Abstract
Tight junctions between vascular endothelial cells help to create the blood-brain and blood-retinal barriers. Breakdown of the retinal tight junction complex is problematic in several disease states including diabetic retinopathy. Glucocorticoids can restore and/or preserve the endothelial barrier to paracellular permeability, although the mechanism remains unclear. We show that glucocorticoid treatment of primary retinal endothelial cells increases content of the tight junction proteins occludin and claudin-5, co-incident with an increase in barrier properties of endothelial monolayers. The glucocorticoid receptor antagonist RU486 reverses both the glucocorticoid-stimulated increase in occludin content and the increase in barrier properties. Transcriptional activity from the human occludin and claudin-5 promoters increases in retinal endothelial cells upon glucocorticoid treatment, and is dependent on the glucocorticoid receptor (GR) as demonstrated by siRNA. Deletion analysis of the occludin promoter reveals a 205 bp sequence responsible for the glucocorticoid response. However, this region does not posses a canonical glucocorticoid response element and does not bind to the GR in a chromatin immunoprecipitation (ChIP) assay. Mutational analysis of this region revealed a novel 40 bp occludin enhancer element (OEE), containing two highly-conserved regions of 10 and 13 base pairs, that is both necessary and sufficient for glucocorticoid-induced gene expression in retinal endothelial cells. These data suggest a novel mechanism for glucocorticoid induction of vascular endothelial barrier properties through increased occludin and claudin-5 gene expression.
Keywords: Tight junctions, Occludin, Claudin, Glucocorticoids
1. Introduction
The tight junction (TJ) is part of the junctional complex in epithelial and endothelial cells that confers cell adhesion and allows tissue compartmentalization by the creation of barriers (Aijaz et al., 2006). The TJ is essential to establish the tight barrier between endothelial cells in the retinal and brain blood vessels in order to maintain the proper environment for neuronal function (Antonetti et al., 1998; Erickson et al., 2007). Breakdown of this blood-retinal barrier (BRB) is a major causative factor in diabetic retinopathy (DR) and correlates directly with the macular edema leading to vision loss (Aiello et al., 1998; Antonetti et al., 1998). Therefore, understanding this TJ complex and how it is regulated is essential to new therapies for diabetic retinopathy. Intraocular glucocorticoid (GC) injections have been widely used off-label to treat the macular edema associated with DR although side effects are problematic. Understanding the mechanism by which glucocorticoids induce endothelial barrier properties may lead to novel therapies to reintroduce the blood-retinal barrier with reduced side effects. We report here a novel mechanism for the GC-mediated induction of the TJ complex and the endothelial barrier properties in retinal endothelial cells.
Three families of transmembrane proteins, the claudin family, the JAM family and the MARVEL domain containing proteins tricellulin and occludin are responsible for cell-to-cell attachment in the establishment of the TJ barrier (Aijaz et al., 2006; Erickson et al., 2007; Fanning et al., 1999; Matter and Balda, 2003). Occludin is expressed in epithelial and endothelial cells and its content correlates with the degree of barrier properties (Hirase et al., 1997; Mitic and Anderson, 1998). There are at least 24 claudin isoforms that contribute to the creation of a barrier towards ions and small molecules (Turksen and Troy, 2004). The tissue expression pattern of claudin isoforms confers barrier characteristics (Kiuchi-Saishin et al., 2002; Kollmar et al., 2001; Rahner et al., 2001). Studies using knock-out mouse models have further clarified the roles for transmembrane proteins in TJ function. Claudin-5 is expressed predominantly in endothelial cells (Morita et al., 1999) and a study using a claudin-5-deficient mouse demonstrated it is necessary to preserve the vascular barrier to small (<0.8 kDa) molecules in the brain (Nitta et al., 2003). Claudin-5 is expressed in retinal vasculature (Antonetti et al., 1998) and is likely to contribute a similar function in the BRB. The occludinnull mouse is viable, able to form morphologically intact TJ complexes and maintains a barrier in the intestinal epithelium as measured by trans-epithelial resistance (TER). However, a host of physiological abnormalities exist, including brain calcification, thinning of compact bone and testicular atrophy leading to sterility (Saitou et al., 2000). These studies suggest that, while occludin may not be necessary for the formation of morphologically distinct TJ complexes, occludin does contribute to barrier properties. Indeed, a recent study using siRNA to occludin in epithelial cells determined occludin contributes to the barrier by regulating small organic acid flux (Yu et al., 2005).
The initial cloning of the human occludin promoter showed a responsiveness to TNF-alpha and gamma interferon (Mankertz et al., 2000). A study investigating epithelialmesenchymal transition showed that the transcriptional repressor snail is responsible for silencing occludin gene expression through a specific E-box promoter element (Ikenouchi et al., 2003). Raf 1 mediated epithelial cell transformation is associated with down-regulation of occludin expression (Wang et al., 2005) through Raf 1 induction of the transcriptional repressor slug that inhibits occludin expression also through the occludin promoter E-box (Wang et al., 2006). A similar result was obtained by Kurrey et al. (Kurrey et al., 2005) who showed that decreased occludin expression by snail/slug resulted in increased invasiveness of ovarian tumor cells. Snail and slug also repress expression from a claudin-1 promoter in MDCK cells (Martinez-Estrada et al., 2006). Together these studies demonstrate regulation of occludin promoter activity through transcriptional repressors.
The ability of GCs to increase the barrier properties of endothelial cells and thereby decrease vascular permeability has been appreciated for some time, particularly in the treatment of edema accompanying brain tumors (Kaal and Vecht, 2004; Ruderman and Hall, 1965). Previously, it was demonstrated that GCs reduce retinal endothelial cell permeability to water and solutes and stimulate the synthesis, assembly and dephosphorylation of occludin (Antonetti et al., 2002). GCs elicit their effects by diffusing into the target cells and binding directly to the glucocorticoid receptor (GR), which then sheds previously bound heat shock proteins allowing nuclear localization (Aranda and Pascual, 2001) where the receptor can then activate or repress gene expression depending on the cis-elements in the gene promoters and on interaction with other trans-acting factors. The dimerized GR can bind to specific glucocorticoid response elements (GREs) in the promoters of target genes activating gene transcription, a mechanism termed transactivation (Barnes, 1998; Lieberman and Nordeen, 1997). Conversely, the receptor may also repress gene expression by interacting with other transcription factors, such as AP-1 (Jonat et al., 1990) or NFκB (Scheinman et al., 1995), blocking their activity independent of GR DNA binding (Reichardt et al., 1998). Recently, Forster et al. have shown that glucocorticoids can increase occludin expression in brain endothelial cells and demonstrated steroid induced gene activation from the occludin promoter in Cos cells (Forster et al., 2005). GCs have also been shown to effect TJ organization by down-regulation of fascin gene expression in mammary epithelial tumor cells (Wong et al., 1999). Here we report that GCs regulate occludin and claudin-5 gene expression and endothelial barrier properties through a transactivation mechanism that does not involve direct TJ promoter binding by the GR. Mutational analysis has led to the identification of a novel 40 nucleotide cis-acting occludin enhancer element (OEE) that is both necessary and sufficient for this indirect GC gene activation in primary retinal endothelial cells.
2. Materials and Methods
2.1 Cell culture
Primary bovine retinal endothelial cells (BREC) were isolated as described previously (Antonetti and Wolpert, 2003). Human retinal endothelial cells (HREC) were from Cell Systems (Krikland, WA). Cells were grown in MCDB-131 media (Sigma) containing 10% fetal bovine serum, 10 ng/ml epidermal growth factor, 200 μg/ml Endogro (Vec Technologies, Rensselaer, NY), 90 μg/ml heparin, antibiotics and antimycotics as published previously (Antonetti and Wolpert, 2003).
2.2 Western Blot Analysis
Protein content was measured by Western blot analysis using a urea based cell lysis buffer (Anderson et al., 1988). Total cell lysates (75 mg) were separated on polyacrylamide gels and western blots were performed by standard methods with a rabbit polyclonal occludin primary antibody (Zymed), a rabbit polyclonal antibody against claudin-5/6 (Barber and Antonetti, 2003), or a rabbit polyclonal antibody specific for the GR (Santa Cruz). Blots were normalized by total protein loaded per lane using the Dc Protein Assay (Biorad).
2.3 Transport Assay
Cells were grown to confluence on a 4 μM pore Transwell filter (Corning). Permeability to 70 kDa RITC-dextran was measured exactly as described previously (Antonetti et al., 2002). Data is presented as diffusive permeability (Po) in cm/s. Due to significant changes in the apical chamber concentration of TAMRA, basolateral chamber aliquots were collected every 15 minutes and apical chamber aliquots every 30 minutes for two hours. Apical chamber concentrations were fit to a linear regression and the time averaged apical fluorescence across each 15 minute interval was used to calculate the Po.
