Abstract
The Drosophila DNA topoisomerase type I mutant allele, top1JS is an effective general seizure-suppressor mutation, reverting seizure-sensitive phenotypes of several mutant strains in a genetic model of epilepsy. Seizure-suppression is caused by reduced transcription of the top1 gene (Song et al., 2007). Here, we examine the possibility that pharmaceutical inhibition of Top1 enzymatic activity may also be effective at reducing seizure phenotypes. We investigate the effect of vertebrate Top1 inhibitor camptothecin (CPT) along with two related compounds, apigenin and kaempferol, when fed to seizure-sensitive mutant Drosophila. All three Top1 inhibitors were found to suppress phenotypes in these mutants. In particular, for drug treatments, the recovery time from seizure and paralysis is greatly reduced compared with untreated animals. Intriguingly we find that chronic drug treatments result in a small reduction in seizure sensitivity. Taken together, the results suggest that Top1 inhibitors may have the potential to be developed into effective AEDs, especially for brain tumor patients presenting with epilepsy.
Keywords: topoisomerase I, seizure, suppression, seizure-suppressor gene, camptothecin, epilepsy, Drosophila
Introduction
Human epilepsy is a significant health concern due to the large number of affected individuals, the potentially devastating ramifications of untreated seizure episodes, and the limitations of anti-epileptic drug (AED) options (Shneker and Fountain, 2003). Epilepsy is a major neurological disorder affecting about 1% of the population. AEDs ameliorate symptoms in approximately two-thirds of all patients (NINDS, 2007; Shneker and Fountain, 2003), but for the remaining third, epileptic seizures are intractable and do not respond to current AEDs. Another important concern is the problem of recurring side effects with presently available AEDs. This is due in large part to the fact that current AEDs act on a limited number of targets, mainly voltage-gated Na and Ca channels and elements of GABA inhibition (White et al., 2007). The future of epilepsy therapeutics would benefit substantially from increased AED drug discovery, especially those that act via novel targets. Presently, a limitation in neurological drug discovery comes from a requirement of mammalian organism-based, relatively low throughput assays for drug screening. For example, AED screening commonly uses rodent models with individual animals laboriously tested with a necessarily limited number of putative drugs (Smith, et al., 2007).
Here, we report on an alternative approach utilizing Drosophila suppressor genetics. First, forward genetic screens are utilized to identify mutations that suppress seizure. Genes corresponding to seizure-suppressor mutations are characterized molecularly and then used to define potential AED targets. These targets may help define unexpected classes of compounds that could become effective new treatments for epilepsy: treatments for intractable syndromes or treatments with reduced side effects. We have previously identified one such potential target - DNA topoisomerase 1 (top1 in Drosophila, corresponding to mammalian topo I) (Song et al. 2007). We identified mutations of top1 that suppress seizures in a Drosophila model of epilepsy. The top1JS mutation is an excellent general seizure-suppressor, ameliorating the phenotypes of sda, eas, and bss (Song et al., 2007). For example, the top1JS mutant completely suppresses eas seizure-like behaviors and paralysis by about 63% (Song et al., 2007). The threshold for evoking seizures by ECS is raised about 2.5 fold.
In the top1JS mutant, transcription of top1 is 12.5-fold less than normal and this reduced transcription appears to account for the suppression of seizures (Song et al., 2007). The investigation here examines the possibility that other methods of decreasing Top1 enzymatic activity might also be effective at reducing seizure phenotypes. CPT is a quinoline-based alkaloid with significant Top1 inhibitor activity, derived as a phytochemical from Camptotheca acuminata (Chinese Happy Tree) (Boege et al., 1996; Pommier et al., 1998). It is thought to work by interfering with the re-association of DNA after cleavage by Top1, thereby trapping the enzyme in a covalent DNA complex. CPT and its related Top1 inhibitors form a drug class with interesting potential for AED development. Due to their ability to inhibit cell division by disrupting the cell cycle, these compounds are of great interest as cancer therapeutics. Several chemical relatives are in clinical trials for breast and colon cancers, malignant melanoma, small-cell lung cancer, and leukemia (Wang et al., 1997; Pommier et al., 1999; Li and Liu, 2000). In 1996, topotecan (Hycamtin®, SmithKline Beecham) received FDA approval for treatment of ovarian cancers (Mathijssen et al., 2002). Injectable irinotecan HCl, generically called irinotecan (Camptosar®, Upjohn) received FDA-approval for treatment of colorectal cancer (Mathijssen et al., 2002). These features suggest the attractive possibility that Top1 inhibitors may be especially valuable for co-treatment of certain brain tumors that also present with epilepsy (Moots et al., 1995). We suggest that Top1 inhibitors can be viewed as having interesting potential as a new class of AED. Identifying Top1 inhibitors as AEDs represents the completion of our first attempt to discover a neurological drug using the suppressor genetics approach of Drosophila [disease model → suppressor gene → target → drug].
Materials and methods
Animals
Wild type Drosophila were of the Canton Special (CS) strain. The seizure-sensitive mutants used were eas1, bss1, and sdaiso7.8. The easily shocked gene (eas) is located at map position 1–53.5 and encodes ethanolamine kinase (Pavlidis et al. 1994). The slamdance gene (sda) is located on the third chromosome at 97D8–9 and encodes aminopeptidase N (Zhang, et al., 2002). The bang senseless mutation (bss) is located at 1–54.6; its gene product has not yet been identified. The mutant behavioral phenotypes of seizure and paralysis, the corresponding electrophysiological phenotypes of seizure and synaptic failure, and the thresholds for seizure susceptibility have all been described previously for the alleles utilized here (Ganetzky and Wu, 1982; Kuebler and Tanouye, 2000; Kuebler et al., 2001; Song and Tanouye, 2006).
