Abstract
OBJECTIVE
The purpose of our study was to determine whether the phosphatidylinositol 3-kinase (PI3K)/Akt pathway contributes to expression of pancreatic duodenal homeobox-1 (PDX-1) in duct cells and the cell differentiation during pancreatic regeneration.
METHODS
The role of PI3K in PDX-1 expression and duct cell differentiation with pancreatic regeneration in mice after partial pancreatectomy (Px) was examined using either wortmannin, a pharmacologic PI3K inhibitor, or siRNA directed to the p85α regulatory subunit of PI3K. Akt phosphorylation, a marker of PI3K activation, and PDX-1 expression were assessed by Western blot analysis and immunohistochemistry.
RESULTS
Both PDX-1 levels and Akt phosphorylation were concomitantly increased in pancreatic ducts following partial Px, and, conversely, blocked by treatment with wortmannin or p85α siRNA. Pancreatic duct cell differentiation, as assessed by appearance of insulin-positive cells 3 days after partial Px, was effectively reduced by wortmannin.
CONCLUSIONS
PI3K/Akt activation plays a critical role for both PDX-1 expression and pancreatic duct cell differentiation into insulin-producing cells during pancreatic regeneration.
Keywords: Insulin, PI3K, PDX-1, Pancreatectomy, Pancreatic regeneration
INTRODUCTION
Phosphatidylinositol 3-kinase (PI3K), a ubiquitous lipid kinase involved in receptor signal transduction, is composed of a regulatory subunit, p85, and a catalytic subunit, p110. 1, 2 PI3K catalyzes the production of phosphatidylinositol-3, 4, 5-triphosphate, which recruits a subset of signal proteins with pleckstrin homology domains to the membrane, where they are phosphorylated.1, 2 These proteins include the protein serine-threonine kinase Akt and phosphoinositide-dependent kinase 1. Activation of Akt results in phosphorylation of downstream target proteins that affect cell growth, cell cycle distribution, apoptosis and survival.1, 2 The PI3K pathway plays various important roles in pancreatic function, such as insulin signaling, insulin-stimulated glucose transport and glycogen synthesis.3-7 In addition, we have previously reported that the PI3K pathway is critical for the proliferation of pancreatic acinar cells and plays a major role in pancreatic regeneration after partial pancreatectomy (Px).8
Pancreatic regeneration has been extensively studied in various animal models.9-11 Increases in islet mass and islet neogenesis are noted in the regenerating pancreas after partial Px in the animal models.9, 12 Islet morphogenesis during embryogenesis is thought to occur predominately through the budding of islet cells from pancreatic ducts, followed by their migration away from the duct to form clusters.13, 14 In the normal adult pancreas, islet β-cell mass is replenished by two major mechanisms, β-cell cell replication and neogenesis from progenitor cells.15-18 The origin of β-cell progenitor cells is not clearly understood at present; pancreatic duct cells represent likely candidates for these progenitor cells since insulin within the pancreatic duct epithelium has been well documented in the regenerating pancreas.19, 20
Pancreatic duodenal homeobox-1 (PDX-1), which is also known as IDX-1, IPF-1 and STF-1, is a key regulator of β-cell differentiation and function.21 It is expressed in the primitive gut during the early embryonic stage, diminished after birth,11 and predominantly expressed in β-cells of adult pancreatic islets.22 PDX-1 plays critical roles in endocrine pancreatic function through its regulatory action on the expression of functional pancreatic genes including insulin.23, 24 PDX-1 is also essential for β-cell neogenesis as demonstrated by several in vitro models of cell differentiation to insulin-producing cells.25-29 Expression of PDX-1 increases in the duct during β-cell neogenesis in an animal model of pancreatic regeneration after partial Px.12 These findings strongly suggest that PDX-1 is an important mediator and marker of ductal cell differentiation into β-cells. However, the molecular mechanisms contributing to PDX-1 expression during β-cell neogenesis are not fully understood. In the present study, we examined whether the PI3K/Akt pathway contributes to PDX-1 expression and β-cell differentiation in the pancreatic ducts.
