Abstract
A patent ductus arteriosus is due in large part to increased sensitivity of the premature ductus to PGE2. Following PGE2 stimulation, cAMP concentrations are higher in the immature than in the mature ductus. cAMP concentrations depend on the rates of adenyl cyclase production and phosphodiesterase (PDE) mediated degradation. We used ductus from immature (n=25) and mature (n=21) fetal sheep to investigate whether a developmental increase in PDE activity could explain the diminished cAMP accumulation that follows PGE2 stimulation in the mature ductus. With advancing gestation, mRNA expression of the smooth muscle PDE isoforms (PDE1A, 1B, 1C, 3A, 3B, 4D, and 5A) increased in the ductus as did their hydrolytic activities. Selective inhibitors of PDE1, PDE3, and PDE4 relaxed the mature and immature ductus in the presence of inhibitors of prostaglandin and nitric oxide production. The mature ductus required higher concentrations of each of the PDE inhibitors to inhibit its tension to the same extent as in the immature ductus. There were no developmental changes in PDE expression in the fetal aorta. In conclusion, we observed a developmental increase in cAMP and cGMP PDE activity that contributes to the decreased sensitivity of the late gestation ductus arteriosus to vasodilators like PGE2.
Keywords: ovine, sheep, cyclic AMP, cyclic GMP, PDE1A, PDE1B, PDE1C, PDE3A, PDE3B, PDE4D, PDE5A, prostaglandins, nitric oxide, calcium, calmodulin, cilostamide, rolipram, MY5445
Preterm infants have a higher incidence of patent ductus arteriosus (PDA) than term infants. In large part, this is due to the increased sensitivity of the premature ductus to Prostaglandin (PG) E2 (1, 2). In the lamb, pig, and baboon ductus arteriosus, PGE2 exerts its effects through 3 selective G-protein coupled receptors (EP2, EP3D and EP4) that activate adenyl cyclase (3, 4). The increased sensitivity of the immature ductus appears to be due to differences in cAMP signaling: 1) EP receptor stimulation in the immature ductus produces higher concentrations of cAMP and Protein Kinase A-mediated relaxation than are found in the mature ductus, and, 2) the immature ductus loses its increased sensitivity to PGE2 and behaves like the mature ductus in the presence of inhibitors of Protein Kinase A (3).
Intracellular levels of cAMP are regulated by adenyl cyclases and cyclic nucleotide phosphodiesterases (PDE). We recently reported that the increased concentration of cAMP that follows PGE2 stimulation of the immature ductus does not appear to be due to increased EP receptor expression or increased adenyl cyclase activity (3). In the current study, we investigated whether alterations in PDE-mediated cAMP breakdown might play a role in the increased cAMP concentrations that result from PGE2 stimulation of the immature ductus.
There are at least 11 distinct PDE families that encode more than 50 different PDE enzyme variations (5, 6). Depending on the species, the major PDEs present in vascular smooth muscle are: PDE1A, 1B, and 1C, PDE3A and 3B, PDE4D, and PDE5A (7, 8). These PDEs are differentially controlled by cAMP, cGMP, and calcium/calmodulin (7, 9). The PDE1 isoforms are stimulated by calcium/calmodulin; they include PDE1A and 1B (which selectively hydrolyze cGMP), and PDE1C (which hydrolyzes cAMP and cGMP equally well). The PDE3 isoforms, 3A and 3B, preferentially hydrolyze cAMP; their hydrolytic activities are competitively inhibited by cGMP since their catalytic sites have similar affinities for cAMP and cGMP (5). This allows vasodilators like nitric oxide, that activate guanyl cyclase, to synergize with adenyl cyclase activating agents like PGE2. PDE4D isoforms specifically hydrolyze cAMP and are not regulated by cGMP. The PDE5A isoform selectively hydrolyzes cGMP and effectively controls cGMP signaling under conditions of low calcium. Under conditions of elevated calcium, PDE1A, 1B, and 1C are likely to play an increasingly important role.
Previous studies have shown that PDE inhibition can relax the ductus areriosus in animals as well as humans (Paradisis M, Evans N, Kluckow M, Osborn DA, unpublished observations), both in vitro (10) and in vivo (11, 12). In the following study, we used ductus from immature (n=25) and mature (n=21) fetal sheep to investigate whether a developmental increase in PDE activity could explain the diminished cAMP accumulation that follows PGE2 stimulation in the mature ductus.
