Hepatic fibrosis represents a significant global health problem for which no adequate therapy exists.1,2 Alcoholic fibrosis is the liver’s wound-healing response to injury and it can lead to cirrhosis characterized by scar accumulation and nodule formation. Thus far, there is no proven therapy for hepatic fibrosis, which can be further complicated by hepatocellular carcinoma. Hence, prevention of liver fibrosis could help to ameliorate the complications of cirrhosis and improve the quality of life of many patients worldwide.1,2
Uncontrolled production of collagen I is the main feature of liver fibrosis.1-13 Following a fibrogenic stimulus such as alcohol, hepatic stellate cells (HSC) transform into an activated collagen-producing cell. In alcoholic liver disease, numerous changes in gene expression are associated with HSC activation, including the induction of several intracellular signaling cascades, which help maintain the activated phenotype and control the fibrogenic and proliferative state of the cell.1-16 Detailed analyses for understanding the molecular basis of the collagen I gene regulation have revealed a complex process involving reactive oxygen species (ROS) as key mediators.1,2 Less is known, however, about the contribution of reactive nitrogen species (RNS). In addition, a series of cytokines, growth factors, and chemokines, which activate extracellular matrix (ECM)-producing cells through paracrine and autocrine loops, contribute to the fibrogenic response.17
RELEVANCE OF OXIDATIVE STRESS IN LIVER DISEASE
Following alcohol consumption, cholestasis, and iron overload, ROS and lipid peroxidation products are generated in large amounts, leading to Kupffer cell activation,18-23 a key event in the liver inflammatory and profibrogenic response, and to the secretion of a myriad of growth factors, cytokines, and prostaglandins.24,25 Among other stimuli, hepatocyte-derived lipid peroxidation induced by ethanol plays an important role in the pathogenesis of liver fibrosis by up-regulating collagen I synthesis.26 Acetaldehyde is the first metabolite of ethanol and is a profibrogenic agent that stimulates intracellular accumulation of H2O2.27 In addition, H2O2 has been also implicated in the onset of scleroderma, an event consistent with earlier clinical evidence for ROS participation in disease pathology.28,29 Furthermore, ROS-sensitive cytokines contribute to HSC activation during inflammatory through paracrine signals released from immune cells.30
Alcohol metabolism by cytochrome P450 2E1 (CYP2E1) generates ROS.31 Binge drinking triggers steatosis, a fibrogenic response, and apoptosis in rats fed a choline-deficient diet through increased oxidant stress, elevated phosphorylation of p38, and down-regulation of ECM proteolytic enzymes.32 To better understand how HSC become activated in the presence of oxidative stress and to evaluate whether CYP2E1-derived ROS could play a role in HSC activation, our laboratory co-incubated primary HSC with HepG2 cells overexpressing or not CYP2E1.8,9 There were enhanced proliferation rates and induction of a-smooth muscle actin, intracellular and secreted collagen I protein, and intra- and extracellular H2O2 and lipid peroxidation products in HSC co-incubated with HepG2 cells overexpressing CYP2E1 compared with HSC incubated with control HepG2 cells.8,9 These effects were prevented by antioxidants and by CYP2E1 inhibitors, suggesting a role for CYP2E1-derived diffusible mediators on these effects.8,9
A causal relationship also exists between oxidative stress and chronic alcohol-induced liver injury, with sustained activation of Kupffer cells and HSC. A recent study4 demonstrated that Kupffer cells induced a more activated phenotype, greater proliferation rates, and increased intra- and extracellular collagen I protein, H2O2, and IL-6 in HSC co-cultured with Kupffer cells when compared with HSC cultured alone. All these features were prevented by catalase, indicating a role for H2O2.4 In addition, MMP-13, which degrades extracellular collagen I, decreased in the co-culture of HSC with Kupffer cells, while there was up-regulation of tissue inhibitor of metalloproteinase 1 (TIMP1), a MMP-13 inhibitor. A novel dual mechanism mediated by H2O2 and IL-6 was proposed for how Kupffer cells could modulate the fibrogenic response in HSC.4
Supporting the role of oxidative stress in fibrosis, a novel study showed a correlation between cellular activation and oxidative stress in the GRX cell line and how vitamin E and N-acetylcysteine prevented the activation.33 Parola and colleagues34 also have demonstrated that activated HSC are more vulnerable than quiescent HSC to aldehyde end products.
SYNERGISM BETWEEN REACTIVE OXYGEN SPECIES AND GROWTH FACTORS
The role of pro-fibrogenic cytokines and growth factors is central for the development of liver fibrosis because they allow cross-talk between ECM-producing cells.3-6 ROS play a decisive role in the initial phase of fibrosis by integrating different profibrotic stimuli independently of TGFβ. By contrast, progression to the subsequent stage depends on TGFβ production and the canonical Smad pathway.35 Liver fibrosis involves elevated expression of TGFβ in rodents36,37 and in humans.38 In normal liver and in CCl4-induced fibrosis, TGFβ1 is mainly produced by Kupffer cells and HSC although a small amount is generated by endothelial cells.36,39 TGFβ is secreted as a latent complex that is trapped among matrix fibbers, from which it is released and activated during tissue remodeling.40,41 Activated TGFβ signals through transmembrane serine/threonine kinases and intracellular Smad proteins, which translocate into the nucleus, activate gene transcription by binding the “CAGA” consensus sequence, and interact with promoter-specific transcription factors and general co-activators42 to initiate and perpetuate the fibrogenic response.43 TGFβ1 is a redox sensitive gene;44 indeed, ROS (eg, H2O2) up-regulate TGFβ1 expression in rat HSC, which is blocked by catalase.45 In addition, TGFβ per se increases O2· − production in fibroblasts.46 Other studies have described how TGFβ increases ROS production by activating the membrane-bound enzyme NADPH oxidase47,48 and by impairing complex IV in the mitochondrial respiratory chain.49 The canonical signal transduction pathway of TGFβ plays a central role in fibrosis42,43,50,51 even though phosphatidylinositol 3-kinase (PI3K)/Akt is a well-documented example of an alternative pathway that is induced by TGFβ in different cell lines and profibrogenic conditions.35,52
PDGF is the most potent mitogen for transdifferentiated HSC during liver fibrosis, and the expression of PDGF receptors is important for mitogenesis and chronic inflammation of the liver,53-55 and particularly chemo-attraction of mononuclear cells.56 The expression of PDGF is also influenced by intracellular redox changes.57 TGFβ1 regulates the PDGFRb in human HSC.58 Moreover, the PDGF-dependent activation of HSC is followed by phosphorylation of PI3K.59,60 PI3K activation is essential for both mitogenesis and chemotaxis induced by PDGF during liver injury in vivo.61 The 70-kDa ribosomal S6 kinase is activated in a PI3K-dependent manner and plays an important role in HSC proliferation, collagen expression, and cell cycle control, representing a potential therapeutic target for liver fibrosis.62 FoxO1 inhibits PDGF-induced HSC proliferation via G1 cell cycle arrest suggesting that FoxO1 is a crucial downstream target of the PI3K/Akt pathway in regulating HSC proliferation.60 PI3K is induced by oxidative stress in rat HSC following exposure to CCl4.63 HSC isolated from CCl4-treated rats or from acute liver damage in vivo show increased ERK activity, which modulates HSC proliferation and chemotaxis and regulates nuclear signaling.64
The proliferative effect of PDGF requires the activation of PDGFRb.65,66 Furthermore, the activation of cultured rat HSC by Kupffer cell-conditioned medium directly enhances matrix synthesis and stimulates HSC proliferation via induction of PDGF receptors.65 The myristoylated alanine-rich protein kinase C substrate is a downstream effector in PDGF-induced motility of activated human HSC.67 Different compounds that activate the AMPK pathway inhibit PDGF-stimulated proliferation and migration of human HSC and reduce the secretion of monocyte chemoattractant protein-1, suggesting that AMPK negatively modulates the activated phenotype of HSC.68 Nitric oxide (NO·) donors also exert a direct antifibrogenic action by inhibiting PDGF-induced proliferation, motility, and contractility in HSC, in addition to lowering ECM proteins.69 A regulatory mechanism of reactive aldehydes on PDGFRb signaling and biologic actions is relevant to liver fibrosis.70
TNFα secreted by macrophages, Kupffer cells, and HSC, as well as IFNγ secreted by T cells, are well-known antifibrogenic signals.71,72 TNFα reduces ECM deposition by inhibiting the synthesis of structural components, including elastin, osteocalcin, and collagen I.73-76 TNFα counteracts TGFβ-stimulation of collagen I gene in different cell types.77,78 Both TNFα and IFNγ blunt the TGFβ-mediated up-regulation of the COL1A2 promoter by interfering with the formation of the TGFβ1-responsive element complex.79-81 TGFβ antagonism by TNFα involves c-Jun N-terminal kinase-1 phosphorylation of c-Jun, which leads to off-DNA interference of Smad3 binding to the cognate DNA site and/or interaction with the p300/CBP co-activators.82 Moreover, a previous report has shown that p38 MAPK is a key mediator of the antifibrogenic effect of TNFα in regulating the expression of col1A1 in HSC in response to cytokines.83
In addition, interferon regulatory factor-binding site IF3 is a novel target of the pathways elicited by IFNγ to blunt COL1A2 promoter transcription.84 IFNγ promotes occupancy of the COL1A2 transcription start site by the RFX5/CIITA complex, which interacts with CBF/NFY and/or YB-1.79 These antifibrogenic cytokines down-regulate COL1A2 transcription and antagonize TGFβ through multiple pathways, which converge on the same promoter elements.85,86 TNFα–mediated down-regulation of the mouse col1a1 gene is associated with the activation and binding of C/EBPδ-and C/EBPβ-containing complexes to the -370 to -344 region of the mouse col1a1 promoter (Table 1).87
Table 1.