2.4 Cloning and Plasmid Constructs
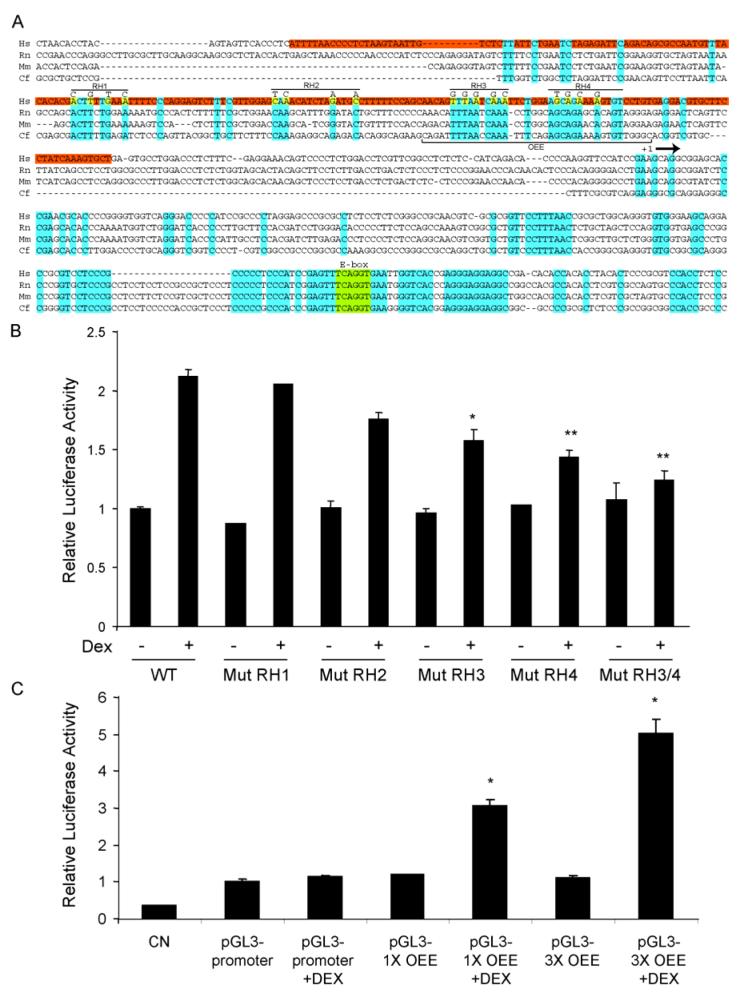
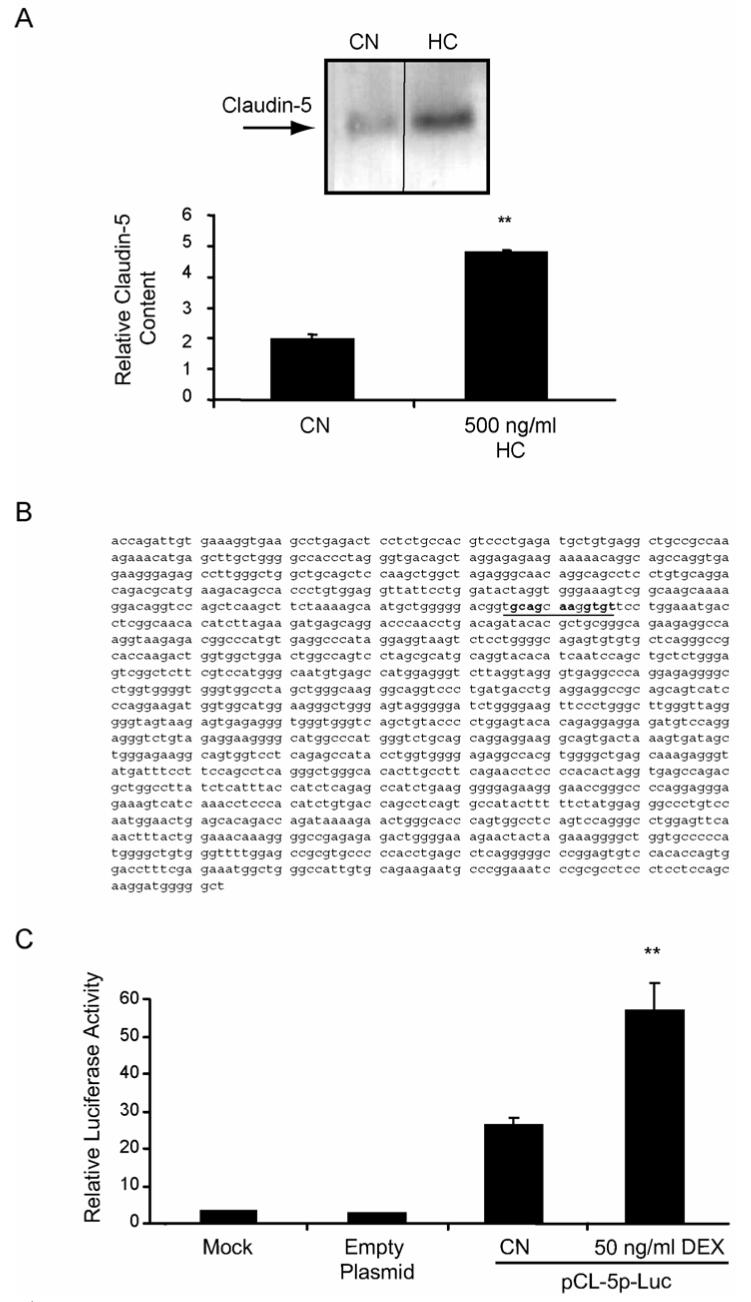
Luciferase reporter constructs were pGL3-Basic, pRL-SV (Renilla luciferase) and pSV40-Luc (Promega). The 1853 bp occludin promoter sequence has been published (Genbank AF246304) and was amplified from human genomic DNA using 5′ and 3′ primers designed from the sequence. The promoter was cloned into the pGL3-basic plasmid using Kpn I and Bgl II restriction sites and was termed plasmid occludin promoter luciferase (pOccp-Luc). Cloning of 1500 bp of the upstream claudin-5 promoter sequence was accomplished using the GenomeWalker system (Clontech) with a human genomic DNA library from the kit and a primer, 5′GCACGCCAGGATCAGAC3′, corresponding to the 5′ end of the claudin-5 cDNA. The resulting fragment was sub-cloned into the pGL3-basic plasmid using the Sma I restriction site, sequenced and termed plasmid claudin-5 promoter luciferase (pCL5p-Luc). Site-directed mutagenesis of the occludin promoter was carried out using the Quick-Change PCR-based mutagenesis kit (Invitrogen) to introduce Spe I restriction sites at regular intervals, approximately 220 bp or 100 bp apart and then these sites were used to delete the desired fragments. Point-mutational analysis was achieved using a similar method in the pOccp-Luc -256-+342 background with primers carrying the desired mutation. pGL3-1XOEE and pGL3-3XOEE were constructed by inserting one or three different pairs of annealed oligonucleotides (Integrated DNA Technologies, Coralville, IA) corresponding to the human promoter sequence (AACAGTTTAATCAAATTCTGGAAGCAGAAAAGTGTCCTGT) which we have termed the occludin enhancer element or OEE. For the 3XOEE, the dimerized oligonucleotides possessed overhangs corresponding to restriction fragment ends for Kpn I/Sac I, Sac I/Nhe I and Nhe I/Xho I (figure 7A) allowing cloning into the plasmid pGL3-promoter (Promega) which contains the SV-40 core promoter, while the 1XOEE was cloned using Kpn I and Xho I.
Fig. 7.
Identification of a novel occludin enhancer element (OEE) that responds to glucocorticoids in the occludin promoter. (A) Alignment of the occludin promoter from four mammalian species showing the 205 bp GC-responsive region in human (red) and bases conserved across all four species (blue). Point mutations made in the four regions of homology (RH1-4) are shown in yellow. (B) The indicated point mutants of Occp-Luc were used to transfect BREC and cells were treated with 50 ng/ml Dex for 24 hours. Relative luciferase activiy was measured and corrected for Renilla luciferase. (C) The occludin enhancer element (OEE) sequence indicated in (A) was subcloned as a monomer (pGL3-1XOEE) or in triplicate (pGL3-3XOEE) into the SV40 core promoter plasmid pGL3-promoter and used to transfect BREC as above. Graphs represent the mean +/- s.e.m. of two independent experiments with n=6 with analysis by ANOVA with Tukey post-test, * p < 0.05, ** p < 0.01.
2.5 Endothelial Cell Transfection and Luciferase Assays
The Amaxa Nucleofector System (Amaxa, Germany) was used to transfect endothelial cells using the suggested human coronary artery endothelial cell (HCAEC) kit conditions. Transfection efficiency was determined with an eGFP plasmid. Treatments of cells were carried out 24 hours after transfection. Luciferase assays were performed using the Dual Luciferase Assay Kit (Promega) according to kit instructions. Values were adjusted for Renilla luciferase activity.
2.6 ChIP Assay
The chromatin Immunoprecipitation (ChIP) assay was completed according to manufacturer protocols (Upstate) using HREC and antibody against the human GR. PCR primers for the occludin promoter 205 bp fragment and 400 bp IκB GRE-containing promoter fragments were used for amplification.
2.7 Statistical Analysis
Data was analyzed using ANOVA with a Tukey post-test analysis using GraphPad Prism 4.0 software. A p value <0.05 was considered significant.