MATERIALS AND METHODS
Materials
Anti-pAkt (Ser473) (#9271) and anti-Akt (#9272) antibodies for Western blot analysis were purchased from Cell Signaling (Beverly, MA). Anti-pAkt (Ser473) (#sc-7985-R) antibodies for immunohistochemistry were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit anti-PI3K p85α antibody was purchased from NeoMarker (Fremont, CA). Rabbit anti-PDX-1 antibody (#AB3243) was purchased from Chemicon International (Temecula, CA). EnVision+ system and anti-insulin (A0564) antibodies were purchased from DAKO Cytomation (Carpinteria, CA). Secondary antibodies (goat anti-rabbit or mouse IgG) for immunoblotting were obtained from Upstate (Waltham, MA). Wortmannin, type IV collagenase, monoclonal anti-β-actin antibody (#A5441; clone AC-15) and other molecular biology grade reagents were purchased from Sigma (St. Louis, MO). The siSTABLE SMARTpool siRNA directed to PI3K p85α regulatory subunit and non-specific control siRNA duplexes were synthesized by Dharmacon (Lafayette, CO). To prevent recognition and cleavage of unintended mRNA targets (off-target effect),30 this siRNA was modified by an ON-TARGET technique from Dharmacon. Trans IT In Vivo Gene Delivery System and Trans TKO transfection reagent was purchased from Mirus (Madison, WI).
Animals and Partial Px model
Male C57BL/6 and Swiss-Webster mice (7-8 wks old) were obtained from Charles Rivers Laboratories (Wilmington, MA) or Harlan (Indianapolis, IN). Before experiments, mice were acclimated for at least 7 days in an environment with controlled temperature (21-23°C), and lighting (12 h light/12 h dark) with free access to tap water and regular chow diet. A detailed procedure of 75% partial Px in mice has been described previously.8 All procedures were approved by the University of Texas Medical Branch (UTMB) Institutional Animal Care and Use Committee (IACUC).
Immunohistochemical analysis
Immunohistochemical staining was performed by the dextran polymer method using Dako EnVision+ system as we have described previously.8
Protein extraction and Western blot analysis
Protein extraction and Western blot analysis were performed as previously described 8. Dilution factor for primary antibodies was 1:1000 for antibodies against phosphorylation of Akt (pAkt), Akt, and p85α, 1:5000 for anti-PDX-1 antibody, and 1:2000 for anti-β-actin antibody, respectively.
siRNA in vivo delivery
Non-targeting control or p85α siRNA (20 μg/mouse) was delivered to Swiss-Webster mice by hydrodynamic tail vein injection31, 32 using Trans IT In Vivo Gene Delivery System as described previously8. siSTABLE SMARTpool in vivo siRNA from Dharmacon was injected into the mice, and 2 days later, the mice underwent either partial Px or sham operation. We have previously shown that administration of siRNA targeting the PI3K p85α subunit effectively blocked PI3K/Akt activation and pancreatic regeneration after partial Px.8
Statistical analysis
The results of insulin-positive duct cell index in vivo were analyzed using a two-group t-test. The effect was assessed at the 0.05 level of significance as the experiment-wise error rates. Data analysis was conducted using Statview software for Windows version 5.0 from SAS (Cary, NC).