Methods
Twenty-one late-gestation sheep fetuses (mixed Western breed: 137 ± 5 d gestation, term=145d, mean±SD) and 25 immature fetuses (105 ± 3 d) were delivered by Cesarean section and anesthetized with ketamine HCl (30 mg/kg IV) before rapid exsanguination. These procedures were approved by the Committee on Animal Research at the University of California, San Francisco.
Preparation of total RNA, reverse transcription, and quantitative polymerase chain reaction
Total RNA was isolated from the frozen ductus and aorta of 7 immature and 7 mature fetuses as described elsewhere (4). We used the TaqMan Universal PCR master mix of PE Applied Biosystems (Foster City, CA) to quantify the expression of the PDE isoforms (PDE1A, 1B, 3A, 3B, 4D, 5A). Taqman probes were designed using the Primer Express program and labeled with fluorophores FAM (6-caboxy-fluorescein) and TAMRA (6 carboxy-tetramethyl-rhodamine) as reporter and quencher dyes, respectively. An ABI PRISM 7500 Sequence detection system was used to determine the cycle threshold (CT). Reactions were carried out in triplicates. Data were analyzed using the Sequence Detector version 1.6.3 program. Malate dehydrogenase (MDH) was used as an internal control to normalize the data (3).
Phophodiesterase activity measurements
Ductus from 5 immature and 5 mature fetuses were homogenized on ice in lysis buffer containing 50 mM HEPES (pH7.4), 1% Triton X-100 and protease inhibitor cocktail from Sigma (Catalog Number:P8340), and spun at 3000 × g for 10 min at 4 °C. The resulting supernatant was used for protein and PDE assays. Protein concentration was determined by Bio-Rad Detergent Compatible Protein assay kit.
Ten micrograms of protein from the supernatant were used for the cAMP PDE or cGMP PDE assays. The assay mixture (100 micro liters volume containing 50×10−3 M HEPES (pH 7.4), 10×10−3 M MgCl2, 1×10−3 M EDTA, 0.1×10−3 M EGTA, and 1×10−6 M 3H-cAMP (30,000 CPM) or 1×10−6 M 3H-cGMP (30,000 CPM)) was incubated at 30 °C for 20 min. The assays were terminated by adding 50 micro liters of ice-cold, 0.5 M EDTA (pH 8.0). After diluting the samples with 0.3 ml of buffer (0.1 M NaCl, 0.1 M HEPES (pH 8.5)), the reaction product, either 5′-[3H] AMP or 5′-[3H] GMP, was purified by polyacrylamide-boronate gel chromatography (affi-Gel 601, 1-ml bed volume) and quantified by liquid scintillation counting.
PDE activity, for each of the PDE families, was determined as that portion of total cAMP or cGMP hydrolysis that was inhibited by selective PDE inhibitors (1×10−6 M cilostamide for PDE3 activity, 5×10−6 M rolipram for PDE4 activity, and 5×10−6 M MY5445 (1-(3-chlorophenylamino)-4-phenylphthalazine) for PDE5 activity) or activators (4×10−3 M CaCl2 and 10 units of calmodulin for PDE1 activity). No EGTA was added to the assay mixture when calcium/calmodulin stimulated PDE1 activity was being measured.
In vitro isometric contractility studies
The ductus arteriosus from 13 immature (106±2 days gestation) and 9 mature (139±3 days) fetuses were divided into 1 mm-thick rings (2 – 3 rings per animal) and isometric tension was measured in a Krebs-bicarbonate solution ([×10−3 M]: 118 NaCl, 4.7 KCl, 2.5 CaCl2, 0.9 MgSO4, 1 KH2PO4, 11.1 glucose, 23 NaHCO3 (pH 7.4)) containing indomethacin (5 × 10−6 M) and Nω-nitro-arginine methyl ester (L-NAME, 10−4 M), to inhibit endogenous prostaglandin and nitric oxide production, respectively (13). The bath solution was equilibrated with 5% CO2/30% O2 balance N2 and was changed every 20 minutes. Rings were stretched to initial lengths (preterm = 5.4 ± 0.3 mm; late-gestation = 6.7 ± 0.6 mm) that produce maximal contractile responses when exposed to potassium-(K+-Krebs)-Kreb’s solution (containing 0.1 M KCl substituted for an equimolar amount of NaCl), equilibrated with 95% O2 (14). After the rings reached a steady-state tension (approximately 100–120 min), K+-Krebs solution was used to measure the maximal contraction that could be developed by the ductus (maximal contraction).
