Abstract
Giant cell myocarditis (GCM) is a rare and highly lethal disorder. The only multi-center case series with treatment data lacked data on cardiac function and had a retrospective design. We conducted a prospective, multi-center study of immunosuppression including cyclosporine and steroids for acute, microscopically-confirmed GCM. From June 1999 to June 2005, 11 subjects received high dose steroids, cyclosporine, and in 9 cases muromonab-CD3 in a standard protocol. Among these, 7 of 11 were female, the mean age was 60±15 years, and the mean time from symptom onset to presentation was 27±33 days. During one year of treatment, one subject died of respiratory complications at day 178, and 2 subjects received heart transplantations on days 2 and 27 respectively. Serial endomyocardial biopsies revealed that after 4 weeks of treatment the degree of necrosis, cellular inflammation and giant cells decreased (P=.001). One subject, who completed the trial, subsequently died of a fatal GCM recurrence after withdrawal of immunosuppression. Her case demonstrates for the first time that there is a risk of recurrent, sometimes fatal GCM after cessation of immunosuppression. In conclusion, this prospective study of immunosuppression for GCM confirms retrospective case reports that such therapy improves long-term survival. Additionally, withdrawal of immunosuppression can be associated with fatal GCM recurrence.
Keywords: giant cell myocarditis, dilated cardiomyopathy, immunosuppression, cyclosporine, myocarditis
Introduction
Until 1987 all published cases of giant cell myocarditis (GCM) were diagnosed at autopsy or heart transplantation after a brief illness.1,2 In 1997 a multicenter international registry of GCM characterized 63 cases from 36 medical centers in 9 countries.3 The main findings of this registry were that median transplant-free survival from symptom onset is poor at 5.5 months, but that in patients diagnosed by biopsy, early immunosuppressive treatment that included cyclosporine, extended median transplant-free survival from 3.0 to 12.4 months. The data from the GCM registry did not include left ventricular function, the effect of immunosuppression on cardiac histology, or an assessment of immunosuppressive treatment risks. To fill these gaps in the knowledge of GCM treatment, a multicenter GCM study was designed to test the hypothesis that 1 year of treatment with cyclosporine given in combination with steroids and 10 days of muromonab-CD3 would improve transplant-free survival in biopsy-proven cases of GCM with less than 3 months symptom duration. The rationale for muromonab-CD3 and cyclosporine-based immunosuppression is founded on the mechanistic assumption from a Lewis rat model that GCM is a T-cell-mediated disease.4,5 Recruitment difficulties precluded patient randomization to a non-immunosuppression arm as originally intended and thus we modified our study design accordingly. Here we report the response of cardiac function and histologic findings in a prospective observational study of 11 subjects with acute GCM.
Methods
The initial design of the study was a multicenter, randomized, open label, 2 arm trial and parallel prospective treatment registry. The active treatment group received 10 days of muromonab-CD3, and 1 year of cyclosporine and steroids as described below. The control group received “usual care” that could include no immunosuppression, or steroids and/or azathioprine at the discretion of the site principal investigator. A prospective treatment registry was an option for subjects who declined to be randomized. In the registry, subjects received the exact treatment and assessments as in the active treatment arm of the trial without randomization.
After 2 years, 8 subjects enrolled in the registry and no subject chose to be randomized and risk not receiving immunosuppression. Therefore, the study was modified and the usual care arm of the trial was replaced with cyclosporine and steroids as in the active treatment arm. The active treatment arm which included muromonab-CD3 was not changed. After an additional 4 years, 4 subjects were randomized, 2 to active treatment and 2 to cyclosporine and steroids without muromonab-CD3. On July 31st, 2005, the study was closed to enrollment due to low accrual with a final total enrollment of 12 patients. This report is the summary of these 12 subjects experience.
