Abstract
Purpose
Neurotensin (NT), a gut tridecapeptide, acts as a potent cellular mitogen for various colorectal and pancreatic cancers which possess high-affinity NT receptors (NTR). Cytokine/chemokine proteins are increasingly recognized as important local factors which play a role in the metastasis and invasion of multiple cancers. The purpose of this study was to: (i) determine the effect of NT on cytokine/chemokine gene expression and cell migration in human cancer cells and, (ii) assess the effect of curcumin, a natural dietary product, on NT-mediated processes.
Experimental Design
The human colorectal cancer, HCT116, was treated with NT, with or without curcumin, and IL-8 expression and protein secretion was measured. Signaling pathways, which contribute to the effects of NT, were assessed. Finally, the effect of curcumin on NT-mediated HCT116 cell migration was analyzed.
Results
We show that NT, acting through the native high-affinity NTR, induced IL-8 expression in human colorectal cancer cells in a time- and dose-dependent fashion. This stimulation involves Ca2+-dependent PKC, ERK-dependent AP-1 and ERK-independent NF-κB pathways. Curcumin inhibited NT-mediated AP-1 and NF-κB activation and Ca2+ mobilization. Moreover curcumin blocked NT-stimulated IL-8 gene induction and protein secretion and, at a low concentration (ie, 10 μM), blocked NT-stimulated colon cancer cell migration.
Conclusions
NT-mediated induction of tumor cell IL-8 expression and secretion may contribute to the procarcinogenic effects of NT on GI cancers. Furthermore a potential mechanism for the chemopreventive and chemotherapeutic effects of curcumin on colon cancers may be through the inhibition of GI hormone (eg, NT)-induced chemokine expression and cell migration.
Keywords: Neurotensin, IL-8, Curcumin, Signal transduction, GI cancers
INTRODUCTION
The endocrine control of cancer growth is well-established, predominantly in breast and prostate cancers, where hormonal or anti-hormonal therapy represents a mainstay of treatment (1). In a manner analogous to breast and prostate cancers, certain gastrointestinal (GI) and pancreatic cancers possess native receptors for GI hormones; growth of these receptor-positive cancers can be altered by the specific peptide analog or the receptor antagonist (2, 3). GI hormones, which are released from specialized endocrine cells in the gut and pancreas by ingestion of nutrients, can affect tumor growth in an endocrine, paracrine and/or autocrine fashion (2, 3).
Our laboratory is focused on the mechanisms and downstream effector proteins which regulate proliferation of colorectal and pancreatic cancers by the GI hormone neurotensin (NT). NT, a tridecapeptide predominantly found in the distal small bowel (4), is potently released by ingestion of fats (5). Physiologic functions of NT include stimulation of GI motility and secretion (4) and stimulation of normal intestinal cell growth (2, 6). In addition, NT is known to stimulate proliferation of human colorectal and pancreatic cancers which possess high-affinity NT receptors (NTR) (7, 8). The high-affinity NTR (designated NTR1), a member of the G-protein coupled receptor (GPCR) family, is present in a majority of human pancreatic and colorectal cancers (7, 9, 10), suggesting that NT may act in an endocrine fashion to affect tumor growth. Acting through the NTR1, NT is known to stimulate various signal transduction pathways, including intracellular calcium ([Ca2+]i), the mitogen-activated protein kinases (MAPKs), ERK and JNK, and various PKC isoforms (9, 11, 12). Ultimately, this stimulation results in the activation of various transcription factors, which can alter the expression of a number of tumor-promoting genes (9, 12-14).
A plethora of growth factors, secreted either locally or by the cancer cells, may affect various aspects of tumor progression. Increasingly, an important role for cytokines/chemokines in tumor growth and metastasis is being recognized (15). Chemokines and their receptors have recently been shown to act at all stages of tumor development and progression including the neoplastic transformation of cells, the promotion of aberrant angiogenesis and clonal expansion and growth (16). In particular, interleukin-8 (IL-8 or CXCL8), an inflammatory component originally identified as a chemotactic factor for leukocytes (17), has been shown to affect cancer progression through mitogenic, angiogenic and motogenic effects (18). The expression of IL-8 and its receptors has been noted in a variety of human cancers including colorectal and pancreatic cancers (19). In fact, some studies suggest that IL-8 may act as an autocrine and/or paracrine growth factor in human colorectal and pancreatic cancers (20) and that expression correlates with the aggressiveness and metastatic potential of these cancers (21). It has been reported that NT induces the expression of MIP-2, MCP-1, IL-1β and TNFα in murine microglial cells and stimulates IL-8 secretion in a non-transformed colon epithelial cell line stably transfected with the NTR (13); however, to our knowledge, the effect of NT on cytokine/chemokine gene expression has not been analyzed in human cancer cells with native NTR.
Curcumin, a naturally occurring polyphenolic pigment isolated from the rhizomes of the plant Curcuma longa, is commonly used as a coloring and flavoring agent in food products. Current studies are assessing the role of curcumin as a chemopreventive and/or chemotherapeutic agent for certain cancers (22). Curcumin has been shown to improve cholecystokinin (CCK)-induced pancreatitis and inhibit CCK-induced IL-8, TNFα and chemokine KC expression in a rat pancreatitis model (23); the effect of curcumin on cytokine/chemokine gene regulation by NT and other GI hormones has not been investigated in GI cancer cells. In this study, we tested the effects of the GI hormone NT, which is released by dietary fat, and curcumin, a natural product of dietary origin, on cytokine/chemokine gene regulation in human colon cancer cells. Importantly, we found that NT selectively stimulated IL-8 gene expression and protein secretion in human colon cancer cells with native, high-affinity NTR; curcumin inhibited NT-induced IL-8 production and migration of HCT116 human colon cancer cells.
MATERIALS AND METHODS
Materials
NT and curcumin were purchased from Sigma Chemical Co. (St. Louis, MO). A23187 and ionomycin were from Alexis Biochemicals (San Diego, CA). [α-32P] UTP (3000 Ci/mmol) was from Amersham Pharmacia Biotech (Piscataway, NY). U0126 was from Promega (Madison, WI). Adenovirus vector encoding hemagglutinin-tagged IκB-α super-repressor (Ad5IκB-AA) and its control vector (Ad5GFP) were gifts from Dr. Christian Jobin (University of North Carolina, Chapel Hill, NC). The NTR antagonists, SR48692 and SR142948A, were generous gifts from Dr. Danielle Gully (Sanofi Recherche, Toulouse, France). All other reagents were from Calbiochem (San Diego, CA).
Cell culture
Human colon cancer cell line (HCT116 and HT29) and the human pancreatic cancer cell line (MIA PaCa-2) were from American Type Culture Collection (Manassas, VA). MIA PaCa-2 cells were incubated in Dulbecco’s modified Eagle’s medium with 10% fetal bovine serum (FBS), HCT116 and HT29 were maintained in McCoy’s 5A supplemented with 10% FBS. The human colon cancer cell line KM20 was obtained from Dr. Isaiah Fidler (M.D. Anderson Cancer Center, Houston, TX), and grown in minimum Eagle’s medium supplemented with 1% sodium pyruvate and 10% FBS.
RNA isolation and RNase protection assay
RNA was isolated from cultured cells using Ultraspec RNA reagent (Biotecx Laboratories, Houston, TX) according to the manufacturer’s protocol. A 32P-UTP labeled antisense RNA probe was prepared using the hCK-5 multi-probe template set (BD PharMingen, San Diego CA) and MAXI-Script SP6/T7 in vitro transcription kit (Ambion, Austin, TX). RNase protection assays were performed using the RPA III Ribonuclease Protection kit (Ambion, Austin, TX) according to the manufacturer’s recommendations and as we have previously described (24). Finally, samples were analyzed by electrophoresis on 5% denaturing polyacrylamide gel and detected by autoradiography.
IL-8 measurement
The concentration of IL-8 from conditioned media was determined using a Human IL-8 ELISA Kit (Pierce Biotechnology, Rockford, IL). Results were expressed as mean ± S.D. (pg/mL). At least three independent experiments were performed for each experimental condition, each with triplicate measurements.