Following the maximal contraction, the K+-Krebs solution was removed from the baths and the rings relaxed to a steady-state tension (baseline tension). We measured the ability of the PDE inhibitors to relax the ductus from the baseline tension. Cumulative dose response curves were constructed for 8-methoxymethyl 3-isobutyl-1-methylxanthine (8-MM-IBMX, a PDE1 inhibitor), cilostamide (a PDE3 inhibitor), milrinone (a PDE3 inhibitor), rolipram (a PDE4 inhibitor), and MY5445 (a PDE5 inhibitor). The concentrations that produced 50% of maximal relaxation (EC50 values) were determined from each dose-response curve. In all experiments, we allowed the tension in the rings to reach a new steady-state plateau after addition of a drug and before another concentration was added to the bath. Sodium nitroprusside (10−4 M) was added to each ring at the end of the experiment to determine its minimal tension. After the experiment, the tissues were removed from the baths and blotted dry and their wet weights determined. The immature ductus rings had a similar wet weight (30±6 mg) as the mature rings (34±10 mg).
The difference in tensions between the maximal tension (produced by K+-Krebs/95 % O2) and the minimal tension (with sodium nitroprusside) was considered the maximal active tension (MAT) that the rings were capable of developing. The immature ductus rings developed significantly lower amounts of MAT than the mature ductus rings (18.8±2.5 gm compared with 23.1±4.2 gm, respectively, p<0.01).
The difference in tensions between the baseline tension (prior to the PDE inhibitor dose response curve) and the minimal tension (with sodium nitroprusside) was considered the baseline Net Tension for the dose response curve. The baseline Net Tensions for the dose response curves were similar in the immature and mature ductus (immature baseline Net Tension = 75±10% of MAT; mature = 81±9% of MAT).
MY5445 and 8-MM-IBMX were obtained from Biomol International (Plymouth Meeting, PA). All other chemicals were from Sigma Chemical (St Louis, MO).
Statistics
Statistical analyses of unpaired and paired data were performed by the appropriate t-test and by analysis of variance. Scheffe’s test was used for post hoc analysis. Values are expressed as mean ± standard deviation. Drug concentrations refer to their final molar concentration in the bath.
Results
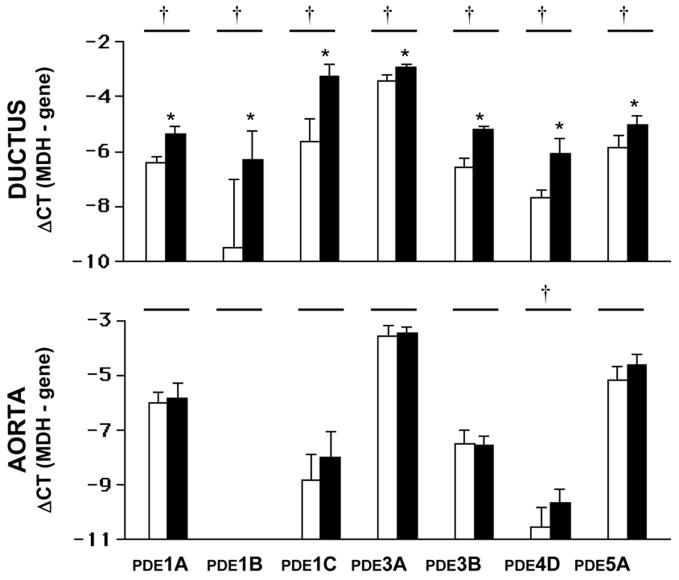
The fetal ductus arteriosus expressed several PDE family isoforms (PDE1, PDE3, PDE4 and PDE5) (Figure 1). The expression of each isoform increased with advancing gestation. In contrast, in the fetal aorta, there was no significant change in PDE expression with advancing gestation. The expression of most PDE isoforms was lower in the near term aorta than in the near term ductus (Fig 1).
Figure 1.
Real Time PCR measurements of PDE isoforms (PDE1A, 1B, 3A, 3B, 4D, 5A) are greater in the mature (■) than in the immature ductus (□) arteriosus. ΔCT(MDH-gene) represents the difference in CT between the expression of the housekeeping gene MDH and the gene of interest. Each unit of ΔCT(MDH-gene) represents a 2-fold increase in a gene’s mRNA. The more negative the ΔCT(MDH-gene), the fewer the number of starting copies of a gene (mRNA). N= number of separate animals used (immature = 7; mature = 7). (=): p<0.05, immature compared with mature. (*): p<0.05, mature ductus compared with mature aorta.