Subjects could be included if they had heart failure and/or arrhythmia of less than 3 months duration, an endomyocardial biopsy diagnostic of giant cell myocarditis, and gave written consent. All subjects who enrolled in the registry or who were randomized to receive muromonab-CD3 received the following immunosuppressive regimen: Muromonab-CD3 5mg daily for 10 days, and cyclosporine, titrated to achieve a target serum level of 150–300 ng/mL measured by high performance liquid chromatography-tandem mass spectroscopy (HPLC-MS/MS). Cyclosporine was continued for one year after randomization. Eleven hour trough cyclosporine levels were recorded at the 1-, 3-, 6-, and 12-month study visits. Intravenous methylprednisolone 10mg/kg preceded the first three doses of muromonab-CD3 by 1 to 4 hours. Beginning on the fourth day, prednisone was administered according to the once daily schedule: 1mg/kg for 4 days, followed by 0.5mg/kg for 1 week, followed by 0.25mg/kg for 1 week, followed by 10mg for 1 week, followed by 5mg for 48 weeks. Patients randomized to the group not receiving muromonab-CD3, received only cyclosporine and steroids in the same schedule.
Seventeen medical centers participated in screening. Subjects in whom the diagnosis of GCM was considered possible were asked to give written consent to screening. Screening included a baseline blood draw for research purposes and collection of clinical data. If the endomyocardial biopsy was positive, subjects were asked to sign a second consent form describing the study protocol and procedures. This study complies with the Declaration of Helsinki. The respective institutional review boards and ethical committees at all sites approved the protocol.
Changes in the left ventricular ejection fraction from baseline to 4 weeks after treatment were compared in the 10 subjects who had baseline studies and had not undergone transplantation by 4 weeks after treatment. The observed difference in the distributions of left ventricular ejection fraction was assessed for significance using the paired Wilcoxon signed rank test.
Equilibrium radionuclide angiography was performed using a modified in vivo red blood cell labeling method. A 20% energy window was set over the 140 keV photopeak. All data were acquired in electrocardiogram synchronized frame mode over 16 frames per cardiac cycle. Global left ventricular ejection fraction was determined from the left anterior oblique image. In subjects deemed too unstable to undergo equilibrium radionuclide angiography, an echocardiogram could be used to determine left ventricular ejection fraction.
Histologic features were semi-quantitatively scored (0, 1+, 2+, 3+), including number of giant cells, overall degree of inflammation, and the presence of myocyte necrosis, fibrosis, eosinophils and granuloma formation, by two pathologists (H.D.T. and W.D.E.). The microscopic slides were coded and randomly sorted to blind the panelists to treatment status. Changes in the myocardial inflammatory infiltrate from baseline to 4 weeks after treatment were compared in the subjects who had not undergone heart transplantation. The observed differences in the average scores from baseline to 4 weeks after start of treatment were assessed for significance using the paired Wilcoxon signed rank test.
Enzyme-linked immunosorbent assays (ELISA) were performed to assess for anti-human cardiac myosin, anti-human β1 receptor (β1) and anti-human β2 (β2) receptor antibodies on 8 serum samples from GCM subjects prior to immunosuppressive treatment. β1 and β2 adrenergic receptor antigens were purchased from Perkin Elmer, Boston, MA as cloned β-adrenergic receptor subtype 1 produced in Sf9 cells and cloned β-adrenergic receptor subtype 2 produced in Sf9 cells. Human cardiac myosin was purified from normal human myocardium according to a previously published procedure.6 Antigens were coated onto Immunlon 4 microtiter plates at 10ug/ml in coating buffer and serum dilutions were tested against the antigens in a standard ELISA protocol.6 Plates were washed with PBS tween buffers using a Bio-Tek Bio-Stack Microplate Washer from Bio-Tek Instruments, Winooski, VT. Plates were developed using anti-human IgG gamma chain specific conjugated with alkaline phosphatase from Sigma Chemical Co, St Louis, MO and optical density of the serum reaction was read in an Dynex Opsys MR ELISA microplate reader. Control sera consisted of healthy subjects with no known cardiac disease. Titers of the sera were calculated at the last serum dilution ot read at an optical density of 0.1. All serum dilutions were tested in duplicate and each assay was controlled using a known positive and negative serum standard.
Adverse events and serious adverse events were recorded, and a data and safety monitoring committee (membership listed in the Appendix) consisting of a biostatistician and 2 cardiologists with expertise in myocarditis reviewed all serious adverse events periodically during the study.
Results
The clinical performance sites and principal investigators that participated in study enrollment are listed in the Appendix. Participation is defined as having IRB approval for the study and all required contractual and regulatory documentation necessary for screening and enrollment. Enrollment refers to subjects with GCM who signed consent.