Ca2+ ratio imaging
Real-time recording of [Ca2+]i was performed in single cells as we have previously described (9). In brief, cells grown on glass coverslips (Carolina Biological, Burlington, NC) were washed with a physiological medium (KRH) then loaded with 2 μM fura-2 AM for 50 min at 25°C to minimize dye compartmentalization. Loaded cells were washed three times with KRH and incubated for 60 min at 25°C in the dark with KRH 0.1%BSA. Loaded cells attached to coverslips were mounted on a Leiden Cover Slip Dish and placed in an Open Perfusion Micro-Incubator (Medical Systems Corp., NY) covered with 3 ml KRH with 0.1% BSA. The Ca2+ variations at the single-cell level were monitored using a Nikon Diaphot inverted microscope (Garden City, NY), equipped with a Nikon 40× (1.3 N.A.) oil immersion objective, coupled to a dual monochrometer system via a fiberoptic cable (Photon Technology International [PTI]), South Brunswick, NJ). Fura-2 intracellular fluorescence was measured at an emission wavelength of 510 nm by alternating the excitation wavelength between 340 and 380 nm. Full ratio images were obtained at 1 image per 1.5 seconds. Images were processed using ImageMaster software (PTI).
Preparation of nuclear extracts and electrophoretic mobility shift assay (EMSAs)
The nuclear extracts were prepared from HCT116 cells using NE-pER Nuclear and Cytoplasmic Extraction Reagents (PIERCE, Rockford, IL) according to the manufacturer’s protocol. EMSAs were performed as described previously (25) with minor modifications. Nuclear extracts (10 μg) were incubated with a 32P-labeled oligonucleotide (4 × 104 cpm) containing consensus AP-1 or NF-κB binding sites (Promega, Madison, WI) and 2 μg of poly (dA·dT) in a buffer containing 10% glycerol, 100 mM KCl, 5 mM MgCl, 12.5 mM HEPES (pH 7.9), 1 mM EDTA and 1 mM dithiothreitol in a final volume of 20 μl, for 15 min at room temperature. For supershift studies, 2 μl of antiserum (Santa Cruz Biotechnology, Santa Cruz, CA) was added to the reaction mixture 1 h prior to the addition of labeled probe. The reaction mixture was fractionated on 6% nondenaturing polyacrylamide gels.
Cell migration assay
The Costar Transwell System (8-μm pore size polycarbonate membrane, 6.5-mm diameter, Corning Inc.) was used to evaluate cell migration. Both sides of each Transwell membrane were coated with 15 μg/ml collagen (Cohesion Technology) by immersion for 30 min at 37°C. Cells (50,000 in 100 μl serum-free medium) were added to the upper well, and 500 μl serum-free medium was added to the lower chamber. NT, curcumin and vehicle were added to the lower chamber. At the end of the 16 h incubation at 37°C/5%CO2, cells on the top of the membrane were removed by swiping with a damp cotton swab, and cells that had migrated to the lower surface were fixed in methanol for 15 min at room temperature and stained with 1% crystal violet. The migration activity was quantified by counting the migrated cells on the lower surface of the membrane of at least 7 fields per chamber using a 10x objective.
Statistical analysis
Data that were from experiments with only two treatment groups (eg, NT only and the control) were analyzed using the two-sample t test. Due to heterogeneous variability among treatment groups, data from experiments with more than two treatments were transformed using logarithm to the base 10 for the data analysis purpose. Then the logarithm transformed data was analyzed using one-way classification analysis of variance. All tests were assessed at the 0.05 level of significance. The Fisher’s least significant difference procedure was used for multiple comparisons with Bonferroni adjustment for the number of comparisons. All statistical computations were conducted using the SAS® system, Release 9.1.
RESULTS
NT stimulates IL-8 gene expression and protein secretion in HCT116 colon cancer cells
To determine the potential effect of NT on cytokine/chemokine expression in cancer cells, HCT116 human colon cancer cells, which possess the high-affinity NTR (NTR1), were treated with NT (100 nM) over a time course. RNA was extracted for RPA using a multi-probe (hCK-5, BD Pharmingen) containing cDNAs for a number of cytokine/chemokine genes (Ltn, RANTES, IP-10, MIP-1β, MIP-1α, MCP-1, IL-8 and I-309); the constitutively-expressed genes, L32 and GAPDH, are included as controls for RNA loading equality (Fig. 1). NT increased IL-8 gene expression by 1 h with maximal stimulation occurring at 2 h after treatment; IL-8 expression returned to control levels by 24 h (Fig.1A, left panel). We next determined the range of concentrations of NT which affect IL-8 expression at 2 h after treatment. Induction of IL-8 was noted with 12.5 nM NT; maximal induction occurred at dosages of 25 to 200 nM (Fig.1B, right panel). The induction of IL-8 mRNA expression was also noted in the colon cancer cells, HT29, KM20, and the pancreatic cancer cell line MIA PaCa-2, all of which possess the high-affinity NTR, thus indicating that NT-mediated IL-8 induction is not limited to HCT116 cells (data not shown).
Figure 1. NT stimulates IL-8 mRNA and protein in HCT116.
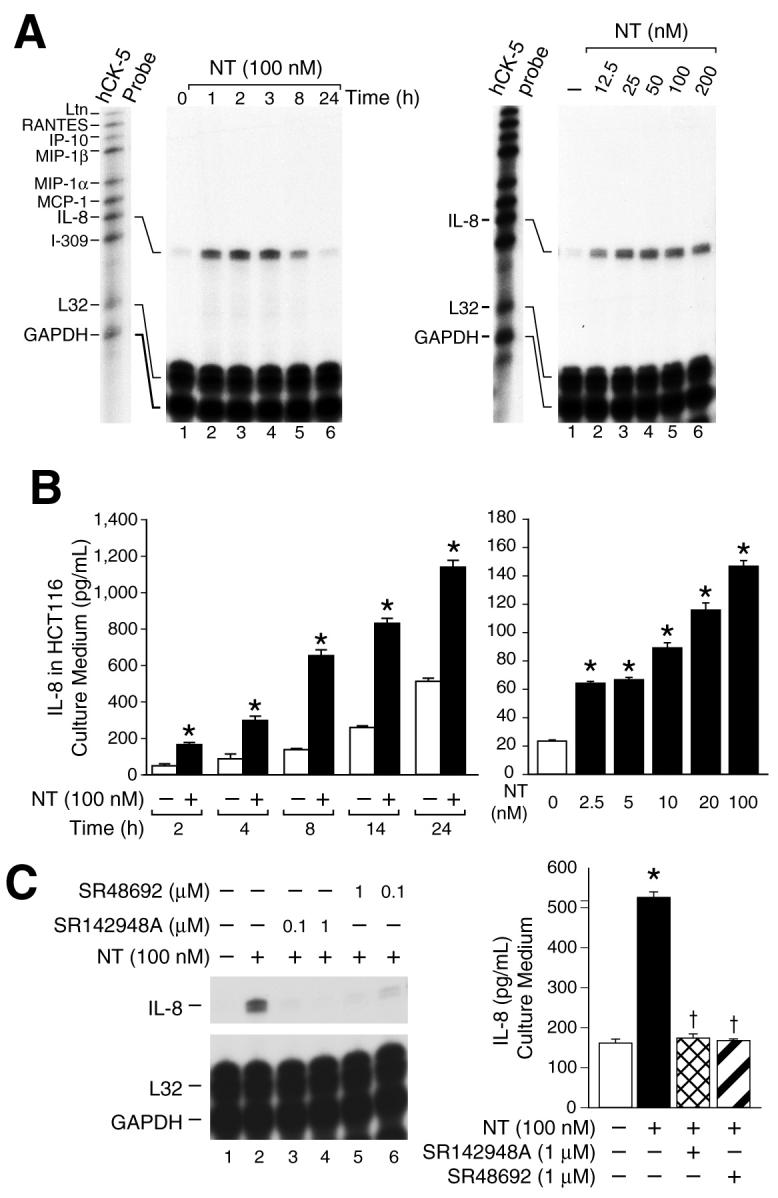
A. HCT116 human colon cancer cells were incubated in McCoy’s 5A media without FBS for 24 h, then treated with NT (100 nM) or vehicle (PBS) over a time course; total RNA was isolated and analyzed by RNase protection assay (RPA) using a 32P-labeled multi-probe template set (hCK-5, BD Pharmingen) (left panel). HCT116 cells were treated with various concentrations of NT or vehicle for 2 h; RNA was isolated and analyzed by RPA using the hCK-5 multi-probe (right panel). B. HCT116 cells were seeded in plates with McCoy’s 5A media and FBS. One day later, media was changed to McCoy’s 5A media without FBS for 24 h and then treated with NT (100 nM) or vehicle for the indicated time points (left panel) or various concentrations of NT for 8 h (* = p < 0.05 vs control at each time point) (right panel). IL-8 protein (pg/ml) released from HCT116 cells into the culture medium was assessed by ELISA (Pierce Biotechnology) (* = p < 0.05 vs control). C. HCT116 cells were pretreated with the NTR inhibitors SR48692 or SR142948A at the indicated concentrations for 45 min, and then treated with NT (100 nM) for 2 h; RNA was isolated and analyzed by RPA using the multi-probe hCK-5 (left panel). HCT116 cells were seeded in plates, using McCoy’s 5A media with FBS. One days later, the media was changed to McCoy’s 5A media without FBS for 24 h, pretreated with of SR48692 (1 μM) or SR142948A (1 μM) for 45 min, and then treated with NT (100 nM) for 8 h; IL-8 secretion was measured in the media (right panel). (Results are shown as mean ± SD and are representative of three separate experiments; * = p < 0.05 vs control, † = p < 0.05 vs NT only).