Drugs
Camptothecin (CPT, Sigma C9911), apigenin (Sigma, A3145), and kaempferol (Sigma, K0133) are plant phytochemicals with significant Top1 inhibitor activities (Pommier et al., 1998; Snyder and Gillies, 2002; Boege et al., 1996). Concentrations of drugs indicated in the text were diluted from stock solutions of 1mM in 5% sucrose. Valproate (Na-valproate, Sigma, P4543) is a short-branched fatty acid that is the most widely used antiepileptic drug for the treatment of generalized and partial epilepsy (Johannessen, 2000; Loscher, 1999). Valproate has been shown to be effective at suppressing seizure-like activity in Drosophila by direct injection of drug into the brain of bss1 and sdaiso7.8 mutants (Kuebler and Tanouye, 2002). Stock solution of valproate was 500mM in 5% sucrose. KBr (Sigma P5510) is an AED that has been shown to be effective at ameliorating phenotypes in bss1 mutants previously in drug feeding experiments (Tan et al., 2004). Stock solution of KBr was 50mM in 5% sucrose.
Drug feeding
Feeding methods are adapted from Reynolds et al. (2004). For acute feeding experiments, drugs were diluted with 5% sucrose to the appropriate concentration and then applied to glass microfiber feeding filters. Drug-soaked filters and five newly-emerged flies are placed into feeding vials with seizure sensitivity testing beginning the following day (designated as 1d-old flies) with repeat testing of the same flies for each of the following two days (designated as 2d and 3d-old tests, respectively). Data from several vials are pooled for behavioral experiments such that n = ~100 flies for each treatment. To test for persistence of drug effects, 3d-old (2d drug + 1d sucrose) flies were utilized. Flies were fed drug for 2 days, followed by one day of sucrose-only feeding prior to behavioral testing. These flies are compared with 3d-old (3d drug) flies to determine if drug effect persists through the 1d of sucrose-only feeding while keeping the age of the flies constant. Similar experiments were conducted to determine the effect of 1d drug feeding. Flies were fed sucrose-only for 2 days, then fed drug for one day prior to behavioral testing. For acute experiments with Top1 inhibitors, lethality is observed at dosages approaching 1mM (data not shown). For the experiments reported here, 100µM is the highest concentrations used. Chronic drug treatment is used to determine drug efficacy on adult flies that have been reared since they were embryos on drug-containing medium. The methods used were similar to those reported by Reynolds et al. (2004). Drug solution is mixed with Formula 4–24 Blue Drosophila Medium (Carolina Biological Supply) in a 1:1 dry food-to-drug solution ratio to create the desired medium. Adult females are placed into vials containing medium, allowed to lay eggs, and their progeny reared on the drug-containing food. Adult progeny are tested each day after eclosion from pupal cases for 3d.
Behavioral testing
For bang-sensitive (BS) behavioral tests, flies requiring anesthetization with CO2 for collection were allowed to recover for at least 24h before testing. For testing, 5 flies were placed in a food vial and mechanically stimulated with a VWR vortex mixer at maximum speed for 10s. The recovery time was measured for each fly from the end of the vortex stimulation until it resumed an upright standing position. The mean recovery time (MRT) was the average time taken for a fly exhibiting bang-sensitive behavior to recover in a population. These values were compared by one-way ANOVA with different drugs as single factor. Separate analyses were performed for different test days and different genotypes. Statistical significance was set at p<0.05. For treatments where the null hypothesis was rejected by overall F-ratio, multiple comparison of MRT was performed by Fisher's LSD with t-test significance set at p<0.05.
Electrophysiology
Flies were mounted without anesthesia by immobilizing them with a vacuum line, and then attaching them to a mounting pin with super glue gel (Scotch®). All electrodes were uninsulated tungsten electrodes. For stimulation, two electrodes were inserted into the brain. An electrode inserted into the abdomen served as ground. For recording, one electrode was inserted into a dorsal longitudinal muscle (DLM) fiber to monitor the activity of the single motoneuron that innervates it (Pavlidis and Tanouye, 1995). To elicit seizures, high frequency (HF) stimuli were delivered (0.5 ms pulses at 200Hz for 300 ms). Stimulation voltage was gradually increased and seizure threshold, a measure of seizure-susceptibility, was defined as the minimum voltage required for HF stimulation to become an electroconvulsive shock (ECS); that is, to induce seizure activity. During the course of each experiment, the giant fiber (GF) circuit was continuously monitored as an indication of general nervous system function, especially synaptic failure (Pavlidis and Tanouye, 1995). To drive the GF circuit, single-pulse stimuli (0.2 ms duration, 0.5 Hz) were delivered to the brain, To track the synaptic failure duration time, two stimulators were used: one for delivery of single pulse stimuli; the other for the delivery of HF stimulation. GF responses are evoked at 2X GF threshold. A seizure was evoked by HF stimulation. Recovery from failure is defined by two successive GF test stimuli eliciting normal latency responses (Tanouye and Wyman, 1980; Pavlidis and Tanouye, 1995). Statistical significance for seizure threshold difference was by Student's t-test at p<.05.
Results
Comparisons of feeding two AEDs: valproate and potassium bromide
For control comparisons, we examined two AEDs, valproate and KBr fed to BS mutant Drosophila. Bromides are historically important AEDs (Friedlander, 2000) that remain an important treatment for canine epilepsy (Podell and Fenner, 1993). Previously, we have shown that feeding KBr causes 0.016 µg of bromide accumulation per fly head as measured by ion chromatography (Tan, et al., 2004). Bromide at this concentration ameliorates bss mutant phenotypes, mainly recovery time from behavioral paralysis. In this section, we contrast KBr feeding with valproate feeding on BS mutant phenotypes. Valproate is a widely-used AED (Johannessen, 2000; Loscher, 1999) that we have previously shown increases seizure-thresholds of sda and bss mutants to wild-type levels when injected directly into the brain (Kuebler and Tanouye, 2002).