A total of 28 subjects signed consent for screening prior to endomyocardial biopsy. Of these 28 screened subjects, 8 subjects (29%) had GCM and subsequently consented to be enrolled in the treatment phase of the study. In addition to these 8 subjects, 4 subjects were diagnosed without prior screening consent. These 4 subjects did not sign a screening consent either because the biopsy was performed at an institution not participating in the GCM trial or because the biopsy was performed for reasons other than GCM screening. These 4 subjects subsequently signed consent to participate in the treatment phase of the study.
Of the 12 subjects who signed consent for the treatment phase of the study, one of was never treated due to the site investigator’s decision and is not included in the following analysis. Of the remaining 11 subjects, 7 entered the registry and 4 were randomized. 2 patients were randomized to receive muromonab-CD3, cyclosporine and steroids and 2 received cyclosporine and steroids without muromonab-CD3. Three subjects had associated disorders: Guillain-Barré syndrome (1), hypothyroidism (1), and celiac disease and chronic lymphocytic leukemia (1). The other 9 patients were previously well. The demographics, treatment assignments, and outcomes are summarized in Table 1.
Table 1.
Demographics, Treatment and Outcome of 11 Subjects with Giant Cell Myocarditis
| Subject Number | Gender | Age at entry | Duration of symptoms (days) | Treatment | Baseline LVEF (Percent) | Outcome |
|---|---|---|---|---|---|---|
| 4 | M | 39 | 19 | OKT3, C, S | 47 | Alive |
| 9 | F | 45 | 4 | OKT3, C, S | 50 | Alive |
| 10 | F | 48 | 6 | OKT3, C, S | 15 | Transplant |
| 1 | M | 49 | 64 | OKT3, C, S | 25 | Transplant |
| 2 | M | 51 | 4 | OKT3, C, S | 48 | Alive |
| 7 | F | 58 | 1 | OKT3, C, S | 43 | Alive |
| 3 | F | 70 | 40 | OKT3, C, S | 54 | Alive |
| 6 | M | 71 | 1 | C, S | 17 | Died |
| 8 | F | 76 | 24 | OKT3, C, S | 67 | Alive |
| 5 | F | 79 | 5 | C, S | 19 | Alive |
| 11 | F | 81 | 1 | OKT3, C, S | 68 | Alive |
OKT3, muromonab-CD3; C, cyclosporine; S, corticosteroids; LVEF, left ventricular ejection fraction
The subjects received standard medications and devices used for the management of heart failure and arrhythmias. Nine of eleven subjects received beta-blockers. Eight of eleven subjects received angiotensin converting enzyme inhibitors (ACEI), angiotensin receptor blockers (ARB) or hydralazine. Seven of eleven subjects received digoxin. Six of eleven subjects received amiodarone for ventricular tachycardia. The explanation for the less than universal use of ACEI and ARB is that only 7 of the 11 subjects had ejection fractions of less than 50%. Only 5 subjects presented with typical symptoms of acute heart failure, three of whom required intravenous inotropic support. Six subjects had implantable defibrillators placed for ventricular tachycardia. No subjects required intra-aortic balloon pump counterpulsation or a ventricular assist device.
All patients received cyclosporine, methylprednisolone and subsequently prednisone per protocol. Nine subjects received muromonab-CD3 per protocol. The average trough cyclosporine levels in subjects who were alive without transplant at 1, 3, 6, and 12 months were 169, 194, 126, and 294 ng/mL respectively.
In this sick population there were 15 serious adverse events (SAE), most of which were related to the underlying disease and a few of which were related to the immunosuppressive treatment. SAE possibly caused by the immunosuppressive treatment included a case of pulmonary cryptococcus with subsequent respiratory arrest, tracheostomy, and death from a mucus plug. Six subjects developed transient renal insufficiency (creatinine >1.5 mg/dl) related to cyclosporine use, including one associated with dehydration which required hospitalization and discontinuation of cyclosporine with substitution of sirolimus after 4 months of treatment. The average creatinine increased from 1.1±0.1 to 1.9±0.8 (p=.01 by 2-tailed T test). No subject developed diabetes or had significant weight gain despite the use of steroids.