IL-8 functions as a paracrine and/or autocrine growth factor in a number of cancer cells; the secretion of IL-8 protein from cancer cells is a key step for these effects (20). To determine whether the NT-stimulated induction of IL-8 mRNA expression was accompanied by an increase in secretion of IL-8 protein into the culture medium, IL-8 protein levels were measured by ELISA after NT treatment. As shown in Fig.1B, (left panel), NT (100 nM) significantly increased IL-8 protein levels in HCT116 cell medium over a time course with maximal increases noted at 8 h after treatment compared with vehicle treatment (control). Next, HCT116 cells were treated with various concentrations of NT and IL-8 protein levels quantitated in the medium. A concentration of 2.5 nM produced a 2-fold increase in IL-8 secretion; a concentration of 100 nM resulted in a 6-fold increase in IL-8 protein secretion into the media (Fig 1B, right panel).
To determine the specific effect of NT, we tested whether the effects of NT on IL-8 expression are mediated through the native, high-affinity NTR. HCT116 cells were pretreated with SR48692, a selective non-peptide NTR1 antagonist which binds with high affinity to NTR1 and has proven to be extremely useful for delineating the functions of NTR1 (26), or SR142948A, another selective non-peptide NTR1 antagonist, and then treated with NT (100 nM). Both NTR antagonists blocked NT-mediated induction of IL-8 mRNA (Fig.1C, left panel). Furthermore, both SR48692 and SR142948A blocked NT-mediated stimulation of IL-8 protein secretion into the culture medium Fig. 1C, right panel). These findings suggest that the cellular effects of NT on IL-8 induction are mediated through the native high-affinity NTR.
Curcumin inhibits IL-8 stimulation by NT
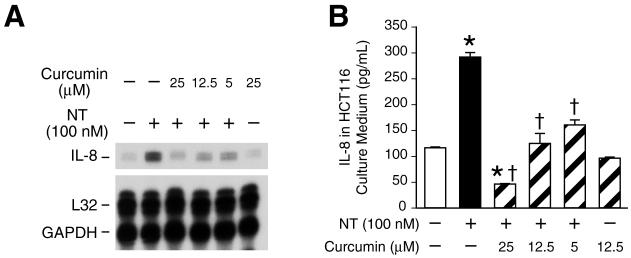
Curcumin, a therapeutic phytochemical of dietary and medicinal origin, has been shown to possess anti-inflammatory and cancer chemotherapy effects. Therefore, we postulated that curcumin may inhibit the effects of NT on IL-8 expression and secretion. We found that the pretreatment of curcumin inhibited NT-induced IL-8 mRNA expression in a dose-dependent fashion (Fig. 2A). In contrast, neither caffeic acid phenethyl ester (CAPE; another naturally occurring product), MG132, PDTC nor sulfasalazine affected NT-mediated IL-8 gene expression (data not shown). Furthermore, curcumin inhibited NT-induced IL-8 protein secretion in a dose-dependent fashion (Fig. 2B). The inhibitory effects of curcumin on IL-8 protein secretion were more pronounced than for IL-8 mRNA expression, thus suggesting the possibility that additional mechanisms are involved in the inhibition of IL-8 secretion by curcumin.
Figure 2. Curcumin inhibits NT-mediated IL-8 mRNA and secretion.
A. HCT116 cells were pretreated with curcumin at the indicated concentrations for 25 min, and then treated with NT (100 nM) for 2 h. RNA was isolated and analyzed by RPA using the hCK-5 multi-probe. B. HCT116 cells were seeded in plates in McCoy’s 5A media with FBS. One days later, the media was changed to serum free for 24 h, pretreated with curcumin at the indicated concentrations for 30 min, and then treated with NT (100 nM) for 8 h. IL-8 secretion was measured in the conditioned media by ELISA. (Results are shown as mean ± SD and representative of three separate experiments; * = p < 0.05 vs control, † = p < 0.05 vs NT only).
The effect of NT is dependent on [Ca2+]i stimulation and PKC activation
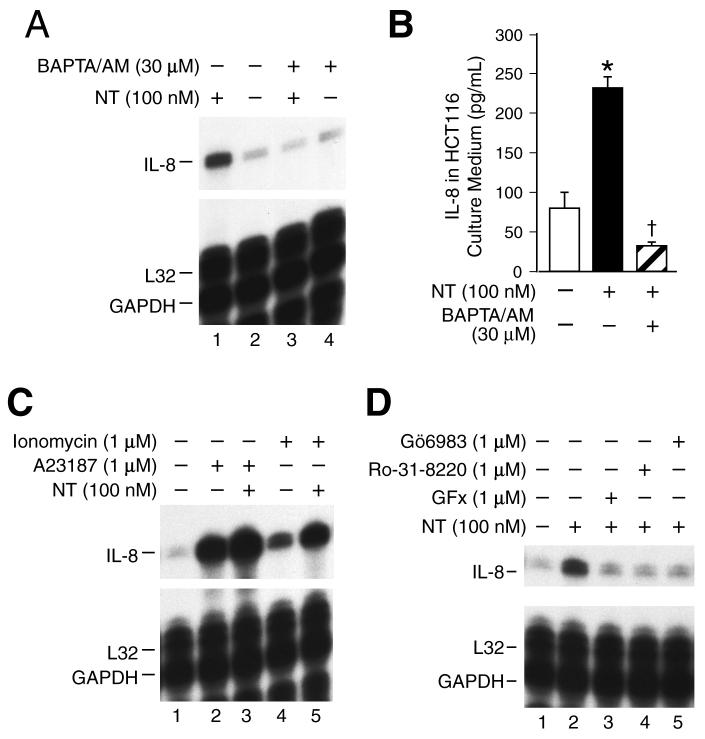
NT can activate the [Ca2+]i and PKC signaling pathways in cells with native NTR (11, 12); these pathways are likewise implicated in IL-8 gene regulation (27). To better delineate the signaling pathways acting downstream of NTR to induce IL-8 gene expression, we first determined the role of [Ca2+]i on the effects of NT. Pretreatment of HCT116 cells with the cell permeable Ca2+ chelator, BAPTA-AM (30 μM), completely blocked NT-induced IL-8 expression (Fig. 3A) and protein secretion (Fig. 3B). In addition, treatment with Ca2+ ionophores, ionomycin and A23187, which increase [Ca2+]i, significantly enhanced IL-8 gene induction in combination with NT compared with NT treatment alone (Fig. 3C). Also, either ionomycin or A23187 alone increased IL-8 mRNA expression compared with vehicle treatment.