Considering the effectiveness of valproate at increasing seizure threshold with brain-injection (Kuebler and Tanouye, 2002), feeding of valproate was surprisingly ineffective. For example, bss flies fed valproate (10mM) for 3d responded especially poorly (Table 1). A number of flies (11%) died after mechanical shock stimulation. For those that survived, all showed paralysis and actually took significantly longer to recover from paralysis than sucrose-fed control flies; mean recovery time (MRT) of 189 ± 99s compared to 113 ± 46s for control (p<10−6; Student's t-test). Roughly similar results were observed with eas mutants in 3d feedings of valproate (Table 1). 4% of flies tested died after mechanical shock stimulation. All survivors displayed paralysis and had a slightly longer, albeit not significant, mean recovery time (MRT) than control eas flies (p<0.23; Student's t-test). Lethality was not observed at shorter feeding times (1d and 2d feedings), but there was no amelioration of bss and eas phenotypes compared to non-drug treated controls (Table 1).
Table 1.
Age- and drug-dependence of BS mutants in acute drug feeding
| MRT (s) | |||
|---|---|---|---|
| Treatment condition | 1d | 2d | 3d |
| bss sucrose (5%) | 60 ± 24 (n = 125) | 93 ± 40 | 113 ± 46 |
| bss valproate (10mM) | 53 ± 22 (n = 82)(p<0.07) | 83 ± 47 (p<0.11) | 189 ± 99 (p<10−6)* |
| bss KBr (20mM) | 55 ± 19 (n = 130)(p<0.18) | 75 ± 24 (p<10−3)* | 86 ± 23 (p<10−5)* |
| bss apigenin (100mM) | 59 ± 19 (n = 92)(p<0.79) | 72 ± 31 (p<10−4)* | 78 ± 29 (p<10−3)* |
| bss kaempferol (100mM) | 56 ± 20 (n = 89)(p<0.22) | 70 ± 28 (p<10−6)* | 94 ± 35 (p<0.055) |
| bss CPT (100mM) | 53 ± 20 (n = 90)(p<0.058) | 68 ± 26 (p<10−7)* | 72 ± 31 (p<10−9)* |
| eas sucrose (5%) | 33± 9 (n = 118) | 40 ± 15 | 46 ± 15 |
| eas valproate (10mM) | 34 ± 11 (n = 80)(p<0.56) | 40 ± 11 (p <0.94) | 53 ± 35 (p<0.23) |
| eas apigenin (100mM) | 29 ± 8 (n = 95)(p<10−2)* | 32 ± 8 (p<10−3)* | 40 ± 12 (p<0.02)* |
| eas kaempferol (100mM) | 28 ± 6 (n = 81)(p<10−4)* | 34 ± 11 (p<0.02)* | 35 ± 12 (p<10−3)* |
| eas CPT (100mM) | 28 ± 6 (n = 97)(p<10−4)* | 32 ± 6* (p<10−3)* | 34 ± 10 (p<10−4)* |
Newly-emerged adult mutant flies were fed drug + sucrose or control sucrose solution acutely and tested for behavioral paralytic recovery times on 1, 2 and 3 days of age. Mean recovery time (MRT) ± SD is given with the number of flies tested (n) indicated for 1d. Values of p indicated are compared with age-matched sucrose control.
Asterisk (*) indicates a significant difference of p<0.05 determined by Fisher's PLSD following ANOVA for Top1 inhibitors or by Student's t-test for valproate and KBr. Note that comparisons between different days may not be significant because the measurements are not completely independent.
In contrast, acute feeding of KBr was found to ameliorate bss phenotypes as previously demonstrated for it and other AEDs (Reynolds et al., 2004; Tan et al., 2004). MRT was decreased by KBr treatment in both 2d and 3d-old bss flies (Table 1). In 3d-old flies, MRT in drug-treated flies was 86 ± 23s compared with 113 ± 46s in sucrose-fed bss controls (p<10−5; Student's t-test). Acute feeding of KBr did not eliminate seizure-like behaviors or paralysis in BS mutants, similar to findings reported previously (Tan et al., 2004).
Investigation of a new class of drug with AED potential: top1 inhibitor drugs inspired by seizure-suppressor top1JS mutants
We tested the ability of three compounds with known Top1 inhibitory activity, CPT, apigenin and kaempferol, to suppress seizures or reduce MRT in BS mutant flies (Pommier et al., 1998; Snyder and Gillies, 2002; Boege et al., 1996). We find that acute feeding of Top1 inhibitors does not eliminate seizure-like behaviors, but did reduce the MRT of BS mutants, similar to MRT reductions seen previously with AED treatments (Reynolds et al., 2004; Tan et al., 2004). All three Top1 inhibitors significantly reduced MRT compared to the sucrose control in 3d-old bss flies fed at 100 µM concentrations (F3,217=17.33; p<10−8; ANOVA). The largest suppression observed was for 3d-old bss flies fed CPT (100µM); MRT was reduced from 113 ± 46s without drug to 72 ± 31s with CPT treatment (p<10−9; Fisher's LSD) (Table 1). Under these conditions, apigenin shows a similar reduction to CPT (78 ± 29s, p<10−3; Fisher's LSD). Kaempferol shows a slight reduction that is not significant (94 ± 35s, p=0.055; Fisher's LSD) (Table 1, figure 2). These correspond to a reduction in MRT of 36%, 31% and 17% respectively. In younger bss control flies, MRTs are generally shorter, with or without drug treatment, however all of the Top1 inhibitors still cause significant reductions in MRTs of 2d-old bss flies (F3,479= 15.90; p<10−8; ANOVA). For 2d-old bss flies, MRT was reduced from 93 ± 40s without drug to 68 ± 26s (p<10−7; Fisher's LSD) for CPT, to 70 ± 28s (p<10−6; Fisher's LSD) for kaempferol, and to 72 ± 31s (p<10−4; Fisher's LSD)(Table 1). There is a slight reduction of MRT in 1d-old bss flies (Table 1), but recovery times in these flies are more variable and the reduction is not significant (F3,355=1.93;p>0.24; ANOVA).