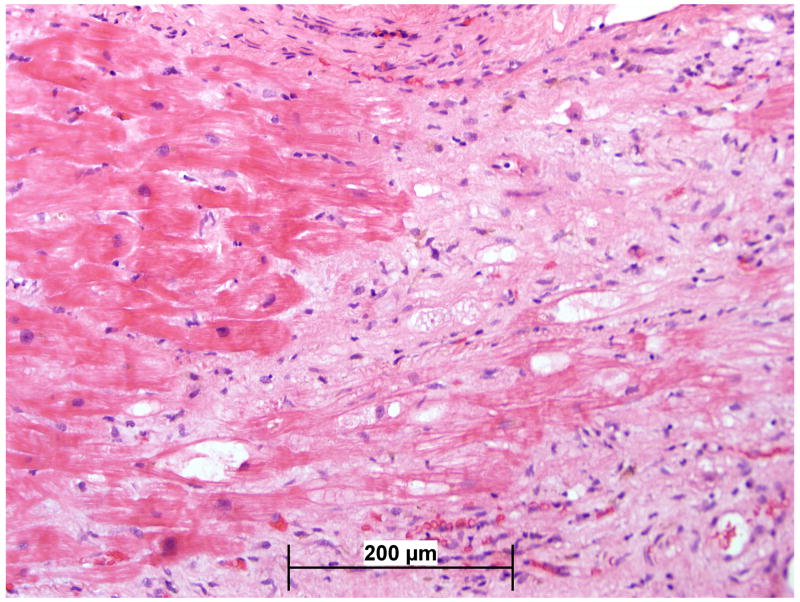
Endomyocardial biopsy was performed twice during this study, at study entry and 4 weeks after the start of treatment. Between the baseline and 4 week post-treatment samples, the degree of necrosis, eosinophils, giant cells, and foci of lymphocytic myocarditis decreased significantly (p=.001). The degree of fibrosis increased, but this change did not reach statistical significance. The comparison between the baseline and 4 week histopathology is illustrated in Figures 1 and 2.
Figure 1.
Average histologic scores by blinded analysis at baseline and day 30 in subjects enrolled in the GCM Treatment Trial.* p<0.001, †p=0.43, ‡p=0.01
Figure 2.
Representative histologic findings of widespread inflammatory infiltrate including giant cells at baseline (2a) is largely resolved with some replacement fibrosis after 30 days of therapy (2b). Hematoxylin and Eosin 100x.
All ten subjects who were alive without transplant at 4 weeks had both baseline and 4 week assessments of LVEF. The mean left ventricular ejection fraction at baseline was only mildly reduced at 44±18% and did not change significantly after 1 month of treatment (47%±15% p=.60). The subject-specific ejection fractions at baseline and after 4 weeks of treatment are illustrated in Figure 3.
Figure 3.
Change in ejection fraction for 10 subjects with baseline and 30 day assessments. p=NS
In the eight subjects studied, only one (subject 10) had a significant elevation in antibody titers against human cardiac myosin and the β1 receptor after screening in the ELISA(Table 2) These data suggest that the presence of antibodies directed against common cardiac antigens is heterogenous in GCM, and that most GCM subjects have normal or only mildly increased autoantibody titers. Normal healthy subjects demonstrated a human cardiac myosin normal titer range from <100 to 800 and a β1 and β2 titer range from 400 to 3200.
Table 2.
Serum Antibody Titers in Acute Giant Cell Myocarditis
| Subject ID | Anti-Human Cardiac Myosin | Anti-β1 Adrenergic Receptor | Anti-β2 Adrenergic Receptor |
|---|---|---|---|
| 1 | 1:100 | 1:400 | 1:400 |
| 2 | 1:100 | 1:3200 | 1:1600 |
| 3 | 1:200 | 1:6400 | 1:3200 |
| 4 | 1:1600 | 1:1600 | 1:1600 |
| 5 | <1:100 | 1:3200 | 1:3200 |
| 8 | 1:800 | 1:6400 | 1:3200 |
| 10 | 1:6400 | 1:25600 | 1:12800 |
| 11 | 1:100 | 1:3200 | 1:3200 |
| positive control | 1:6400 | 1:25600 | 1:25600 |
| negative control | 1:100 | 1:800 | 1:800 |
Only 1 patient died and 2 required heart transplantation during 1 year in this prospectively treated cohort. Notably, the one subject who stopped all immunosuppression at the end of the one year study period died of biopsy-proven, recurrent GCM. This subject’s biopsy after 4 weeks of treatment demonstrated “resolving minimal lymphocytic myocarditis with no giant cells.” Eight months after the end of the study, the subject developed chest pressure, dyspnea, and increased abdominal girth of one week duration and presented with NYHA class IV heart failure and polymorphic ventricular tachycardia. Her right ventricular biopsy showed 6 of 6 pieces involved with diffuse GCM. This subject’s death due to GCM after withdrawal of immunosuppression demonstrates for the first time that there is a risk of recurrent, sometimes fatal GCM in the native heart.