Figure 3. The effect of NT is dependent on [Ca2+]i stimulation and PKC activation.
A. HCT116 cells were pretreated with BAPTA/AM (30 μM) for 20 min, and then treated with of NT (100 nM) for 2 h; RNA was extracted and analyzed by RPA using the hCK-5 multi-probe. B. HCT116 cells were seeded in plates in McCoy’s 5A medium with FBS. Two days later, the media was changed to serum free for 24 h, pretreated with BAPTA/AM (30 μM) for 20 min, and then treated with NT (100 nM) for 6 h. The conditioned media was collected and IL-8 secretion was measured by ELISA. (Results are shown as mean ± SD and representative of three separate experiments; * = p < 0.05 vs control, † = p < 0.05 vs NT only). C and D. HCT116 cells were pretreated with 1 μM of ionomycin, A23187, Gö6983, Ro-31-8220 or GFx for 20 min, and then treated with NT (100 nM) for 2 h. RNA was extracted and analyzed by RPA using the hCK-5 multi-probe.
The conventional PKCs, which include PKCα, βI, βII and γ are dependent on Ca2+ signaling. To examine the PKC isoforms contributing to IL-8 induction by NT, HCT116 cells were pretreated with PKC isoform-selective inhibitors. GFx (which inhibits PKCα, βI, βII, γ, δ and ζ), Ro-31-8220 (which inhibits PKCα, βI, βII, γ, and ε), and Gö6983 (which inhibits PKCα, β, γ, δ and ζ) blocked NT-induced IL-8 mRNA expression (Fig.3D). Similar to the inhibition of IL-8 expression, pretreatment with GFx and Gö6983 completely blocked NT-mediated IL-8 secretion; however, rottlerin, which is considered a relatively selective PKCδ inhibitor, exhibited no inhibitory effects on NT-mediated IL-8 stimulation (data not shown). Collectively, these results suggest that Ca2+-dependent PKC isoforms contribute to IL-8 regulation by NT.
Involvement of NF-κB and the MEK/AP-1 pathway on NT-mediated IL-8 stimulation
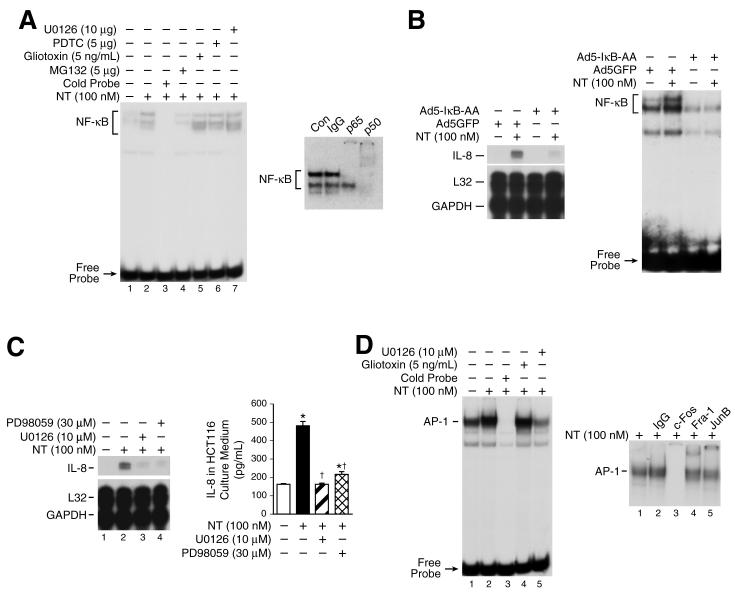
A well-described mechanism contributing to IL-8 gene induction is the activation of NF-κB which binds the IL-8 promoter to stimulate IL-8. Moreover, NT can increase NF-κB activation in certain cells (13). As shown in Fig. 4A, (left panel), we found that NT (100 nM) increased NF-κB binding activity; in contrast, NT had no effect on the DNA-binding activity of NFAT, a transcription factor that also binds the proximal IL-8 promoter (data not shown). The proteasome inhibitor MG132 inhibited NF-κB activation, whereas gliotoxin, PDTC or the MEK inhibitor, U0126, had no effect on NT-induced NF-κB binding activity indicating NT-mediated NF-κB induction is specific, and ERK-independent. Supershift analysis showed that the upper of the two retarded DNA-protein complexes was supershifted with either p65 or p50 antibodies and the lower band was shifted with the p50 antibody (Fig. 4A, right panel); addition of IgG (control) did not affect NT-mediated NF-κB activation.
Figure 4. NF-κB activation and the MEK/AP-1 pathway play a role in IL-8 regulation by NT.
A. HCT116 cells were pretreated with vehicle or U0126 (10 μM), PDTC (5 μM), Giotoxin (5 ng/mL), MG132 (5 μM) for 25 min, and then treated with NT (100 nM) for 30 min. Cells were extracted for nuclear protein and analyzed by EMSA using a 32P-labeled NF-κB probe as described in “Materials and Methods” (left panel). Nuclear protein (10 μg per lane) from HCT116 treated with NT (100 nM) were preincubated with specific antibodies (p65 and p50) or IgG prior to the addition of 32P-labeled NF-κB probe and then DNA-binding activity was assessed by EMSA (right panel). B. HCT116 cells were infected with adenovirus encoding the super-repressor of IκBα (Ad5IκB-AA) or the adenovirus control vector encoding GFP (Ad5GFP) for 1h, washed and replated in McCoy’s 5A without FBS for 24 h and then treated with NT (100 nM) for 2 h. RNA was isolated and analyzed by RPA using the hCK-5 multi-probe (left panel). To confirm inhibition of NF-κB activation, cells were extracted for nuclear protein and analyzed by EMSA using a 32P-labeled NF-κB probe as described in “Materials and Methods” (right panel). C. HCT116 cells were pretreated with the MEK/ERK inhibitors PD98059 (30 μM) or U0126 (10 μM) for 20 min, and then treated with NT (100 nM) for 2 h; RNA was extracted and analyzed by RPA using the hCK-5 multi-probe(left panel). HCT116 cells were seeded in plates in McCoy’s 5A media with FBS. Twenty-four h later, media was changed to serum free and cells pretreated with PD98059 (30 μM) or U0126 (10 μM) at the indicated concentrations for 25 min, then treated with NT (100 nM) for 8 h. IL-8 secretion was measured in the conditioned media by ELISA(right panel). (Results are shown as mean ± SD and representative of three separate experiments; * = p < 0.05 vs control, † = p < 0.05 vs NT only). D. HCT116 cells were pretreated with U0126 (1 μM) or gliotoxin (5 ng/ml) for 25 min and then treated with NT (100 nM) for 30 min. Cells were extracted for nuclear protein and analyzed by EMSA using a 32P-labeled AP-1 probe as described in “Materials and Methods”(left panel). Nuclear extracts (10 μg per lane) from HCT116 cells were preincubated with specific antibodies (c-Fos, Fra-1 or JunB) prior to the addition of 32P-labeled AP-1 probe and then DNA-binding activity was assessed by EMSA(right panel).
To determine whether NT-mediated NF-κB induction is important for IL-8 gene regulation, HCT116 cells were infected with adenovirus encoding the super-repressor of IκBα (IκB-AA) and the adenoviral control vector encoding GFP (28). HCT116 cells were then treated with NT (100 nM) or vehicle, and RNA extracted for RNase protection assay. Overexpression of IκB-AA significantly, but not completely, reduced NT-mediated IL-8 mRNA expression compared with the control vector (Fig. 4B, left panel). EMSA analysis confirmed that overexpression of IκB-AA blocked NT-induced NF-κB activity and strongly inhibited basal NF-κB activity compared with the control vector (Fig. 4B, right panel). These data suggest that NF-κB-dependent and NF-κB-independent pathways are involved in the regulation of IL-8 gene expression by NT.