Summary of different factors that modulate collagen I expression
| Collagen I | |
|---|---|
| ROS(O2· − and H2O2) 11,29,47,64,91,133,157-160 | ↑ |
| IL-4100 | ↑ |
| IL-6101,102 | ↑ |
| Acetaldehyde29,106,128-131 | ↑ |
| Arachidonic acid11 | ↑ |
| Malondialdehyde134 | ↑ |
| PDGF61,62,67 | ↑ |
| Nitric oxide71 | ↓ |
| TGFβ47,112,113 | ↑ |
| TNFα78,85,89 | ↓ |
| INFγ81,83,86 | ↓ |
| Fli-1120-122 | ↓ |
| Sp1/Sp3118,119 | ↑ |
| Adiponectin123,124 | ↓ |
| Leptin125-127 | ↑ |
EFFECTS OF REACTIVE OXYGEN SPECIES ON THE COL1A1 AND COL1A2 PROMOTERS
Collagen I, the most abundant collagen type found in liver fibrosis, is a heterotrimeric protein composed of two α1 chains and one α2 chain forming a triple helical structure. The human α-chains genes are located as single copies on different chromosomes. The α1(I) chain gene is on chromosome 17q21-22 whereas the α2(I) chain gene is located on 7q21-22.88,89 In normal tissue, both genes are co-ordinately expressed,90 whereas a homotrimer of three α1(I) chains occasionally occurs in tumors91 and in cultured cells.92 Structural and metabolic deficiencies of the collagen I chains lead to several heritable and acquired disorders of connective tissue,93 in general, and impair liver function, in particular.94
The IL-4-induced transcriptional activator STAT6 binds to various sequences within the COL1A1 and COL1A2 promoters.95 An AP-2 site adjacent to the reverse-oriented STAT6 consensus motif TTCN3/4 GCT is located within 205 bp from the transcription start site and seems to support the moderate IL-4-induced COL1A1 gene activation. Furthermore, IL-6 up-regulates the expression of type I collagen in vivo96 and in cultured HSC.97-99 HSC respond to IL-6 with a transient increase in col1a1 mRNA expression.97,100 A TGFβ1-responsive element is found in the -370 to -344 bp region of the mouse col1a1 gene.45 TGFβ1 induces the activation and binding to the TGFβ1-responsive element of a protein complex that contains C/EBPβ, and H2O2 acts as a second messenger for the TGFβ1-mediated col1a1 gene up-regulation.45 In fact, acetaldehyde induces col1a1 up-regulation via H2O2.101 However, IFNγ and TNFα down-regulate transcription of the COL1A1 promoter.95 The TNFα-mediated down-regulation of the col1a1 gene is associated with activation and binding of C/EBPδ- and C/EBPβ-containing complexes to the -370 to -344 bp region of the mouse col1a1 promoter. Indeed, over-expression of C/EBPδ or p20C/EBPβ down-regulates the expression of a reporter construct driven by the -412 to +110 bp sequence of the col1a1 promoter, validating the relevance of these two transcription factors in the TNFα-mediated col1a1 down-regulation.87
The functional properties of the proximal promoter of the COL1A2 gene have been studied in transfection experiments,102 which have described the minimal upstream sequence directing high and cell type-specific expression of the CAT-reporter gene. The proximal promoter of the COL1A2 spans from −380 to +54 bp relative to the transcription start site and contains several overlapping DNA elements that are bound by ubiquitous transcription factors. Constitutive transcription of the proximal promoter of the COL1A2 is under the control of four clusters of cis-acting elements, which are involved in mediating the transcriptional response to cytokines implicated in tissue remodeling and fibrosis.103 Recent studies have underscored the importance of the proximal promoter in proper COL1A2 expression, describing, in addition, that a far-upstream enhancer and a downstream silencer are also part of the regulatory network of the COL1A2 gene.104,105 A model has been proposed whereby COL1A2 expression is the result of combinatorial interactions amongst trans-acting factors bound within the promoter, enhancer, and repressor sequences.85 The downstream repressor resides within the first intron of COL1A2 and contains a DNase I hypersensitive site located around a cluster of three cis-acting elements, which contain binding sites for ‘GATA’ (FIi1 an FIi2) and IRF (FIi3) nuclear proteins.105
Many nuclear factors that have been implicated in regulating the COL1A2 proximal promoter in fibroblasts, including Sp1/Sp3, NFkB, C/EBPδ and β, AP1, Fli-1/Ets-1, CBF/NFY, YB-1 and the Smads 3/4 and RFX5/CIITA complexes.76,79,81,106 Specifically, either single or multiple “CAGA” boxes are present in the TGFβ -responsive element on the COL1A2 gene; hence, the Smad3/4 complex represents a common mediator of the TGFβ signaling for ECM accumulation.107 The ubiquitous transcription factor Sp1, the Smad3/4 complex, and the co-activators p300/CBP mediate the response of the COL1A2 promoter to TGFβ stimulation.85,108,109 A Smad complex may represent the alleged Sp1 co-factor involved in COL1A2 trans-activation.110 Transient over-expression of Smad3 and Smad4 can transactivate the COL1A2 promoter.111 Sp1 and Smad3/Smad4 cooperate synergistically in transactivating the COL1A2 promoter after binding to the TGFβ1-responsive element. Furthermore, there is additional evidence for a critical role of Sp1 in constitutive COL1A2 expression, and in integrating the transcriptional responses of the gene to antagonistic cytokines.112 Sp1/Sp3 proteins bound to the COL1A2 promoter interact with CBF/NFY to strengthen promoter activity and patterned transgene expression.113,114 Fli-1, a member of Ets transcriptional factors, is a negative regulator of the COL1A2 gene expression in dermal fibroblasts.115 Competition between Fli-1 and Ets-1 for binding to the same promoter sequence is associated both with modulating constitutive COL1A2 activity and inhibiting TGFβ signaling.116 In addition, a recent study has shown that TGFβ -dependent acetylation and inhibition of Fli-1 may represent the principal mechanisms responsible for the TGFβ -induced dissociation of Fli-1 from the COL1A2 promoter.117
Several studies indicate that adiponectin has antifibrotic properties because hepatic fibrosis produced by chronic CCl4 was enhanced in adiponectin-knockout mice as compared with wild-type mice,118,119 while leptin has an opposite effect of enhancing fibrogenesis.120-122
Acetaldehyde up-regulates type I collagen in HSC123-125 and the COL1A1 and COL1A2 induction occurs through a TGFβ-dependent mechanism.126 In addition, acetaldehyde stimulates intracellular accumulation of H2O2 and COL1A2 promoter activity in HSC,29 strongly suggesting that the early stage of ethanol-induced liver fibrosis induces a H2O2-dependent loop, which triggers and amplifies autocrine TGFβ production, via activation of the Sp1-Smad3/4 complex, conceivably through the PI3K pathway.29,127 Another report has implicated H2O2 in the pathology of scleroderma, demonstrating that PDGF treatment of primary human fibroblasts triggers an intracellular loop involving Ha-Ras, ERK1/2, and ROS, ultimately leading to COL1A2 up-regulation.128 The TGFβRI-dependent program and the up-regulation of collagen I does not involve Smad2/3 activation but is mediated by ALK1/Smad1 and ERK1/2 pathways in scleroderma fibroblasts.128 The involvement of H2O2 in the induction of the COL1A1 promoter under TGFβ treatment has been defined.45,76,101 Primary Kupffer cells in co-culture with HSC induce a profibrogenic response mediated by H2O2 that leads to up-regulation of COL1A1 and COL1A2 trans-activation and simultaneously prevents collagen I protein degradation via an IL-6-dependent mechanism.4 Work from our group has described a role for H2O2 in the up-regulation of COL1A2 expression by ethanol and arachidonic acid whereby COX-2 appears to mediate the arachidonic acid-mediated induction of COL1A2 expression (see Table 1).11
STABILITY AND DEGRADATION OF COLLAGEN I: ROLE OF METALLOPROTEINASES
Liver fibrosis is characterized by activation of HSC and subsequent ECM synthesis, along with insufficient degradation. Matrix remodeling occurs mainly due to the action of MMPs, a multidomain family of zinc-dependent endopeptidases. These enzymes are secreted into the extracellular space as zymogens that require activation by a variety of stimuli. The active enzymes can, in turn, be inhibited by the family of tissue inhibitors of metalloproteinases (TIMPs). ECM remodeling is, therefore, highly regulated under physiologic conditions. Alteration of the balance between MMPs plays a role in scarring.129,130
The three most relevant MMPs are gelatinase A (MMP-2), gelatinase B (MMP-9), and stromelysin (MMP-3). In liver fibrosis, the expression of the MMPs involved in fibrillar collagen degradation (eg, MMP-1 in humans and rodents and MMP-13 also in rodents) is limited, whereas the expression of MMP-2 is markedly increased.131 TGFβ1 modulates MMP-13 expression in HSC by complex mechanisms involving p38 MAPK, PI3K/AKT and p70-ribosomal S6 kinase.132 MMP-2 can degrade several components of the subendothelial matrix, including collagen IV, laminin, and fibronectin, and it may be important in the remodeling of matrix during tissue repair processes.133 A recent study has shown that oxidative stress induces MMP-2 expression, proliferation, and invasiveness of HSC; these effects could be prevented by specific MMP inhibitors and antioxidants.62 Increased expression of pro-gelatinase and formation of active enzyme occurs in human liver disease and in animal models of liver fibrosis.134,135 Sustained over-expression of MMPs like gelatinase A, with the consequent degradation of basement-membrane collagen IV, represents a basic mechanism in the remodeling of the space of Disse with capillarization of the sinusoids.136 In progressive liver fibrosis, the overall MMP activity decreases,137 due to increased expression of TIMPs and other anti-proteases expressed by HSC and hepatocytes.62,138 In liver fibrosis, hepatic TIMP-1 expression is markedly up-regulated both in humans and in murine fibrosis models.130,139,140 Both TIMP-1 and TIMP-2 are released by fully activated HSC.141 The increased expression of TIMPs is important in advanced liver fibrosis both in rodents and in humans.142,143 In fact, increased TIMP-1 and TIMP-2 mRNA levels have been demonstrated by in situ hybridization in CCl4-induced rat fibrosis,144 as well as in primary biliary cirrhosis and biliary atresia.140
Plasmin is a broad-spectrum protease capable of directly degrading matrix components, including fibronectin, laminin, and proteoglycans145 and also participates in matrix degradation indirectly by activating MMP-13, MMP-1, MMP-3, interstitial collagenase, and stromelysin.146,147 PAI-1, a physiologic inhibitor of plasminogen activator, inhibits protease-dependent fibrinolytic activity and subsequent ECM degradation. PAI-1 expression is up-regulated in a variety of fibrotic diseases as well as in experimental animal models such as CCl4-induced liver fibrosis,148 and PAI-1-deficient mice develop less severe fibrosis in lung,149 suggesting a role for PAI-1 in the progression of fibrosis. Furthermore, GSH inhibits TGFβ -induced collagen I accumulation by blocking TGFβ-induced PAI-1 expression, and thus stimulating collagen degradation.150 Many other studies have also described TGFβ as an inducer of ROS production and ROS mediate PAI-1 induction by different stimuli.151-154
SOURCES OF REACTIVE NITROGEN SPECIES IN THE LIVER
Nearly all cell types in the liver, including hepatocytes, Kupffer cells, HSC, and endothelial cells, have the capacity to generate NO·.155 The reactivity of NO· per se has been greatly overestimated in vitro because no drain is provided to remove NO·.156 NO· remains in solution for several minutes in micromolar concentrations before it reacts with O2 to form much stronger oxidants like nitrogen dioxide and others.157 Most biological actions of NO· appear to be mediated by interactions with paramagnetic centers in effector proteins, such as heme- or iron-sulfur centers, but NO· is also known to react rapidly, via radical termination reactions, with other targets that carry unpaired electrons.158 These reactions include interactions with ROS such as O2· −, or with radical intermediates in proteins or lipids.156 Furthermore, NO· reacts with O2 to form higher oxides of nitrogen, in a relatively slow reaction (Fig. 1).159 The eventual biologic fate of NO· is oxidation to nitrite and nitrate, end products of NO· metabolism that are rapidly distributed throughout the body and excreted in urine.158
Fig. 1.
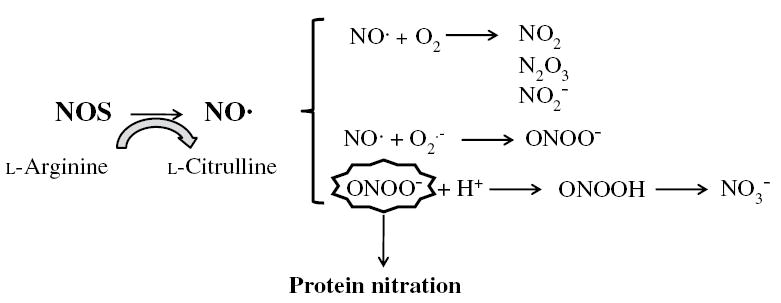
Generation of NO· by NOS in liver cells. NO· is relatively unstable in the presence of O2 and will rapidly and spontaneoulsy auto-oxidize to yield a variety of nitrogen oxides.NO· also reacts with O2· − to generate ONOO−. Although ONOO− is relatively stable, it has a pKa of 6.8, which implies that substantial amounts of ONOO− will be protonated at physiologic pH to yield peroxynitrous acid. This conjugate acid rapidly decomposes to yield NO3−. Nitration of tyrosine residues by ONOO− forms the stable product, 3-nitrotyrosine (3-NT, the footprint for ONOO−) by addition of a nitro group to the 3-position adjacent to the hydroxyl group of tyrosine.