The top1JS mutation acts as a general seizure suppressor, reducing the seizure susceptibility of multiple bang sensitive mutations including bss, eas, and sda (Song et al, 2007). To determine if Top1 inhibitors compounds can also act as general seizure suppressors, we examined the efficacy of acute drug treatment on eas MRT. The eas gene encodes an ethanolamine kinase with altered levels of phosphatidylethanolamine (Pavlidis et al., 1994); and is thought to cause seizure-sensitivity through a different mechanism than bss (Parker and Tanouye, unpublished results). Control eas flies have shorter MRTs than bss flies at each of the three days examined (Table 1). For 3d-old eas flies, all three Top1 inhibitors significantly reduced MRT compared with sucrose control (F3,163=9.02; p<10−4; ANOVA). The greatest effect observed was for 3d-old eas flies fed CPT (100µM); MRT was reduced from 46 ± 15s without drug to 34 ± 10s (p<10−4; Fisher's LSD) with CPT treatment, corresponding to a 26% reduction (Table 1). Under these conditions, apigenin is less effective than CPT, showing an MRT of 40 ± 12s (p<0.02; Fisher's LSD) (13% reduction), however kaempferol is similar to CPT, with an MRT of 35 ± 12s (p<10−3; Fisher's LSD)(24% reduction) (Table 1). As in bss flies, younger eas flies display shorter MRTs, however all of the Top1 inhibitors still result in significant MRT reductions in 1d-old (F3,263=8.60; p<10−5; ANOVA) and 2d-old (F3,260=7.01; p<10−4; ANOVA) eas flies (Table 1).
Dosage-response characteristics of Top1 inhibitor compounds were investigated by comparing treatments using drugs of different concentrations on 2d-old bss flies (Table 2). The lower dosages of apigenin and kaempferol used here (30µM, 8µM) showed no significant effects in reducing bss MRTs (Table 2); recovery times are not significantly different from sucrose controls (for apigenin: F2,263=0.45; p>0.63; ANOVA; for kaempferol: F2,261=3.02; p>0.05; ANOVA). CPT on bss recovery time decreased MRT significantly at lower dosages and appeared to show dosage-response features (F3,406=14.54; p<10−9; ANOVA). Compared to sucrose control MRT (93 ± 40s), 30µM CPT was 71 ± 26 (p<10−5; Fisher's LSD) and 8µM CPT was 76 ± 33s (p<0.003; Fisher's LSD).
Table 2.
Dose-dependence of top1 inhibitors in bss1 flies in acute feeding
| Treatment | control | 30µM | 8µM |
|---|---|---|---|
| No drug | 93 ± 40 | N/A | N/A |
| Apigenin | 88 ± 36 (p<0.35) | 91 ± 38 (p<0.76) | |
| Kaempferol | 82 ± 30 (p<0.03) | 83 ± 33 (p<0.10) | |
| CPT | 71 ± 26 (p<10−5)* | 76 ± 33 (p<0.003)* |
Newly-emerged bss1 flies were fed drug + sucrose at various concentrations (8 or 30 µM) and tested for behavioral paralytic recovery times at 2d. Values are MRT ± standard deviation. Values of p indicated are compared with age-matched sucrose control.
Asterisk (*) indicates a significant difference of p<0.05 compared with sucrose control determined by Fisher's PLSD following ANOVA. Note that the MRT for kaempferol (30µM) is not significant as determined by ANOVA. For each condition, n ≥ 80 flies.
Persistence of Top1 inhibitor activity during acute treatment
We tested whether Top1 inhibitor activity could ameliorate seizure severity even after withdrawal of the drug. Mutant bss flies were fed drug for 2 days, followed by one day of sucrose feeding prior to behavioral testing (2d drug + 1d sucrose). We found that all three Top1 inhibitors show activity-that persists in reducing MRT compared to sucrose controls (F3,344=15.47; p<10−8; ANOVA). The MRT for CPT-fed flies (2d drug + 1d sucrose) was 70 ± 28s, significantly shorter than untreated bss controls (113 ± 46s) (p<10−6; Fisher's LSD). The persistent effect on MRT is similar to recovery time for bss flies continuously fed CPT (3d drug)(72 ± 31s) (p>0.38; Student's t-test). The MRT for apigenin-fed flies (2d drug + 1d sucrose)(78 ± 26s) was also significantly shorter than bss controls (p<10−7; Fisher's LSD). Similarly, the MRT for kaempferol-fed flies (2d drug + 1d sucrose)(80 ± 30s) was shorter than control (p<10−5; Fisher's LSD). Thus, the effects of Top1 inhibition appear to be relatively long lasting with respect to seizure amelioration.