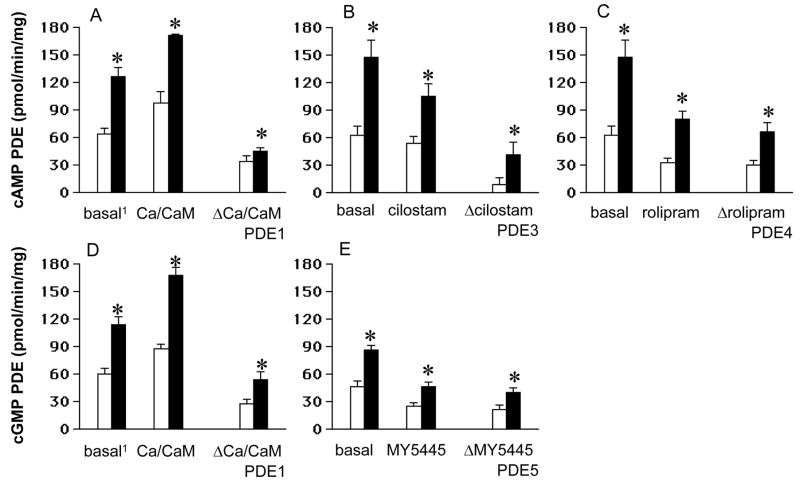
Basal cAMP and cGMP PDE activities were measured in ductus homogenates in the presence of 1×10−6 M cAMP and 1×10−6 M cGMP, respectively. The nonselective PDE antagonist, 3-isobutyl-1-methylxanthine (IBMX), inhibited more than 97% of the ductus’ basal PDE activity (n=3, data not shown). Both the basal cAMP and basal cGMP PDE activities, as well as the activities attributed to PDE3 (Δcilostamide), PDE4 (Δrolipram), and PDE5 (ΔMY5445) were significantly increased in the mature ductus compared with the immature ductus (Figure 2). To assess the presence of PDE1 in ductus homogenates, we tested the effect of calcium/calmodulin on both cAMP and cGMP hydrolytic activities. Calcium/calmodulin produced a greater stimulation of both cAMP and cGMP hydrolytic activities (ΔCa/CaM) in the mature ductus than in the immature ductus (Figure 2).
Figure 2.
cAMP and cGMP PDE activities are greater in the mature (■) than in the immature ductus (□) arteriosus. Ductus homogenates were incubated in assay buffer containing either 3H-cAMP (panels A, B, and C) or 3H-cGMP (panels D and E). PDE activity was measured either in the absence (basal) or presence of selective PDE inhibitors (cilostamide 1×10−6 M, for PDE3; rolipram 5×10−6 M, for PDE4; and MY5445 5×10−6 M, for PDE5); or activators (CaCl2/calmodulin (4×10−3 M)/10 units, respectively), for PDE1). We determined the PDE activity for each PDE family (Δ) by subtracting the basal activity from the activity in the presence of inhibitor or activator. Basal1: no EGTA was added to the assay buffer in CaCl2/calmodulin experiments (see Methods). N= number of separate animals used (immature = 5; mature = 5). (*): p<0.05, immature compared with mature.
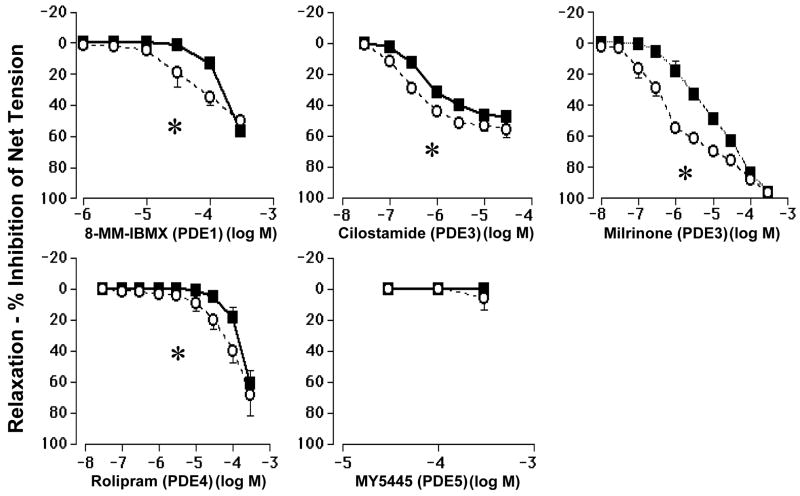
Under normoxic conditions (30% O2), in the presence of inhibitors of prostaglandin and nitric oxide production, the ductus arteriosus developed an active tension that was approximately 75 – 80% of its Maximal Active Tension (see Methods). Selective inhibitors of PDE1 (8-MM-IBMX), PDE3 (cilostamide and milrinone), and PDE4 (rolipram) relaxed the ductus in both the mature and immature ductus (figure 3). The selective PDE5 inhibitor MY5445 had no effect on ductus tension. Higher concentrations of 8-MM-IBMX (PDE1), cilostamide, milrinone (PDE3), and rolipram (PDE4) were needed to inhibit tensions in the mature ductus than in the immature ductus (Figure 3).
