Abstract
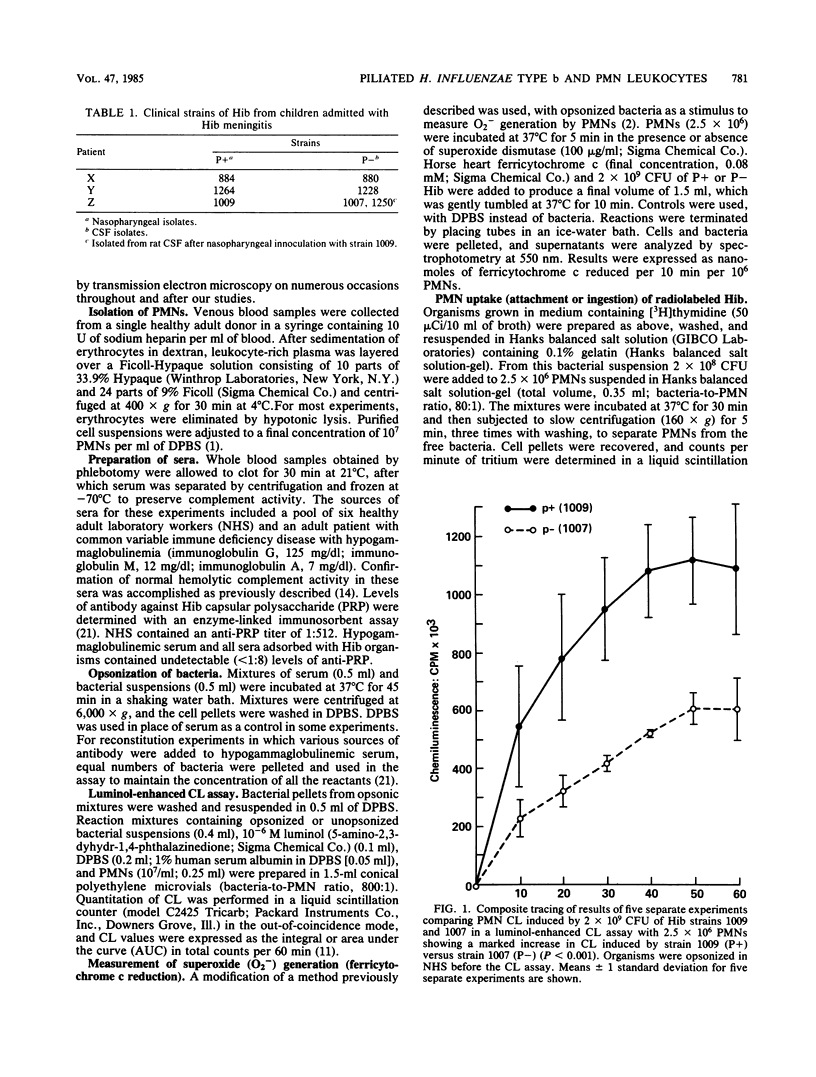
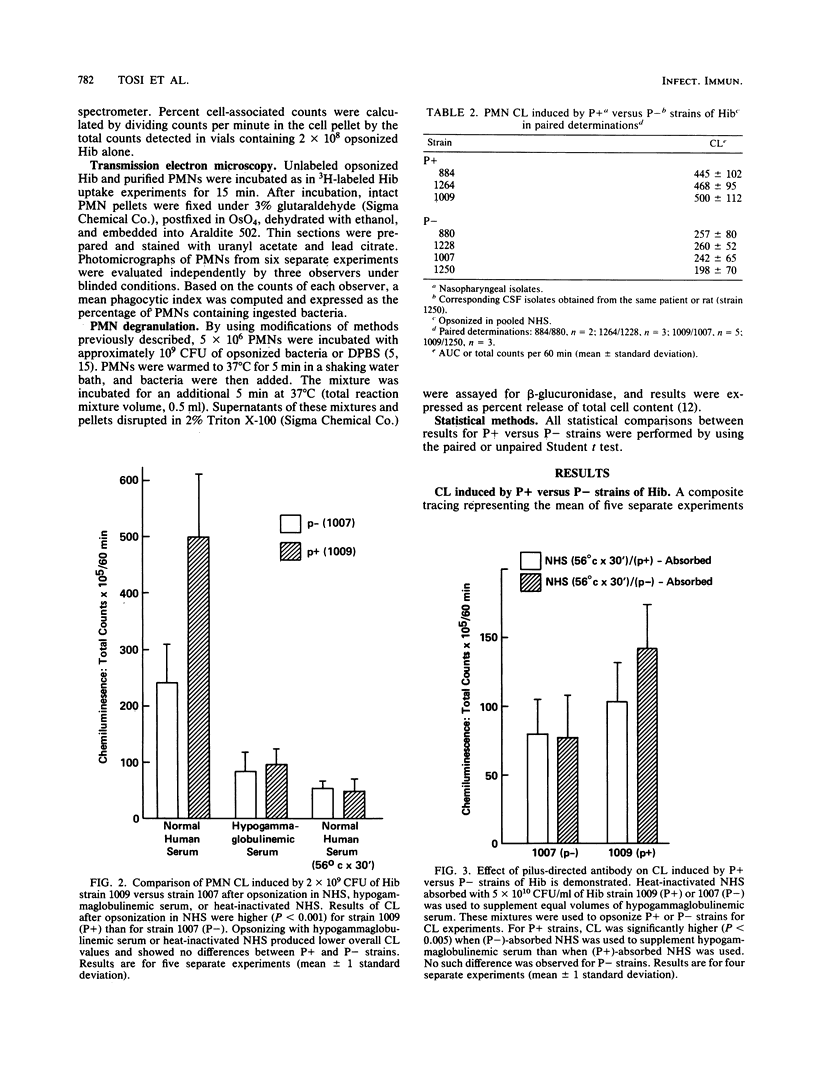
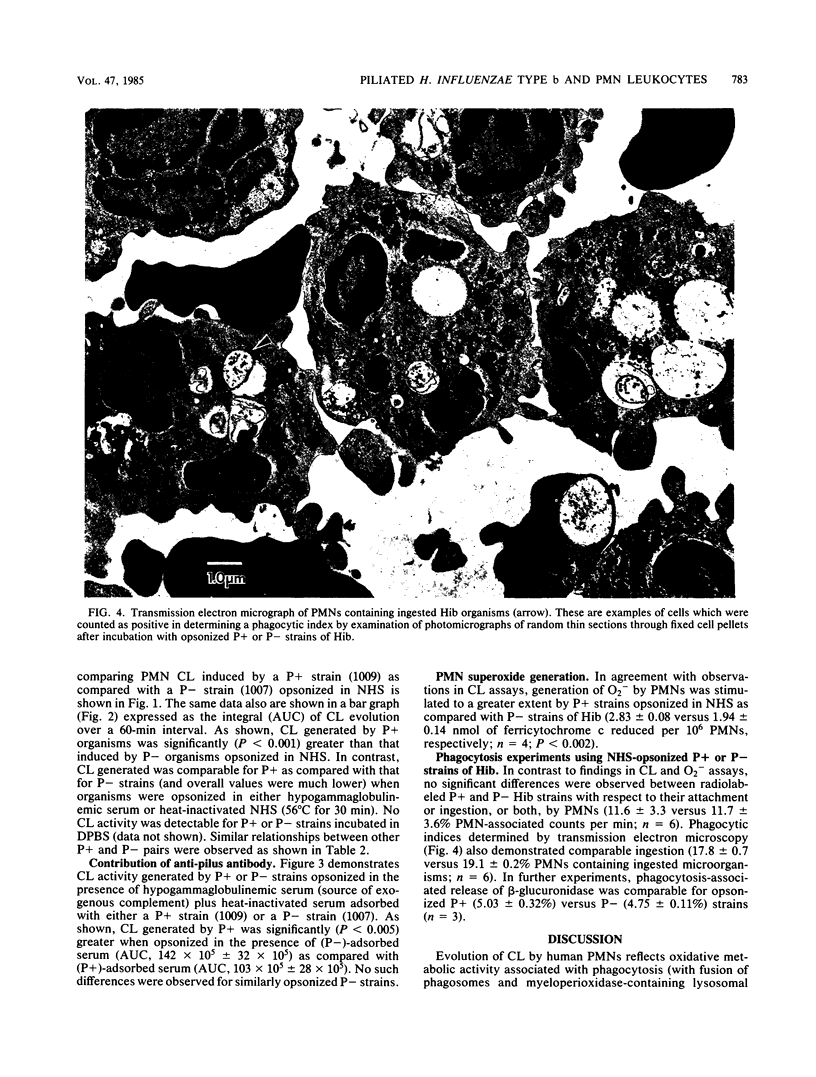
Piliated, adherent (P+) and nonpiliated, nonadherent (P-) strains of Haemophilus influenzae type b (Hib) were compared with respect to their ability to induce polymorphonuclear leukocyte (PMN) chemiluminescence (CL) and superoxide (O2-) generation and their susceptibility to phagocytosis by PMNs. P+ strains opsonized in normal human serum (NHS) induced significantly greater CL than did P- strains (500 X 10(5) +/- 112 X 10(5) versus 242 X 10(5) +/- 65 X 10(5) total counts per 60 min; P less than 0.001) when reacted with normal PMNs. Contributions of immunoglobulin and complement to CL activity in these mixtures were shown by findings of lower overall levels of CL when hypogammaglobulinemic serum or heat-inactivated NHS was used to opsonize either P+ or P- organisms. Results obtained with mixtures of hypogammaglobulinemic plus adsorbed heat-inactivated NHS (with P+ or P- organisms) suggested a role for an antipilus antibody in the enhancement of CL by these strains. NHS-opsonized P+ strains also induced significantly greater (P less than 0.002) O2- generation than did P- strains (2.83 +/- 0.08 versus 1.94 +/- 0.14 nmol of ferricytochrome c reduced per 10 min/10(6) PMN). Comparable ingestion of P+ or P- strains opsonized in NHS by PMNs was demonstrated by a radiolabeled uptake technique and transmission electron microscopy, and primary granule release (beta-glucuronidase) was comparable during ingestion of P+ or P- strains. The basis for the observed enhanced capacity of P+ Hib to stimulate PMN oxidative metabolism as compared with P- organisms is uncertain. Possible clinical implications of these findings deserve further study.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. C., Hughes B. J., Smith C. W. Abnormal mobility of neonatal polymorphonuclear leukocytes. Relationship to impaired redistribution of surface adhesion sites by chemotactic factor or colchicine. J Clin Invest. 