We also tested how rapidly Top1 inhibitors can affect seizure behavior. To do this we took bss flies which were fed sucrose for 2 days, followed this by one day of CPT feeding prior to testing (2d sucrose + 1d drug). Recovery time for 2d sucrose + 1d drug flies was 83 ± 44s, a significant reduction compared to control flies (113 ±46s)(p<10−3; Student's t-test) however it is not as effective as continuous feeding (72 ± 31s) or 2d drug + 1d sucrose (70 ± 28s) bss flies. It is possible that while CPT acts relatively rapidly, the level of the drug in the fly brain builds up over the course of 2–3 days to increase effectiveness.
Top1 inhibitor effectiveness in chronic drug treatment
Many AEDs currently used to treat humans start to work only after several weeks of treatment, as the drug must build up in the nervous system to an effective level. As the anti-epileptic mode of action of Top1 inhibitors is likely to be during DNA replication or transcription, and not synaptic transmission, it is also possible that a longer exposure to the drug could improve effectiveness. In order to test the effects of longer drug exposure, we utilized a chronic treatment method described by Reynolds et al. (2004) whereby flies are raised, and thereby continuously exposed to throughout larval development. Flies are then tested for BS phenotypes as adults (Table 3). Chronic drug treatment experiments utilized apigenin and kaempferol at 100µM, but CPT was found to be toxic at this concentration. Thus, CPT was reduced to 1µM for chronic drug treatment.
Table 3.
Age- and drug-dependence of BS mutants in chronic drug exposure
| Treatment | MRT (s) | ||
|---|---|---|---|
| 1d | 2d | 3d | |
| bss control | 63 ± 29 (n=103) | 83 ± 38 | 101± 62 |
| bss apigenin (100mM) | 59 ± 21 (n=72)(p<0.22) | 68 ± 25 (p<0.0009)* | 84 ± 36 (p<.01)* |
| bss kaempferol (100mM) | 59 ± 26 (n=68)(p<0.30) | 72 ± 34 (p<0.05)* | 85 ± 35 (p<0.03)* |
| bss CPT (1mM) | 68 ± 30 (n=74)(p<0.40) | 79 ± 28 (p<0.56) | 78 ± 27 (p<0.004)* |
Mutants were reared in drug-containing food (chronic drug treatment) and tested for behavioral paralytic recovery times on 1, 2, and 3 days of age. MRT ± SD is shown with the number of flies tested indicated in parenthesis on 1d.
Asterisk (*) indicates a significance value of p < 0.05 compared with age-matched normal food control determined by Fisher's PLSD following ANOVA. Note that comparisons between different days may not be significant because the measurements are not completely independent. Note that because of lethality, CPT concentration is 1µM.
In general, the results of chronic drug treatment resembled those of acute treatment, but did not appear to be better. Top1 inhibitors applied chronically were found to reduce MRT significantly in 3d-old bss flies compared to untreated flies (F3,350=3.54; p<0.015; ANOVA). The largest effect on MRT appeared to be in bss flies treated with CPT (78 ± 27s) (p<0.004; Fisher's LSD), significantly shorter than untreated controls (101 ± 62s)(Table 3). Comparable effects on MRT were seen in 3d-old bss flies treated with apigenin and kaempferol (Table 3). Chronic treatment with Top1 inhibitors (Table 3) reduced bss MRT significantly in 2d-old flies (F3,350; p<0.009; ANOVA), but not in 1dold flies (F3,348=1.66; p>0.17; ANOVA).
Overall, we find that chronic feeding of Top1 inhibitors results in amelioration of bss phenotypes. However, contrary to our initial expectations, chronic feeding did not appear to be better than acute feeding; it may actually have been less effective. One possibility is that chronic feeding led to an up-regulation of fly detoxification mechanisms such that after a few days the chronically-fed larva had smaller amounts of the drug in their system as compared to what the acute exposure flies had.
Eletrophysiological data corroborate behavioral results
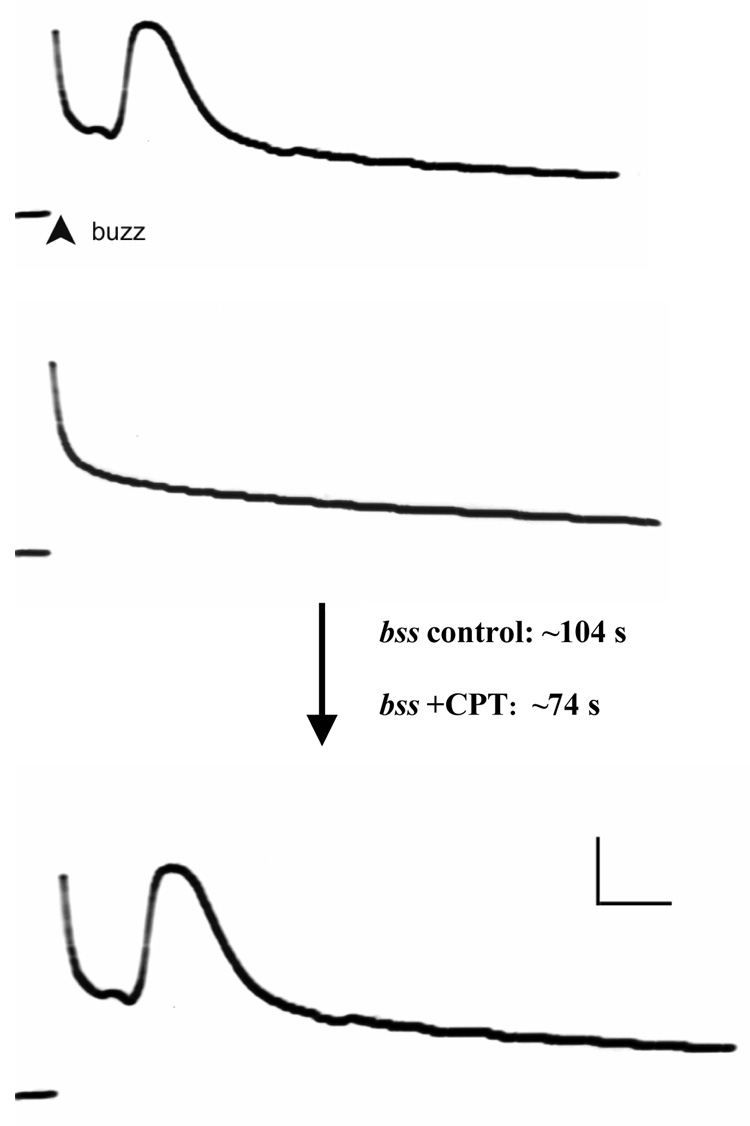
Electrophysiological recordings indicated that acute CPT feeding results in a change in synaptic failure time (Figure 1). Following acute treatment with CPT (100µM), 3d-old bss flies recovered function in GF system synapses in 74 ± 23s (n=10). In contrast, control bss flies recovered GF system function only after a significantly (p<0.007; Student's t-test) greater time period 104 ± 15s (n=10). The differences between drug-treated and control synaptic failure duration are proportionally similar to behavioral data on recovery from BS paralysis for bss, suggesting that Top1 inhibitors are acting on synaptic mechanisms to ameliorate the processes underlying behavioral paralysis. In contrast, Top1 inhibitor treatment had a very small effect on seizure threshold. We have previously observed a much greater effect by genetically suppressing top1 expression using the top1JS mutant, resulting in a 2.5-fold increase in seizure threshold (Song et al., 2007).
