Abstract
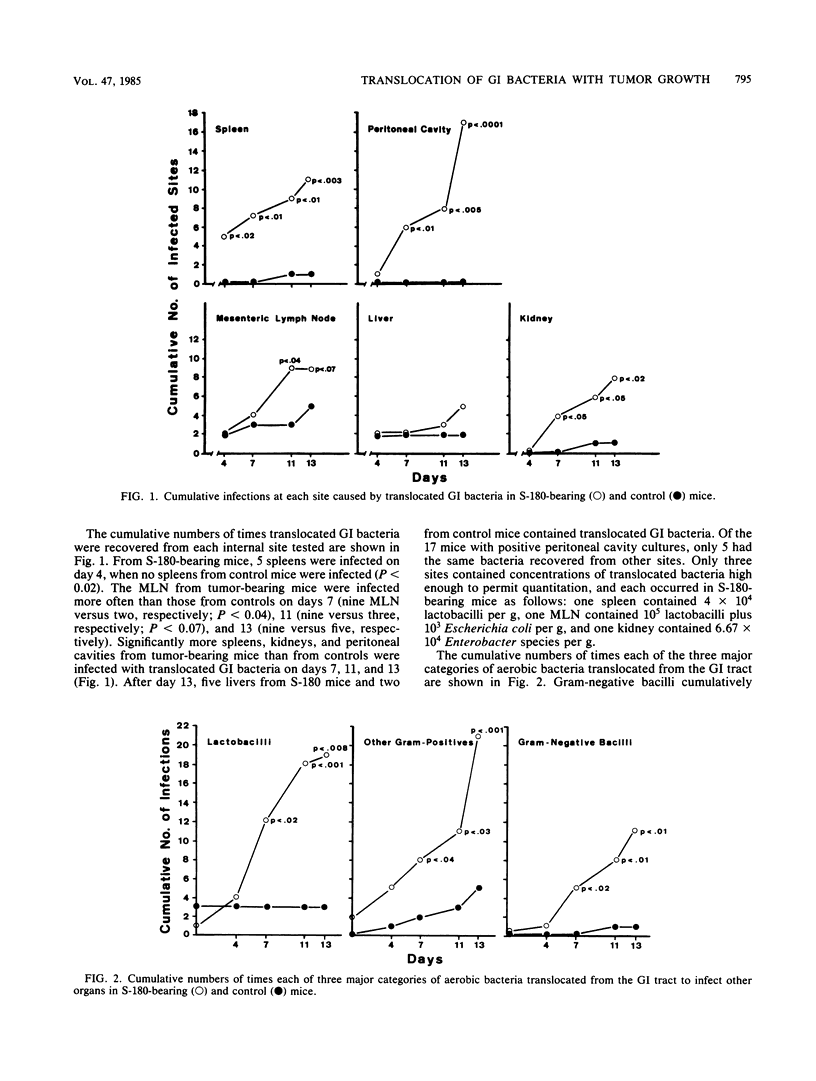
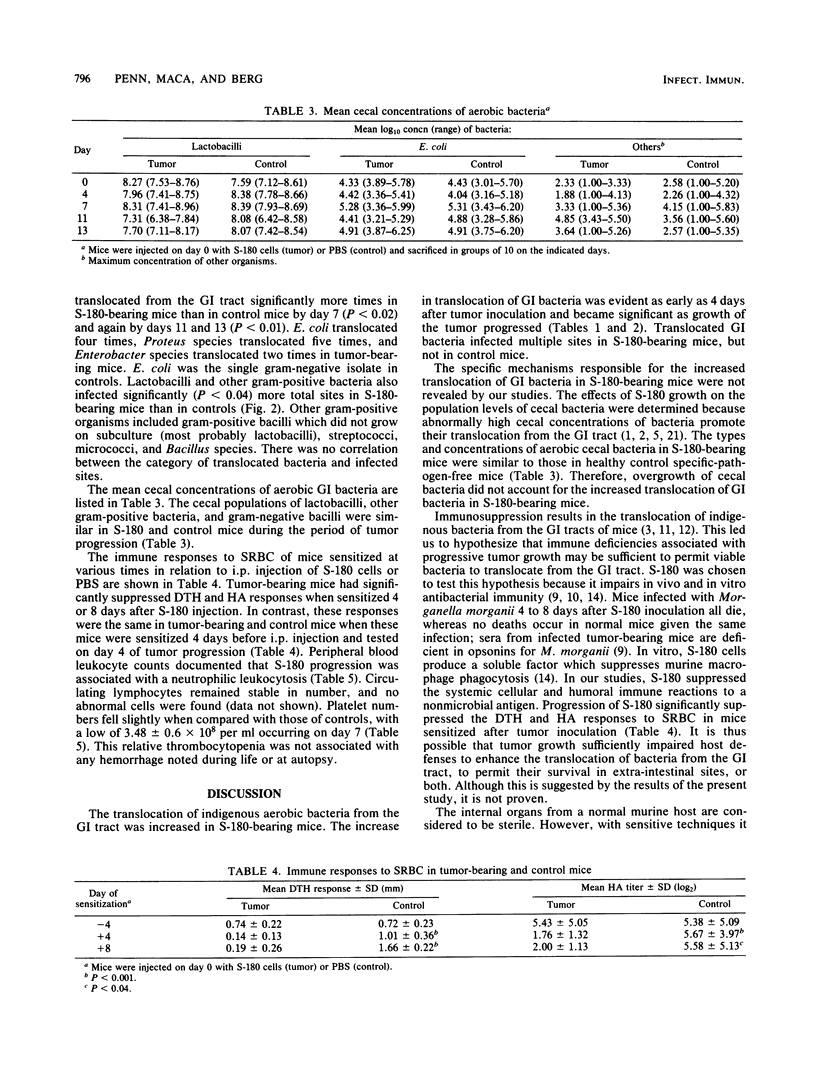
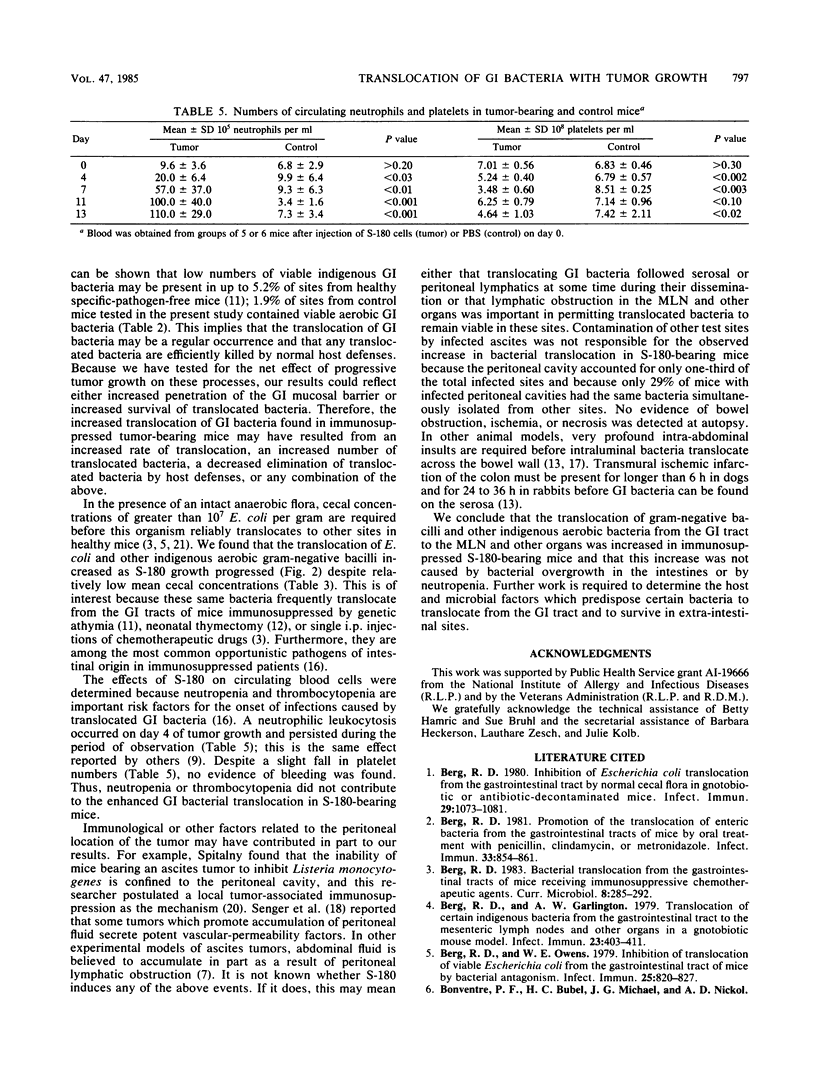
Aerobic gram-negative bacilli and other indigenous gastrointestinal (GI) bacteria are important opportunistic pathogens in immunosuppressed cancer patients. These same bacteria frequently translocate from the GI tracts of mice immunosuppressed by single injections of certain anticancer drugs or by T-lymphocyte impairments. Since similar cellular and humoral immune deficiencies may be present in the tumor-bearing host, we sought to determine if progressive growth of a tumor alone would be sufficient to enhance the translocation of indigenous bacteria from the murine GI tract. Pathogen-free DBA/2 mice were injected intraperitoneally with 10(6) viable sarcoma 180 (S-180) cells or 0.5 ml of sterile buffer. Mesenteric lymph nodes, livers, spleens, and kidneys were tested for the presence of translocated aerobic GI bacteria on various days after tumor injection. Immunity was assessed by measuring footpad delayed-type hypersensitivity and serum hemagglutinins to sheep erythrocytes. Overall, translocated aerobic GI bacteria infected 33 of 92 S-180-bearing mice (36%) and only 9 of 99 control mice (9%) (P less than 10(-6)). Cumulatively, 50 of 460 sites (10.9%) in S-180-bearing mice were infected with translocated GI bacteria as opposed to only 9 of 485 sites (1.9%) in control animals (P less than 10(-7)). GI bacteria often translocated to infect more than one site in tumor-bearing mice, but not in controls. Aerobic gram-negative bacilli translocated 11 times in tumor-bearing mice, but only once in controls, even though the mean cecal population levels of these bacteria were relatively low (range, 4.33 to 5.28 log10 bacteria per g). The population levels of cecal aerobic bacteria were similar in S-180 and control mice throughout the period of observation. S-180 mice had significantly suppressed (P less than 0.04) delayed-type hypersensitivity and serum hemagglutinin responses when sensitized 4 or 8 days after S-180 injection. S-180 growth was associated with a neutrophilic leukocytosis and a slight drop in platelet counts; no bleeding was detected. Thus, the translocation of gram-negative bacilli and other indigenous aerobic bacteria from the GI tract to the mesenteric lymph nodes and other organs was increased in immunosuppressed S-180-bearing mice, and this increase was not caused by bacterial overgrowth in the intestines or by neutropenia.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg R. D., Garlington A. W. Translocation of certain indigenous bacteria from the gastrointestinal tract to the mesenteric lymph nodes and other organs in a gnotobiotic mouse model. Infect Immun. 1979 Feb;23(2):403–411. doi: 10.1128/iai.23.2.403-411.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg R. D. Inhibition of Escherichia coli translocation from the gastrointestinal tract by normal cecal flora in gnotobiotic or antibiotic-decontaminated mice. Infect Immun. 1980 Sep;29(3):1073–1081. doi: 10.1128/iai.29.3.1073-1081.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg R. D., Owens W. E. Inhibition of translocation of viable Escherichia coli from the gastrointestinal tract of mice by bacterial antagonism. Infect Immun. 1979 Sep;25(3):820–827. doi: 10.1128/iai.25.3.820-827.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg R. D. Promotion of the translocation of enteric bacteria from the gastrointestinal tracts of mice by oral treatment with penicillin, clindamycin, or metronidazole. Infect Immun. 