RESULTS
Akt phosphorylation and PDX-1 expression are concomitantly increased in the ducts of regenerating pancreas
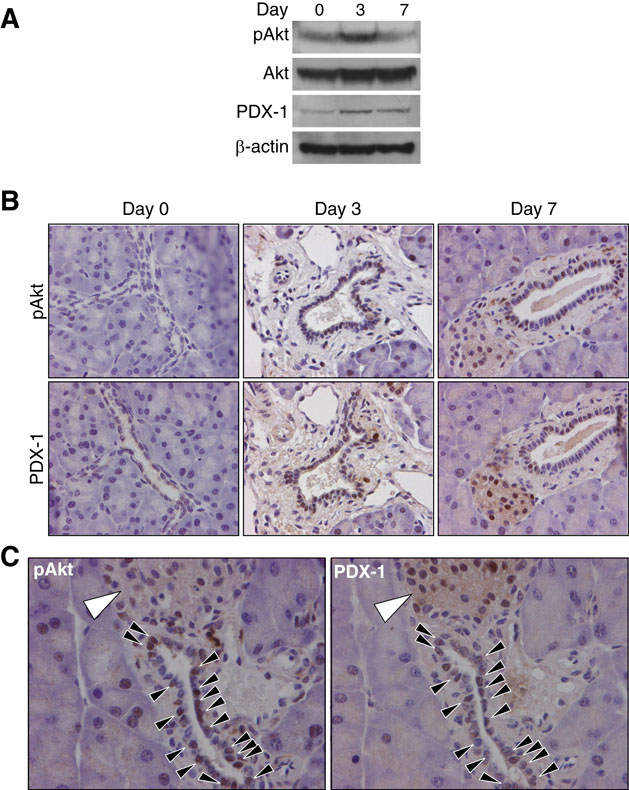
To determine whether PI3K/Akt activation may play a role in PDX-1 expression, we first assessed the remnant pancreas from mice after partial 75% Px for expression of both pAkt and PDX-1 by Western blot and immunohistochemistry. Western blot analysis on total protein samples from the remnant pancreas detected an increased expression of pAkt and PDX-1 after partial Px (Fig. 1A) confirming previous observations.8,12 Immunohistochemical analysis found that PDX-1 and pAkt were increased in pancreatic ducts on day 3 compared to day 0 after partial Px; both PDX-1 and pAkt were still detected in several ducts on day 7 after partial Px (Fig. 1B). Increased pAkt was also detected on day 3 in acinar cells as we have previously reported8 (data not shown). Analysis of serial sections of the remnant pancreas revealed that the majority of PDX-1 positive cells after partial Px were also positive for pAkt (Fig. 1C). These results demonstrate concomitant PI3K activation and PDX-1 induction in the ducts of regenerating pancreas.
Figure 1. Concomitant PDX-1 expression with Akt phosphorylation in remnant pancreas during pancreatic regeneration.
(A) Western blot analysis assessing expression of pAkt and PDX-1 in remnant pancreas at Day 0 (before surgery), and Day 3 and 7 after 75% partial Px. This figure shows one experiment testing 3 mice per group; results were similar in a repeated experiment. (B) The expression of pAkt and PDX-1 in remnant pancreas was immunohistochemically determined on Day 0, 3 and 7 after partial Px (magnification × 400). (C) Simultaneous expression of pAkt and PDX-1 in pancreatic duct cells was immunohistochemically determined on serial sections of remnant pancreatic tissues after partial Px. Closed arrow heads indicate pAkt or PDX-1 positive duct cells. Islets, which contain pAkt or PDX-1 positive cells, are indicated by large open arrow heads. Slides were counterstained with hematoxylin.
PI3K inhibition effectively blocks PDX-1 expression in regenerating pancreas
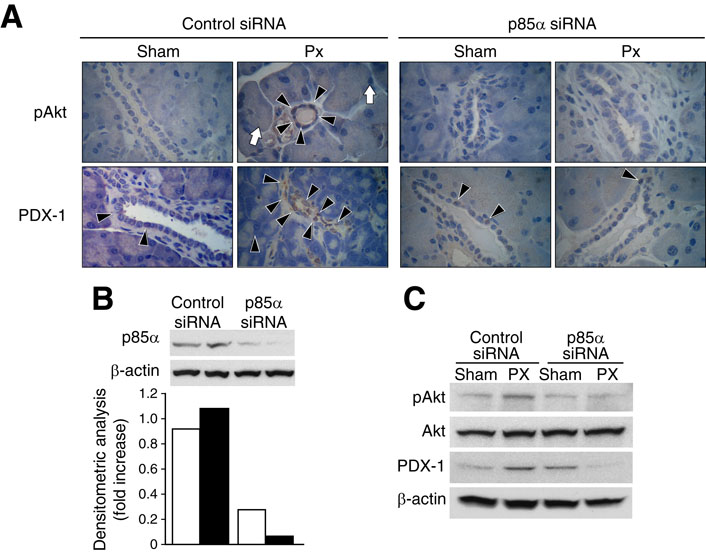
To examine whether inhibition of PI3K can block PDX-1 expression in pancreatic ducts during pancreatic regeneration, mice received either control siRNA or p85α siRNA and underwent either partial 75% Px or sham operation (n=3 per group). Both PDX-1 and pAkt were increased in the remnant pancreas of mice treated with control siRNA 3 days after partial Px; these increases were blocked by p85α siRNA (Fig. 2A). To confirm that treatment with p85α siRNA effectively suppressed pancreatic protein levels of p85α and pAkt, Western blot analysis was performed on total protein extracts. Treatment with p85α siRNA effectively reduced pancreatic p85α protein levels by approximately 80% (Fig. 2B). Moreover, p85α siRNA effectively blocked induction of pAkt and PDX-1 levels in the remnant pancreas of mice after partial Px (Fig. 2C). Taken together, these results demonstrate that the PI3K p85α regulatory subunit is important for PDX-1 expression in the remnant pancreas during pancreatic regeneration.