Figure 1. DLM motor neuron function during GF-evoked response failure.
An example of the DLM recordings before and after ECS stimulation (buzz). Drug-treated flies have shorter synaptic failure duration time than untreated flies: control bss1 flies have an average synaptic failure duration time of 104 s; while CPT-treated bss1 flies have an average synaptic failure duration time of 74s (p<0.007; Student's t-test). Following a buzz, GF stimulation was resumed to test for the failure and then recovery of evoked muscle potentials. Recovery from failure is defined here by two successive GF test stimuli giving rise to normal latency responses (Tanouye and Wyman, 1980; Pavlidis and Tanouye, 1995). Calibration: 20 mV, 2 ms. n ≥ 10 for electrophysiology.
Discussion
This study investigates a novel approach for identifying AED candidates through the use of suppressor genetics in Drosophila. The approach relies on first reverting the phenotype of seizure-sensitive mutants with suppressor mutations identified from forward genetics screens. Potential drug targets are then defined by molecular identification of the genes corresponding to the suppressor mutations. The present investigation is the culmination of our first attempt at utilizing this approach. It is based on the recent identification of the top1JS mutation that was found to be an especially effective seizure-suppressor (Song et al., 2007). The top1JS mutation results in a strong reduction in transcription of Topoisomerase I. In the present study, compounds that inhibit Top1 enzymatic activity are evaluated for seizure-suppression capabilities by feeding them to seizure-sensitive flies.
The Top1 inhibitors examined in this study were found to cause significant reduction in the time a fly takes to recover from synaptic failure, or mean recovery time (MRT), in Drosophila seizure-sensitive mutants. Previous studies using these mutants have shown that a reduction in MRT can be used to assess AED efficacy (Reynolds et al., 2004; Tan et al., 2004; Song and Tanouye, 2006). Phenytoin, gabapentin, and KBr have all been shown to be effective AEDs in the Drosophila model. Using the criterion of reduction in MRT, it would appear that the Top1 inhibitors examined in this study, CPT, apigenin, and kaempferol, are comparable to these AEDs in their efficacy.
The identification of the top1JS mutation and Top1 inhibitors as seizure suppressors is a novel finding, since DNA topoisomerases have not been previously associated with seizure or seizure control. Type 1 DNA topoisomerase is thought to resolve the torsional tension associated with replication and transcription by binding DNA and relaxing the helix (Champoux, 2001). A one explanation for the inhibitory effect on seizures seen in top1JS mutants and Top1 inhibitors is that transcription in neurons generates supercoiled DNA that must be continuously relaxed to sustain high levels of RNA synthesis. Reduced levels of Top1 activity might lead to partial inhibition of transcription of neuronal genes required for efficient synaptic transmission. Alternatively, previous studies on the top1JS mutation provided several lines of evidence that neuronal apoptosis may be the explanation for seizure suppression seen in double mutants between top1JS and various seizure-sensitive mutants (Song et al., 2007). TUNEL (terminal deoxynucleotidyl transferase-mediated biotinylated UTP nick end labeling) assay showed that the top1JS mutation caused considerable amounts of cell death. Furthermore, overexpression of Drosophila Inhibitor of Apoptosis (DIAP1) gene in the nervous system resulted in the restoration of seizure-sensitivity in top1JS eas double mutants suggesting increased apoptosis in the top1JS mutant is the cause of seizure suppression, thereby pointing to a novel function for Top1 in construction and/or maintenance of circuits required for seizure propagation in vivo. Similar to the genetic results, neuronal death through apoptosis may be a possible explanation for acute and chronic Top1 inhibitor treatment. Indeed, Top1 inhibitors are currently subject to intense study as potential cancer treatments. Their role in apoptosis is an important concern when considering their suitability as future AEDs. Treatment of pediatric seizures with these particular drugs would obviously be inadvisable given the likelihood of neuronal cell death. However in cases where seizures are a secondary result of brain tumors, Top1 inhibitors could be a highly valuable therapeutic tool, treating not only the seizures but the underlying cancer as well.