1981 Sep;33(3):854–861. doi: 10.1128/iai.33.3.854-861.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman G. B., Knapp R. C., Order S. E., Hellman S. The role of lymphatic obstruction in the formation of ascites in a murine ovarian carcinoma. Cancer Res. 1972 Aug;32(8):1663–1666. [PubMed] [Google Scholar]
- GRAY D. F., JENNINGS P. A. Allergy in experimental mouse tuberculosis. Am Rev Tuberc. 1955 Aug;72(2):171–195. doi: 10.1164/artpd.1955.72.2.171. [DOI] [PubMed] [Google Scholar]
- Kawaharajo K., Shitoh K., Kazuno Y., Sekizawa Y. Infections in compromised hosts: modified susceptibility of tumor-bearing mice to experimental infection with Proteus morganii strain 1510. Nihon Juigaku Zasshi. 1982 Oct;44(5):743–749. doi: 10.1292/jvms1939.44.743. [DOI] [PubMed] [Google Scholar]
- Matsuo K., Takeya K., Nomoto K., Shimotori S., Terasaka R. T-cell-independent activation of macrophages by viable BCG in tumor-bearing mice. Cell Immunol. 1981 Jan 15;57(2):293–306. doi: 10.1016/0008-8749(81)90088-5. [DOI] [PubMed] [Google Scholar]
- Owens W. E., Berg R. D. Bacterial translocation from the gastrointestinal tract of athymic (nu/nu) mice. Infect Immun. 1980 Feb;27(2):461–467. doi: 10.1128/iai.27.2.461-467.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papa M., Halperin Z., Rubinstein E., Orenstein A., Gafin S., Adar R. The effect of ischemia of the dog's colon on transmural migration of bacteria and endotoxin. J Surg Res. 1983 Sep;35(3):264–269. doi: 10.1016/s0022-4804(83)80013-4. [DOI] [PubMed] [Google Scholar]