There are a number of RNS derived from NO·.160 Of these, peroxynitrite (ONOO−) is the best characterized and appears to have the highest biological activity.160 ONOO− is formed by the bi-radical reaction of NO· and O2· −. The reaction is extremely fast and will occur at a near diffusion-limited rate.161 NO· is the only biological molecule produced in concentrations large enough to compete with superoxide dismutase for O2· −.156 ONOO− reacts relatively slowly with most biological molecules, which defines it as a selective oxidant. Effects of ONOO− may also be beneficial or detrimental depending on the concentration and local environment, the level of cellular activation, and the endogenous GSH pool, which acts as a natural ONOO− scavenger.162 On the other hand, direct in vivo and in vitro evidence that, at low concentrations, ONOO− is actively involved in triggering cellular survival signals has been reported in studies demonstrating protection against myocardial ischemia-reperfusion injury and neuronal apoptosis.163,164
REACTIVE NITROGEN SPECIES AND HEPATOTOXICITY
NO· is a short-life gaseous free radical known to exert many actions in the liver as well as in other tissues and organs.165 This review summarizes only the major notions, with special reference to interactions with ROS at the molecular level leading to collagen I regulation, and effects at the cellular level (ie, HSC activation). In normal liver, low fluxes of NO· are produced by constitutive endothelial nitric oxide synthase (eNOS, mainly in endothelial cells) and are considered sufficient to maintain perfusion of liver sinusoids by acting on vascular tone (ie, vasodilatation) and on vascular permeability.166 NO· regulates leukocyte adhesion to sinusoidal endothelium and inhibits platelet adhesion and aggregation.167 In pathologic conditions, including endotoxemia and chronic inflammation, nitric oxide synthase 2 (NOS2) is up-regulated in almost all liver cells, including HSC,168 by several mediators and, consequently, NO· generation increases.166 Under these conditions, NO· acts either as cytoprotective or as cytotoxic depending on the cellular microenvironment.169
The molecular regulation of NOS2 expression is complex and occurs at multiple levels. NOS2 expression requires the transcription factor NFκB and is down-regulated by steroids, TGFβ, the heat shock response, p53, and NO· itself.169 NO· also presents a protective effect both in vivo and in vitro by blocking TNFα-induced apoptosis and hepatotoxicity, in part by a thiol-dependent inhibition of caspase-3-like protease activity.170 These studies demonstrate the cytoprotective effects of NO· in the liver and suggest that hepatic NOS2 expression may function as an adaptive response to minimize inflammatory injury.170 Thus, numerous mechanisms have evolved to regulate NOS2 expression during hepatocellular injury.171 The activation of NO· synthesis can be considered as an early adaptive response, which may become a mediator of tissue damage in excess. Whether or not NO· or secondary oxidants generated from NO· act as mediators of tissue injury or protect against toxicity will likely depend on the precise targets of these RNS, the levels of O2· −, and the extent to which tissue injury is mediated by ROS.155
SYNERGISM BETWEEN REACTIVE NITROGEN SPECIES AND REACTIVE OXYGEN SPECIES
ROS may synergize with or antagonize RNS during liver injury and inflammation. ROS and RNS are important in the process of energy generation, lipid peroxidation, protein and DNA oxidation, nitration, nitrosation, or nitrosylation and catecholamine response.172 ROS and RNS also strongly interact with reactive sulfur species, ie, derivatives of reduced thiols (RSH) including the thiolate anion (RS−), thiyl radical (RS·) , sulfenic acid (R-SOH), sulfinic acid (R-SOO), and sulfonic acid (R-SOOH) derivatives.
Among the many types of oxidative modifications induced by ONOO− and other RNS are the characteristic addition or substitution products in which NO· is essentially incorporated into the target molecule (ie, nitrosation and nitration reactions).158 For instance, reactions with thiol residues to form S-nitrosothiols have been proposed as a mechanism of either enzyme regulation or NO· transport, and may provide a unique signaling mechanism induced by nitrosative stress. S-Nitrosothiols in proteins (eg, albumin) or in low-molecular-weight thiols, such as GSH, have been detected in the circulation, bile, as well as in respiratory tract lining fluids.158 There is a mechanism for pro-MMPs activation caused by S-glutathiolation whereby the GSH adduct of pro-MMP may be produced through disulfide S-oxide formation involving generation of S-nitrosoglutathione (GSNO2) by ONOO−.173
The amino acid tyrosine appears to be a particularly susceptible target for nitration, and the formation of free or protein-associated 3-nitrotyrosine has received much recent interest as a potential biomarker for the generation of RNS in vivo.158 Furthermore, there is considerable evidence in the protein chemistry literature that nitration of essential tyrosine residues can inactivate many enzymes or prevent phosphorylation of tyrosine kinase substrates,174 and these findings have supported the hypothesis that tyrosine nitration might result not only in the formation of inactive “footprints” of RNS but might also be functionally related to the pathobiology of inflammatory diseases.175 For example, ONOO− promoted nitration and/or phosphorylation of regulatory sites at tyrosine kinase receptors coupled to the well-known anti-apoptotic pathways involving PI3K/Akt or MAPK.176,177 Moreover, one of the most interesting protective effects of NO· is represented by the NO-dependent blocking of hepatocyte apoptosis induced either by removal of growth factors or by exposure to TNFα or anti-Fas antibody. This anti-apoptotic effect has been ascribed to S-nitrosylation of caspase-3 and -8, with the subsequent inhibition of their activity.178
REACTIVE NITROGEN SPECIES AND HEPATIC STELLATE CELL ACTIVATION
Paracrine signaling is also important for nitrosative stress as it is for oxidative stress. Kupffer cells also produce NO·, which can counterbalance the stimulatory effects of ROS by reducing HSC proliferation, contractility, and collagen I production.179
Neutrophils are an important source of ROS, which have a direct stimulatory effect on HSC collagen I synthesis. Activated neutrophils increased HSC collagen synthesis 3-fold over control levels. O2· − was identified as the principal mediator of the neutrophils’ effect. Activated neutrophils also produce NO·, which dampened the effect of O2· − on collagen I expression but did not abrogate it completely.180
Activated HSC have contractile features181 that may contribute to increased intrahepatic portal hypertension via constriction of the sinusoid or by contraction of fibrous ECM rich in collagen I with concomitant disruption of lobular architecture.182 Endothelin and NO· play a major role in the modulation of HSC contractility, and are therefore important in the pathogenesis of intrahepatic portal hypertension.182
Therefore, NO· and NO· donors are capable of preventing or reducing proliferative responses of activated HSC. NO· donors can efficiently inhibit PDGF-dependent proliferation and chemotaxis in activated human HSC by activating an ibuprofen-sensitive, prostaglandin E2 and cAMP-dependent pathway which interferes negatively with PDGF signaling.69 Similar results (ie, inhibition of stimulated proliferation of HSC by NO· donors) have been found with angiotensin II as proliferative stimulus.183
There is recent work showing lipopolysaccharide-induced synthesis of IL-6, TNFα, and NO· via NOS2 in HSC.184 This group presented evidence that activation of p38 by lipopolysaccharide initiates signaling via NFκB and ROS (eg, H2O2) leading to the induction of NOS2 and expression of IL-6 and TNFα, major players in hepatic hemodynamic regulation, inflammation, and immune responses.
REACTIVE NITROGEN SPECIES AND COLLAGEN I
Oxidative stress may represent a direct or indirect relevant profibrogenic stimulus for HSC, as suggested by in vivo experimental studies in which administration of antioxidants prevents oxidative stress, lipid peroxidation, and liver fibrosis.185 Furthermore, oxidative stress and lipid peroxidation are concomitant or precede HSC activation and collagen I deposition.4 Exposure of cultured human or rat HSC to pro-oxidants or to medium containing products released from hepatocytes undergoing oxidative stress (ie, to mimic a possible paracrine effect by damaged parenchymal cells) is followed by increased pro-collagen I gene expression.10
In contrast to ROS, which have been typically considered pro-fibrogenic agents,4,11 NO· may be anti-fibrogenic.69,186 In the wound-healing response that restores tissue integrity, NO· is synthesized in the early phase by inflammatory cells, mainly macrophages.187,188 However, many cells participate in NO· synthesis during the proliferative phase after wounding. NO· released via NOS2 regulates collagen formation, cell proliferation, and wound contraction in distinct ways in animal models of wound healing. Although NOS2 gene deletion delays, and arginine and NO· administration, improve healing, the exact mechanisms of action of NO· on wound-healing parameters are still unknown.187,188
ONOO− can down-regulate type I collagen in dermal and cardiac fibroblasts,189 smooth muscle cells,190 and other cell types.191 In addition, ONOO− can act as a potent antifibrotic effector in animal models of experimental fibrosis,192 and in the long-term inhibition of NOS2 in rats.186 However, in early traumatic wound-healing conditions, ONOO− favors collagen synthesis and the formation of granulation tissue.193 There are different mechanisms to explain the inhibition of collagen by ONOO−, ie, ONOO− may act through direct inhibition of collagen synthesis by proline hydroxylation,191 stimulation of MMPs,194,195 reduced production of TGFβ,189,195 initiation of fibroblast apoptosis,196 and/or neutralization of profibrogenic ROS.156,164
Recent data from our group indicate that ONOO− and its secondary products may have potential beneficial effects in the early fibrogenic response of HSC, which are exposed to reactive species (ie, O2· − and NO·) per se and also able to generate them (Fig. 2). The authors found a time- and dose-dependent down-regulation of intra- and extracellular collagen I protein along with an up-regulation of MMP-1 and TNFα in HSC treated with either pure ONOO− or a ONOO− donor, which were blocked by ONOO− scavengers. The addition of ONOO− increased nitration of MMP-1 and MMP-13 leading to increased activity as the cleaved active isoforms 22/25 kDa for MMP-1 and 44/48 kDa for MMP-13 were undetected in the absence of ONOO−. However, the protective role of ONOO− occurs only in the early fibrogenic response, and it is lost at more advanced stages of the disease when other factors may hit in a synergistic way.197
Fig. 2.
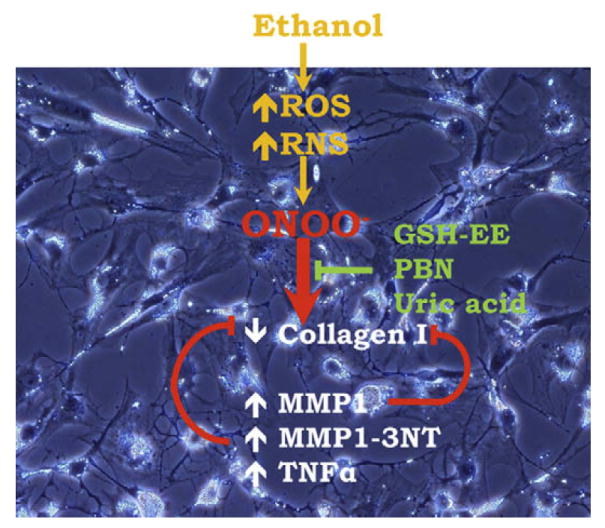
In liver injury, ROS and RNS are generated in all liver cells. O2· − can react with NO· to generate ONOO− and other metabolites that may impact the HSC fibrogenic response in the early stages of cellular activation. ONOO− and its metabolites lower collagen I protein by increasing TNFα and inducing nitration of MMP1 with the subsequent cleavage of collagen I. These effects can be reverted by ONOO− chelating agents.