3. Results
3.1 Glucocorticoids increase the barrier properties of retinal endothelial cells
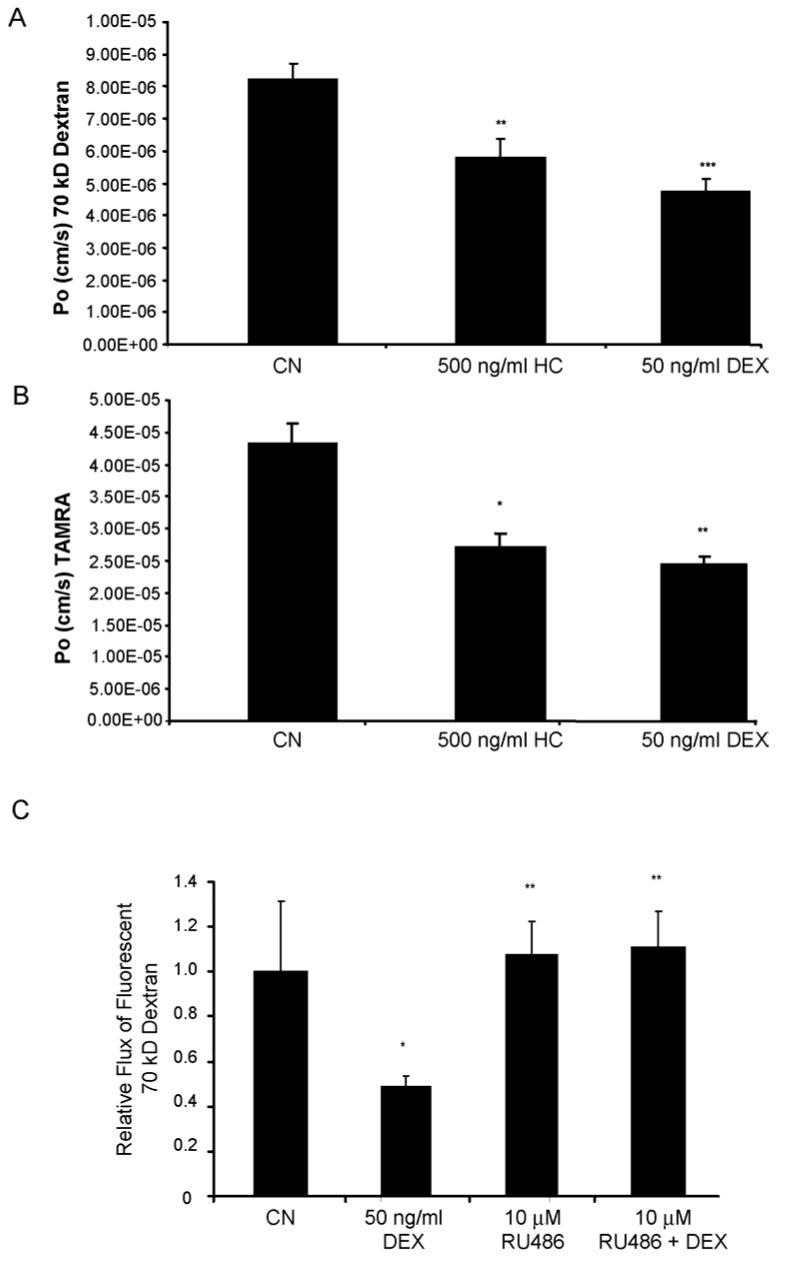
To determine the effect of GCs on endothelial cell monolayer permeability, an endothelial cell transport assay was employed as previously described (Antonetti et al., 2002). Primary human retinal endothelial cells (HREC) were grown to confluence on Transwell filters and treated with 500 ng/ml hydrocortisone (HC) or 50 ng/ml dexamethasone (Dex) for 24 hours. Fluorescent labeled 70 kDa RITC-dextran (70 kDa RITC-Dex) (Fig. 1A) or 467 Dalton TAMRA (Fig. 1B) was added to the apical chamber at 10 μM, media was collected from the basolateral chamber at 30 minute intervals and quantified on a fluoroimager. The endothelial permeability under diffusive flux conditions was calculated as described (Antonetti et al., 2002). Both HC and Dex significantly reduced the rate of flux of both 70 kDa dextran and 467 Dalton TAMRA, suggesting the effect of GCs on stimulating barrier properties is significant for both large and small molecules. To determine if the observed GC effects on endothelial barrier properties is a result of transcriptional transactivation by the GR, we pretreated bovine retinal endothelial cells (BREC) with 10 μM RU486, which has been shown to specifically inhibit GR transactivation of gene expression (Mahajan and London, 1997). Cells were pretreated with RU486 for 1 hour prior to 50 ng/ml Dex treatment for 24 hours and the permeability of 70 kDa RITC-dextran across endothelial monolayers was measured. Dex treatment reduced permeability through the BREC monolayer by approximately 2-fold while pretreatment with RU486 completely blocked this effect (Fig. 1C). Cells treated with RU486 did not appear morphologically different from control cells when examined under phase-contrast microscopy (data not shown). These results suggest that GCs induce endothelial barrier properties through a mechanism that involves gene transactivation by the GR and also demonstrate that the observed effect is similar for both human and bovine endothelial cells.
Fig. 1.
Glucocorticoids regulate endothelial barrier properties. HREC (A&B) or BREC (C) were grown to confluence on 0.4 μm Transwell filters then treated with GC as indicated. (C) Cells were pretreated with 10 μM RU486 for 1 hour prior to GC treatment. After 24 hours, permeability of 70 kDa RITC-dextran (A&C) or 467 Dalton TAMRA (B) was determined as described. All graphs represent the mean +/- s.e.m. with analysis by ANOVA with Tukey post-test (n=4), * p < 0.05, ** p < 0.01, *** p < 0.001.
3.2 Glucocrticoid treatment increases content of the tight junction protein occludin
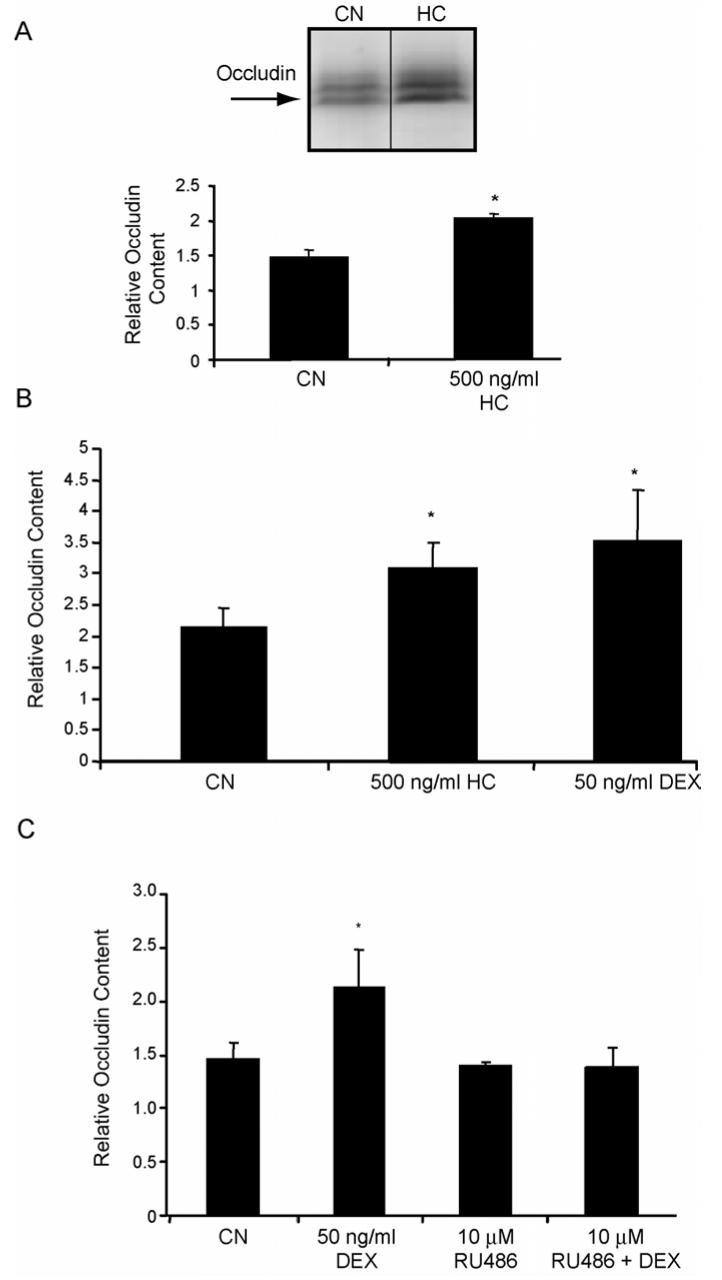
To assess the effect of GCs on tight junction protein content, BREC were isolated as previously reported (Antonetti and Wolpert, 2003) and grown in culture to confluence, switched to media without serum and treated with 500 ng/ml HC. After 24 hours, cell lysates were analyzed by western blot using a polyclonal antibody specific for occludin. After 24 hrs of HC treatment, we observed a significant increase in occludin protein content (Fig. 2A). HREC were treated with either 500 ng/ml HC or 50 ng/ml Dex and analyzed for occludin content as above (Fig. 2B). Pretreatment of BREC with the GR antagonist RU486 for 1 hour reversed the effect of Dex on occludin content (Fig. 2C) suggesting transactivation is necessary for the GC induction of occludin protein. Content of the intracellular TJ protein ZO-1 was unchanged by GC treatment (data not shown).
Fig. 2.
Glucocorticoids increase the content of occludin protein in retinal endothelial cells through a transactivation mechanism. BREC (A&C) or HREC (B) were grown to confluence then switched to media without serum (CN) or without serum plus 500 ng/ml hydrocortisone (HC) or 50 ng/ml dexamethasone (DEX) for 24 hours. (C) BREC were pretreated for 1 hour with 10 μM RU486 prior to Dex treatment. Whole-cell lysates were made in urea extraction buffer and western blots were probed with a primary antibody against occludin and quantified. Representative blots are shown with n=3. Graphs represent the mean +/- s.e.m. with analysis by ANOVA with Tukey post-test. * p < 0.05.