Figure 2. PI3K p85α regulatory subunit siRNA suppresses PDX-1 expression in remnant pancreas during pancreatic regeneration.
(A) Non-targeting control or p85α siRNA (20 μg/mouse) was delivered to mice by hydrodynamic tail vein injection 2 days prior to either partial Px or sham operation. All mice were sacrificed 3 days after the operation, and the remnant pancreas of Px-mice or the remnant equivalent tissue segments of sham-operated mice were collected, and Akt phosphorylation (pAkt) and PDX-1 expression assessed by immunohistochemistry (n=3 per group). Typical positive areas are indicated as follows: arrow head = pAkt or PDX-1 positive duct cell (magnification × 400), white arrow=pAkt positive acinar cells. Slides were counterstained with hematoxylin. (B) To confirm whether p85α siRNA effectively reduces target protein, p85α protein levels were assessed by Western blot; the blot was stripped and reprobed with β-actin. Densitometric analysis of the blot is shown in the bottom panel. Mice were sacrificed 2 days after siRNA delivery. (C) Western blot analysis of pAkt, Akt and PDX-1 expression in remnant pancreas was assessed 3 days after partial Px. Each lane represents pooled samples from 2 mice at the indicated times.
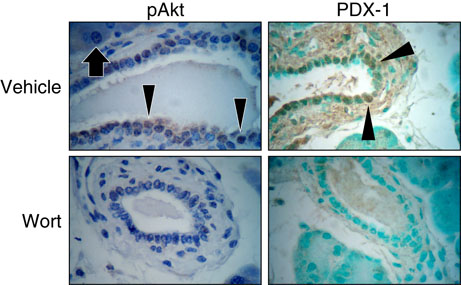
Using wortmannin, we confirmed the effect of PI3K inhibition on PDX-1 expression in the pancreatic ducts during pancreatic regeneration. Mice were treated with either wortmannin or vehicle, underwent partial Px or sham operation, and sacrificed 3 days later; pAkt and PDX-1 expression were determined in the remnant pancreas by immunohistochemistry (n=3 per group). In the vehicle-injected group, pAkt and PDX-1 were abundantly localized to duct and islet cells of the remnant pancreas 3 days after Px (Fig. 3). However, treatment with wortmannin effectively blocked both pAkt and induction of PDX-1 in the ducts of the remnant pancreas (Fig. 3); pAkt and PDX-1 expression in islet cells was similar to the control (i.e., vehicle injection) group (data not shown).
Figure 3. PI3K inhibitor wortmannin suppresses PDX-1 expression in remnant pancreas during pancreatic regeneration.
The effect of PI3K inhibition by wortmannin (Wort) on pAkt and PDX-1 expression in duct cells of remnant pancreas was assessed by immunohistochemistry as described in Fig. 1. Mice underwent partial Px and sacrificed 3 days after operation (n=3 per group). Arrow heads indicate typical pAkt or PDX-1 positive duct cell (magnification × 400). Arrows indicates pAkt-positive acinar cell. Slides were counterstained with hematoxylin.
Taken together, these complementary techniques utilizing the chemical inhibitor wortmannin and p85α siRNA demonstrate that PI3K activation plays an important role in induction of PDX-1 expression in the pancreatic ducts.