The temporal expression profiles of profibrogenic genes in HSC and their coordination concerning cell proliferation in alcoholic liver disease still needs further clarification. Stress-derived mediators may activate seemingly contradictory signaling pathways and the ultimate outcome may be dependent on the balance between these stress-activated pathways because they could determine whether the cell proliferates or undergoes a fibrogenic response.
KEYWORDS
- CYP2E1
Cytochrome P450 2E1
- ECM
Extracellular matrix
- ERK 1/2
Extracellular signal-regulated kinase 1/2
- GSH
Glutathione
- HSC
Hepatic stellate cells
- H2O2
Hydrogen peroxide
- IFNγ
Interferon γ
- IL
Interleukin
- MMPs
Matrix metalloproteinases
- MAPK
Mitogen-activated protein kinase
- NO·
Nitric oxide
- NOS2
Nitric oxide synthase 2
- ONOO−
Peroxynitrite
- PI3K
Phosphatidylinositol 3-kinase
- PDGF
Platelet-derived growth factor
- RNS
Reactive nitrogen species
- ROS
Reactive oxygen species
- O2· −
Superoxide anion
- TIMPs
Tissue inhibitor of metalloproteinases
- TGFβ
Transforming growth factor-beta
References
- 1.Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88:125–72. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friedman SL. Mechanisms of disease: mechanisms of hepatic fibrosis and therapeutic implications. Nat Clin Pract Gastroenterol Hepatol. 2004;1:98–105. doi: 10.1038/ncpgasthep0055. [DOI] [PubMed] [Google Scholar]
- 3.Nieto N. Ethanol and fish oil induce NFkappaB trans-activation of the collagen alpha2(I) promoter through lipid peroxidation-driven activation of the PKC-PI3K-Akt pathway. Hepatology. 2007;45:1433–45. doi: 10.1002/hep.21659. [DOI] [PubMed] [Google Scholar]
- 4.Nieto N. Oxidative-stress and IL-6 mediate the fibrogenic effects of rodent Kupffer cells on stellate cells. Hepatology. 2006;44:1487–501. doi: 10.1002/hep.21427. [DOI] [PubMed] [Google Scholar]
- 5.Nieto N, Cederbaum AI. S-adenosylmethionine blocks collagen I production by preventing transforming growth factor-beta induction of the COL1A2 promoter. J Biol Chem. 2005;280:30963–74. doi: 10.1074/jbc.M503569200. [DOI] [PubMed] [Google Scholar]
- 6.Nieto N, Cederbaum AI. Increased Sp1-dependent trans-activation of the LAMgamma 1 promoter in hepatic stellate cells co-cultured with HepG2 cells overexpressing cytochrome P450 2E1. J Biol Chem. 2003;278:15360–72. doi: 10.1074/jbc.M206790200. [DOI] [PubMed] [Google Scholar]
- 7.Nieto N, Dominguez-Rosales JA, Fontana L, et al. Rat hepatic stellate cells contribute to the acute-phase response with increased expression of alpha1(I) and alpha1(IV) collagens, tissue inhibitor of metalloproteinase-1, and matrix-metalloproteinase-2 messenger RNAs. Hepatology. 2001;33:597–607. doi: 10.1053/jhep.2001.22520. [DOI] [PubMed] [Google Scholar]
- 8.Nieto N, Friedman SL, Cederbaum AI. Stimulation and proliferation of primary rat hepatic stellate cells by cytochrome P450 2E1-derived reactive oxygen species. Hepatology. 2002;35:62–73. doi: 10.1053/jhep.2002.30362. [DOI] [PubMed] [Google Scholar]
- 9.Nieto N, Friedman SL, Cederbaum AI. Cytochrome P450 2E1-derived reactive oxygen species mediate paracrine stimulation of collagen I protein synthesis by hepatic stellate cells. J Biol Chem. 2002;277:9853–64. doi: 10.1074/jbc.M110506200. [DOI] [PubMed] [Google Scholar]
- 10.Nieto N, Friedman SL, Greenwel P, et al. CYP2E1-mediated oxidative stress induces collagen type I expression in rat hepatic stellate cells. Hepatology. 1999;30:987–96. doi: 10.1002/hep.510300433. [DOI] [PubMed] [Google Scholar]
- 11.Nieto N, Greenwel P, Friedman SL, et al. Ethanol and arachidonic acid increase alpha 2(I) collagen expression in rat hepatic stellate cells overexpressing cytochrome P450 2E1. Role of H2O2 and cyclooxygenase-2. J Biol Chem. 2000;275:20136–45. doi: 10.1074/jbc.M001422200. [DOI] [PubMed] [Google Scholar]
- 12.Anania FA, Womack L, Potter JJ, et al. Acetaldehyde enhances murine alpha2(I) collagen promoter activity by Ca2+-independent protein kinase C activation in cultured rat hepatic stellate cells. Alcohol Clin Exp Res. 1999;23:279–84. [PubMed] [Google Scholar]
- 13.Saxena NK, Saliba G, Floyd JJ, et al. Leptin induces increased alpha2(I) collagen gene expression in cultured rat hepatic stellate cells. J Cell Biochem. 2003;89:311–20. doi: 10.1002/jcb.10494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu J, Zern MA. Hepatic stellate cells: a target for the treatment of liver fibrosis. J Gastroenterol. 2000;35:665–72. doi: 10.1007/s005350070045. [DOI] [PubMed] [Google Scholar]
- 15.Rippe RA, Brenner DA. From quiescence to activation: gene regulation in hepatic stellate cells. Gastroenterology. 2004;127:1260–2. doi: 10.1053/j.gastro.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 16.Safadi R, Friedman SL. Hepatic fibrosis–role of hepatic stellate cell activation. MedGenMed. 2002;4:27. [PubMed] [Google Scholar]
- 17.Gressner AM. Cytokines and cellular crosstalk involved in the activation of fat-storing cells. J Hepatol. 1995;22:28–36. [PubMed] [Google Scholar]
- 18.Balasubramaniyan V, Shukla R, Murugaiyan G, et al. Mouse recombinant leptin protects human hepatoma HepG2 against apoptosis, TNF-alpha response and oxidative stress induced by the hepatotoxin-ethanol. Biochim Biophys Acta. 2007;1770:1136–44. doi: 10.1016/j.bbagen.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 19.Wu J, Danielsson A, Zern MA. Toxicity of hepatotoxins: new insights into mechanisms and therapy. Expert Opin Investig Drugs. 1999;8:585–607. doi: 10.1517/13543784.8.5.585. [DOI] [PubMed] [Google Scholar]
- 20.Wu D, Zhai Q, Shi X. Alcohol-induced oxidative stress and cell responses. J Gastroenterol Hepatol. 2006;21(Suppl 3):S26–9. doi: 10.1111/j.1440-1746.2006.04589.x. [DOI] [PubMed] [Google Scholar]
- 21.Wang AL, Wang JP, Wang H, et al. A dual effect of N-acetylcysteine on acute ethanol-induced liver damage in mice. Hepatol Res. 2006;34:199–206. doi: 10.1016/j.hepres.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 22.Videla LA, Fernandez V, Tapia G, et al. Oxidative stress-mediated hepatotoxicity of iron and copper: role of Kupffer cells. Biometals. 2003;16:103–11. doi: 10.1023/a:1020707811707. [DOI] [PubMed] [Google Scholar]
- 23.Perez MJ, Velasco E, Monte MJ, et al. Maternal ethanol consumption during pregnancy enhances bile acid-induced oxidative stress and apoptosis in fetal rat liver. Toxicology. 2006;225:183–94. doi: 10.1016/j.tox.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 24.Kunkel SL, Chensue SW, Phan SH. Prostaglandins as endogenous mediators of interleukin 1 production. J Immunol. 1986;136:186–92. [PubMed] [Google Scholar]
- 25.Funaki N, Arii S, Monden K, et al. Chemical mediators released from hepatic macrophages in primary culture–basic characteristics of human hepatic macrophages and changes in liver cirrhosis. J Surg Res. 1993;54:222–9. doi: 10.1006/jsre.1993.1035. [DOI] [PubMed] [Google Scholar]
- 26.Kamimura S, Gaal K, Britton RS, et al. Increased 4-hydroxynonenal levels in experimental alcoholic liver disease: association of lipid peroxidation with liver fibrogenesis. Hepatology. 1992;16:448–53. doi: 10.1002/hep.1840160225. [DOI] [PubMed] [Google Scholar]
- 27.Rojkind M, Dominguez-Rosales JA, Nieto N, et al. Role of hydrogen peroxide and oxidative stress in healing responses. Cell Mol Life Sci. 2002;59:1872–91. doi: 10.1007/PL00012511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murrell DF. A radical proposal for the pathogenesis of scleroderma. J Am Acad Dermatol. 1993;28:78–85. doi: 10.1016/0190-9622(93)70014-k. [DOI] [PubMed] [Google Scholar]
- 29.Svegliati-Baroni G, Inagaki Y, Rincon-Sanchez AR, et al. Early response of alpha2(I) collagen to acetaldehyde in human hepatic stellate cells is TGF-beta independent. Hepatology. 2005;42:343–52. doi: 10.1002/hep.20798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Friedman SL. Cytokines and fibrogenesis. Semin Liver Dis. 1999;19:129–40. doi: 10.1055/s-2007-1007105. [DOI] [PubMed] [Google Scholar]
- 31.Ekstrom G, Ingelman-Sundberg M. Rat liver microsomal NADPH-supported oxidase activity and lipid peroxidation dependent on ethanol-inducible cytochrome P-450 (P-450IIE1) Biochem Pharmacol. 1989;38:1313–9. doi: 10.1016/0006-2952(89)90338-9. [DOI] [PubMed] [Google Scholar]
- 32.Nieto N, Rojkind M. Repeated whiskey binges promote liver injury in rats fed a choline-deficient diet. J Hepatol. 2007;46:330–9. doi: 10.1016/j.jhep.2006.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guimaraes EL, Franceschi MF, Grivicich I, et al. Relationship between oxidative stress levels and activation state on a hepatic stellate cell line. Liver Int. 2006;26:477–85. doi: 10.1111/j.1478-3231.2006.01245.x. [DOI] [PubMed] [Google Scholar]
- 34.Parola M, Robino G. Oxidative stress-related molecules and liver fibrosis. J Hepatol. 2001;35:297–306. doi: 10.1016/s0168-8278(01)00142-8. [DOI] [PubMed] [Google Scholar]
- 35.Svegliati-Baroni G, Ridolfi F, Di Sario A, et al. Intracellular signaling pathways involved in acetaldehyde-induced collagen and fibronectin gene expression in human hepatic stellate cells. Hepatology. 2001;33:1130–40. doi: 10.1053/jhep.2001.23788. [DOI] [PubMed] [Google Scholar]
- 36.De Bleser PJ, Niki T, Rogiers V, et al. Transforming growth factor-beta gene expression in normal and fibrotic rat liver. J Hepatol. 1997;26:886–93. doi: 10.1016/s0168-8278(97)80257-7. [DOI] [PubMed] [Google Scholar]
- 37.Poli G, Parola M. Oxidative damage and fibrogenesis. Free Radic Biol Med. 1997;22:287–305. doi: 10.1016/s0891-5849(96)00327-9. [DOI] [PubMed] [Google Scholar]
- 38.Annoni G, Weiner FR, Zern MA. Increased transforming growth factor-beta 1 gene expression in human liver disease. J Hepatol. 1992;14:259–64. doi: 10.1016/0168-8278(92)90168-o. [DOI] [PubMed] [Google Scholar]
- 39.Gressner AM, Bachem MG. Molecular mechanisms of liver fibrogenesis–a homage to the role of activated fat-storing cells. Digestion. 1995;56:335–46. doi: 10.1159/000201257. [DOI] [PubMed] [Google Scholar]
