]