Discussion
Several observations in this study add meaningfully to the very limited knowledge regarding GCM treatment. First, immunosuppressive treatment with the combinations of agents used in this study resulted in relatively low one-year mortality. Further, abrupt withdrawal of immunosuppression can result in recurrent, fatal GCM. Finally, the rate of adverse events probably has an acceptable safety profile in this high-risk population.
We observed a marked improvement in the inflammatory infiltrate accompanied by an increase in fibrosis after 4 weeks of treatment. The increase in fibrosis and the relatively mild cardiac dysfunction at study entry may explain the lack of significant improvement in left ventricular ejection fraction. As is the case with other forms of myocarditis, subjects in our series with lower ejection fractions had a greater likelihood of death or transplantation.
The mean age in our sample, 60 years, is older than has previously reported in acute GCM. The reason for this is that the majority of younger patients who had acute GCM during the period of study enrollment had a fulminant course and were diagnosed at time of transplantation, autopsy, or VAD placement. These observations are consistent with the aggressive course of GCM described in recent case series7,8 and confirm that during the timeframe of enrollment in our study, GCM remained a highly lethal disorder.
Although no suitable comparator group is available, for reference, one may compare survival the present series to the 56% overall and 6% transplant-free survival rates in the 16 subjects in the 1995–1997 multicenter GCM registry who were diagnosed by biopsy and were treated with no immunosuppression or steroids as sole immunosuppression (p=.0005 and 0.07 respectively by Log-Rank test). However, we suggest caution because this comparison is subject to uncontrollable biases.
The serum titers of antibodies that recognize HCM, β1 and β2 receptors were normal or only mildly elevated in most GCM subjects. The frequency of elevated antibody titers in GCM was lower than has been reported for lymphocytic myocarditis and dilated cardiomyopathy.9 This observation is consistent with our original hypothesis that GCM is primarily mediated by T lymphocytes and not antibodies.
Finally, our data highlight several unanswered questions and suggest directions for clinical research in the management of GCM. The optimal role of mechanical circulatory support as a bridge to transplantation or recovery,10 the optimal duration of immunosuppression in those patients who recover left ventricular function, and the best management strategy for prevention and treatment of post transplantation GCM recurrence have yet to be addressed.
Acknowledgments
Funding: US Food and Drug Administration (FD-RO-1-001986); Leder Family Philanthropic Grant; Muromonab-CD3 was supplied by Ortho-Biotek, Inc.
We especially thank Adita Mascaro-Blanco for performing the anti-HCM, anti-β1 and anti-β2 ELISAs in this study.
Appendix
Performance Sites and Principal Investigators for the GCM Treatment Trial
| Institution | Principal Investigator | Enrollment |
|---|---|---|
| 1. Mayo Clinic, Rochester, MN | Leslie T. Cooper, MD | 6 |
| 2. University of Kentucky | Santosh Menon, MD | 2 |
| 3. New York Presbyterian Hospital Medical Center, New York, NY | Mario Deng, MD | 1 |
| 4. Loyola University Medical Center | G. Martin Mullin, MD | 1 |
| 5. The Cleveland Clinic Foundation | Randall Starling, MD | 1 |
| 6. Sharp Memorial Hospital | Brian Jaski, MD | 1 |
The following institutions and investigators participated also in screening: University of Alabama, Robert Bourge, MD; Massachusetts General Hospital, G. Wiliam Dec, MD; The Johns Hopkins Hospital, Joshua Hare, MD; Hospital of the University of Pennsylvania, Andrew Kao, MD Ohio State University Medical Center; Carl Leier, MD University of Cincinnati, Lynne Wagoner, MD; Newark Beth Israel Medical Center, Marl Zucker, MD North Shore Medical Center, Hal Skopecki, MD, PhD; University of Pittsburgh Medical Center, Srinivas Murali, MD; St. Luke’s Medical Center, Milwaukee, WI, Joe Mendez, MD; University of Padova, Padova, IT, Alida Caforio, MD.