3.3 Glucocorticoids increase the activity on the occludin promoter in BREC
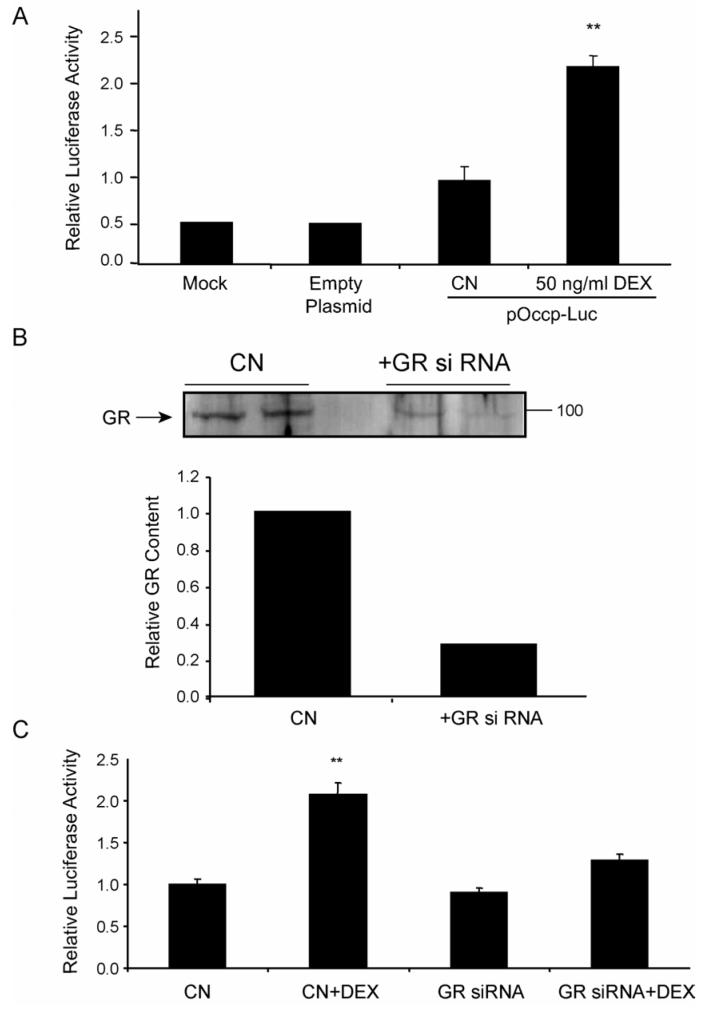
To directly measure the effect of GCs on occludin gene expression, the occludin promoter was cloned for promoter activity assays. Based on the previously published sequence of a 1853 bp occludin promoter region (Mankertz et al., 2000), PCR primers were designed to clone this promoter region, consisting of a fragment corresponding to basepairs -1511 to + 342 of the human occludin promoter sequence, from human genomic DNA. The PCR amplicon obtained was subcloned into the pGL-3-Basic luciferase reporter plasmid, sequenced and used to transfect BREC in culture. High transfection efficiencies, approximately 70-80%, were obtained as assessed with a peGFP green fluorescent protein expression plasmid (data not shown). BREC were transfected with the occludin-luciferase reporter plasmid, pOccp-Luc, along with a Renilla luciferase reporter, pRL-SV, to correct for transfection efficiency. After transfection, cells were treated with 50 ng/ml Dex for 24 hours or maintained in serum-free condition as controls, and relative luciferase activity was assayed from whole cell lysates. The occludin promoter possessed basal activity compared to vector alone (Fig. 3A). Furthermore, cells treated with Dex had a 2-3 fold higher luciferase activity compared to untreated controls, demonstrating a significant increase in occludin promoter activity with GC treatment. These results reveal the occludin promoter is responsive to GC treatment and this transcriptional control can account for the observed increase in protein content (Fig. 2A).
Fig. 3.
Occludin promoter activity is increased with glucocorticoid treatment dependent on the GR. (A) BREC were co-transfected with pGL3-Luc (empty Plasmid) or pOccp-Luc together with pRL-SV, treated with 50 ng/ml Dex for 24 hours and relative luciferase activity was determined. (B) HREC were transfected with siRNA specific for the human GR, cells were grown for 48 hours then cell lysates were assayed for GR by western blot and quantified. (C) HREC were cotransfected with GR siRNA and pOccp-Luc and treated with 50 ng/ml Dex for 24 hrs as indicated. Relative luciferase activity was determined as above. Data represents two independent experiments with n=6. Graphs represent the mean +/- s.e.m. with analysis by ANOVA with Tukey post-test ** p < 0.01.
In order to confirm that the effect of RU486 was specific for the GR and not other receptors of the steroid super-family (Mahajan and London, 1997), a small inhibitory RNA (siRNA) targeting the human GR was used to reduce the content of the GR in endothelial cells. HREC were co-transfected with the pOccp-Luc plasmid, the Renilla control plasmid and siRNA to the GR and cells were tested for GC responsiveness of the occludin promoter. Cells treated with siRNA for 40 hours were harvested in lysis buffer and a western blot was performed to determine relative amounts of GR protein present in cells with and without the siRNA. Samples that included the GR siRNA showed a 78% reduction in the amount of the 97 kDa GR protein (Fig. 3B) compared to cells transfected with a scrambled siRNA. Luciferase assays demonstrated that the GR was required for GC induction of occludin promoter activity. Upon treatment with Dex, samples without GR siRNA showed a 2 to 3-fold increase in luciferase activity observed previously (Fig. 3A), however samples that included GR siRNA were not significantly different from controls (Fig. 3C). This result demonstrates that the observed increase in occludin promoter activity with GC treatment depends on the presence of the GR, through either a direct or indirect mechanism.
3.4 Glucocorticoids increase the expression of the TJ protein claudin-5 through activation of the claudin-5 gene promoter
Claudin-5 is expressed in endothelial cells (Morita et al., 1999) and required for the blood-brain barrier (Liebner et al., 2000). To determine if GCs increase protein content of claudin-5, BREC were treated with HC and whole-cell lysates were teseted by western blot for content of the claudin-5 protein. Treatment of cells with 500 ng/ml HC for 24 hours resulted in an approximately 2.5-fold increase in the content of claudin-5 in these cells (Fig. 4A).
Fig. 4.
Glucocorticoids increase the content of claudin-5 protein and the activity of the claudin-5 promoter in retinal endothelial cells. BREC were grown to confluence then switched to media without serum (CN) or without serum plus 500 ng/ml hydrocortisone (HC) for 24 hours. Whole-cell lysates were made in urea extraction buffer and western blots were probed with a primary antibody against claudin-5 and quantified. (B) Sequence of the 1500 bp upstream claudin-5 promoter sequence (sequence with homology to occludin RH4 highlighted). (C) BREC were transfected with pCL-5p-Luc and assayed for luciferase activity as above. Data represents three independent experiments with n=9. Graphs represent the mean +/- s.e.m. with analysis by ANOVA with Tukey post-test **p < 0.01.
The claudin-5 promoter was cloned and its responsiveness to GC treatment was tested. Using the published sequence of the claudin-5 cDNA, a primer was designed to be used in a genome walking method to clone the claudin-5 upstream promoter sequence from a human genomic DNA library. A 1500 bp clone upstream of the extreme 5′ region of the claudin-5 cDNA was cloned and sequenced (Fig. 4B). This fragment was subcloned into the pGL-3-Basic luciferase reporter plasmid and used to transfect primary BREC as described above. The 1500 bp clone possessed basal promoter activity and treatment of transfected cells with 50 ng/ml Dex increased luciferase activity by 2 to 3 fold demonstrating GC responsiveness of the claudin-5 gene (Fig. 4C). This result was very similar to that obtained with the occludin promoter (Fig. 3A). These data demonstrate that the claudin-5 promoter, like the occludin promoter, is responsive to GC treatment through transactivation.
3.5 Identification of a glucocorticoid responsive sequence of the occludin promoter
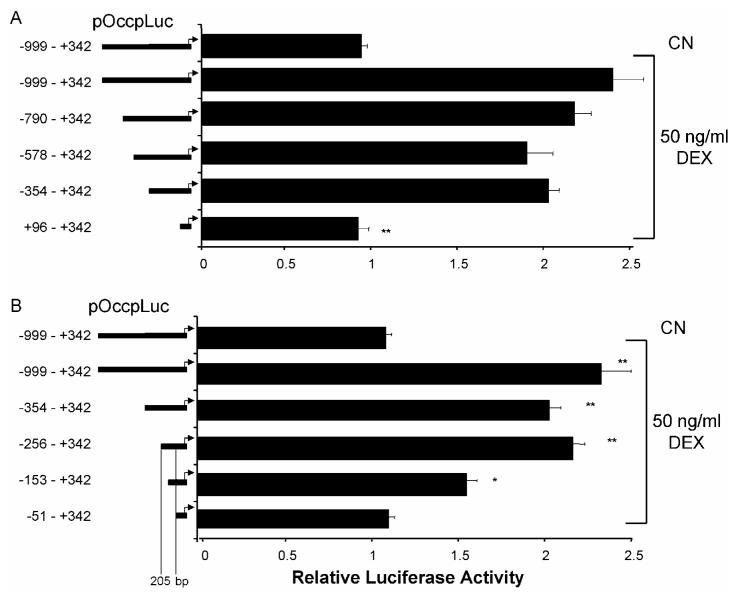
To determine the mechanism of GC activation of TJ genes, we chose to examine the responsiveness of the occludin promoter in greater detail. Upon analysis of the occludin promoter sequence, no canonical GRE could be identified, although potential half-sites with partial homology were identified as discussed below. The occludin promoter was therefore subjected to systematic deletion mutagenesis to determine the minimal sequence necessary for a GC response. Initially, site-directed mutagenesis was used to introduce Spe I restriction sites at approximately 220 bp intervals along the length of the promoter sequence which were used to make a series of promoter deletions (Fig. 5A). Plasmids were cotransfected into BREC with Renilla pRL-SV plasmid as before and treated for 24 hr with 50 ng/ml Dex. Luciferase activity of cell lysates was determined and adjusted for transfection efficiency based on Renilla activity. None of the deletion constructs tested through bp -354 to +342 was different from the full length promoter sequence. However, pOccp-Luc +96 - +342 maintained basal activity but lost GC responsiveness suggesting a sequence between bases -345 and +96 (450 bp) is required for GC responsiveness (Fig. 5A).