- 40.Ross JJ, Tranquillo RT. ECM gene expression correlates with in vitro tissue growth and development in fibrin gel remodeled by neonatal smooth muscle cells. Matrix Biol. 2003;22:477–90. doi: 10.1016/s0945-053x(03)00078-7. [DOI] [PubMed] [Google Scholar]
- 41.Shi Y, O’Brien JE, Jr, Fard A, et al. Transforming growth factor-beta 1 expression and myofibroblast formation during arterial repair. Arterioscler Thromb Vasc Biol. 1996;16:1298–305. doi: 10.1161/01.atv.16.10.1298. [DOI] [PubMed] [Google Scholar]
- 42.Massague J, Seoane J, Wotton D. Smad transcription factors. Genes Dev. 2005;19:2783–810. doi: 10.1101/gad.1350705. [DOI] [PubMed] [Google Scholar]
- 43.Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB J. 2004;18:816–27. doi: 10.1096/fj.03-1273rev. [DOI] [PubMed] [Google Scholar]
- 44.Leonarduzzi G, Scavazza A, Biasi F, et al. The lipid peroxidation end product 4-hydroxy-2,3-nonenal up-regulates transforming growth factor beta1 expression in the macrophage lineage: a link between oxidative injury and fibrosclerosis. FASEB J. 1997;11:851–7. doi: 10.1096/fasebj.11.11.9285483. [DOI] [PubMed] [Google Scholar]
- 45.Garcia-Trevijano ER, Iraburu MJ, Fontana L, et al. Transforming growth factor beta1 induces the expression of alpha1(I) procollagen mRNA by a hydrogen peroxide-C/EBPbeta-dependent mechanism in rat hepatic stellate cells. Hepatology. 1999;29:960–70. doi: 10.1002/hep.510290346. [DOI] [PubMed] [Google Scholar]
- 46.Liu RM, Liu Y, Forman HJ, et al. Glutathione regulates transforming growth factor-beta-stimulated collagen production in fibroblasts. Am J Physiol Lung Cell Mol Physiol. 2004;286:L121–8. doi: 10.1152/ajplung.00231.2003. [DOI] [PubMed] [Google Scholar]
- 47.Thannickal VJ, Day RM, Klinz SG, et al. Ras-dependent and -independent regulation of reactive oxygen species by mitogenic growth factors and TGF-beta1. FASEB J. 2000;14:1741–8. doi: 10.1096/fj.99-0878com. [DOI] [PubMed] [Google Scholar]
- 48.Thannickal VJ, Fanburg BL. Activation of an H2O2-generating NADH oxidase in human lung fibroblasts by transforming growth factor beta 1. J Biol Chem. 1995;270:30334–8. doi: 10.1074/jbc.270.51.30334. [DOI] [PubMed] [Google Scholar]
- 49.Yoon YS, Lee JH, Hwang SC, et al. TGF beta1 induces prolonged mitochondrial ROS generation through decreased complex IV activity with senescent arrest in Mv1Lu cells. Oncogene. 2005;24:1895–903. doi: 10.1038/sj.onc.1208262. [DOI] [PubMed] [Google Scholar]
- 50.Verrecchia F, Mauviel A. TGF-beta and TNF-alpha: antagonistic cytokines controlling type I collagen gene expression. Cell Signal. 2004;16:873–80. doi: 10.1016/j.cellsig.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 51.Abraham DJ, Varga J. Scleroderma: from cell and molecular mechanisms to disease models. Trends Immunol. 2005;26:587–95. doi: 10.1016/j.it.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 52.Runyan CE, Schnaper HW, Poncelet AC. The phosphatidylinositol 3-kinase/Akt pathway enhances Smad3-stimulated mesangial cell collagen I expression in response to transforming growth factor-beta1. J Biol Chem. 2004;279:2632–9. doi: 10.1074/jbc.M310412200. [DOI] [PubMed] [Google Scholar]
- 53.Vlahos CJ, Kriauciunas TD, Gleason PE, et al. Platelet-derived growth factor induces proliferation of hyperplastic human prostatic stromal cells. J Cell Biochem. 1993;52:404–13. doi: 10.1002/jcb.240520405. [DOI] [PubMed] [Google Scholar]
- 54.Grappone C, Pinzani M, Parola M, et al. Expression of platelet-derived growth factor in newly formed cholangiocytes during experimental biliary fibrosis in rats. J Hepatol. 1999;31:100–9. doi: 10.1016/s0168-8278(99)80169-x. [DOI] [PubMed] [Google Scholar]
- 55.Marra F, Choudhury GG, Pinzani M, et al. Regulation of platelet-derived growth factor secretion and gene expression in human liver fat-storing cells. Gastroenterology. 1994;107:1110–7. doi: 10.1016/0016-5085(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 56.Kinnman N, Hultcrantz R, Barbu V, et al. PDGF-mediated chemoattraction of hepatic stellate cells by bile duct segments in cholestatic liver injury. Lab Invest. 2000;80:697–707. doi: 10.1038/labinvest.3780073. [DOI] [PubMed] [Google Scholar]
- 57.Ruef J, Rao GN, Li F, et al. Induction of rat aortic smooth muscle cell growth by the lipid peroxidation product 4-hydroxy-2-nonenal. Circulation. 1998;97:1071–8. doi: 10.1161/01.cir.97.11.1071. [DOI] [PubMed] [Google Scholar]
- 58.Pinzani M, Gentilini A, Caligiuri A, et al. Transforming growth factor-beta 1 regulates platelet-derived growth factor receptor beta subunit in human liver fat-storing cells. Hepatology. 1995;21:232–9. [PubMed] [Google Scholar]
- 59.Lechuga CG, Hernandez-Nazara ZH, Hernandez E, et al. PI3K is involved in PDGF-beta receptor upregulation post-PDGF-BB treatment in mouse HSC. Am J Physiol Gastrointest Liver Physiol. 2006;291:G1051–61. doi: 10.1152/ajpgi.00058.2005. [DOI] [PubMed] [Google Scholar]
- 60.Adachi M, Osawa Y, Uchinami H, et al. The forkhead transcription factor FoxO1 regulates proliferation and transdifferentiation of hepatic stellate cells. Gastroenterology. 2007;132:1434–46. doi: 10.1053/j.gastro.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 61.Marra F, Gentilini A, Pinzani M, et al. Phosphatidylinositol 3-kinase is required for platelet-derived growth factor’s actions on hepatic stellate cells. Gastroenterology. 1997;112:1297–306. doi: 10.1016/s0016-5085(97)70144-6. [DOI] [PubMed] [Google Scholar]
- 62.Galli A, Svegliati-Baroni G, Ceni E, et al. Oxidative stress stimulates proliferation and invasiveness of hepatic stellate cells via a MMP2-mediated mechanism. Hepatology. 2005;41:1074–84. doi: 10.1002/hep.20683. [DOI] [PubMed] [Google Scholar]
- 63.Pinzani M, Marra F, Carloni V. Signal transduction in hepatic stellate cells. Liver. 1998;18:2–13. doi: 10.1111/j.1600-0676.1998.tb00120.x. [DOI] [PubMed] [Google Scholar]
- 64.Marra F, Arrighi MC, Fazi M, et al. Extracellular signal-regulated kinase activation differentially regulates platelet-derived growth factor’s actions in hepatic stellate cells, and is induced by in vivo liver injury in the rat. Hepatology. 1999;30:951–8. doi: 10.1002/hep.510300406. [DOI] [PubMed] [Google Scholar]
- 65.Friedman SL, Arthur MJ. Activation of cultured rat hepatic lipocytes by Kupffer cell conditioned medium. Direct enhancement of matrix synthesis and stimulation of cell proliferation via induction of platelet-derived growth factor receptors. J Clin Invest. 1989;84:1780–5. doi: 10.1172/JCI114362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wong L, Yamasaki G, Johnson RJ, et al. Induction of beta-platelet-derived growth factor receptor in rat hepatic lipocytes during cellular activation in vivo and in culture. J Clin Invest. 1994;94:1563–9. doi: 10.1172/JCI117497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rombouts K, Lottini B, Caligiuri A, et al. MARCKS is a downstream effector in platelet-derived growth factor-induced cell motility in activated human hepatic stellate cells. Exp Cell Res. 2008;314(7):1444–54. doi: 10.1016/j.yexcr.2008.01.029. [DOI] [PubMed] [Google Scholar]
- 68.Caligiuri A, Bertolani C, Guerra CT, et al. Adenosine monophosphate-activated protein kinase modulates the activated phenotype of hepatic stellate cells. Hepatology. 2008;47:668–76. doi: 10.1002/hep.21995. [DOI] [PubMed] [Google Scholar]
- 69.Failli P, De FR, Caligiuri A, et al. Nitrovasodilators inhibit platelet-derived growth factor-induced proliferation and migration of activated human hepatic stellate cells. Gastroenterology. 2000;119:479–92. doi: 10.1053/gast.2000.9354. [DOI] [PubMed] [Google Scholar]
- 70.Robino G, Parola M, Marra F, et al. Interaction between 4-hydroxy-2,3-alkenals and the platelet-derived growth factor-beta receptor. Reduced tyrosine phosphorylation and downstream signaling in hepatic stellate cells. J Biol Chem. 2000;275:40561–7. doi: 10.1074/jbc.M007694200. [DOI] [PubMed] [Google Scholar]
- 71.Riches DW, Chan ED, Winston BW. TNF-alpha-induced regulation and signalling in macrophages. Immunobiology. 1996;195:477–90. doi: 10.1016/s0171-2985(96)80017-9. [DOI] [PubMed] [Google Scholar]
- 72.Bach EA, Aguet M, Schreiber RD. The IFN gamma receptor: a paradigm for cytokine receptor signaling. Annu Rev Immunol. 1997;15:563–91. doi: 10.1146/annurev.immunol.15.1.563. [DOI] [PubMed] [Google Scholar]
- 73.Sivakumar P, Gupta S, Sarkar S, et al. Upregulation of lysyl oxidase and MMPs during cardiac remodeling in human dilated cardiomyopathy. Mol Cell Biochem. 2008;307:159–67. doi: 10.1007/s11010-007-9595-2. [DOI] [PubMed] [Google Scholar]
- 74.Zhang XH, Sun HM, Yuan JQ. Extracellular matrix production of lens epithelial cells. J Cataract Refract Surg. 2001;27:1303–9. doi: 10.1016/s0886-3350(00)00833-6. [DOI] [PubMed] [Google Scholar]
- 75.Li YY, Feng YQ, Kadokami T, et al. Myocardial extracellular matrix remodeling in transgenic mice overexpressing tumor necrosis factor alpha can be modulated by anti-tumor necrosis factor alpha therapy. Proc Natl Acad Sci U S A. 2000;97:12746–51. doi: 10.1073/pnas.97.23.12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Greenwel P, Tanaka S, Penkov D, et al. Tumor necrosis factor alpha inhibits type I collagen synthesis through repressive CCAAT/enhancer-binding proteins. Mol Cell Biol. 2000;20:912–8. doi: 10.1128/mcb.20.3.912-918.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Czaja MJ, Weiner FR, Flanders KC, et al. In vitro and in vivo association of transforming growth factor-beta 1 with hepatic fibrosis. J Cell Biol. 1989;108:2477–82. doi: 10.1083/jcb.108.6.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Son G, Iimuro Y, Seki E, et al. Selective inactivation of NF-kappaB in the liver using NF-kappaB decoy suppresses CCl4-induced liver injury and fibrosis. Am J Physiol Gastrointest Liver Physiol. 2007;293:G631–9. doi: 10.1152/ajpgi.00185.2007. [DOI] [PubMed] [Google Scholar]