Activation of MEK/ERK by NT and downstream induction of AP-1 transcription factors contributes to the proliferative effects of NT (9, 12). In addition, the MEK/AP-1 pathway has been shown to contribute to IL-8 gene regulation (29). To determine the role of ERK1/2 on NT-mediated IL-8 stimulation, HCT116 cells were pretreated with the MEK inhibitors PD98059 (30 μM) or U0126 (10 μM) and then treated with NT (100 nM). Treatment with both U0126 and PD98059, at concentrations which block ERK activation in HCT116 cells (30), inhibited NT-induced IL-8 mRNA expression (Fig. 4C, left panel) and protein secretion (Fig.4C, right panel), indicating that the MEK/ERK pathway is involved in IL-8 regulation by NT. It is interesting to note that neither U0126 nor PD98059 completely blocked NT-induced IL-8 mRNA indicating ERK-dependent and ERK-independent regulation (such as NF-κB) of the IL-8 gene by NT in HCT116 cells. In contrast, neither the p38 inhibitor SB203580 nor the JNK inhibitor SP600125 affected NT-mediated IL-8 gene induction (data not shown).
The AP-1 family of transcription factors can be activated by the upstream MEK/ERK pathway. c-Fos is a direct substrate of ERK in vitro and in vivo (31-33). Therefore, we speculated that one mechanism for NT-mediated MEK/ERK pathway regulation of IL-8 gene expression is through AP-1 transcription factor activation. We treated HCT116 cells with NT and determined AP-1 DNA-binding activity by EMSA. NT treatment for 30 min increased AP-1 DNA-binding activity; this increase was prevented by the ERK inhibitor U0126, suggesting NT-mediated AP-1 activation is regulated by the MEK/ERK pathway (Fig.4D, left panel). In contrast, gliotoxin had no effect on AP-1 binding activity. Supershift analyses were performed to delineate the AP-1 proteins in the binding complex. Preincubation with antibodies against JunB and Fra-1 resulted in supershifts of the DNA-protein band whereas c-Fos antibody completely blocked the retarded band indicating interaction of these proteins with the labeled AP-1 probe; in contrast, antibodies to FosB, c-Jun and Fra-2 did not result in either a supershifted band or diminution of the AP-1 complex (Fig. 4D, right panel). These results suggest that ERK-dependent AP-1 activation by NT may play a role in NT-mediated IL-8 regulation.
Curcumin inhibits NT-stimulated NF-κB and AP-1 activity and NT-activated Ca2+ mobilization
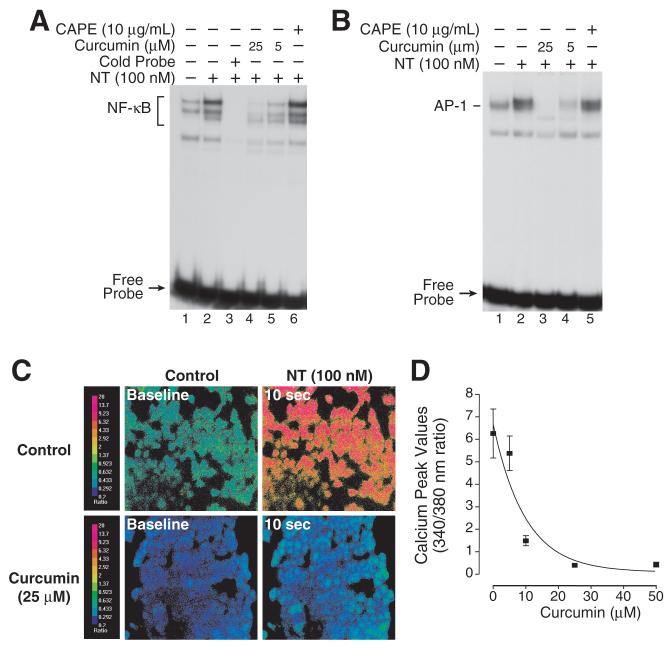
Curcumin inhibits both NF-κB and AP-1 activation in different cells types. We next determined the effect of curcumin on NT-stimulated AP-1 and NF-κB activation. Curcumin, at dosages of 5 and 25 μM, blocked NT-mediated NF-κB induction (Fig. 5A); however, CAPE, which has been reported to suppress NF-κB activation in certain cell lines, had no inhibitory effect on NT-mediated NF-κB induction in HCT116 cells. NT increases AP-1 DNA-binding activity, which involves c-Fos, JunB, and Fra-1 proteins. Hahm et al (34) reported that curcumin can inhibit the formation of the Fos-Jun DNA complex, thus suppressing AP-1 regulated gene expression. As shown in Fig. 5B, NT-mediated AP-1 activation was blocked by curcumin, but not CAPE, in HCT116 cells which suggests that the inhibition of AP-1 activation by curcumin is involved in IL-8 regulation. Taken together, NT-mediated ERK-dependent AP-1 activation and ERK-independent NF-κB activation were blocked by curcumin, but not by CAPE, gliotoxin, MG132 or PDTC in HCT116 cells. These data may explain the findings that NT-mediated IL-8 expression was inhibited by curcumin, but not by other agents.
Figure 5. Curcumin inhibits NT-mediated IL-8 stimulation.
A. HCT116 cells were pretreated with CAPE (10 μg/ml) or curcumin (either 5 or 25 μM) for 25 min and then treated with NT (100 nM) for 30 min. Cells were extracted for nuclear protein and analyzed by EMSA using a 32P-labeled NF-κB probe as described in “Materials and Methods”. B. HCT116 cells were pretreated with curcumin (either 5 or 25 μM) or CAPE (10 μg/mL) for 25 min and then treated with NT (100 nM) for 30 min. Cells were extracted for nuclear protein and analyzed by EMSA using a 32P-labeled AP-1 probe as described in “Materials and Methods”. C. Pseudo-color images of HCT116 cells loaded with the Ca2+ sensitive dye, Fura-2. The cells were pretreated with vehicle or curcumin (25 mM) for 2 min, and then treated with NT (100 nM). The red color indicates the highest level of intracellular calcium while the blue-green color represents baseline levels. D. Pretreatment with increasing concentrations of curcumin decreases the peak [Ca2+]i response to NT (100 nM). (Each data point represents the average 340/380 nm ratio from 40 individual cells ± SD).
The inhibitory effects of curcumin on IL-8 protein secretion were more pronounced than noted for IL-8 mRNA expression. We showed that IL-8 protein secretion was more sensitive to Ca2+ than IL-8 mRNA induction (Fig. 3A and 3B). A number of studies have shown that curcumin suppresses Ca2+ activation by a variety of stimuli (35, 36); therefore, we examined the effects of curcumin on Ca2+ signaling changes in HCT116 cells (Fig. 5C and 5D). Curcumin strongly inhibited NT-induced Ca2+ mobilization; a dosage of 10 μM almost completely blocked NT-induced Ca2+ activation and 25 μM of curcumin decreased basal levels of Ca2+ activity. Collectively, our findings indicate that curcumin inhibited NT-induced IL-8 production likely through the inhibition of AP-1 and NF-κB activity as well as NT-induced [Ca2+]i mobilization.