Wortmannin blocks appearance of insulin-positive duct cells in remnant pancreas during pancreatic regeneration
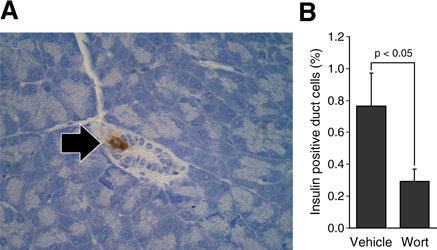
Next, we examined the effect of PI3K inhibition on the differentiation of pancreatic duct cells to insulin-positive cells. Young adult mice underwent either partial 75% Px or sham operation (n=12 per group); each group was further subdivided to receive either vehicle (n=5) or wortmannin (n=7), an irreversible PI3K inhibitor. Mice were sacrificed 3 days after the surgery, and insulin-positive duct cells were immunohistochemically analyzed in the remnant pancreas from the Px groups and the remnant equivalent tissues from the sham-operated groups (Fig. 4). As previously reported,20 insulin-positive duct cells are observed in the remnant pancreas of mice after partial Px and saline injections; however, wortmannin significantly reduced the number of insulin-positive duct cells in the remnant pancreas (p<0.05). In total, 4761 and 9209 duct cells were counted in the control (ie, saline injection) and wortmannin groups with 35 (0.73%) and 25 (0.27%) duct cells positive for insulin staining, respectively. There were no insulin-positive duct cells in the pancreas of sham-operated mice. These findings suggest that the PI3K signaling pathway plays a role in the differentiation of pancreatic duct cells to insulin-positive cells during pancreatic regeneration.
Figure 4. Wortmannin, a pharmacological PI3K inhibitor, significantly reduced the induction of insulin-positive duct cells in remnant pancreas after partial Px.
(A) An example of immunohistochemistry detecting an insulin-positive cell (arrow) in the duct of regenerating remnant pancreas 3 days after partial Px. (B) Insulin-positive duct cell index was calculated in pancreatic sections from mice after partial Px mice that were injected either with wortmannin (wort: 0.75mg/kg, i.p.) (n = 7) or vehicle (physiological saline with 5% ethanol) (n = 5) 2 h before the operation and then every 12 h thereafter. Mice were sacrificed 3 days after the surgery, and three independent sections were prepared from each mouse. All insulin-positive duct cells were counted in one section and insulin-positive duct cell index was calculated by division of all pancreatic duct cells in the same section (Values are mean ± SEM; * = p < 0.05).
DISCUSSION
The molecular mechanisms of pancreatic duct cell differentiation into islet cells in adult animals have not been clearly defined. Although PI3K is a critical step for proliferation of various cell types and insulin signaling, its role in duct differentiation into endocrine cells is not known. pAkt, an indicator of PI3K activation, is significantly increased in the remnant pancreas during pancreatic regeneration after partial Px.8 Expression of PDX-1 is increased in remnant pancreas a few days after partial Px.12 The PI3K pathway, but not the p38 MAPK pathway, has an important role in glucose-stimulated preproinsulin gene transcription and nuclear trans-location of PDX-1 in islet β-cells.33 Since duct-specific pAkt and induction of PDX-1 in remnant pancreas has not been studied, we examined whether pAkt and induction of PDX-1 occurs in duct cells during pancreatic regeneration. We found that PI3K/Akt activation (as assessed by Akt phosphorylation) and PDX-1 induction occurred in the ducts concomitantly during pancreatic regeneration, and that inhibition of PI3K suppressed expression PDX-1 in duct cells during pancreatic regeneration.
Partial Px induces pancreatic regeneration and islet neogenesis,34 insulin-positive cells are detected in the duct of the remnant pancreas during pancreatic regeneration.20 Given the role of PI3K/Akt in acinar cell regeneration noted in our previous study,8 we next hypothesized that PI3K activation is important for duct-derived islet cell neogenesis during partial Px-induced pancreatic regeneration. Our result demonstrated that inhibition of PI3K effectively blocked duct cell differentiation into insulin-producing cells in the regenerating pancreas. These results clearly show that activation of the PI3K pathway plays a critical role in PDX-1 induction in pancreatic ducts during pancreatic regeneration. PDX-1 is an important factor for the pancreatic duct cell differentiation; pancreatic ductal-derived cancer cells, Panc-1, differentiated to insulin-producing cells upon forceful expression of exogenous PDX-1.25 Thus, our results also suggest a possible involvement of the PI3K pathway in duct cell differentiation into insulin-producing cells.