- 79.Xu Y, Wang L, Buttice G, et al. Major histocompatibility class II transactivator (CIITA) mediates repression of collagen (COL1A2) transcription by interferon gamma (IFN-gamma) J Biol Chem. 2004;279:41319–32. doi: 10.1074/jbc.M404174200. [DOI] [PubMed] [Google Scholar]
- 80.Ghosh AK, Bhattacharyya S, Mori Y, et al. Inhibition of collagen gene expression by interferon-gamma: novel role of the CCAAT/enhancer binding protein beta (C/EBPbeta) J Cell Physiol. 2006;207:251–60. doi: 10.1002/jcp.20559. [DOI] [PubMed] [Google Scholar]
- 81.Higashi K, Inagaki Y, Fujimori K, et al. Interferon-gamma interferes with transforming growth factor-beta signaling through direct interaction of YB-1 with Smad3. J Biol Chem. 2003;278:43470–9. doi: 10.1074/jbc.M302339200. [DOI] [PubMed] [Google Scholar]
- 82.Feinberg MW, Cao Z, Wara AK, et al. Kruppel-like factor 4 is a mediator of proinflammatory signaling in macrophages. J Biol Chem. 2005;280:38247–58. doi: 10.1074/jbc.M509378200. [DOI] [PubMed] [Google Scholar]
- 83.Varela-Rey M, Montiel-Duarte C, Oses-Prieto JA, et al. p38 MAPK mediates the regulation of alpha1(I) procollagen mRNA levels by TNF-alpha and TGF-beta in a cell line of rat hepatic stellate cells(1) FEBS Lett. 2002;528:133–8. doi: 10.1016/s0014-5793(02)03276-3. [DOI] [PubMed] [Google Scholar]
- 84.Tanaka S, Ramirez F. The first intron of the human alpha2(I) collagen gene (COL1A2) contains a novel interferon-gamma responsive element. Matrix Biol. 2007;26:185–9. doi: 10.1016/j.matbio.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 85.Ramirez F, Tanaka S, Bou-Gharios G. Transcriptional regulation of the human alpha2(I) collagen gene (COL1A2), an informative model system to study fibrotic diseases. Matrix Biol. 2006;25:365–72. doi: 10.1016/j.matbio.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 86.Kahari VM, Chen YQ, Su MW, et al. Tumor necrosis factor-alpha and interferon-gamma suppress the activation of human type I collagen gene expression by transforming growth factor-beta 1. Evidence for two distinct mechanisms of inhibition at the transcriptional and posttranscriptional levels. J Clin Invest. 1990;86:1489–95. doi: 10.1172/JCI114866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Iraburu MJ, Dominguez-Rosales JA, Fontana L, et al. Tumor necrosis factor alpha down-regulates expression of the alpha1(I) collagen gene in rat hepatic stellate cells through a p20C/EBPbeta- and C/EBPdelta-dependent mechanism. Hepatology. 2000;31:1086–93. doi: 10.1053/he.2000.5981. [DOI] [PubMed] [Google Scholar]
- 88.Dalgleish R, Trapnell BC, Crystal RG, et al. Copy number of a human type I alpha 2 collagen gene. J Biol Chem. 1982;257:13816–22. [PubMed] [Google Scholar]
- 89.Huerre C, Junien C, Weil D, et al. Human type I procollagen genes are located on different chromosomes. Proc Natl Acad Sci U S A. 1982;79:6627–30. doi: 10.1073/pnas.79.21.6627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hata R. Transfection of normal human skin fibroblasts with human alpha 1(I) and alpha 2(I) collagen gene constructs and evidence for their coordinate expression. Cell Biol Int. 1995;19:735–41. doi: 10.1006/cbir.1995.1124. [DOI] [PubMed] [Google Scholar]
- 91.Moro L, Smith BD. Identification of collagen alpha1(I) trimer and normal type I collagen in a polyoma virus-induced mouse tumor. Arch Biochem Biophys. 1977;182:33–41. doi: 10.1016/0003-9861(77)90280-6. [DOI] [PubMed] [Google Scholar]
- 92.Gan YB. Expression of type I and type III procollagen genes in human scirrhous carcinoma of breast by in situ hybridization. Zhonghua Zhong Liu Za Zhi. 1992;14:348–50. in Chinese. [PubMed] [Google Scholar]
- 93.Colige A, Li SW, Sieron AL, et al. cDNA cloning and expression of bovine procollagen I N-proteinase: a new member of the superfamily of zinc-metalloproteinases with binding sites for cells and other matrix components. Proc Natl Acad Sci U S A. 1997;94:2374–9. doi: 10.1073/pnas.94.6.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Myllyharju J, Kivirikko KI. Collagens and collagen-related diseases. Ann Med. 2001;33:7–21. doi: 10.3109/07853890109002055. [DOI] [PubMed] [Google Scholar]
- 95.Buttner C, Skupin A, Rieber EP. Transcriptional activation of the type I collagen genes COL1A1 and COL1A2 in fibroblasts by interleukin-4: analysis of the functional collagen promoter sequences. J Cell Physiol. 2004;198:248–58. doi: 10.1002/jcp.10395. [DOI] [PubMed] [Google Scholar]
- 96.Greenwel P, Iraburu MJ, Reyes-Romero M, et al. Induction of an acute phase response in rats stimulates the expression of alpha 1(I) procollagen messenger ribonucleic acid in their livers. Possible role of interleukin-6. Lab Invest. 1995;72:83–91. [PubMed] [Google Scholar]
- 97.Greenwel P, Rubin J, Schwartz M, et al. Liver fat-storing cell clones obtained from a CCl4-cirrhotic rat are heterogeneous with regard to proliferation, expression of extracellular matrix components, interleukin-6, and connexin 43. Lab Invest. 1993;69:210–6. [PubMed] [Google Scholar]
- 98.Solis-Herruzo JA, Brenner DA, Chojkier M. Tumor necrosis factor alpha inhibits collagen gene transcription and collagen synthesis in cultured human fibroblasts. J Biol Chem. 1988;263:5841–5. [PubMed] [Google Scholar]
- 99.Armendariz-Borunda J, Katayama K, Seyer JM. Transcriptional mechanisms of type I collagen gene expression are differentially regulated by interleukin-1 beta, tumor necrosis factor alpha, and transforming growth factor beta in Ito cells. J Biol Chem. 1992;267:14316–21. [PubMed] [Google Scholar]
- 100.Greenwel P, Schwartz M, Rosas M, et al. Characterization of fat-storing cell lines derived from normal and CCl4-cirrhotic livers. Differences in the production of interleukin-6. Lab Invest. 1991;65:644–53. [PubMed] [Google Scholar]
- 101.Greenwel P, Dominguez-Rosales JA, Mavi G, et al. Hydrogen peroxide: a link between acetaldehyde-elicited alpha1(I) collagen gene up-regulation and oxidative stress in mouse hepatic stellate cells. Hepatology. 2000;31:109–16. doi: 10.1002/hep.510310118. [DOI] [PubMed] [Google Scholar]
- 102.Boast S, Su MW, Ramirez F, et al. Functional analysis of cis-acting DNA sequences controlling transcription of the human type I collagen genes. J Biol Chem. 1990;265:13351–6. [PubMed] [Google Scholar]
- 103.Florin L, Knebel J, Zigrino P, et al. Delayed wound healing and epidermal hyperproliferation in mice lacking JunB in the skin. J Invest Dermatol. 2006;126:902–11. doi: 10.1038/sj.jid.5700123. [DOI] [PubMed] [Google Scholar]
- 104.Antoniv TT, De Val S, Wells D, et al. Characterization of an evolutionarily conserved far-upstream enhancer in the human alpha 2(I) collagen (COL1A2) gene. J Biol Chem. 2001;276:21754–64. doi: 10.1074/jbc.M101397200. [DOI] [PubMed] [Google Scholar]
- 105.Antoniv TT, Tanaka S, Sudan B, et al. Identification of a repressor in the first intron of the human alpha2(I) collagen gene (COL1A2) J Biol Chem. 2005;280:35417–23. doi: 10.1074/jbc.M502681200. [DOI] [PubMed] [Google Scholar]
- 106.Inagaki Y, Truter S, Ramirez F. Transforming growth factor-beta stimulates alpha 2(I) collagen gene expression through a cis-acting element that contains an Sp1-binding site. J Biol Chem. 1994;269:14828–34. [PubMed] [Google Scholar]
- 107.Dennler S, Itoh S, Vivien D, et al. Direct binding of Smad3 and Smad4 to critical TGF beta-inducible elements in the promoter of human plasminogen activator inhibitor-type 1 gene. EMBO J. 1998;17:3091–100. doi: 10.1093/emboj/17.11.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Poncelet AC, Schnaper HW. Sp1 and Smad proteins cooperate to mediate transforming growth factor-beta 1-induced alpha 2(I) collagen expression in human glomerular mesangial cells. J Biol Chem. 2001;276:6983–92. doi: 10.1074/jbc.M006442200. [DOI] [PubMed] [Google Scholar]
- 109.Verrecchia F, Rossert J, Mauviel A. Blocking sp1 transcription factor broadly inhibits extracellular matrix gene expression in vitro and in vivo: implications for the treatment of tissue fibrosis. J Invest Dermatol. 2001;116:755–63. doi: 10.1046/j.1523-1747.2001.01326.x. [DOI] [PubMed] [Google Scholar]
- 110.Greenwel P, Inagaki Y, Hu W, et al. Sp1 is required for the early response of alpha2(I) collagen to transforming growth factor-beta1. J Biol Chem. 1997;272:19738–45. doi: 10.1074/jbc.272.32.19738. [DOI] [PubMed] [Google Scholar]
- 111.Chen SJ, Yuan W, Mori Y, et al. Stimulation of type I collagen transcription in human skin fibroblasts by TGF-beta: involvement of Smad 3. J Invest Dermatol. 1999;112:49–57. doi: 10.1046/j.1523-1747.1999.00477.x. [DOI] [PubMed] [Google Scholar]
- 112.Zhang W, Ou J, Inagaki Y, et al. Synergistic cooperation between Sp1 and Smad3/Smad4 mediates transforming growth factor beta1 stimulation of alpha 2(I)-collagen (COL1A2) transcription. J Biol Chem. 2000;275:39237–45. doi: 10.1074/jbc.M003339200. [DOI] [PubMed] [Google Scholar]
- 113.Ihn H, Ohnishi K, Tamaki T, et al. Transcriptional regulation of the human alpha2(I) collagen gene. Combined action of upstream stimulatory and inhibitory cis-acting elements. J Biol Chem. 1996;271:26717–23. doi: 10.1074/jbc.271.43.26717. [DOI] [PubMed] [Google Scholar]
- 114.Tanaka S, Antoniv TT, Liu K, et al. Cooperativity between far upstream enhancer and proximal promoter elements of the human {alpha}2(I) collagen (COL1A2) gene instructs tissue specificity in transgenic mice. J Biol Chem. 2004;279:56024–31. doi: 10.1074/jbc.M411406200. [DOI] [PubMed] [Google Scholar]
- 115.Czuwara-Ladykowska J, Shirasaki F, Jackers P, et al. Fli-1 inhibits collagen type I production in dermal fibroblasts via an Sp1-dependent pathway. J Biol Chem. 2001;276:20839–48. doi: 10.1074/jbc.M010133200. [DOI] [PubMed] [Google Scholar]
- 116.Czuwara-Ladykowska J, Sementchenko VI, Watson DK, et al. Ets1 is an effector of the transforming growth factor beta (TGF-beta) signaling pathway and an antagonist of the profibrotic effects of TGF-beta. J Biol Chem. 2002;277:20399–408. doi: 10.1074/jbc.M200206200. [DOI] [PubMed] [Google Scholar]