Fig. 5.
The occludin promoter contains a 205 bp minimal glucocorticoid-responsive sequence. The indicated truncation mutants of the occludin promoter were synthesized using site-directed mutagenesis and used to transfect BREC. After 24 hours, cells were treated with 50 ng/ml Dex for 24 hrs. Relative luciferase activiy was measured and corrected for Renilla luciferase. Graphs represent the mean +/- s.e.m. with analysis by ANOVA with Tukey post-test of two independent experiments with n=6, ** p < 0.01.
Identification of a 450 bp sequence of the occludin promoter that is responsive to GCs prompted the synthesis of a second series of truncation mutants, removing approximately 100 bp sequentially from the previously identified minimally responsive fragment. Response to Dex was tested following transfection into BREC as above. The pOccp-Luc -256 to +342 plasmid responded to Dex at a level similar to the full-length promoter. However, the pOccp-Luc -51 to +342 construct was not significantly different from the untreated control. The pOccp-Luc -153 to +342 construct showed an intermediate activity that was statistically different from both the control and the full-length sequence (Fig. 5B). These results demonstrate that the full GC responsive sequence of the occludin promoter lies within a 205 bp fragment between nucleotides -256 and -51.
3.6 The 205 bp GC-responsive occludin promoter fragment does not bind the GR by ChIP and potential GRE half-sites are not responsible for GC stimulation
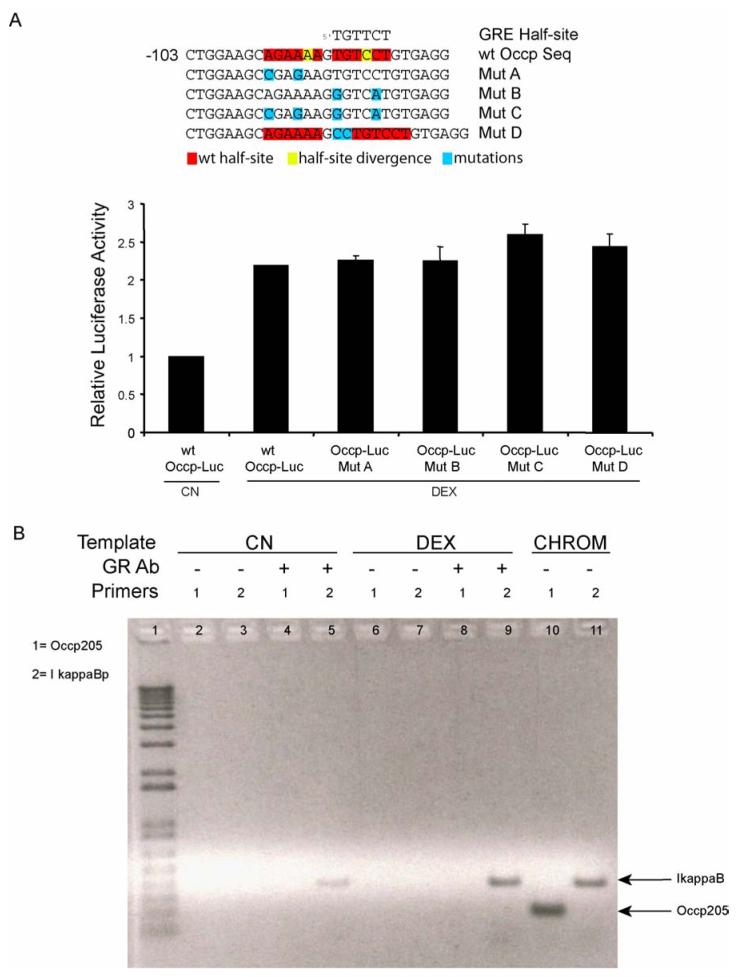
Upon sequence analysis of the 205 bp minimal fragment obtained from the promoter deletion studies shown in figure 5, we found that two potential GRE half-sites reside in this region starting at bases -95 and -88 in the promoter (Fig. 6A). Although these sequences were not exact matches with the canonical GRE half-site, TGTTCT, and had only one basepair of spacing instead of the typical three, they were each only one base divergent and the GR is known to bind to non-canonical GRE sites (Aranda and Pascual, 2001). Therefore, in order to determine whether these sites contribute to the GC induction of promoter activity, the sites were mutated for activity analysis. Site-directed mutagenesis was used to mutate two bases in each of the two half-sites, both mutants together, and finally, the proper spacing of three bases was introduced between the wild-type sequences to determine if a functional GRE with greater activity could be synthesized from the wild-type sequence. Mutants were transfected into BREC and tested for GC-responsiveness in a luciferase assay, as above, and compared to the wild-type sequence (Fig. 6A). Activity of the four mutants was not different from that of the wild-type promoter sequence suggesting that these putative GRE half-sites were not responsible for the observed effects of GC on occludin promoter activity.
Fig. 6.
GR does not directly bind the glucocorticoid responsive region of the occludin promoter. (A) Potential GRE half-sites are not necessary for the glucocorticoid response. Potential GRE half-sites within the occludin promoter 205 bp GC-responsive sequence identified by a cis-element search were mutated as indicated and used to transfect BREC cells as above. Transfected cells were treated with 50 ng/ml Dex and a dual luciferase assay was performed as described, n=3. (B) Confluent HREC were treated with 50 ng/ml Dex. A chromatin immunoprecipitation (ChIP) experiment was performed using primary antibody against the GR. Primers specific for the 205 bp GC-responsive fragment of the occludin promoter (primer set 1) or for a 400 bp GC-responsive fragment of the Iκ-B promoter (primer set 2) were used to perform PCR reactions from the precipitated DNA. CHROM is unprecipitated chromatin samples as a control for PCR. Graph represents the mean +/- s.e.m. with analysis by ANOVA with Tukey post-test of two independent experiments with n=6.
Although the minimal GC-responsive fragment of the occludin promoter does not contain a canonical GRE, and attempts to assign function to potential non-canonical sites were negative (Fig. 6A), it is possible for the GR to synergize with other transcription factors through a crosstalk mechanism that does not require direct binding of the GR to the DNA (Gottlicher et al., 1998). Therefore, to determine if the GR forms a complex with the occludin promoter 205 bp sequence under conditions of GC stimulation of retinal endothelial cells, a chromatin immunoprecipitation assay (ChIP) with an antibody specific for the human GR was performed. HREC were grown to confluence in culture then treated with and without 50 ng/ml DEX for 24 hours, conditions under which occludin promoter activity is stimulated. DNA protein complexes were then cross-linked with formaldehyde, chromatin was sheared, and immunoprecipitated with the anti-GR antibody. Resulting DNA fragments then served as a template for PCR using primers to amplify the 205 bp GC-responsive occludin promoter fragment. As a positive control for GR binding, we chose the Iκ-B promoter, which is regulated by direct GR binding (Auphan et al., 1995). Non-precipitated chromatin DNA served as a positive control for primer amplification. Figure 6B shows that both sets of primers amplified products from chromatin controls (lanes 10 & 11) and that the GR ChIP successfully precipitated a DNA fragment containing the Iκ-B promoter from GC stimulated cells (lane 9). Interestingly, a weaker amplicon was detected with the Iκ-B primers in the absence of stimulation, suggesting the possibility of a low level of GR occupancy at this promoter in these cells without GC stimulation. However, no amplicons were detectable using primers specific for the occludin 205 bp GC-responsive fragment under basal (lane 4) or GC-stimulated conditions (lane 8) suggesting the GR is not part of the complex at the occludin promoter site.