Curcumin blocks NT-dependent migration of HCT116 cells
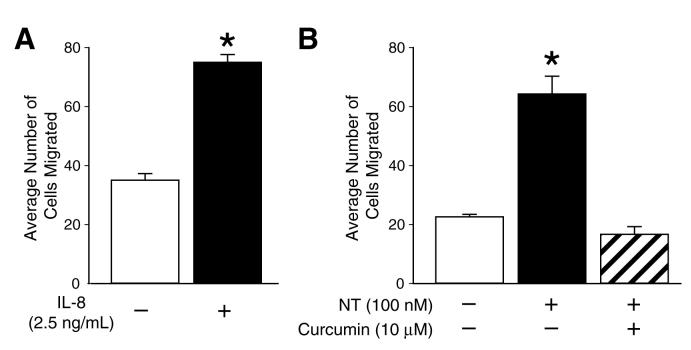
IL-8 belongs the CXC chemokine family, and its action is mediated by membrane receptors, CXCR-1 and-2. Expression of these receptors and an autocrine effect of IL-8 in HCT116 cells was reported by Brew et al (20). To address whether IL-8 plays a role in HCT116 cell migration, we treated HCT116 cells with recombinant IL-8 and migration assays were performed using collagen coated Costar Transwell membranes. Treatment with IL-8 (2.5 ng/mL) resulted in an approximate 2-fold increase in HCT116 cell migration indicating that IL-8 can increase the migration of HCT116 colon cancer cells (Fig. 6A). Our previous results showed that curcumin blocks NT-induced IL-8 expression and secretion (Fig. 2A and 2B). The inhibition of colon cancer cell growth by curcumin has been well described (37). Therefore, we next determined whether NT stimulates HCT116 cell migration and if curcumin could block this effect. Treatment of HCT116 cells with NT (100 nM) significantly increased HCT116 cell migration (∼ 3-fold) compared with vehicle treatment; pretreatment with curcumin (10 μM) blocked the stimulatory effect of NT on HCT116 cell migration (Fig.6B). These results suggest that curcumin suppresses NT-induced HCT116 cell migration, at least partially through the inhibition of IL-8 expression.
Figure 6. IL-8 and NT increase HCT116 cell migration.
HCT116 cells (5 ×104) were seeded in the upper well of the Costar Transwell System as described in “Materials and Methods”; vehicle, IL-8 (2.5 ng/mL) (A), NT (100 nM) or NT + curcumin (10 μM). (B) were added to the lower well. After 16 h incubation, the cells on the lower surface of the well were fixed in methanol and stained with 1% crystal violet. The average number of migrated cells (n = 3) was quantified by counting under a microscope in at least 7 different viewing fields at 10x magnification. (Results are shown as mean ± SD and representative of separate experiments; * = p < 0.05 vs control).
DISCUSSION
NT, an important intestinal hormone for multiple physiologic functions in the GI tract, stimulates proliferation of normal intestine and NTR positive colorectal and pancreatic cancers through mechanisms that are not entirely understood (2). In our present study, we show that NT selectively increases the expression and secretion of IL-8, a chemokine increasingly recognized as contributing to increased tumor invasion, progression and angiogenesis (16). These findings identify a potentially important mechanism contributing to enhanced tumor growth by NT through the stimulation of multiple signaling pathways leading ultimately to the downstream regulation of tumor-secreted proteins such as IL-8. In addition, we find that the natural product curcumin, which has been evaluated as an anti-inflammatory and anti-tumor agent, blocked NT-mediated IL-8 gene induction, protein secretion and cell migration.
The predominant proliferative effects of NT appear to be mediated through the high-affinity NTR1 which is present in a number of colorectal, pancreatic and prostate cancers. (38). We have used two non-peptide NTR antagonists to further demonstrate that the effects of NT are acting directly through the NTR1. Consistent with our findings, Zhao et al, (13) demonstrated increased IL-8 promoter activity and protein secretion by NT in nontransformed human colonocytes stably transfected with NTR1. Therefore, our study using cancer cells with native NTR1 and the previous studies utilizing an artificially-derived cell line (13), show that NT selectively regulates IL-8 activity through the NTR1. Since we (8) and others (7, 39) have shown that SR48692 is nontoxic and effective in vivo to block NT-mediated tumor growth, our findings have important implications in the possible development of novel therapeutic strategies for the treatment of human cancers based on NTR1 receptor blockade.
The signaling pathways and transcription factors regulating IL-8 expression have been described in different cell types in response to various stimuli. Similar to other physiological agents (eg, histamine, endothelin-1 and bradykinin), NT acts through Ca2+/PKC, ERK activation and the ubiquitous AP-1 and NF-κB transcription factors to induce IL-8 gene expression in HCT116 cells. Most studies have shown that NF-κB plays the predominant role in IL-8 regulation. We found that both ERK-dependent AP-1 and ERK-independent NF-κB activation was necessary for NT-mediated IL-8 mRNA and secretion in HCT116 cells. Consistent with our findings, AP-1 and NF-κB co-operatively regulate IL-8 expression in other cell types. For example, the activation of both AP-1 and NF-κB was essential for IL-8 induction in human breast cancer cells in response to glutamine deprivation (40), NF-κB-dependent AP-1 activity regulates VEGF expression (41). Furthermore, ERK-dependent AP-1 activation synergizes with p65/NF-κB to stimulate IL-8 gene transcription in human epidermal carcinoma cells. Our findings suggest that either receptor blockade, or alternatively, an agent that inhibits both AP-1 and NF-κB activation would be required to block the effects of NT on IL-8 gene induction and protein secretion.
Curcumin, a component of curry spice turmeric, possesses anti-inflammatory and anti-cancer effects through the inhibition of NF-κB and AP-1 activation. We found that curcumin effectively inhibited NT-induced AP-1 and NF-κB induction and subsequent IL-8 gene expression and protein secretion in HCT116 cells. Similar to our findings, Nakamura et al (42) found that curcumin decreased both AP-1 and NF-κB activation in prostate cancer cell lines. Moreover, curcumin markedly inhibited the activation of AP-1 and NF-κB DNA-binding induced by LPS, H2O2 or TNFα (43, 44). The inhibition of AP-1 and NF-κB by curcumin effectively suppressed IL-8 release in alveolar epithelial cells (43) which corroborates our findings in HCT116 cells. IL-8, regulated by AP-1 and NF-κB transcription factors in various cancer cells, is becoming increasingly recognized as an important local factor in tumorigenesis and metastasis. The results from our study and those of others (22, 45) suggest that an important mechanism for the anti-tumor effects of curcumin may be through the suppression of chemokines induced by GI hormone or other stimuli. Moreoever, Zhao et al (13) found that NT stimulates IL-8 secretion through NF-κB activation and plays a role in colonic inflammation, while numerous other studies showed that curcumin possesses anti-inflammatory effects through inhibition of NF-κB, and that colonic inflammation increases the risk for developing colorectal cancer (46). Our findings further suggest that curcumin may be useful for colon cancer treatment as well as potential colon cancer prevention. In fact, curcumin is currently being evaluated in clinical trials as a cancer chemotherapeutic and chemopreventive agent for colorectal cancers (47). Our findings provide additional evidence that curcumin may be beneficial in the suppression of certain chemokines (eg, IL-8) which play a role in tumor progression.
While a number of groups have confirmed that NT stimulates GI cancer cell growth and that curcumin suppresses the growth of various cancer types, few studies have analyzed the effect of NT or curcumin on colon cancer cell migration and/or metastasis. In our current study, we found that NT significantly increased HCT116 cells migration by ∼3 fold and that curcumin blocked NT-induced HCT116 cell migration at a concentration (10 μM) which does not cause cell death as demonstrated in our current experiments and by Kang et al (44). The increase in HCT116 migration by IL-8 treatment suggested that the effect of curcumin on NT-mediated HCT116 migration may be through the inhibition of IL-8. Numerous studies have shown that IL-8 can function as a motility factor for tumor cells, which is relevant to tumor invasion and metastasis. This concept was first shown in human melanoma cells (48), and subsequently demonstrated in human colon carcinoma cells (49). HCT116 migration was increased ∼2-fold by IL-8 and ∼3-fold by NT. We speculate that IL-8 is one of the motility factors in HCT116 cells which is stimulated by NT. In addition, NT has been shown to stimulate other pro-tumor factors, such as COX-2 (50), c-myc and matrix metalloproteinases (QD Wang, preliminary results) which may also contribute to the enhanced migration. Taken together, our current study has identified additional effects of NT which may enhance GI carcinogenesis. In addition to stimulating proliferation, NT can promote the induction and secretion of IL-8, a proinvasive factor and can increase tumor cell migration. The fact that curcumin, a natural product, can block these effects is appealing for future treatment strategies.