- 117.Asano Y, Czuwara J, Trojanowska M. Transforming growth factor-beta regulates DNA binding activity of transcription factor Fli1 by p300/CREB-binding protein-associated factor-dependent acetylation. J Biol Chem. 2007;282:34672–83. doi: 10.1074/jbc.M703907200. [DOI] [PubMed] [Google Scholar]
- 118.Kamada Y, Tamura S, Kiso S, et al. Enhanced carbon tetrachloride-induced liver fibrosis in mice lacking adiponectin. Gastroenterology. 2003;125:1796–807. doi: 10.1053/j.gastro.2003.08.029. [DOI] [PubMed] [Google Scholar]
- 119.Wang Y, Xu A, Knight C, et al. Hydroxylation and glycosylation of the four conserved lysine residues in the collagenous domain of adiponectin. Potential role in the modulation of its insulin-sensitizing activity. J Biol Chem. 2002;277:19521–9. doi: 10.1074/jbc.M200601200. [DOI] [PubMed] [Google Scholar]
- 120.Honda H, Ikejima K, Hirose M, et al. Leptin is required for fibrogenic responses induced by thioacetamide in the murine liver. Hepatology. 2002;36:12–21. doi: 10.1053/jhep.2002.33684. [DOI] [PubMed] [Google Scholar]
- 121.Potter JJ, Rennie-Tankesley L, Mezey E. Influence of leptin in the development of hepatic fibrosis produced in mice by Schistosoma mansoni infection and by chronic carbon tetrachloride administration. J Hepatol. 2003;38:281–8. doi: 10.1016/s0168-8278(02)00414-2. [DOI] [PubMed] [Google Scholar]
- 122.Saxena NK, Ikeda K, Rockey DC, et al. Leptin in hepatic fibrosis: evidence for increased collagen production in stellate cells and lean littermates of ob/ob mice. Hepatology. 2002;35:762–71. doi: 10.1053/jhep.2002.32029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Anania FA, Potter JJ, Rennie-Tankersley L, et al. Activation by acetaldehyde of the promoter of the mouse alpha2(I) collagen gene when transfected into rat activated stellate cells. Arch Biochem Biophys. 1996;331:187–93. doi: 10.1006/abbi.1996.0297. [DOI] [PubMed] [Google Scholar]
- 124.Casini A, Cunningham M, Rojkind M, et al. Acetaldehyde increases procollagen type I and fibronectin gene transcription in cultured rat fat-storing cells through a protein synthesis-dependent mechanism. Hepatology. 1991;13:758–65. [PubMed] [Google Scholar]
- 125.Moshage H, Casini A, Lieber CS. Acetaldehyde selectively stimulates collagen production in cultured rat liver fat-storing cells but not in hepatocytes. Hepatology. 1990;12:511–8. doi: 10.1002/hep.1840120311. [DOI] [PubMed] [Google Scholar]
- 126.Chen A. Acetaldehyde stimulates the activation of latent transforming growth factor-beta1 and induces expression of the type II receptor of the cytokine in rat cultured hepatic stellate cells. Biochem J. 2002;368:683–93. doi: 10.1042/BJ20020949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Svegliati-Baroni G, Saccomanno S, van Goor H, et al. Involvement of reactive oxygen species and nitric oxide radicals in activation and proliferation of rat hepatic stellate cells. Liver. 2001;21:1–12. doi: 10.1034/j.1600-0676.2001.210101.x. [DOI] [PubMed] [Google Scholar]
- 128.Pannu J, Nakerakanti S, Smith E, et al. Transforming growth factor-beta receptor type I-dependent fibrogenic gene program is mediated via activation of Smad1 and ERK1/2 pathways. J Biol Chem. 2007;282:10405–13. doi: 10.1074/jbc.M611742200. [DOI] [PubMed] [Google Scholar]
- 129.Arthur MJ. Fibrosis and altered matrix degradation. Digestion. 1998;59:376–80. doi: 10.1159/000007492. [DOI] [PubMed] [Google Scholar]
- 130.Arthur MJ, Mann DA, Iredale JP. Tissue inhibitors of metalloproteinases, hepatic stellate cells and liver fibrosis. J Gastroenterol Hepatol. 1998;13(Suppl):S33–8. doi: 10.1111/jgh.1998.13.s1.33. [DOI] [PubMed] [Google Scholar]
- 131.Milani S, Herbst H, Schuppan D, et al. Differential expression of matrix-metalloproteinase-1 and -2 genes in normal and fibrotic human liver. Am J Pathol. 1994;144:528–37. [PMC free article] [PubMed] [Google Scholar]
- 132.Lechuga CG, Hernandez-Nazara ZH, Dominguez Rosales JA, et al. TGF-{beta}1 modulates matrix metalloproteinase-13 expression in hepatic stellate cells by complex mechanisms involving p38MAPK, PI3K, AKT and p70S6K. Am J Physiol Gastrointest Liver Physiol. 2004;287(5):G974–87. doi: 10.1152/ajpgi.00264.2003. [DOI] [PubMed] [Google Scholar]
- 133.Wang JC. Importance of plasma matrix metalloproteinases (MMP) and tissue inhibitors of metalloproteinase (TIMP) in development of fibrosis in agnogenic myeloid metaplasia. Leuk Lymphoma. 2005;46:1261–8. doi: 10.1080/10428190500126463. [DOI] [PubMed] [Google Scholar]
- 134.Takahara T, Furui K, Funaki J, et al. Increased expression of matrix metalloproteinase-II in experimental liver fibrosis in rats. Hepatology. 1995;21:787–95. [PubMed] [Google Scholar]
- 135.Takahara T, Furui K, Yata Y, et al. Dual expression of matrix metalloproteinase-2 and membrane-type 1-matrix metalloproteinase in fibrotic human livers. Hepatology. 1997;26:1521–9. doi: 10.1002/hep.510260620. [DOI] [PubMed] [Google Scholar]
- 136.Burggraf D, Martens HK, Dichgans M, et al. Matrix metalloproteinase (MMP) induction and inhibition at different doses of recombinant tissue plasminogen activator following experimental stroke. Thromb Haemost. 2007;98:963–9. doi: 10.1160/th07-03-0194. [DOI] [PubMed] [Google Scholar]
- 137.Okazaki I, Maruyama K. Collagenase activity in experimental hepatic fibrosis. Nature. 1974;252:49–50. doi: 10.1038/252049a0. [DOI] [PubMed] [Google Scholar]
- 138.Giannelli G, Bergamini C, Marinosci F, et al. Antifibrogenic effect of IFN-alpha2b on hepatic stellate cell activation by human hepatocytes. J Interferon Cytokine Res. 2006;26:301–8. doi: 10.1089/jir.2006.26.301. [DOI] [PubMed] [Google Scholar]
- 139.Iredale JP. Tissue inhibitors of metalloproteinases in liver fibrosis. Int J Biochem Cell Biol. 1997;29:43–54. doi: 10.1016/s1357-2725(96)00118-5. [DOI] [PubMed] [Google Scholar]
- 140.Benyon RC, Iredale JP, Goddard S, et al. Expression of tissue inhibitor of metalloproteinases 1 and 2 is increased in fibrotic human liver. Gastroenterology. 1996;110:821–31. doi: 10.1053/gast.1996.v110.pm8608892. [DOI] [PubMed] [Google Scholar]
- 141.Roeb E, Graeve L, Hoffmann R, et al. Regulation of tissue inhibitor of metalloproteinases-1 gene expression by cytokines and dexamethasone in rat hepatocyte primary cultures. Hepatology. 1993;18:1437–42. [PubMed] [Google Scholar]
- 142.Nie QH, Zhang YF, Xie YM, et al. Correlation between TIMP-1 expression and liver fibrosis in two rat liver fibrosis models. World J Gastroenterol. 2006;12:3044–9. doi: 10.3748/wjg.v12.i19.3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hsieh CS, Chuang JH, Huang CC, et al. Evaluation of matrix metalloproteinases and their endogenous tissue inhibitors in biliary atresia-associated liver fibrosis. J Pediatr Surg. 2005;40:1568–73. doi: 10.1016/j.jpedsurg.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 144.Herbst H, Wege T, Milani S, et al. Tissue inhibitor of metalloproteinase-1 and -2 RNA expression in rat and human liver fibrosis. Am J Pathol. 1997;150:1647–59. [PMC free article] [PubMed] [Google Scholar]
- 145.Werb Z, Banda MJ, Jones PA. Degradation of connective tissue matrices by macrophages. I. Proteolysis of elastin, glycoproteins, and collagen by proteinases isolated from macrophages. J Exp Med. 1980;152:1340–57. doi: 10.1084/jem.152.5.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.He CS, Wilhelm SM, Pentland AP, et al. Tissue cooperation in a proteolytic cascade activating human interstitial collagenase. Proc Natl Acad Sci U S A. 1989;86:2632–6. doi: 10.1073/pnas.86.8.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Murphy G, Stanton H, Cowell S, et al. Mechanisms for pro matrix metalloproteinase activation. APMIS. 1999;107:38–44. doi: 10.1111/j.1699-0463.1999.tb01524.x. [DOI] [PubMed] [Google Scholar]
- 148.Zhang Z, Shen HM, Zhang QF, et al. Critical role of GSH in silica-induced oxidative stress, cytotoxicity, and genotoxicity in alveolar macrophages. Am J Physiol. 1999;277:L743–8. doi: 10.1152/ajplung.1999.277.4.L743. [DOI] [PubMed] [Google Scholar]
- 149.Eitzman DT, Krauss JC, Shen T, Ginsburg, et al. Lack of plasminogen activator inhibitor-1 effect in a transgenic mouse model of metastatic melanoma. Blood. 1996;87:4718–22. [PubMed] [Google Scholar]
- 150.Vayalil PK, Olman M, Murphy-Ullrich JE, et al. Glutathione restores collagen degradation in TGF-beta-treated fibroblasts by blocking plasminogen activator inhibitor-1 expression and activating plasminogen. Am J Physiol Lung Cell Mol Physiol. 2005;289:L937–45. doi: 10.1152/ajplung.00150.2005. [DOI] [PubMed] [Google Scholar]
- 151.Ferroni P, Guagnano MT, Manigrasso MR, et al. Increased plasminogen activator inhibitor-1 levels in android obesity: correlation with oxidative stress. J Thromb Haemost. 2005;3:1086–7. doi: 10.1111/j.1538-7836.2005.01395.x. [DOI] [PubMed] [Google Scholar]
- 152.Jiang Z, Seo JY, Ha H, et al. Reactive oxygen species mediate TGF-beta1-induced plasminogen activator inhibitor-1 upregulation in mesangial cells. Biochem Biophys Res Commun. 2003;309:961–6. doi: 10.1016/j.bbrc.2003.08.102. [DOI] [PubMed] [Google Scholar]
- 153.Lee EA, Seo JY, Jiang Z, et al. Reactive oxygen species mediate high glucose-induced plasminogen activator inhibitor-1 up-regulation in mesangial cells and in diabetic kidney. Kidney Int. 2005;67:1762–71. doi: 10.1111/j.1523-1755.2005.00274.x. [DOI] [PubMed] [Google Scholar]
- 154.Swiatkowska M, Szemraj J, Al-Nedawi KN, et al. Reactive oxygen species upregulate expression of PAI-1 in endothelial cells. Cell Mol Biol Lett. 2002;7:1065–71. [PubMed] [Google Scholar]
- 155.Feder L, Todaro JA, Laskin DL. Characterization of interleukin-1 and interleukin-6 production by hepatic endothelial cells and macrophages. J Leukoc Biol. 1993;53:126–32. doi: 10.1002/jlb.53.2.126. [DOI] [PubMed] [Google Scholar]