1981 Oct;68(4):863–874. doi: 10.1172/JCI110341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkstén B., Wadström T. Interaction of Escherichia coli with different fimbriae and polymorphonuclear leukocytes. Infect Immun. 1982 Oct;38(1):298–305. doi: 10.1128/iai.38.1.298-305.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenstock E., Jann K. Adhesion of piliated Escherichia coli strains to phagocytes: differences between bacteria with mannose-sensitive pili and those with mannose-resistant pili. Infect Immun. 1982 Jan;35(1):264–269. doi: 10.1128/iai.35.1.264-269.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor E. M., Loeb M. R. A hemadsorption method for detection of colonies of Haemophilus influenzae type b expressing fimbriae. J Infect Dis. 1983 Nov;148(5):855–860. doi: 10.1093/infdis/148.5.855. [DOI] [PubMed] [Google Scholar]
- DeChatelet L. R., Long G. D., Shirley P. S., Bass D. A., Thomas M. J., Henderson F. W., Cohen M. S. Mechanism of the luminol-dependent chemiluminescence of human neutrophils. J Immunol. 1982 Oct;129(4):1589–1593. [PubMed] [Google Scholar]
- Densen P., Mandell G. L. Gonococcal interactions with polymorphonuclear neutrophils: importance of the phagosome for bactericidal activity. J Clin Invest. 1978 Dec;62(6):1161–1171. doi: 10.1172/JCI109235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edén C. S., Hansson H. A. Escherichia coli pili as possible mediators of attachment to human urinary tract epithelial cells. Infect Immun. 1978 Jul;21(1):229–237. doi: 10.1128/iai.21.1.229-237.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan S. L., Mason E. O., Jr, Wiedermann B. L. Role of adherence in the pathogenesis of Haemophilus influenzae type b infection in infant rats. Infect Immun. 1983 Nov;42(2):612–617. doi: 10.1128/iai.42.2.612-617.