3.7 Identification of the Occudin Enhancer Element that controls responsiveness of the occludin gene to GCs
The lack of an identifiable GRE in the 205 bp GC-responsive sequence of the occludin promoter, together with the lack of GR binding to the sequence, prompted an exploration for an alternative mechanism of activation. Sequence alignment was used to identify conserved regions that may be necessary for GC responsiveness. Figure 7A shows an alignment between the human, rat, mouse, and dog occludin promoter sequences encompassing the 205 bp GC-responsive region (red). Nucleotides identical across the four species are noted throughout the sequence (blue). The conserved regions in the occludin promoter includes an area of particularly high homology in a region downstream of the transcriptional start site encompassing the E-box (green), a cis-element that represses occludin promoter activity by the transcription factor snail or slug during the epithelial to mesenchymal transition (Wang et al., 2006). Also noted were four regions of homology within the 205 bp GC responsive region, named regions of homology 1-4 (RH1-4). Site-directed mutagenesis was used to mutate conserved bases within each of the four regions of homology (yellow). Each resulting mutant was sequence-verified and used to transfect BREC to test for GC responsiveness in luciferase reporter assays as above. Mutations within RH1 or RH2 did not significantly affect the GC responsiveness of the 205 bp region, however mutations in either RH3 or RH4 did significantly reduce the GC response (Fig. 7B). Based on these results, we determined that a 40 bp region encompassing RH3 and RH4 was necessary for the observed GC activation of the occludin promoter in primary retinal endothelial cells (Fig. 7A). This 40 bp region has been termed the occludin enhancer element (OEE).
To determine if the OEE identified in figure 7B can function as a GC-responsive enhancer in another promoter context, we synthesized plasmids containing either one copy (pGL3-1XOEE) or three copies (pGL3-3XOEE) of the OEE, upstream of the enhancerless SV-40 core promoter in the vector pGL3-promoter. The resulting plasmids were used to transfect BREC as above and tested for GC responsiveness in luciferase reporter assays. In the absense of GC stimulation, pGL3-1XOEE and pGL3-3XOEE were not different from empty vector (Fig. 7C). However, treatment with 50 ng/ml Dex for 24 hours increased activity from the single OEE plasmid approximately 3-fold and from the triple OEE plasmid approximately 5-fold. These results demonstrate that a novel 40 bp OEE of the human occludin promoter is both necessary and sufficient to direct GC responsiveness in primary retinal endothelial cells.
4. Discussion
Overall the results presented here suggest a mechanism for GC stimulation of the endothelial barrier in retinal vasculature that involves, at least in part, increased expression of the TJ proteins occludin and claudin-5 at the level of gene expression. Further, a novel 40 bp cis-acting element that lies between bases -148 and -111 of the occludin promoter is both necessary and sufficient for steroid induction of occludin gene expression. Although E-box sequence has been previously shown to repress occludin gene activation (Wang et al., 2006), we believe this is the first report of a positive acting cis-element specifically driving occludin gene expression. The lack of a functional GRE and the lack of GR binding to this sequence suggest that activation of a secondary, trans-acting factor that interacts with the OEE controls occludin gene expression in response to GCs. Elucidating the mechanism of GC action may lead to novel therapies to treat vascular permeability.
The correlation between loss of TJ complex components and decreased barrier properties of endothelial cells has prompted the investigation of GCs as potential pro-barrier agents. The therapeutic effects of GCs on clinical conditions involving increased vascular permeability such as brain tumors has been long established (Ruderman and Hall, 1965) although the mechanism underlying these effects was unknown. The classic anti-inflammatory properties of GCs involve transcriptional transrepression by which the transcription of pro-inflammatory genes is blocked by nuclear-localized GR forming heterodimers with transcription factors such as NFκ-B (Caldenhoven et al., 1995). The transactivation mechanism of the ligand-bound receptor homodimer is associated with the up-regulation of genes such as those involved in hepatic gluconeogenesis. It is interesting to note that while the increased expression of the TJ genes occludin and claudin-5 is the result of the transactivation by the GR, the physical outcome could be considered anti-inflammatory, through a decrease in solute flux and edema in the tissues surrounding the vasculature.
Previous studies have investigated mechanisms of tight junction formation in response to glucocorticoids. Firestone and colleagues have shown that GCs induce the reorganization of the tight junction complex in mammary epithelial tumor cells and that this effect is dependent on a functional Ras pathway (Guan et al., 2002; Woo et al., 1999). It was also shown that GC-mediated down-regulation of the actin-bundling protein fascin was required for the GC-induced formation of the tight junctions from pre-formed components in these cells (Wong et al., 1999), and that the regulation of fascin expression may be a point of cross-talk between the Ras and GC pathways in these cells (Guan et al., 2002). Other studies implicated the helix-loop-helix protein Id-1, a non-DNA binding repressor of other helix-loop-helix transcritpion factors, as enhancing the GC-induced reorganization of junctional complexes in mammary epithelial cells (Woo et al., 2000). Taken together, these studies shed interesting light on the GC-mediated control of TJ organization in a culture model of mammary epithelium.
In this report, we provide evidence that glucocorticoids upregulate the tight junction transmembrane proteins occludin and claudin 5 in primary retinal endothelial cells through transactivation of the promoters for these genes. Further, a specific occludin enhancer element has been identified that is both necessary and sufficient for the glucocorticoid response. The pathways identified in the mammary epithelial cells and these studies may complement one another but future studies are required to determine whether important differences exist between mammary epithelial cells and vascular endothelial cells regarding the response to glucocorticoids.
The increase in TJ protein content in endothelial cells upon GC treatment correlates with reduced water and solute permeability in these cells (Antonetti et al., 2002). Although a recent study in which occludin expression was inhibited in MDCK cells showed no effect on permeability of large solutes, permeability to small, positively charged organic acids was increased with occludin knock-down. Further, changes in signaling between the TJ and the actin cytoskeleton were observed suggesting a role for occludin in regulating junctional organization (Yu et al., 2005). Other studies from our laboratory suggest that occludin is required for an adaptive response to changes in hydrostatic pressure (data not shown). The permeability of TAMRA in retinal pigment epithelial cells after occludin knock-down was increased 15% under diffusive conditions but was increased 50% under 10 cm of water pressure compared to controls. A number of studies demonstrate that peptides to the external loops of occludin increase solute or ion permeability (Lacaz-Vieira et al., 1999; Nusrat et al., 2005; Tavelin et al., 2003; Vietor et al., 2001; Wong and Gumbiner, 1997).
Several studies have shown that claudins regulate movement of solutes and ions through the junction and that these proteins may be responsible for the character of the junction (Nitta et al., 2003; Turksen and Troy, 2002; Van Itallie et al., 2003). In particular, claudin-5 regulates the permeability of molecules < 200 angstroms across the blood-brain barrier as demonstrated in mice with claudin 5 gene deletion (Nitta et al., 2003). The results presented here, showing a similar response of the claudin-5 and occludin genes to GCs in both time course and magnitude, suggest a coordinated effect of GCs on these transmembrane tight junction proteins.
Despite the fact that both the occludin and claudin-5 gene promoters are activated by GCs and that occludin gene responsiveness is blocked with GR siRNA and RU486, sequence analysis does not reveal a canonical GRE in either promoter. Foester et al. recently showed that the occludin promoter is GC responsive in Cos-7 cells (Forster et al., 2005), similar to our results in retinal endothelial cells. They suggest that the GR may utilize a non-canonical pentameric half-site as has been previously published for somatastatin gene (Kraus et al., 1999). However, the sequences suggested by these authors have a statistical probability of randomly occurring in a sequence of this size and no other evidence for their utilization is offered. The authors also concede lack of a traditional palandromic GRE in the occludin promoter. Sequence analysis of the 205 bp minimally responsive fragment identified here does reveal other near-consensus GRE hexameric half-sites. However, when mutated, these sequences had no effect on the GC-responsiveness of the promoter region. Also, these near-GRE sequences fall within the RH4 of the OEE, but key bases know to be indispensable for GC activation (Nordeen et al., 1990) are not conserved across species as shown in the alignment (Fig. 7A). Our data support an alternative hypothesis that a common factor, stimulated by GCs, directs occludin and claudin-5 gene expression. This hypothesis is further supported by the 24 hours required for GC response.
The identification of the 40 bp OEE sequence as a functional enhancer element responsive to GCs in primary retinal endothelial cells suggests further that a secondary factor is responsible for the observed effects. Experiments in which the OEE is moved to another core promoter context and still responds to GCs (Fig. 7C) are particularly important as they demonstrate that this sequence is capable of functioning in the absence of other occuldin promoter elements. It is interesting that inserting three copies of the OEE did increase the response above one OEE but did not lead to a three-fold activation when compared to the single OEE clone. However, enhancer responses are often not linear in this regard. Also, the fact that both RH3 and RH4 seem to contribute to the full responsiveness of the region suggests that cooperativity between multiple trans-acting factors may be required for a full response. However RH4 appears critical for a large quantity of the GC response as indicated by the mutational analysis. Searches to identify potential known transcription factor binding sites within the OEE did not return any matches with factors known to be GC-responsive. However, GCs may induce a transcription factor or coactivators by de novo synthesis or alternatively GCs may lead to allosteric modification or recompartmentalization of a trans-acting factor leading to gene activation. Future studies identifying trans-acting factors that interact with the OEE will shed more light on this system.
The OEE may act on genes other than occludin. A genome blast search using the 13 bp RH4 returned 92 matches in the human genome with 100% homology, more than double the 44 matches that what would be expected from a random occurrence of this sequence in the genome, suggesting a potential role for the sequence in the genome. In fact, the sequence encompassing RH4 (-126 to -114) was found upstream of the gene that codes for cadherin-9, an adherens junction protein which is reported to be upregulated by glucocorticoids (Foty et al., 1998). Importantly, alignment of the occludin OEE with the human claudin-5 promoter sequence revealed a sequence at -1159 to -1147 (Fig.4B, underlined, bold sequence) with 77% identity to the 13 bp occudin RH4 suggesting that a common mechanism may be responsible for activation of both genes. Thus, a novel enhancer that responds indirectly to GCs was identified in the occludin promoter and regions of homology to this element have been identified in the GC-responsive junctional genes claudin-5 and cadherin-9 suggesting a common mechanism of GC control.