Figure 3.
Higher concentrations of PDE1, 3 and 4 inhibitors are required to inhibit isometric tension in the mature ductus arteriosus (■) than in the immature ductus (○). Ductus rings were precontracted with 30% oxygen, indomethacin and L-NAME (see Methods). Dose responses for 8-MM-IBMX, cilostamide, milrinone, rolipram, and MY5445 were studied. Tension is expressed as a percentage of baseline Net Tension (see Methods). (*): p<0.05, EC50 (immature) < EC50 (mature). n= number of separate animals used. 8-MM -IBMX: EC50 (immature) = 0.4±0.4 × 10−4M (n= 6); EC50 (mature) = 1.5±0.7 × 10−4M (n= 5), p<0.05. Cilostamide: EC50 (immature) = 2.9±0.8 × 10−7M (n= 4); EC50 (mature) = 8.4±1.5 × 10−7M (n= 4), p<0.05. Milrinone: EC50 (immature) = 0.7±0.1 × 10−6M (n= 6); EC50 (mature) = 9.4±2.2 × 10−6M (n= 7), p<0.05. Rolipram: EC50 (immature) = 0.7±0.1 × 10−4M (n= 6); EC50 (mature) = 1.3±0.1 × 10−4M (n= 7), p<0.05. MY5445: EC50 (immature) = not measurable (n= 4); EC50 (mature) = not measurable (n= 4).
Discussion
An increase in cAMP or cGMP is generally associated with smooth muscle relaxation through both calcium-dependent and calcium independent processes (15, 16) (17). Consequently, an increase in PDE activity should result in a blunted relaxation. Our findings support the hypothesis that increasing PDE activity may contribute to the decreased ability of PGE2 to relax the ductus with advancing gestation. In the mature ductus, there was both increased expression (Figure 1) and increased activity (Figure 2) of all of the PDE isoforms: PDE1, 3, 4, and 5. In parallel with the increase in PDE activity, higher concentrations of PDE inhibitors were needed to produce the same degree of relaxation with advancing gestation (Figure 3). Our observation that the mature ductus, in vitro, requires higher concentrations of PDE inhibitors to produce a similar relaxation as in the immature ductus is consistent with a prior report for PDE3 inhibition in the fetal rat, in vivo (11).
Several of our findings deserve further discussion. Although PDE1A and PDE1B, which hydrolyze cGMP, have frequently been observed in adult and newborn vascular tissues, PDE1C which hydrolyzes both cAMP and cGMP has not (18). In contrast, fetal vessels appear to express PDE1C (Figure 1) (19). With advancing gestation, we observed both increased expression of PDE1C and increased calcium/calmodulin stimulation of cAMP hydrolysis in the ductus. This is consistent with increased PDE1C activity. Currently, it is difficult to assess the relative role of PDE1C in ductus contractility since available inhibitors of PDE1, like 8-MM-IBMX, have poor selectivity among the different PDE1 isoforms and are less potent against the PDE1C enzyme than against PDE1A or PDE1B (20, 21). However, the increased expression of PDE1C in the ductus (compared with the aorta) may make it a useful target for selective regulation of cyclic nucleotide signaling after birth.
PDE3 plays an important role in coordinating and amplifying the vasodilatory effects of agents that increase cAMP and cGMP. Uninhibited PDE3 activity has been found to promote vasoconstriction and neointima accumulation (5, 22–25). The increase in ductus arteriosus PDE3 activity with advancing gestation (Figures 1 and 2) may contribute to the vasoconstriction and neointima formation that occur as term approaches (26).