There may be two distinct roles for PI3K activation in the ducts during pancreatic regeneration. One role is to promote differentiation of pancreatic duct cells into insulin-producing cells. This function requires induction of PDX-1 in the duct cells and is clearly supported by our results with wortmannin and p85α-siRNA. The second role for PI3K activation in the ducts may be to promote proliferation of duct cells for tissue regeneration. As we have previously reported, activation of the PI3K pathway is critical for acinar cell proliferation during pancreatic regeneration.8 Thus, it is reasonable to speculate that PI3K has a similar function for duct cells. In our previous study, we also found increased phosphorylation of ERK in the ducts during pancreatic regeneration raising a possibility that MAPK/ERK plays a role in the ducts during pancreatic regeneration. The involvement of PI3K and/or MAPK pathway in the duct cell proliferation during pancreatic regeneration is currently being evaluated.
PI3K is divided into three classes (ie, Class I, II and III). Class IA PI3K, which is composed of a p85 regulatory and p110 catalytic subunit in mammalian cells, regulates cell proliferation, cell survival and apoptosis in various cell types.1 In this study, we clearly demonstrate that Class IA PI3K plays a central role in PDX-1 expression in pancreatic duct cell during pancreatic regeneration. At present, it is not known whether other classes of PI3K also mediate PDX-1 expression in duct cells. Blocking specific PI3K subunits by RNA interference techniques should be able to answer this question.
The physiological significance of the differentiation of pancreatic duct cells into insulin-producing cells is not currently clear. While islet β-cell mass is replenished mostly by β-cell replication, a certain role for the neogenesis of β-cells from stem cells cannot be excluded.15-18 The occasional detection of insulin in the pancreatic duct epithelium by a number of investigators has led to the hypothesis that the duct cells possess a stem cell-like function to differentiate into endocrine cells under certain conditions (eg, regeneration).19, 20 Detection of insulin-positive duct cells only after partial Px noted in our study supports this hypothesis.
Our current study strongly suggests that activation of PI3K, especially Class IA PI3K, plays a critical role in PDX-1 expression and differentiation of pancreatic duct cells into insulin-secreting cells. These findings provide a further understanding of the molecular mechanisms of pancreatic duct cell differentiation in the adult pancreas following physiologic stimulation. Consistent with our previous findings8, it is possible that PI3K/Akt activation is critical for not only pancreatic acinar cell regeneration but also duct cell proliferation following partial pancreatectomy. These findings further support the important role of PI3K in the regulation of physiologically stimulated growth of various organs including the pancreas, intestinal mucosa and heart.8, 35-38
ACKNOWLEDGEMENTS
We thank Ms. Karen Martin for manuscript preparation and Mr. Tatsuo Uchida for statistical analysis. This work was supported from grants from P01DK35608, R37AG10885, R01DK48498 and R01AG025273 from the National Institutes of Health.
REFERENCES
- 1.Vanhaesebroeck B, Leevers SJ, Ahmadi K, et al. Synthesis and function of 3-phosphorylated inositol lipids. Annu Rev Biochem. 2001;70:535–602. doi: 10.1146/annurev.biochem.70.1.535. [DOI] [PubMed] [Google Scholar]
- 2.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 3.Burks DJ, White MF. IRS proteins and beta-cell function. Diabetes. 2001;50(Suppl 1):S140–145. doi: 10.2337/diabetes.50.2007.s140. [DOI] [PubMed] [Google Scholar]
- 4.Hugl SR, White MF, Rhodes CJ. Insulin-like growth factor I (IGF-I)-stimulated pancreatic beta-cell growth is glucose-dependent. Synergistic activation of insulin receptor substrate-mediated signal transduction pathways by glucose and IGF-I in INS-1 cells. J Biol Chem. 1998;273:17771–17779. doi: 10.1074/jbc.273.28.17771. [DOI] [PubMed] [Google Scholar]