The use of glucocorticoids is under investigation for the treatment of diabetic retinopathy and other disorders that include vascular permeability in retinal biology. Ocular injection of the GC triamcinolone have shown some promise in preventing the macular edema associated with vision loss (Massin et al., 2004; Sutter et al., 2004). However, long term steroid treatment is associated with adverse outcomes including cataract formation and increased intraocular pressure, potentially leading to glaucoma (Ciardella et al., 2004; Lam et al., 2004) (Moshfeghi et al., 2003; Sutter and Gillies, 2003). Through an understanding of the mechanism by which GCs exert their pro-barrier effects it may be possible to identify new targets for control of the blood-retinal barrier in disease.
Acknowledgements
The authors thank Ellen Wolpert and Brian Leonard for technical assistance. Work was supported by NIH RO1 EY 016413 (DAA) and the Juvenile Diabetes Research Foundation (DAA) and a kind gift from Mr. Jack Turner.
References
- Aiello LP, Gardner TW, King GL, Blankenship G, Cavallerano JD, Ferris FL, 3rd, Klein R. Diabetic retinopathy. Diabetes Care. 1998;21:143–56. doi: 10.2337/diacare.21.1.143. [DOI] [PubMed] [Google Scholar]
- Aijaz S, Balda MS, Matter K. Tight junctions: molecular architecture and function. Int Rev Cytol. 2006;248:261–98. doi: 10.1016/S0074-7696(06)48005-0. [DOI] [PubMed] [Google Scholar]
- Anderson JM, Stevenson BR, Jesaitis LA, Goodenough DA, Mooseker MS. Characterization of ZO-1, a protein component of the tight junction from mouse liver and Madin-Darby canine kidney cells. J Cell Biol. 1988;106:1141–9. doi: 10.1083/jcb.106.4.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonetti DA, Barber AJ, Khin S, Lieth E, Tarbell JM, Gardner TW. Vascular permeability in experimental diabetes is associated with reduced endothelial occludin content: vascular endothelial growth factor decreases occludin in retinal endothelial cells. Penn State Retina Research Group. Diabetes. 1998;47:1953–9. doi: 10.2337/diabetes.47.12.1953. [DOI] [PubMed] [Google Scholar]
- Antonetti DA, Wolpert EB. Isolation and characterization of retinal endothelial cells. Methods Mol Med. 2003;89:365–74. doi: 10.1385/1-59259-419-0:365. [DOI] [PubMed] [Google Scholar]
- Antonetti DA, Wolpert EB, DeMaio L, Harhaj NS, Scaduto RC., Jr. Hydrocortisone decreases retinal endothelial cell water and solute flux coincident with increased content and decreased phosphorylation of occludin. J Neurochem. 2002;80:667–77. doi: 10.1046/j.0022-3042.2001.00740.x. [DOI] [PubMed] [Google Scholar]
- Aranda A, Pascual A. Nuclear hormone receptors and gene expression. Physiol Rev. 2001;81:1269–304. doi: 10.1152/physrev.2001.81.3.1269. [DOI] [PubMed] [Google Scholar]
- Auphan N, DiDonato JA, Rosette C, Helmberg A, Karin M. Immunosuppression by glucocorticoids: inhibition of NF-kappa B activity through induction of I kappa B synthesis. Science. 1995;270:286–90. doi: 10.1126/science.270.5234.286. [DOI] [PubMed] [Google Scholar]
- Barber AJ, Antonetti DA. Mapping the Blood Vessels with Paracellular Permeability in the Retinas of Diabetic Rats. Invest Ophthalmol Vis Sci. 2003;44:5410–5415. doi: 10.1167/iovs.03-0244. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. Anti-inflammatory actions of glucocorticoids: molecular mechanisms. Clin Sci (Lond) 1998;94:557–72. doi: 10.1042/cs0940557. [DOI] [PubMed] [Google Scholar]
- Caldenhoven E, Liden J, Wissink S, Van de Stolpe A, Raaijmakers J, Koenderman L, Okret S, Gustafsson JA, Van der Saag PT. Negative cross-talk between RelA and the glucocorticoid receptor: a possible mechanism for the antiinflammatory action of glucocorticoids. Mol Endocrinol. 1995;9:401–12. doi: 10.1210/mend.9.4.7659084. [DOI] [PubMed] [Google Scholar]
- Ciardella AP, Klancnik J, Schiff W, Barile G, Langton K, Chang S. Intravitreal triamcinolone for the treatment of refractory diabetic macular oedema with hard exudates: an optical coherence tomography study. Br J Ophthalmol. 2004;88:1131–6. doi: 10.1136/bjo.2004.041707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KK, Sundstrom JM, Antonetti DA. Vascular permeability in ocular disease and the role of tight junctions. Angiogenesis. 2007;10:103–17. doi: 10.1007/s10456-007-9067-z. [DOI] [PubMed] [Google Scholar]
- Fanning AS, Mitic LL, Anderson JM. Transmembrane proteins in the tight junction barrier. J Am Soc Nephrol. 1999;10:1337–45. doi: 10.1681/ASN.V1061337. [DOI] [PubMed] [Google Scholar]
- Forster C, Silwedel C, Golenhofen N, Burek M, Kietz S, Mankertz J, Drenckhahn D. Occludin as direct target for glucocorticoid-induced improvement of blood-brain barrier properties in a murine in vitro system. J Physiol. 2005;565:475–86. doi: 10.1113/jphysiol.2005.084038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foty RA, Corbett SA, Schwarzbauer JE, Steinberg MS. Dexamethasone up-regulates cadherin expression and cohesion of HT-1080 human fibrosarcoma cells. Cancer Res. 1998;58:3586–9. [PubMed] [Google Scholar]
- Gottlicher M, Heck S, Herrlich P. Transcriptional cross-talk, the second mode of steroid hormone receptor action. J Mol Med. 1998;76:480–9. doi: 10.1007/s001090050242. [DOI] [PubMed] [Google Scholar]
- Guan Y, Woo PL, Rubenstein NM, Firestone GL. Transforming growth factor-alpha abrogates the glucocorticoid stimulation of tight junction formation and reverses the steroid-induced down-regulation of fascin in rat mammary epithelial tumor cells by a Ras-dependent pathway. Exp Cell Res. 2002;273:1–11. doi: 10.1006/excr.2001.5415. [DOI] [PubMed] [Google Scholar]
- Hirase T, Staddon JM, Saitou M, Ando-Akatsuka Y, Itoh M, Furuse M, Fujimoto K, Tsukita S, Rubin LL. Occludin as a possible determinant of tight junction permeability in endothelial cells. J Cell Sci. 1997;110(Pt 14):1603–13. doi: 10.1242/jcs.110.14.1603. [DOI] [PubMed] [Google Scholar]
- Ikenouchi J, Matsuda M, Furuse M, Tsukita S. Regulation of tight junctions during the epithelium-mesenchyme transition: direct repression of the gene expression of claudins/occludin by Snail. Journal of Cell Science. 2003;116:1959–1967. doi: 10.1242/jcs.00389. [DOI] [PubMed] [Google Scholar]
- Jonat C, Rahmsdorf HJ, Park KK, Cato AC, Gebel S, Ponta H, Herrlich P. Antitumor promotion and antiinflammation: down-modulation of AP-1 (Fos/Jun) activity by glucocorticoid hormone. Cell. 1990;62:1189–204. doi: 10.1016/0092-8674(90)90395-u. [DOI] [PubMed] [Google Scholar]
- Kaal EC, Vecht CJ. The management of brain edema in brain tumors. Curr Opin Oncol. 2004;16:593–600. doi: 10.1097/01.cco.0000142076.52721.b3. [DOI] [PubMed] [Google Scholar]
- Kiuchi-Saishin Y, Gotoh S, Furuse M, Takasuga A, Tano Y, Tsukita S. Differential expression patterns of claudins, tight junction membrane proteins, in mouse nephron segments. J Am Soc Nephrol. 2002;13:875–86. doi: 10.1681/ASN.V134875. [DOI] [PubMed] [Google Scholar]
- Kollmar R, Nakamura SK, Kappler JA, Hudspeth AJ. Expression and phylogeny of claudins in vertebrate primordia. Proc Natl Acad Sci USA. 2001;98:10196–201. doi: 10.1073/pnas.171325898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus J, Woltje M, Hollt V. Regulation of mouse somatostatin receptor type 2 gene expression by glucocorticoids. FEBS Lett. 1999;459:200–204. doi: 10.1016/s0014-5793(99)01236-3. [DOI] [PubMed] [Google Scholar]
- Kurrey NK, K A, Bapat SA. Snail and Slug are major determinants of ovarian cancer invasiveness at the transcription level. Gynecol Oncol. 2005;97:155–65. doi: 10.1016/j.ygyno.2004.12.043. [DOI] [PubMed] [Google Scholar]
- Lacaz-Vieira F, Jaeger MM, Farshori P, Kachar B. Small synthetic peptides homologous to segments of the first external loop of occludin impair tight junction resealing. J Membr Biol. 1999;168:289–97. doi: 10.1007/s002329900518. [DOI] [PubMed] [Google Scholar]
- Lam DS, Chan CK, Tang EW, Li KK, Fan DS, Chan WM. Intravitreal triamcinolone for diabetic macular oedema in Chinese patients: six-month prospective longitudinal pilot study. Clin Experiment Ophthalmol. 2004;32:569–72. doi: 10.1111/j.1442-9071.2004.00903.x. [DOI] [PubMed] [Google Scholar]
- Lieberman BA, Nordeen SK. DNA intersegment transfer, how steroid receptors search for a target site. J Biol Chem. 1997;272:1061–8. doi: 10.1074/jbc.272.2.1061. [DOI] [PubMed] [Google Scholar]
- Liebner S, Kniesel U, Kalbacher H, Wolburg H. Correlation of tight junction morphology with the expression of tight junction proteins in blood-brain barrier endothelial cells. Eur J Cell Biol. 2000;79:707–17. doi: 10.1078/0171-9335-00101. [DOI] [PubMed] [Google Scholar]
- Mahajan DK, London SN. Mifepristone (RU486): a review. Fertil Steril. 1997;68:967–76. doi: 10.1016/s0015-0282(97)00189-1. [DOI] [PubMed] [Google Scholar]
- Mankertz J, Tavalali S, Schmitz H, Mankertz A, Riecken EO, Fromm M, Schulzke JD. Expression from the human occludin promoter is affected by tumor necrosis factor alpha and interferon gamma. J Cell Sci. 2000;113(Pt 11):2085–90. doi: 10.1242/jcs.113.11.2085. [DOI] [PubMed] [Google Scholar]
- Martinez-Estrada OM, Culleres A, Soriano FX, Peinado H, Bolos V, Martinez FO, Reina M, Cano A, Fabre M, Vilaro S. The transcription factors Slug and Snail act as repressors of Claudin-1 expression in epithelial cells. Biochem J. 2006;394:449–57. doi: 10.1042/BJ20050591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massin P, Audren F, Haouchine B, Erginay A, Bergmann JF, Benosman R, Caulin C, Gaudric A. Intravitreal triamcinolone acetonide for diabetic diffuse macular edema: preliminary results of a prospective controlled trial. Ophthalmology. 2004;111:218–24. doi: 10.1016/j.ophtha.2003.05.037.; discussion 224-5.