The results of our contractility studies differed significantly from those of previous reports (10, 12) with regard to the effects of inhibition of PDE5 on ductus tension. We found that even though PDE5 was expressed in the wall of the fetal ductus (Figures 1 and 2), inhibition of PDE5 had almost no effect on ductus contractility. Our contractility studies, however, were performed under different conditions than the previous studies (10, 12). We examined the contractility of ductus rings in the presence of inhibitors of endogenous prostaglandin and nitric oxide production (13). Both prostaglandin and nitric oxide production in the ductus increase significantly with advancing gestation (13, 27, 28). Our intention was to examine the effects of PDE inhibition on ductus contractility during development, without the confounding effects of changes in prostaglandin or nitric oxide production. We found that, in the presence of an inhibitor of nitric oxide production, PDE5 inhibition had no effect on ductus tension (Figure 3). Several endogenous vasodilators (e.g., nitric oxide, carbon monoxide, atrial natriuretic peptide, etc) are capable of regulating smooth muscle tone in the ductus by activating guanyl cyclase and increasing cGMP production (27, 29, 30). Our findings suggest that among these cGMP-producing vasodilators, nitric oxide appears to be the most important, since in its absence, inhibitors of cGMP degradation no longer have an effect on ductus tone (Figure 3). Inhibition of nitric oxide (and cGMP production) may have a direct effect on PDE5 activity itself. PDE5 inhibitors are most effective when the nitric oxide/cGMP signaling pathway has been activated (31). cGMP binds to a regulatory GAF domain on PDE5; this produces a large stimulatory effect on the PDE5 catalytic domain. Without this effect, PDE5 has only a low intrinsic hydrolytic activity (32).
In conclusion, we observed a developmental increase in both cAMP and cGMP PDEs that contribute to the decreased sensitivity of the late gestation ductus arteriosus to vasodilators. We speculate that the developmental increase in PDEs may enable the ductus to constrict and remodel itself more efficiently after birth.
Acknowledgments
This research was supported by grants from U.S. Public Health Service (NIH grants HL46691, HL56061) and by a gift from the Jamie and Bobby Gates Foundation.
We wish to thank Ms. Christine Roman for her help with the contractility studies and Ms. Francoise Mauray with the manuscript preparation.
Abbreviations
- IBMX
3-isobutyl-1-methylxanthine
- K+-Krebs
potassium Kreb’s solution
- MAT
maximal active tension
- 8-MM-IBMX
8-methoxymethyl 3-isobutyl-1-methylxanthine
- MY5445
1-(3-chlorophenylamino)-4-phenylphthalazine
- PDE
phosphodiesterase
- PGE2
prostaglandin E2
Footnotes
Publisher's Disclaimer: Pediatric Research Articles Ahead of Print contains articles in unedited manuscript form that have been peer-reviewed and accepted for publication. As a service to our readers, we are providing this early version of the manuscript.The manuscript will undergo copyediting, typesetting and review of the resulting proof before it is published in its final definitive form. Please note that during the production process errors may be discovered, which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Clyman RI, Mauray F, Rudolph AM, Heymann MA. Age-dependent sensitivity of the lamb ductus arteriosus to indomethacin and prostaglandins. J Pediatr. 1980;96:94–98. doi: 10.1016/s0022-3476(80)80338-6. [DOI] [PubMed] [Google Scholar]
- 2.Clyman RI, Mauray F, Roman C, Heymann MA, Payne B. Effect of gestational age on ductus arteriosus response to circulating prostaglandin E2. J Pediatr. 1983;102:907–911. doi: 10.1016/s0022-3476(83)80023-7. [DOI] [PubMed] [Google Scholar]
- 3.Waleh N, Kajino H, Marrache AM, Ginzinger D, Roman C, Seidner SR, Moss TJ, Fouron JC, Vazquez-Tello A, Chemtob S, Clyman RI. Prostaglandin E2--mediated relaxation of the ductus arteriosus: effects of gestational age on g protein-coupled receptor expression, signaling, and vasomotor control. Circulation. 2004;110:2326–2332. doi: 10.1161/01.CIR.0000145159.16637.5D. [DOI] [PubMed] [Google Scholar]