Steering Committee:
Leslie T. Cooper, Jr, MD, Chair
Joshua Hare, MD
G William Dec, MD
Richard Rodeheffer, MD
Data and Safety Monitoring Committee:
Jay W. Mason, MD, Chair
Karla V. Ballman, PhD
Bernard J. Gersh, M.B.Ch.B., D.Phil
Pathology Committee:
Henry D. Tazelaar, MD, Chair
William D. Edwards, MD
Footnotes
Disclosures: None of the Authors have any conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Costanzo-Nordin M, Silver M, O’Connell J, Scanlon P, Robinson J. Giant Cell Myocarditis: dramatic hemodynamic histologic improvement with immunosuppressive therapy. Eur Heart J. 1987;(Suppl J):271–274. [Google Scholar]
- 2.Cooper LT, Jr, Berry GJ, Rizeq M, Schroeder JS. Giant cell myocarditis. J Heart Lung Transplant. 1995;14:394–401. [PubMed] [Google Scholar]
- 3.Cooper LT, Berry GJ, Shabetai R. Giant Cell Myocarditis: Natural History and Treatment. N Engl J Med. 1997;336:1860–1866. doi: 10.1056/NEJM199706263362603. [DOI] [PubMed] [Google Scholar]
- 4.Kodama M, Matsumoto Y, Fujiwara M, Masani F, Izumi T, Shibata A. A novel experimental model of giant cell myocarditis induced in rats by immunization with cardiac myosin fraction. Clin Immunol Immunopathol. 1990;57:250–262. doi: 10.1016/0090-1229(90)90039-s. [DOI] [PubMed] [Google Scholar]
- 5.Kodama M, Matsumoto Y, Fujiwara M. In vivo lymphocyte-mediated myocardial injuries demonstrated by adoptive transfer of experimental autoimmune myocarditis. Circulation. 1992;85:1918–1926. doi: 10.1161/01.cir.85.5.1918. [DOI] [PubMed] [Google Scholar]
- 6.Galvin J, Hemric M, Ward K, Cunningham M. Cytotoxic mAb from rheumatic carditis recognizes heart valves and laminin. JCI. 2000;106:217–224. doi: 10.1172/JCI7132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caforio A, Calabrese F, Angelini A, Tona F, Vinci A, Bottaro S, Ramondo A, Carturan E, Iliceto S, Thiene G, Daliento L. A prospective study of biopsy-proven myocarditis: prognostic relevance of clinical and aetiopathogenetic features at diagnosis. European Heart Journal. 2007;28:1326–1333. doi: 10.1093/eurheartj/ehm076. [DOI] [PubMed] [Google Scholar]
- 8.Das B, Recto M, Johnsrude C, Klein L, Orman K, Shoemaker L, Mitchell M, Austin E. Cardiac Transplantation for Pediatric Giant Cell Myocarditis. J Heart Lung Transplant. 2006;25:474–478. doi: 10.1016/j.healun.2005.11.444. [DOI] [PubMed] [Google Scholar]
- 9.Caforio AL, Mahon NJ, Tona F, McKenna WJ. Circulating cardiac autoantibodies in dilated cardiomyopathy and myocarditis: pathogenetic and clinical significance. Eur J Heart Fail. 2002;4:411–417. doi: 10.1016/s1388-9842(02)00010-7. [DOI] [PubMed] [Google Scholar]
- 10.Ankersmit H, Ullrich R, Moser B, Hoetzenecker K, hacker S, German P, Krenn C, Horvat R, Grimm M, Wolner E, Zuckerman A. Recovery from giant cell myocarditis with ECMO support and utilization of polyclonal antithymocyte globulin: a case report. Thorac Cardiovasc Surg. 2006;54:278–280. doi: 10.1055/s-2006-923803. [DOI] [PubMed] [Google Scholar]