- 156.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996;271:C1424–37. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 157.Ohhashi T, Mizuno R, Ikomi F, et al. Current topics of physiology and pharmacology in the lymphatic system. Pharmacol Ther. 2005;105:165–88. doi: 10.1016/j.pharmthera.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 158.van der Vliet A, Eiserich JP, Shigenaga MK, et al. Reactive nitrogen species and tyrosine nitration in the respiratory tract: epiphenomena or a pathobiologic mechanism of disease? Am J Respir Crit Care Med. 1999;160:1–9. doi: 10.1164/ajrccm.160.1.9807044. [DOI] [PubMed] [Google Scholar]
- 159.Beckman JS, Chen J, Ischiropoulos H, et al. Oxidative chemistry of peroxynitrite. Methods Enzymol. 1994;233:229–40. doi: 10.1016/s0076-6879(94)33026-3. [DOI] [PubMed] [Google Scholar]
- 160.Eiserich JP, Patel RP, O’Donnell VB. Pathophysiology of nitric oxide and related species: free radical reactions and modification of biomolecules. Mol Aspects Med. 1998;19:221–357. doi: 10.1016/s0098-2997(99)00002-3. [DOI] [PubMed] [Google Scholar]
- 161.Huie RE, Padmaja S. The reaction of no with superoxide. Free Radic Res Commun. 1993;18:195–9. doi: 10.3109/10715769309145868. [DOI] [PubMed] [Google Scholar]
- 162.Hecker M, Schott C, Bucher B, et al. Increase in serum NG-hydroxy-L-arginine in rats treated with bacterial lipopolysaccharide. Eur J Pharmacol. 1995;275:R1–3. doi: 10.1016/0014-2999(95)00046-n. [DOI] [PubMed] [Google Scholar]
- 163.Laude K, Thuillez C, Richard V. Peroxynitrite triggers a delayed resistance of coronary endothelial cells against ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2002;283:H1418–23. doi: 10.1152/ajpheart.00375.2002. [DOI] [PubMed] [Google Scholar]
- 164.Bolanos JP, Garcia-Nogales P, Almeida A. Provoking neuroprotection by peroxynitrite. Curr Pharm Des. 2004;10:867–77. doi: 10.2174/1381612043452910. [DOI] [PubMed] [Google Scholar]
- 165.De Keulenaer GW, Alexander RW, Ushio-Fukai M, et al. Tumour necrosis factor alpha activates a p22phox-based NADPH oxidase in vascular smooth muscle. Biochem J. 1998;329(Pt 3):653–7. doi: 10.1042/bj3290653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Clemens MG. Nitric oxide in liver injury. Hepatology. 1999;30:1–5. doi: 10.1002/hep.510300148. [DOI] [PubMed] [Google Scholar]
- 167.Pietrangelo A. Iron, oxidative stress and liver fibrogenesis. J Hepatol. 1998;28(Suppl 1):8–13. doi: 10.1016/s0168-8278(98)80368-1. [DOI] [PubMed] [Google Scholar]
- 168.Rockey DC, Chung JJ. Inducible nitric oxide synthase in rat hepatic lipocytes and the effect of nitric oxide on lipocyte contractility. J Clin Invest. 1995;95:1199–206. doi: 10.1172/JCI117769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wink DA, Mitchell JB. Chemical biology of nitric oxide: insights into regulatory, cytotoxic, and cytoprotective mechanisms of nitric oxide. Free Radic Biol Med. 1998;25:434–56. doi: 10.1016/s0891-5849(98)00092-6. [DOI] [PubMed] [Google Scholar]
- 170.Xu Y, Bialik S, Jones BE, et al. NF-kappaB inactivation converts a hepatocyte cell line TNF-alpha response from proliferation to apoptosis. Am J Physiol. 1998;275:C1058–66. doi: 10.1152/ajpcell.1998.275.4.C1058. [DOI] [PubMed] [Google Scholar]
- 171.Hur GM, Ryu YS, Yun HY, et al. Hepatic ischemia/reperfusion in rats induces iNOS gene transcription by activation of NF-kappaB. Biochem Biophys Res Commun. 1999;261:917–22. doi: 10.1006/bbrc.1999.1143. [DOI] [PubMed] [Google Scholar]
- 172.Smith KJ, Kapoor R, Felts PA. Demyelination: the role of reactive oxygen and nitrogen species. Brain Pathol. 1999;9:69–92. doi: 10.1111/j.1750-3639.1999.tb00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Uemura M, Tsujii T, Kikuchi E, et al. Increased plasma levels of substance P and disturbed water excretion in patients with liver cirrhosis. Scand J Gastroenterol. 1998;33:860–6. doi: 10.1080/00365529850171530. [DOI] [PubMed] [Google Scholar]
- 174.Carlucci F, Marinello E, Rosi F, et al. Nitric oxide generation is associated with an unbalance of protein tyrosine phosphatases during liver transplantation. Biomed Pharmacother. 2007;61:216–21. doi: 10.1016/j.biopha.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 175.Hanazawa T, Kharitonov SA, Barnes PJ. Increased nitrotyrosine in exhaled breath condensate of patients with asthma. Am J Respir Crit Care Med. 2000;162:1273–6. doi: 10.1164/ajrccm.162.4.9912064. [DOI] [PubMed] [Google Scholar]
- 176.Srisook K, Kim C, Cha YN. Cytotoxic and cytoprotective actions of O2- and NO (ONOO−) are determined both by cellular GSH level and HO activity in macrophages. Methods Enzymol. 2005;396:414–24. doi: 10.1016/S0076-6879(05)96035-7. [DOI] [PubMed] [Google Scholar]
- 177.Pesse B, Levrand S, Feihl F, et al. Peroxynitrite activates ERK via Raf-1 and MEK, independently from EGF receptor and p21Ras in H9C2 cardiomyocytes. J Mol Cell Cardiol. 2005;38:765–75. doi: 10.1016/j.yjmcc.2005.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Rippe RA, Umezawa A, Kimball JP, et al. Binding of upstream stimulatory factor to an E-box in the 3’-flanking region stimulates alpha1(I) collagen gene transcription. J Biol Chem. 1997;272:1753–60. doi: 10.1074/jbc.272.3.1753. [DOI] [PubMed] [Google Scholar]
- 179.Friedman SL. Liver fibrosis – from bench to bedside. J Hepatol. 2003;38(Suppl 1):S38–53. doi: 10.1016/s0168-8278(02)00429-4. [DOI] [PubMed] [Google Scholar]
- 180.Tan AS, Berridge MV. Superoxide produced by activated neutrophils efficiently reduces the tetrazolium salt, WST-1 to produce a soluble formazan: a simple colorimetric assay for measuring respiratory burst activation and for screening anti-inflammatory agents. J Immunol Methods. 2000;238:59–68. doi: 10.1016/s0022-1759(00)00156-3. [DOI] [PubMed] [Google Scholar]
- 181.Rockey D. The cellular pathogenesis of portal hypertension: stellate cell contractility, endothelin, and nitric oxide. Hepatology. 1997;25:2–5. doi: 10.1053/jhep.1997.v25.ajhep0250002. [DOI] [PubMed] [Google Scholar]
- 182.Colombato L. The role of transjugular intrahepatic portosystemic shunt (TIPS) in the management of portal hypertension. J Clin Gastroenterol. 2007;41:S344–51. doi: 10.1097/MCG.0b013e318157e500. [DOI] [PubMed] [Google Scholar]
- 183.Bataller R, Gines P, Nicolas JM, et al. Angiotensin II induces contraction and proliferation of human hepatic stellate cells. Gastroenterology. 2000;118:1149–56. doi: 10.1016/s0016-5085(00)70368-4. [DOI] [PubMed] [Google Scholar]
- 184.Thirunavukkarasu C, Watkins S, Harvey SA, et al. Superoxide-induced apoptosis of activated rat hepatic stellate cells. J Hepatol. 2004;41:567–75. doi: 10.1016/j.jhep.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 185.Parola M, Pinzani M, Casini A, et al. Stimulation of lipid peroxidation or 4-hydroxynonenal treatment increases procollagen alpha 1 (I) gene expression in human liver fat-storing cells. Biochem Biophys Res Commun. 1993;194:1044–50. doi: 10.1006/bbrc.1993.1927. [DOI] [PubMed] [Google Scholar]
- 186.Ferrini MG, Vernet D, Magee TR, et al. Antifibrotic role of inducible nitric oxide synthase. Nitric Oxide. 2002;6:283–94. doi: 10.1006/niox.2001.0421. [DOI] [PubMed] [Google Scholar]
- 187.Schwentker A, Vodovotz Y, Weller R, et al. Nitric oxide and wound repair: role of cytokines? Nitric Oxide. 2002;7:1–10. doi: 10.1016/s1089-8603(02)00002-2. [DOI] [PubMed] [Google Scholar]
- 188.Witte MH, Borgs P, Way DL, et al. Alcohol, hepatic sinusoidal microcirculation, and chronic liver disease. Alcohol. 1992;9:473–80. doi: 10.1016/0741-8329(92)90083-m. [DOI] [PubMed] [Google Scholar]
- 189.Chu AJ, Prasad JK. Up-regulation by human recombinant transforming growth factor beta-1 of collagen production in cultured dermal fibroblasts is mediated by the inhibition of nitric oxide signaling. J Am Coll Surg. 1999;188:271–80. doi: 10.1016/s1072-7515(98)00303-2. [DOI] [PubMed] [Google Scholar]
- 190.Kolpakov V, Gordon D, Kulik TJ. Nitric oxide-generating compounds inhibit total protein and collagen synthesis in cultured vascular smooth muscle cells. Circ Res. 1995;76:305–9. doi: 10.1161/01.res.76.2.305. [DOI] [PubMed] [Google Scholar]
- 191.Cao M, Westerhausen-Larson A, Niyibizi C, et al. Nitric oxide inhibits the synthesis of type-II collagen without altering Col2A1 mRNA abundance: prolyl hydroxylase as a possible target. Biochem J. 1997;324(Pt 1):305–10. doi: 10.1042/bj3240305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Genovese T, Cuzzocrea S, Di Paola R, et al. Inhibition or knock out of inducible nitric oxide synthase result in resistance to bleomycin-induced lung injury. Respir Res. 2005;6(58):1–17. doi: 10.1186/1465-9921-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Thornton FJ, Schaffer MR, Witte MB, et al. Enhanced collagen accumulation following direct transfection of the inducible nitric oxide synthase gene in cutaneous wounds. Biochem Biophys Res Commun. 1998;246:654–9. doi: 10.1006/bbrc.1998.8681. [DOI] [PubMed] [Google Scholar]
- 194.Trachtman H, Futterweit S, Garg P, et al. Nitric oxide stimulates the activity of a 72-kDa neutral matrix metalloproteinase in cultured rat mesangial cells. Biochem Biophys Res Commun. 1996;218:704–8. doi: 10.1006/bbrc.1996.0125. [DOI] [PubMed] [Google Scholar]
- 195.Rai RM, Lee FY, Rosen A, et al. Impaired liver regeneration in inducible nitric oxide synthasedeficient mice. Proc Natl Acad Sci U S A. 1998;95:13829–34. doi: 10.1073/pnas.95.23.13829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Valente EG, Vernet D, Ferrini MG, et al. L-arginine and phosphodiesterase (PDE) inhibitors counteract fibrosis in the Peyronie’s fibrotic plaque and related fibroblast cultures. Nitric Oxide. 2003;9:229–44. doi: 10.1016/j.niox.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 197.Urtasun R, Cubero FJ, Vera M, et al. Reactive nitrogen species switch on early extracellular matrix remodeling via induction of MMP1 and TNFα. Gastroenterology. doi: 10.1053/j.gastro.2008.12.065. in press. [DOI] [PubMed] [Google Scholar]