- 4.Bouayad A, Kajino H, Waleh N, Fouron JC, Andelfinger G, Varma DR, Skoll A, Vazquez A, Gobeil F, Jr, Clyman RI, Chemtob S. Characterization of PGE2 receptors in fetal and newborn lamb ductus arteriosus. Am J Physiol Heart Circ Physiol. 2001;280:H2342–H2349. doi: 10.1152/ajpheart.2001.280.5.H2342. [DOI] [PubMed] [Google Scholar]
- 5.Manganiello VC, Degerman E. Cyclic nucleotide phosphodiesterases (PDEs): diverse regulators of cyclic nucleotide signals and inviting molecular targets for novel therapeutic agents. Thromb Haemost. 1999;82:407–411. [PubMed] [Google Scholar]
- 6.Soderling SH, Beavo JA. Regulation of cAMP and cGMP signaling: new phosphodiesterases and new functions. Curr Opin Cell Biol. 2000;12:174–179. doi: 10.1016/s0955-0674(99)00073-3. [DOI] [PubMed] [Google Scholar]
- 7.Maurice DH, Palmer D, Tilley DG, Dunkerley HA, Netherton SJ, Raymond DR, Elbatarny HS, Jimmo SL. Cyclic nucleotide phosphodiesterase activity, expression, and targeting in cells of the cardiovascular system. Mol Pharmacol. 2003;64:533–546. doi: 10.1124/mol.64.3.533. [DOI] [PubMed] [Google Scholar]
- 8.Rybalkin SD, Yan C, Bornfeldt KE, Beavo JA. Cyclic GMP phosphodiesterases and regulation of smooth muscle function. Circ Res. 2003;93:280–291. doi: 10.1161/01.RES.0000087541.15600.2B. [DOI] [PubMed] [Google Scholar]
- 9.Polson JB, Strada SJ. Cyclic nucleotide phosphodiesterases and vascular smooth muscle. Annu Rev Pharmacol Toxicol. 1996;36:403–427. doi: 10.1146/annurev.pa.36.040196.002155. [DOI] [PubMed] [Google Scholar]
- 10.Thebaud B, Michelakis E, Wu XC, Harry G, Hashimoto K, Archer SL. Sildenafil reverses O2 constriction of the rabbit ductus arteriosus by inhibiting type 5 phosphodiesterase and activating BK(Ca) channels. Pediatr Res. 2002;52:19–24. doi: 10.1203/00006450-200207000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Toyoshima K, Momma K, Imamura S, Nakanishi T. In vivo dilatation of the fetal and postnatal ductus arteriosus by inhibition of phosphodiesterase 3 in rats. Biol Neonate. 2006;89:251–256. doi: 10.1159/000089954. [DOI] [PubMed] [Google Scholar]
- 12.Momma K, Toyoshima K, Imamura S, Nakanishi T. In vivo dilation of fetal and neonatal ductus arteriosus by inhibition of phosphodiesterase-5 in rats. Pediatr Res. 2005;58:42–45. doi: 10.1203/01.PDR.0000156370.50874.3C. [DOI] [PubMed] [Google Scholar]
- 13.Kajino H, Chen YQ, Seidner SR, Waleh N, Mauray F, Roman C, Chemtob S, Koch CJ, Clyman RI. Factors that increase the contractile tone of the Ductus Arteriosus also regulate its anatomic remodeling. Am J Physiol Regul Integr Comp Physiol. 2001;281:R291–R301. doi: 10.1152/ajpregu.2001.281.1.R291. [DOI] [PubMed] [Google Scholar]
- 14.Clyman RI, Mauray F, Wong L, Heymann MA, Rudolph AM. The developmental response of the ductus arteriosus to oxygen. Biol Neonate. 1978;34:177–181. doi: 10.1159/000241124. [DOI] [PubMed] [Google Scholar]
- 15.Karaki H, Ozaki H, Hori M, Mitsui-Saito M, Amano K, Harada K, Miyamoto S, Nakazawa H, Won KJ, Sato K. Calcium movements, distribution, and functions in smooth muscle. Pharmacol Rev. 1997;49:157–230. [PubMed] [Google Scholar]
- 16.Lee MR, Li L, Kitazawa T. Cyclic GMP causes Ca2+ desensitization in vascular smooth muscle by activating the myosin light chain phosphatase. J Biol Chem. 1997;272:5063–5068. doi: 10.1074/jbc.272.8.5063. [DOI] [PubMed] [Google Scholar]
- 17.Savineau JP, Marthan R. Modulation of the calcium sensitivity of the smooth muscle contractile apparatus: molecular mechanisms, pharmacological and pathophysiological implications. Fundam Clin Pharmacol. 1997;11:289–299. doi: 10.1111/j.1472-8206.1997.tb00841.x. [DOI] [PubMed] [Google Scholar]
- 18.Rybalkin SD, Bornfeldt KE, Sonnenburg WK, Rybalkina IG, Kwak KS, Hanson K, Krebs EG, Beavo JA. Calmodulin-stimulated cyclic nucleotide phosphodiesterase (PDE1C) is induced in human arterial smooth muscle cells of the synthetic, proliferative phenotype. J Clin Invest. 1997;100:2611–2621. doi: 10.1172/JCI119805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rybalkin SD, Rybalkina I, Beavo JA, Bornfeldt KE. Cyclic nucleotide phosphodiesterase 1C promotes human arterial smooth muscle cell proliferation. Circ Res. 2002;90:151–157. doi: 10.1161/hh0202.104108. [DOI] [PubMed] [Google Scholar]