- 5.White MF. The insulin signalling system and the IRS proteins. Diabetologia. 1997;40(Suppl 2):S2–17. doi: 10.1007/s001250051387. [DOI] [PubMed] [Google Scholar]
- 6.Williams JA. Intracellular signaling mechanisms activated by cholecystokinin-regulating synthesis and secretion of digestive enzymes in pancreatic acinar cells. Annu Rev Physiol. 2001;63:77–97. doi: 10.1146/annurev.physiol.63.1.77. [DOI] [PubMed] [Google Scholar]
- 7.Zawalich WS, Tesz GJ, Zawalich KC. Inhibitors of phosphatidylinositol 3-kinase amplify insulin release from islets of lean but not obese mice. J Endocrinol. 2002;174:247–258. doi: 10.1677/joe.0.1740247. [DOI] [PubMed] [Google Scholar]
- 8.Watanabe H, Saito H, Rychahou PG, et al. Aging is associated with decreased pancreatic acinar cell regeneration and phosphatidylinositol 3-kinase/Akt activation. Gastroenterology. 2005;128:1391–1404. doi: 10.1053/j.gastro.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 9.Bonner-Weir S, Trent DF, Weir GC. Partial pancreatectomy in the rat and subsequent defect in glucose-induced insulin release. J Clin Invest. 1983;71:1544–1553. doi: 10.1172/JCI110910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pearson KW, Scott D, Torrance B. Effects of partial surgical pancreatectomy in rats. I. Pancreatic regeneration. Gastroenterology. 1977;72:469–473. [PubMed] [Google Scholar]
- 11.Sumi S, Tamura K. Frontiers of pancreas regeneration. J Hepatobiliary Pancreat Surg. 2000;7:286–294. doi: 10.1007/s005340070050. [DOI] [PubMed] [Google Scholar]
- 12.Sharma A, Zangen DH, Reitz P, et al. The homeodomain protein IDX-1 increases after an early burst of proliferation during pancreatic regeneration. Diabetes. 1999;48:507–513. doi: 10.2337/diabetes.48.3.507. [DOI] [PubMed] [Google Scholar]
- 13.Bouwens L. Islet morphogenesis and stem cell markers. Cell Biochem Biophys. 2004;40:81–88. doi: 10.1385/cbb:40:3:81. [DOI] [PubMed] [Google Scholar]
- 14.Jensen J. Gene regulatory factors in pancreatic development. Dev Dyn. 2004;229:176–200. doi: 10.1002/dvdy.10460. [DOI] [PubMed] [Google Scholar]
- 15.Weir GC, Laybutt DR, Kaneto H, et al. Beta-cell adaptation and decompensation during the progression of diabetes. Diabetes. 2001;50(Suppl 1):S154–159. doi: 10.2337/diabetes.50.2007.s154. [DOI] [PubMed] [Google Scholar]
- 16.Hayashi KY, Tamaki H, Handa K, et al. Differentiation and proliferation of endocrine cells in the regenerating rat pancreas after 90% pancreatectomy. Arch Histol Cytol. 2003;66:163–174. doi: 10.1679/aohc.66.163. [DOI] [PubMed] [Google Scholar]
- 17.Bonner-Weir S. Islet growth and development in the adult. J Mol Endocrinol. 2000;24:297–302. doi: 10.1677/jme.0.0240297. [DOI] [PubMed] [Google Scholar]
- 18.Dor Y, Brown J, Martinez OI, et al. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- 19.Paris M, Tourrel-Cuzin C, Plachot C, et al. Review: pancreatic beta-cell neogenesis revisited. Exp Diabesity Res. 2004;5:111–121. doi: 10.1080/15438600490455079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zenilman ME, Perfetti R, Swinson K, et al. Pancreatic regeneration (reg) gene expression in a rat model of islet hyperplasia. Surgery. 1996;119:576–584. doi: 10.1016/s0039-6060(96)80270-4. [DOI] [PubMed] [Google Scholar]
- 21.Melloul D, Marshak S, Cerasi E. Regulation of pdx-1 gene expression. Diabetes. 2002;51(Suppl 3):S320–325. doi: 10.2337/diabetes.51.2007.s320. [DOI] [PubMed] [Google Scholar]
- 22.Miller CP, McGehee RE, Jr., Habener JF. IDX-1: a new homeodomain transcription factor expressed in rat pancreatic islets and duodenum that transactivates the somatostatin gene. Embo J. 1994;13:1145–1156. doi: 10.1002/j.1460-2075.1994.tb06363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohlsson H, Karlsson K, Edlund T. IPF1, a homeodomain-containing transactivator of the insulin gene. Embo J. 1993;12:4251–4259. doi: 10.1002/j.1460-2075.1993.tb06109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hui H, Perfetti R. Pancreas duodenum homeobox-1 regulates pancreas development during embryogenesis and islet cell function in adulthood. Eur J Endocrinol. 2002;146:129–141. doi: 10.1530/eje.0.1460129. [DOI] [PubMed] [Google Scholar]