In summary, we show that NT, an intestinal hormone that is potently released by fat ingestion (4, 5), acts through its native NTR to stimulate Ca2+/PKC, ERK/AP-1 and NF-κB pathways and ultimately increases expression and secretion of IL-8 and enhances colon cancer cell migration. These effects were blocked by either NTR1 antagonists or curcumin, a diet-derived chemopreventive and/or chemotherapeutic agent which blocks AP-1 and NF-κB induction. In addition to NT, other GI hormones (eg, bombesin, gastrin, GRP and substance-P) have been reported to stimulate expression of various cytokines/chemokines, such as IL-1β, IL-4, IL-6, IL-8, IL-12 and VEGF. Therefore, it is intriguing to speculate that GI hormones, released in response to dietary components, may enhance tumor growth and promote invasion through the increased expression and secretion of cytokines/chemokines and that these effects may be suppressed by curcumin. Our findings have important clinical ramifications since a majority of colorectal and pancreatic cancers possess receptors for various GI hormones, including NT.
ACKNOWLEDGEMENTS
The authors thank Mr. Tatsuo Uchida for statistical analysis, Dr. Kathy O’Connor for helpful advice and assistance with the cell migration studies, Ms. Karen Martin for manuscript preparation and members of the Evers laboratory for helpful comments and discussion.
This work was supported by grants R37 AG10885, R01 DK48498 and P01 DK35608 from the National Institutes of Health.
REFERENCES
- 1.Erlichman C, Loprinzi CL. Hormonal Therapies. In: Devita J, Hellman S, Rosenberg SA, editors. Cancer Principles & Practice of Oncology. Lippincott Williams & Wilkins; Philadelphia: 2001. pp. 478–88. V.T. [Google Scholar]
- 2.Thomas RP, Hellmich MR, Townsend CM, Jr., Evers BM. Role of gastrointestinal hormones in the proliferation of normal and neoplastic tissues. Endocr Rev. 2003;24:571–99. doi: 10.1210/er.2002-0028. [DOI] [PubMed] [Google Scholar]
- 3.Evers BM, Townsend CM., Jr. Growth factors, hormones and receptors in GI cancers. In: Evers BM, editor. Molecular mechanisms in gastrointestinal cancer. Landes Company; Austin: 1999. pp. 1–19. [Google Scholar]
- 4.Reinecke M. Neurotensin. Immunohistochemical localization in central and peripheral nervous system and in endocrine cells and its functional role as neurotransmitter and endocrine hormone. Prog Histochem Cytochem. 1985;16:1–172. [PubMed] [Google Scholar]
- 5.Ferris CF, Carraway RE, Hammer RA, Leeman SE. Release and degradation of neurotensin during perfusion of rat small intestine with lipid. Regul Pept. 1985;12:101–11. doi: 10.1016/0167-0115(85)90191-0. [DOI] [PubMed] [Google Scholar]
- 6.Evers BM, Izukura M, Chung DH, et al. Neurotensin stimulates growth of colonic mucosa in young and aged rats. Gastroenterology. 1992;103:86–91. doi: 10.1016/0016-5085(92)91099-p. [DOI] [PubMed] [Google Scholar]
- 7.Maoret JJ, Anini Y, Rouyer-Fessard C, Gully D, Laburthe M. Neurotensin and a non-peptide neurotensin receptor antagonist control human colon cancer cell growth in cell culture and in cells xenografted into nude mice. Int J Cancer. 1999;80:448–54. doi: 10.1002/(sici)1097-0215(19990129)80:3<448::aid-ijc19>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 8.Iwase K, Evers BM, Hellmich MR, et al. Inhibition of neurotensin-induced pancreatic carcinoma growth by a nonpeptide neurotensin receptor antagonist, SR48692. Cancer. 1997;79:1787–93. doi: 10.1002/(sici)1097-0142(19970501)79:9<1787::aid-cncr22>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 9.Ehlers RA, Zhang Y, Hellmich MR, Evers BM. Neurotensin-mediated activation of MAPK pathways and AP-1 binding in the human pancreatic cancer cell line, MIA PaCa-2. Biochem Biophys Res Commun. 2000;269:704–8. doi: 10.1006/bbrc.2000.2335. [DOI] [PubMed] [Google Scholar]
- 10.Reubi JC, Waser B, Friess H, Buchler M, Laissue J. Neurotensin receptors: a new marker for human ductal pancreatic adenocarcinoma. Gut. 1998;42:546–50. doi: 10.1136/gut.42.4.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guha S, Lunn JA, Santiskulvong C, Rozengurt E. Neurotensin stimulates protein kinase C-dependent mitogenic signaling in human pancreatic carcinoma cell line PANC-1. Cancer Res. 2003;63:2379–87. [PubMed] [Google Scholar]
- 12.Ehlers RA, 2nd, Bonnor RM, Wang X, Hellmich MR, Evers BM. Signal transduction mechanisms in neurotensin-mediated cellular regulation. Surgery. 1998;124:239–46. discussion 46-7. [PubMed] [Google Scholar]
- 13.Zhao D, Keates AC, Kuhnt-Moore S, Moyer MP, Kelly CP, Pothoulakis C. Signal transduction pathways mediating neurotensin-stimulated interleukin-8 expression in human colonocytes. J Biol Chem. 2001;276:44464–71. doi: 10.1074/jbc.M104942200. [DOI] [PubMed] [Google Scholar]
- 14.Amorino GP, Parsons SJ. Neuroendocrine cells in prostate cancer. Crit Rev Eukaryot Gene Expr. 2004;14:287–300. doi: 10.1615/critreveukaryotgeneexpr.v14.i4.40. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka T, Bai Z, Srinoulprasert Y, Yang BG, Hayasaka H, Miyasaka M. Chemokines in tumor progression and metastasis. Cancer Sci. 2005;96:317–22. doi: 10.1111/j.1349-7006.2005.00059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arya M, Patel HR, Williamson M. Chemokines: key players in cancer. Curr Med Res Opin. 2003;19:557–64. doi: 10.1185/030079903125002216. [DOI] [PubMed] [Google Scholar]
- 17.van Eeden SF, Terashima T. Interleukin 8 (IL-8) and the release of leukocytes from the bone marrow. Leuk Lymphoma. 2000;37:259–71. doi: 10.3109/10428190009089427. [DOI] [PubMed] [Google Scholar]
- 18.Xie K. Interleukin-8 and human cancer biology. Cytokine Growth Factor Rev. 2001;12:375–91. doi: 10.1016/s1359-6101(01)00016-8. [DOI] [PubMed] [Google Scholar]
- 19.Brew R, Southern SA, Flanagan BF, McDicken IW, Christmas SE. Detection of interleukin-8 mRNA and protein in human colorectal carcinoma cells. Eur J Cancer. 1996;32A:2142–7. doi: 10.1016/s0959-8049(96)00221-3. [DOI] [PubMed] [Google Scholar]
- 20.Brew R, Erikson JS, West DC, Kinsella AR, Slavin J, Christmas SE. Interleukin-8 as an autocrine growth factor for human colon carcinoma cells in vitro. Cytokine. 2000;12:78–85. doi: 10.1006/cyto.1999.0518. [DOI] [PubMed] [Google Scholar]
- 21.Li A, Varney ML, Singh RK. Expression of interleukin 8 and its receptors in human colon carcinoma cells with different metastatic potentials. Clin Cancer Res. 2001;7:3298–304. [PubMed] [Google Scholar]
- 22.Dorai T, Aggarwal BB. Role of chemopreventive agents in cancer therapy. Cancer Lett. 2004;215:129–40. doi: 10.1016/j.canlet.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 23.Gukovsky I, Reyes CN, Vaquero EC, Gukovskaya AS, Pandol SJ. Curcumin ameliorates ethanol and nonethanol experimental pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2003;284:G85–95. doi: 10.1152/ajpgi.00138.2002. [DOI] [PubMed] [Google Scholar]