- Matter K, Balda MS. Signalling to and from tight junctions. Nat Rev Mol Cell Biol. 2003;4:225–36. doi: 10.1038/nrm1055. [DOI] [PubMed] [Google Scholar]
- Mitic LL, Anderson JM. Molecular architecture of tight junctions. Annu Rev Physiol. 1998;60:121–42. doi: 10.1146/annurev.physiol.60.1.121. [DOI] [PubMed] [Google Scholar]
- Morita K, Sasaki H, Furuse M, Tsukita S. Endothelial claudin: claudin-5/TMVCF constitutes tight junction strands in endothelial cells. J Cell Biol. 1999;147:185–94. doi: 10.1083/jcb.147.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshfeghi DM, Kaiser PK, Scott IU, Sears JE, Benz M, Sinesterra JP, Kaiser RS, Bakri SJ, Maturi RK, Belmont J, et al. Acute endophthalmitis following intravitreal triamcinolone acetonide injection. Am J Ophthalmol. 2003;136:791–6. doi: 10.1016/s0002-9394(03)00483-5. [DOI] [PubMed] [Google Scholar]
- Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol. 2003;161:653–60. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordeen SK, Suh BJ, Kuhnel B, Hutchison CD. Structural determinants of a glucocorticoid receptor recognition element. Mol Endocrinol. 1990;4:1866–73. doi: 10.1210/mend-4-12-1866. [DOI] [PubMed] [Google Scholar]
- Nusrat A, Brown GT, Tom J, Drake A, Bui TT, Quan C, Mrsny RJ. Multiple protein interactions involving proposed extracellular loop domains of the tight junction protein occludin. Mol Biol Cell. 2005;16:1725–34. doi: 10.1091/mbc.E04-06-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahner C, Mitic LL, Anderson JM. Heterogeneity in expression and subcellular localization of claudins 2, 3, 4, and 5 in the rat liver, pancreas, and gut. Gastroenterology. 2001;120:411–22. doi: 10.1053/gast.2001.21736. [DOI] [PubMed] [Google Scholar]
- Reichardt HM, Kaestner KH, Tuckermann J, Kretz O, Wessely O, Bock R, Gass P, Schmid W, Herrlich P, Angel P, et al. DNA binding of the glucocorticoid receptor is not essential for survival. Cell. 1998;93:531–41. doi: 10.1016/s0092-8674(00)81183-6. [DOI] [PubMed] [Google Scholar]
- Ruderman NB, Hall TC. Use of glucocorticoids in the paliative treatment of metastatic brain tumors. Cancer. 1965;18:298–306. doi: 10.1002/1097-0142(196503)18:3<298::aid-cncr2820180306>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Saitou M, Furuse M, Sasaki H, Schulzke JD, Fromm M, Takano H, Noda T, Tsukita S. Complex phenotype of mice lacking occludin, a component of tight junction strands. Molec. Biol. Cell. 2000;11:4131–4142. doi: 10.1091/mbc.11.12.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinman RI, Gualberto A, Jewell CM, Cidlowski JA, Baldwin AS., Jr. Characterization of mechanisms involved in transrepression of NF-kappa B by activated glucocorticoid receptors. Mol Cell Biol. 1995;15:943–53. doi: 10.1128/mcb.15.2.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter FK, Gillies MC. Pseudo-endophthalmitis after intravitreal injection of triamcinolone. Br J Ophthalmol. 2003;87:972–4. doi: 10.1136/bjo.87.8.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter FK, Simpson JM, Gillies MC. Intravitreal triamcinolone for diabetic macular edema that persists after laser treatment: three-month efficacy and safety results of a prospective, randomized, double-masked, placebo-controlled clinical trial. Ophthalmology. 2004;111:2044–9. doi: 10.1016/j.ophtha.2004.05.025. [DOI] [PubMed] [Google Scholar]
- Tavelin S, Hashimoto K, Malkinson J, Lazorova L, Toth I, Artursson P. A new principle for tight junction modulation based on occludin peptides. Mol Pharmacol. 2003;64:1530–40. doi: 10.1124/mol.64.6.1530. [DOI] [PubMed] [Google Scholar]
- Turksen K, Troy TC. Permeability barrier dysfunction in transgenic mice overexpressing claudin 6. Development. 2002;129:1775–84. doi: 10.1242/dev.129.7.1775. [DOI] [PubMed] [Google Scholar]
- Turksen K, Troy TC. Barriers built on claudins. J Cell Sci. 2004;117:2435–47. doi: 10.1242/jcs.01235. [DOI] [PubMed] [Google Scholar]
- Van Itallie CM, Fanning AS, Anderson JM. Reversal of charge selectivity in cation or anion-selective epithelial lines by expression of different claudins. Am J Physiol Renal Physiol. 2003;285:F1078–84. doi: 10.1152/ajprenal.00116.2003. [DOI] [PubMed] [Google Scholar]
- Vietor I, Bader T, Paiha K, Huber LA. Perturbation of the tight junction permeability barrier by occludin loop peptides activates beta-catenin/TCF/LEF-mediated transcription. EMBO Rep. 2001;2:306–12. doi: 10.1093/embo-reports/kve066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Mandell KJ, Parkos CA, Mrsny RJ, Nusrat A. The second loop of occludin is required for suppression of Raf1-induced tumor growth. Oncogene. 2005;24:4412–20. doi: 10.1038/sj.onc.1208634. [DOI] [PubMed] [Google Scholar]
- Wang Z, Wade P, Mandell KJ, Akyildiz A, Parkos CA, Mrsny RJ, Nusrat A. Raf 1 represses expression of the tight junction protein occludin via activation of the zinc-finger transcription factor slug. Oncogene. 2006 doi: 10.1038/sj.onc.1209902. [DOI] [PubMed] [Google Scholar]
- Wong V, Ching D, McCrea PD, Firestone GL. Glucocorticoid down-regulation of fascin protein expression is required for the steroid-induced formation of tight junctions and cell-cell interactions in rat mammary epithelial tumor cells. J Biol Chem. 1999;274:5443–53. doi: 10.1074/jbc.274.9.5443. [DOI] [PubMed] [Google Scholar]
- Wong V, Gumbiner BM. A synthetic peptide corresponding to the extracellular domain of occludin perturbs the tight junction permeability barrier. J Cell Biol. 1997;136:399–409. doi: 10.1083/jcb.136.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo PL, Cercek A, Desprez PY, Firestone GL. Involvement of the helix-loop-helix protein Id-1 in the glucocorticoid regulation of tight junctions in mammary epithelial cells. J Biol Chem. 2000;275:28649–58. doi: 10.1074/jbc.M910373199. [DOI] [PubMed] [Google Scholar]
- Woo PL, Ching D, Guan Y, Firestone GL. Requirement for Ras and phosphatidylinositol 3-kinase signaling uncouples the glucocorticoid-induced junctional organization and transepithelial electrical resistance in mammary tumor cells. J Biol Chem. 1999;274:32818–28. doi: 10.1074/jbc.274.46.32818. [DOI] [PubMed] [Google Scholar]
- Yu AS, McCarthy KM, Francis SA, McCormack JM, Lai J, Rogers RA, Lynch RD, Schneeberger EE. Knockdown of occludin expression leads to diverse phenotypic alterations in epithelial cells. Am J Physiol Cell Physiol. 2005;288:C1231–41. doi: 10.1152/ajpcell.00581.2004. [DOI] [PubMed] [Google Scholar]