- 25.Hui H, Wright C, Perfetti R. Glucagon-like peptide 1 induces differentiation of islet duodenal homeobox-1-positive pancreatic ductal cells into insulin-secreting cells. Diabetes. 2001;50:785–796. doi: 10.2337/diabetes.50.4.785. [DOI] [PubMed] [Google Scholar]
- 26.Petersen HV, Serup P, Leonard J, et al. Transcriptional regulation of the human insulin gene is dependent on the homeodomain protein STF1/IPF1 acting through the CT boxes. Proc Natl Acad Sci U S A. 1994;91:10465–10469. doi: 10.1073/pnas.91.22.10465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferber S, Halkin A, Cohen H, et al. Pancreatic and duodenal homeobox gene 1 induces expression of insulin genes in liver and ameliorates streptozotocin-induced hyperglycemia. Nat Med. 2000;6:568–572. doi: 10.1038/75050. [DOI] [PubMed] [Google Scholar]
- 28.Taniguchi H, Yamato E, Tashiro F, et al. beta-cell neogenesis induced by adenovirus-mediated gene delivery of transcription factor pdx-1 into mouse pancreas. Gene Ther. 2003;10:15–23. doi: 10.1038/sj.gt.3301846. [DOI] [PubMed] [Google Scholar]
- 29.Li M, Miyagawa J, Moriwaki M, et al. Analysis of expression profiles of islet-associated transcription and growth factors during beta-cell neogenesis from duct cells in partially ductligated mice. Pancreas. 2003;27:345–355. doi: 10.1097/00006676-200311000-00013. [DOI] [PubMed] [Google Scholar]
- 30.Scacheri PC, Rozenblatt-Rosen O, Caplen NJ, et al. Short interfering RNAs can induce unexpected and divergent changes in the levels of untargeted proteins in mammalian cells. Proc Natl Acad Sci U S A. 2004;101:1892–1897. doi: 10.1073/pnas.0308698100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song E, Lee SK, Wang J, et al. RNA interference targeting Fas protects mice from fulminant hepatitis. Nat Med. 2003;9:347–351. doi: 10.1038/nm828. [DOI] [PubMed] [Google Scholar]
- 32.Zhang G, Budker V, Wolff JA. High levels of foreign gene expression in hepatocytes after tail vein injections of naked plasmid DNA. Hum Gene Ther. 1999;10:1735–1737. doi: 10.1089/10430349950017734. [DOI] [PubMed] [Google Scholar]
- 33.Rafiq I, da Silva Xavier G, Hooper S, et al. Glucose-stimulated preproinsulin gene expression and nuclear trans-location of pancreatic duodenum homeobox-1 require activation of phosphatidylinositol 3-kinase but not p38 MAPK/SAPK2. J Biol Chem. 2000;275:15977–15984. doi: 10.1074/jbc.275.21.15977. [DOI] [PubMed] [Google Scholar]
- 34.Lipsett M, Finegood DT. beta-cell neogenesis during prolonged hyperglycemia in rats. Diabetes. 2002;51:1834–1841. doi: 10.2337/diabetes.51.6.1834. [DOI] [PubMed] [Google Scholar]
- 35.Luo J, McMullen JR, Sobkiw CL, et al. Class IA phosphoinositide 3-kinase regulates heart size and physiological cardiac hypertrophy. Mol Cell Biol. 2005;25:9491–9502. doi: 10.1128/MCB.25.21.9491-9502.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McMullen JR, Shioi T, Zhang L, et al. Phosphoinositide 3-kinase(p110alpha) plays a critical role for the induction of physiological, but not pathological, cardiac hypertrophy. Proc Natl Acad Sci U S A. 2003;100:12355–12360. doi: 10.1073/pnas.1934654100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ueda J, Saito H, Watanabe H, et al. Novel and quantitative DNA dot-blotting method for assessment of in vivo proliferation. Am J Physiol Gastrointest Liver Physiol. 2005;288:G842–847. doi: 10.1152/ajpgi.00463.2004. [DOI] [PubMed] [Google Scholar]
- 38.Sheng H, Shao J, Townsend CM, Jr., et al. Phosphatidylinositol 3-kinase mediates proliferative signals in intestinal epithelial cells. Gut. 2003;52:1472–1478. doi: 10.1136/gut.52.10.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]