- SCHAEDLER R. W., DUBOS R., COSTELLO R. THE DEVELOPMENT OF THE BACTERIAL FLORA IN THE GASTROINTESTINAL TRACT OF MICE. J Exp Med. 1965 Jul 1;122:59–66. doi: 10.1084/jem.122.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWEINBURG F. B., SELIGMAN A. M., FINE J. Transmural migration of intestinal bacteria; a study based on the use of radioactive Escherichia coli. N Engl J Med. 1950 May 11;242(19):747–751. doi: 10.1056/NEJM195005112421903. [DOI] [PubMed] [Google Scholar]
- Saito H., Tomioka H. Suppressive factor of tumour origin against macrophage phagocytosis of Staphylococcus aureus. Br J Cancer. 1980 Feb;41(2):259–267. doi: 10.1038/bjc.1980.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimpff S. C. Infection prevention during profound granulocytopenia. New approaches to alimentary canal microbial suppression. Ann Intern Med. 1980 Aug;93(2):358–361. doi: 10.7326/0003-4819-93-2-358. [DOI] [PubMed] [Google Scholar]
- Senger D. R., Galli S. J., Dvorak A. M., Perruzzi C. A., Harvey V. S., Dvorak H. F. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983 Feb 25;219(4587):983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- Smith P. B., Tomfohrde K. M., Rhoden D. L., Balows A. API system: a multitube micromethod for identification of Enterobacteriaceae. Appl Microbiol. 1972 Sep;24(3):449–452. doi: 10.1128/am.24.3.449-452.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitalny G. L. Suppression of bactericidal activity of macrophages in ascites tumors. J Reticuloendothel Soc. 1980 Sep;28(3):223–235. [PubMed] [Google Scholar]
- Steffen E. K., Berg R. D. Relationship between cecal population levels of indigenous bacteria and translocation to the mesenteric lymph nodes. Infect Immun. 1983 Mar;39(3):1252–1259. doi: 10.1128/iai.39.3.1252-1259.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]