- 20.Zhao AZ, Yan C, Sonnenburg WK, Beavo JA. Recent advances in the study of Ca2+/CaM-activated phosphodiesterases: expression and physiological functions. Adv Second Messenger Phosphoprotein Res. 1997;31:237–251. [PubMed] [Google Scholar]
- 21.Kakkar R, Raju RV, Sharma RK. Calmodulin-dependent cyclic nucleotide phosphodiesterase (PDE1) Cell Mol Life Sci. 1999;55:1164–1186. doi: 10.1007/s000180050364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Netherton SJ, Jimmo SL, Palmer D, Tilley DG, Dunkerley HA, Raymond DR, Russell JC, Absher PM, Sage EH, Vernon RB, Maurice DH. Altered phosphodiesterase 3-mediated cAMP hydrolysis contributes to a hypermotile phenotype in obese JCR:LA-cp rat aortic vascular smooth muscle cells: implications for diabetes-associated cardiovascular disease. Diabetes. 2002;51:1194–1200. doi: 10.2337/diabetes.51.4.1194. [DOI] [PubMed] [Google Scholar]
- 23.Pan X, Arauz E, Krzanowski JJ, Fitzpatrick DF, Polson JB. Synergistic interactions between selective pharmacological inhibitors of phosphodiesterase isozyme families PDE III and PDE IV to attenuate proliferation of rat vascular smooth muscle cells. Biochem Pharmacol. 1994;48:827–835. doi: 10.1016/0006-2952(94)90062-0. [DOI] [PubMed] [Google Scholar]
- 24.Souness JE, Hassall GA, Parrott DP. Inhibition of pig aortic smooth muscle cell DNA synthesis by selective type III and type IV cyclic AMP phosphodiesterase inhibitors. Biochem Pharmacol. 1992;44:857–866. doi: 10.1016/0006-2952(92)90116-z. [DOI] [PubMed] [Google Scholar]
- 25.Lindgren S, Andersson KE, Belfrage P, Degerman E, Manganiello VC. Relaxant effects of the selective phosphodiesterase inhibitors milrinone and OPC 3911 on isolated human mesenteric vessels. Pharmacol Toxicol. 1989;64:440–445. doi: 10.1111/j.1600-0773.1989.tb00683.x. [DOI] [PubMed] [Google Scholar]
- 26.Clyman RI, Chan CY, Mauray F, Chen YQ, Cox W, Seidner SR, Lord EM, Weiss H, Wale N, Evan SM, Koch CJ. Permanent anatomic closure of the ductus arteriosus in newborn baboons: the roles of postnatal constriction, hypoxia, and gestation. Pediatr Res. 1999;45:19–29. doi: 10.1203/00006450-199901000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Clyman RI, Waleh N, Black SM, Riemer RK, Mauray F, Chen YQ. Regulation of ductus arteriosus patency by nitric oxide in fetal lambs. The role of gestation, oxygen tension and vasa vasorum. Pediatr Res. 1998;43:633–644. doi: 10.1203/00006450-199805000-00012. [DOI] [PubMed] [Google Scholar]
- 28.Baragatti B, Brizzi F, Ackerley C, Barogi S, Ballou LR, Coceani F. Cyclooxygenase-1 and cyclooxygenase-2 in the mouse ductus arteriosus: individual activity and functional coupling with nitric oxide synthase. Br J Pharmacol. 2003;139:1505–1515. doi: 10.1038/sj.bjp.0705391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baragatti B, Brizzi F, Barogi S, Laubach VE, Sodini D, Shesely EG, Regan RF, Coceani F. Interactions between NO, CO and an endothelium-derived hyperpolarizing factor (EDHF) in maintaining patency of the ductus arteriosus in the mouse. Br J Pharmacol. 2007;151:54–62. doi: 10.1038/sj.bjp.0707211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Toyoshima K, Momma K, Imamura S, Nakanishi T. In vivo dilatation of the postnatal ductus arteriosus by atrial natriuretic peptide in the rat. Neonatology. 2007;92:139–144. doi: 10.1159/000101526. [DOI] [PubMed] [Google Scholar]
- 31.Kloner RA. Cardiovascular risk and sildenafil. Am J Cardiol. 2000;86:57F–61F. doi: 10.1016/s0002-9149(00)00895-x. [DOI] [PubMed] [Google Scholar]
- 32.Rybalkin SD, Rybalkina IG, Shimizu-Albergine M, Tang XB, Beavo JA. PDE5 is converted to an activated state upon cGMP binding to the GAF A domain. EMBO J. 2003;22:469–478. doi: 10.1093/emboj/cdg051. [DOI] [PMC free article] [PubMed] [Google Scholar]