- 24.Wang X, Wang Q, Hu W, Evers BM. Regulation of phorbol ester-mediated TRAF1 induction in human colon cancer cells through a PKC/RAF/ERK/NF-kappaB-dependent pathway. Oncogene. 2004;23:1885–95. doi: 10.1038/sj.onc.1207312. [DOI] [PubMed] [Google Scholar]
- 25.Dong Z, Wang X, Evers BM. Site-specific DNA methylation contributes to neurotensin/neuromedin N expression in colon cancers. Am J Physiol Gastrointest Liver Physiol. 2000;279:G1139–47. doi: 10.1152/ajpgi.2000.279.6.G1139. [DOI] [PubMed] [Google Scholar]
- 26.Gully D, Canton M, Boigegrain R, et al. Biochemical and pharmacological profile of a potent and selective nonpeptide antagonist of the neurotensin receptor. Proc Natl Acad Sci U S A. 1993;90:65–9. doi: 10.1073/pnas.90.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsubara M, Tamura T, Ohmori K, Hasegawa K. Histamine H1 receptor antagonist blocks histamine-induced proinflammatory cytokine production through inhibition of Ca2+-dependent protein kinase C, Raf/MEK/ERK and IKK/I kappa B/NF-kappa B signal cascades. Biochem Pharmacol. 2005;69:433–49. doi: 10.1016/j.bcp.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 28.Wang Q, Wang X, Zhou Y, Evers BM. PKCdelta-mediated regulation of FLIP expression in human colon cancer cells. Int J Cancer. 2006;118:326–34. doi: 10.1002/ijc.21373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akhtar M, Watson JL, Nazli A, McKay DM. Bacterial DNA evokes epithelial IL-8 production by a MAPK-dependent, NF-kappaB-independent pathway. Faseb J. 2003;17:1319–21. doi: 10.1096/fj.03-0950fje. [DOI] [PubMed] [Google Scholar]
- 30.Qiao D, Stratagouleas ED, Martinez JD. Activation and role of mitogen-activated protein kinases in deoxycholic acid-induced apoptosis. Carcinogenesis. 2001;22:35–41. doi: 10.1093/carcin/22.1.35. [DOI] [PubMed] [Google Scholar]
- 31.Monje P, Hernandez-Losa J, Lyons RJ, Castellone MD, Gutkind JS. Regulation of the transcriptional activity of c-Fos by ERK. A novel role for the prolyl isomerase PIN1. J Biol Chem. 2005;280:35081–4. doi: 10.1074/jbc.C500353200. [DOI] [PubMed] [Google Scholar]
- 32.Monje P, Marinissen MJ, Gutkind JS. Phosphorylation of the carboxyl-terminal transactivation domain of c-Fos by extracellular signal-regulated kinase mediates the transcriptional activation of AP-1 and cellular transformation induced by platelet-derived growth factor. Mol Cell Biol. 2003;23:7030–43. doi: 10.1128/MCB.23.19.7030-7043.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murphy LO, Smith S, Chen RH, Fingar DC, Blenis J. Molecular interpretation of ERK signal duration by immediate early gene products. Nat Cell Biol. 2002;4:556–64. doi: 10.1038/ncb822. [DOI] [PubMed] [Google Scholar]
- 34.Hahm ER, Cheon G, Lee J, Kim B, Park C, Yang CH. New and known symmetrical curcumin derivatives inhibit the formation of Fos-Jun-DNA complex. Cancer Lett. 2002;184:89–96. doi: 10.1016/s0304-3835(02)00170-2. [DOI] [PubMed] [Google Scholar]
- 35.Logan-Smith MJ, Lockyer PJ, East JM, Lee AG. Curcumin, a molecule that inhibits the Ca2+-ATPase of sarcoplasmic reticulum but increases the rate of accumulation of Ca2+ J Biol Chem. 2001;276:46905–11. doi: 10.1074/jbc.M108778200. [DOI] [PubMed] [Google Scholar]
- 36.Dyer JL, Khan SZ, Bilmen JG, et al. Curcumin: a new cell-permeant inhibitor of the inositol 1,4,5-trisphosphate receptor. Cell Calcium. 2002;31:45–52. doi: 10.1054/ceca.2001.0259. [DOI] [PubMed] [Google Scholar]
- 37.Chen A, Xu J, Johnson AC. Curcumin inhibits human colon cancer cell growth by suppressing gene expression of epidermal growth factor receptor through reducing the activity of the transcription factor Egr-1. Oncogene. 2006;25:278–87. doi: 10.1038/sj.onc.1209019. [DOI] [PubMed] [Google Scholar]
- 38.Vincent JP, Mazella J, Kitabgi P. Neurotensin and neurotensin receptors. Trends Pharmacol Sci. 1999;20:302–9. doi: 10.1016/s0165-6147(99)01357-7. [DOI] [PubMed] [Google Scholar]
- 39.Yamada M, Ohata H, Momose K, Yamada M, Richelson E. Pharmacological characterization of SR 48692 sensitive neurotensin receptor in human pancreatic cancer cells, MIA PaCa-2. Res Commun Mol Pathol Pharmacol. 1995;90:37–47. [PubMed] [Google Scholar]
- 40.Bobrovnikova-Marjon EV, Marjon PL, Barbash O, Vander Jagt DL, Abcouwer SF. Expression of angiogenic factors vascular endothelial growth factor and interleukin-8/CXCL8 is highly responsive to ambient glutamine availability: role of nuclear factor-kappaB and activating protein-1. Cancer Res. 2004;64:4858–69. doi: 10.1158/0008-5472.CAN-04-0682. [DOI] [PubMed] [Google Scholar]
- 41.Fujioka S, Niu J, Schmidt C, et al. NF-kappaB and AP-1 connection: mechanism of NF-kappaB-dependent regulation of AP-1 activity. Mol Cell Biol. 2004;24:7806–19. doi: 10.1128/MCB.24.17.7806-7819.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakamura K, Yasunaga Y, Segawa T, et al. Curcumin down-regulates AR gene expression and activation in prostate cancer cell lines. Int J Oncol. 2002;21:825–30. [PubMed] [Google Scholar]
- 43.Biswas SK, McClure D, Jimenez LA, Megson IL, Rahman I. Curcumin induces glutathione biosynthesis and inhibits NF-kappaB activation and interleukin-8 release in alveolar epithelial cells: mechanism of free radical scavenging activity. Antioxid Redox Signal. 2005;7:32–41. doi: 10.1089/ars.2005.7.32. [DOI] [PubMed] [Google Scholar]
- 44.Kang G, Kong PJ, Yuh YJ, et al. Curcumin suppresses lipopolysaccharide-induced cyclooxygenase-2 expression by inhibiting activator protein 1 and nuclear factor kappab bindings in BV2 microglial cells. J Pharmacol Sci. 2004;94:325–8. doi: 10.1254/jphs.94.325. [DOI] [PubMed] [Google Scholar]
- 45.Hidaka H, Ishiko T, Furuhashi T, et al. Curcumin inhibits interleukin 8 production and enhances interleukin 8 receptor expression on the cell surface:impact on human pancreatic carcinoma cell growth by autocrine regulation. Cancer. 2002;95:1206–14. doi: 10.1002/cncr.10812. [DOI] [PubMed] [Google Scholar]
- 46.Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol. 2004;287:G7–17. doi: 10.1152/ajpgi.00079.2004. [DOI] [PubMed] [Google Scholar]
- 47.Sharma RA, Euden SA, Platton SL, et al. Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin Cancer Res. 2004;10:6847–54. doi: 10.1158/1078-0432.CCR-04-0744. [DOI] [PubMed] [Google Scholar]
- 48.Wang JM, Taraboletti G, Matsushima K, Van Damme J, Mantovani A. Induction of haptotactic migration of melanoma cells by neutrophil activating protein/interleukin-8. Biochem Biophys Res Commun. 1990;169:165–70. doi: 10.1016/0006-291x(90)91449-3. [DOI] [PubMed] [Google Scholar]
- 49.Itoh Y, Joh T, Tanida S, et al. IL-8 promotes cell proliferation and migration through metalloproteinase-cleavage proHB-EGF in human colon carcinoma cells. Cytokine. 2005;29:275–82. doi: 10.1016/j.cyto.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 50.Brun P, Mastrotto C, Beggiao E, et al. Neuropeptide neurotensin stimulates intestinal wound healing following chronic intestinal inflammation. Am J Physiol Gastrointest Liver Physiol. 2005;288:G621–9. doi: 10.1152/ajpgi.00140.2004. [DOI] [PubMed] [Google Scholar]