Genetic and molecular analyses of Drosophila mutants have provided a model for examining fundamentally important problems particularly in developmental and neurobiology. An important lesson from these studies is that fundamental processes and many of the essential genes are conserved across the phylogenetic spectrum. Thus, findings derived from Drosophila are often applicable to mammalian biology. One result of this cross-species conservation is that Drosophila has become an attractive system for developing disease models and evaluating new drug therapies to treat human pathologies. Here is a first attempt to use Drosophila suppressor genetics to identify neurological drug candidates. If further tests confirm the use of Top1 inhibitors in the neurology clinic, we suggest Drosophila will have a major impact on directly improving the human condition through advances of medical treatments in nervous system and other debilitating disorders.
Acknowledgements
We thank Dr. Daria Hekmat-Scafe for discussions throughout the project. We are especially grateful to Dr. Edward Glasscock and Dr. Jeffrey Noebels for discussion and for sharing unpublished results. This work was supported by an NIH research grant and an Epilepsy Foundation grant to M.T.
Abbreviations
- Top1
topoisomerase I
- BS
bang-sensitive
- GF
giant fiber
- DLM
dorsal longitudinal muscle
- HF
high-frequency
- ECS
electroconvulsive shock
- bss
bangsenseless
- eas
easily-shocked
- sda
slamdance
- paraST76
paralyzed
- mlenapts
male-lethal no-action potential
- CPT
camptothecin
- AED
anti-epileptic drug
- GABA
gamma amino butyric acid
- MRT
mean recovery time
- ANOVA
analysis of variance
- LSD
least significant difference
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bazil CW, Pedley TA. Advances in the medical treatment of epilepsy. Annu Rev Med. 1998;49:135–162. doi: 10.1146/annurev.med.49.1.135. [DOI] [PubMed] [Google Scholar]
- Boege F, Straub T, Kehr A, Boesenberg C, Christiansen K, Anderson A, Jacob F, Kohrle J. Selected novel flavones inhibit the DNA binding or the DNA religation step of eukaryotic topoisomerase I. J Biol Chem. 1996;271:2262–2270. doi: 10.1074/jbc.271.4.2262. [DOI] [PubMed] [Google Scholar]
- Friedlander WJ. The rise and fall of bromide therapy in epilepsy. Arch Neurol. 2000;57:1782–1785. doi: 10.1001/archneur.57.12.1782. [DOI] [PubMed] [Google Scholar]
- Champoux JJ. DNA topoisomerases: structure, function and mechanism. Annu Rev Biochem. 2001;70:369–413. doi: 10.1146/annurev.biochem.70.1.369. [DOI] [PubMed] [Google Scholar]
- Ganetzky B, Wu C-F. Indirect suppression involving behavioral mutants with altered nerve excitability in Drosophila melanogaster. Genetics. 1982;100:597–614. doi: 10.1093/genetics/100.4.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasscock E, Singhania A, Tanouye MA. The mei-P26 gene encodes a RING finger B-box coiled-coil-NHL protein that regulates seizure susceptibility in Drosophilia. Genetics. 2005;170(4):1677–1689. doi: 10.1534/genetics.105.043174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hekmat-Scafe DS, Dang KN, Tanouye MA. Seizure suppression by gain-of-function escargot mutations. Genetics. 2005;169:1477–1493. doi: 10.1534/genetics.104.036558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannessen CU. Mechanisms of action of valproate: a commentatory. Neurochem Int. 2000;37:103–110. doi: 10.1016/s0197-0186(00)00013-9. [DOI] [PubMed] [Google Scholar]
- Kuebler D, Tanouye MA. Modifications of seizure susceptibility in Drosophila. J Neurophysiol. 2000;83:998–1009. doi: 10.1152/jn.2000.83.2.998. [DOI] [PubMed] [Google Scholar]
- Kuebler D, Zhang H, Ren X, Tanouye MA. Genetic suppression of seizure susceptibility in Drosophila. J Neurophysiol. 2001;86:1211–1225. doi: 10.1152/jn.2001.86.3.1211. [DOI] [PubMed] [Google Scholar]
- Kuebler D, Tanouye MA. Anticonvulsant valproate reduces seizure-susceptibility in mutant Drosophila. Brain Res. 2002;958:36–42. doi: 10.1016/s0006-8993(02)03431-5. [DOI] [PubMed] [Google Scholar]
- Lee J, Wu C-F. Electroconvulsive seizure behavior in Drosophila: analysis of the physiological repertoire underlying a stereotyped action pattern in bang-sensitive mutants. J Neurosci. 2002;22:11065–11079. doi: 10.1523/JNEUROSCI.22-24-11065.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Liu L. Tumor cell death induced by topoisomerase-targeting drugs. Annu Rev Pharmacol Toxicol. 2000;41:53–77. doi: 10.1146/annurev.pharmtox.41.1.53. [DOI] [PubMed] [Google Scholar]
- Loscher W. Valproate: a reappraisal of it pharmacodynamic properties and mechanism of action. Prog Neurobiol. 1999;58:31–59. doi: 10.1016/s0301-0082(98)00075-6. [DOI] [PubMed] [Google Scholar]
- Loscher W, Schmidt D. New horizons in the development of antiepileptic drugs. Epilepsy Res. 2002;50:3–16. doi: 10.1016/s0920-1211(02)00063-3. [DOI] [PubMed] [Google Scholar]