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan S. L., Umstead C. L., Mason E. O., Anderson D. C., Parke J. C., Jr, Feigin R. D. Assessment of Haemophilus influenzae type b opsonins by neutrophil chemiluminescence. J Clin Microbiol. 1981 Mar;13(3):532–539. doi: 10.1128/jcm.13.3.532-539.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo A., Caimi L., Marchesini S., Goi G. C., Tettamanti G. Enzymes of lysosomal origin in human plasma and serum: assay conditions and parameters influencing the assay. Clin Chim Acta. 1980 Dec 22;108(3):337–346. doi: 10.1016/0009-8981(80)90339-3. [DOI] [PubMed] [Google Scholar]
- Mangan D. F., Snyder I. S. Mannose-sensitive stimulation of human leukocyte chemiluminescence by Escherichia coli. Infect Immun. 1979 Dec;26(3):1014–1019. doi: 10.1128/iai.26.3.1014-1019.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oseas R., Yang H. H., Baehner R. L., Boxer L. A. Lactoferrin: a promoter of polymorphonuclear leukocyte adhesiveness. Blood. 1981 May;57(5):939–945. [PubMed] [Google Scholar]
- Root R. K., Cohen M. S. The microbicidal mechanisms of human neutrophils and eosinophils. Rev Infect Dis. 1981 May-Jun;3(3):565–598. doi: 10.1093/clinids/3.3.565. [DOI] [PubMed] [Google Scholar]
- Silverblatt F. J., Dreyer J. S., Schauer S. Effect of pili on susceptibility of Escherichia coli to phagocytosis. Infect Immun. 1979 Apr;24(1):218–223. doi: 10.1128/iai.24.1.218-223.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. IV. Pili: their role in attachment of gonococci to tissue culture cells. J Exp Med. 1973 Mar 1;137(3):571–589. doi: 10.1084/jem.137.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosi M. F., Kaplan S. L., Mason E. O., Buffone G. J., Anderson D. C. Generation of chemotactic activity in serum by Haemophilus influenzae type b. Infect Immun. 1984 Feb;43(2):593–599. doi: 10.1128/iai.43.2.593-599.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]