- Mathijssen RH, Loos WJ, Verweij J, Sparreboom A. Pharmacology of topoisomerase I inhibitors irinotecan (CPT-11) and topotecan. Curr Cancer Drug Targets. 2002;2(2):103–123. doi: 10.2174/1568009023333890. [DOI] [PubMed] [Google Scholar]
- Moots PL, Maciunas RJ, Eisert DR, Parker RA, Abou-Khalil B. The course of seizure disorders in patients with malignant gliomas. Arch Neurol. 1995;52(7):717–724. doi: 10.1001/archneur.1995.00540310091021. [DOI] [PubMed] [Google Scholar]
- NINDS Website. Seizures and epilepsy: hope through research. 2007 http://www.ninds.nih.gov/disorders/epilepsy/
- Pavlidis P, Ramaswami M, Tanouye MA. The Drosophila easily shocked gene: a mutation in a phospholipid synthetic pathway causes seizure, neuronal failure, and paralysis. Cell. 1994;79:23–33. doi: 10.1016/0092-8674(94)90397-2. [DOI] [PubMed] [Google Scholar]
- Pavlidis P, Tanouye MA. Seizures and failures in the giant fiber pathway of Drosophila bang-sensitive paralytic mutants. J Neurosci. 1995;15:5810–5819. doi: 10.1523/JNEUROSCI.15-08-05810.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podell M, Fenner WR. Bromide therapy in refractory canine idiopathic epilepsy. J Vet Internal Med. 1993;7:318–327. doi: 10.1111/j.1939-1676.1993.tb01025.x. [DOI] [PubMed] [Google Scholar]
- Pommier Y, Pourquier P, Fan Y, Strumberg D. Mechanism of action of eukaryotic DNA topoisomerase I and drugs targeted to the enzyme. Biochim Biophys Acta. 1998;1400:83–105. doi: 10.1016/s0167-4781(98)00129-8. [DOI] [PubMed] [Google Scholar]
- Pommier Y, Pourquier P, Urasaki Y, Wu J, Laco GS. Topoisomerase I inhibitors: selectivity and cellular resistance. Drug Resistance Update. 1999;2:307–318. doi: 10.1054/drup.1999.0102. [DOI] [PubMed] [Google Scholar]
- Reynolds ER, Stauffer EA, Feeney L, Rojahn E, Jacobs B, McKeever C. Treatment with the antiepileptic drugs phenytoin and gabapentin ameliorates seizure and paralysis of Drosophila bang-sensitive mutants. J Neurobiol. 2004;58(4):503–513. doi: 10.1002/neu.10297. [DOI] [PubMed] [Google Scholar]
- Shneker BF, Fountain NB. Epilepsy. Disease-A-Month. 2003;49:426–478. doi: 10.1016/s0011-5029(03)00065-8. [DOI] [PubMed] [Google Scholar]
- Smith M, Wilcox KS, White HS. Discovery of antiepileptic drugs. Neurotherapeutics. 2007;4:12–17. doi: 10.1016/j.nurt.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder RD, Gillies PJ. Evaluation of the clastogenic, DNA intercalative, and topoisomerase II-interactive properties of bioflavonoids in Chinese hamster V79 cells. Env Molec Mutagenesis. 2002;40:266–276. doi: 10.1002/em.10121. [DOI] [PubMed] [Google Scholar]
- Song J, Tanouye MA. Seizure suppression by shakB2, a gap junction mutation in Drosophila. J Neurophysiol. 2006;95(2):627–635. doi: 10.1152/jn.01059.2004. [DOI] [PubMed] [Google Scholar]
- Song J, Hu J, Tanouye MA. Seizure suppression by top1 mutations in Drosophila. J Neurosci. 2007;27(11):2927–2937. doi: 10.1523/JNEUROSCI.3944-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Tanouye MA. A role for para sodium channel gene 3' UTR in the modification of Drosophila seizure susceptibility. Dev Neurobiol. 2007;67(14):1944–1956. doi: 10.1002/dneu.20519. [DOI] [PubMed] [Google Scholar]
- Tan JS, Lin F, Tanouye MA. Potassium bromide, an anticonvulsant, is effective at alleviating seizures in the Drosophila bang-sensitive mutant bang senseless. Brain Res. 2004;1020:45–52. doi: 10.1016/j.brainres.2004.05.111. [DOI] [PubMed] [Google Scholar]
- Tanouye MA, Wyman RJ. Motor outputs of the giant nerve fiber in Drosophila. J Neurophysiol. 1980;44:405–421. doi: 10.1152/jn.1980.44.2.405. [DOI] [PubMed] [Google Scholar]
- Wang HK, Morris-Natschke SL, Lee KH. Recent advances in discovery and development of topoisomerase inhibitors as antitumor agents. Med Res Rev. 1997;17:367–425. doi: 10.1002/(sici)1098-1128(199707)17:4<367::aid-med3>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- White HS, Woodhead JH, Wilcox KS, Kupferberg HJ, Wolf HH. Discovery and preclinical development of anitepileptic drugs. In: Levy RH, Mattson RH, Meldrum BS, Perucca E, editors. Antiepileptic Drugs. Lippincott: Williams & Wilkins; 2002. pp. 36–48. [Google Scholar]
- White HS, Smith MD, Wilcox KS. Mechanisms of action of antiepileptic drugs. Int Rev Neurobiol. 2007;81:85–110. doi: 10.1016/S0074-7742(06)81006-8. [DOI] [PubMed] [Google Scholar]
- Yang Y, Frankel WN. Genetic approaches to studying mouse models of human seizure disorders. Adv Exptl Med Biol. 2004;548:1–11. doi: 10.1007/978-1-4757-6376-8_1. [DOI] [PubMed] [Google Scholar]
- Zhang HG, Tan J, Reynolds E, Kuebler D, Faulhaber S, Tanouye MA. The Drosophila slamdance gene: a mutation in an aminopeptidase can cause seizure, paralysis and neuronal failure. Genetics. 2002;162:1283–1299. doi: 10.1093/genetics/162.3.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]