Abstract
Periodic pulses of the insect steroid molting hormone 20-hydroxyecdysone (20E), acting via its nuclear receptor complex (EcR/USP), control gene expression at many stages throughout Drosophila development. However, during the last larval instar of some lepidopteran insects, subtle changes in titers of ecdysteroids have been documented, including the so-called "commitment peak". This small elevation of 20E reprograms the larva for metamorphosis to the pupa. Similar periods of ecdysteroid immunoreactivity have been observed during the last larval instar of Drosophila. However, due to low amplitude and short duration, along with small body size and staging difficulties, their timing and ecdysteroid composition have remained uncertain. Employing a rigorous regimen of Drosophila culture and a salivary gland reporter gene, Sgs3-GFP, we used RP-HPLC and differential ecdysteroid RIA analysis to determine whole body titers of 20E during the last larval instar. Three small peaks of 20E were observed at 8, 20 and 28 hr following ecdysis, prior to the well-characterized large peak around the time of pupariation. The possible regulation of 20E levels by biosynthetic P450 enzymes and the roles of these early peaks in coordinating gene expression and late larval development are discussed.
Keywords: ecdysteroid, ecdysone-responsive genes, Sgs3, GFP, CYP enzyme, Disembodied, Phantom, metamorphosis, RP-HPLC, RIA
INTRODUCTION
The importance of the arthropod molting hormone, 20-hydroxyecdysone (20E), to the success of insects and crustaceans is well accepted, as is the concept that this hormone basically directs the development of every tissue in the arthropod body. Exactly how 20E achieves this monumental task is not known with certainty despite a great deal of research on Drosophila melanogaster, much of which has focused on 20E regulation of specific transcriptional activities via the nuclear receptor dimer, EcR/USP (see Henrich, 2005). These studies utilized the application of 20E both in vivo and in vitro, but the precise titer of 20E at critical developmental stages is not known with certainty.
There have been numerous ecdysteroid (but not 20E) titers reported for Drosophila, usually using immunoassay with one of several available antisera made to various regions of the ecdysone (E) or 20E molecules (see Warren and Gilbert, 1988). This approach has limitations since the antibodies have different affinities for specific ecdysteroids, including 20E and E, the latter being the immediate precursor of 20E. Furthermore, conditions for raising the insects varied considerably and, in general, staging efforts failed to maintain developmental synchronization during the four days of larval development.
Thus, it is not surprising that the published studies present ecdysteroid titers with different numbers and developmental timing (see Richards, 1981; Berreur et al., 1979, 1984; Schwartz et al., 1984; Parvy et al., 2005). The number, timing and composition of low-amplitude elevations during the feeding stage of the last (third) larval instar are of importance because one or more may be analogous to the commitment peak of Lepidoptera (Bollenbacher et al., 1975; Riddiford, 1976; Wolfgang and Riddiford, 1986; Parlak et al., 1992; Dai et al., 1995). Although relatively small, the commitment peak is of great physiological significance because it is required to reprogram cells and tissues, so that when challenged by the subsequent large ecdysteroid peak, a metamorphic molt is initiated.
To determine if Drosophila too has a commitment peak by physiological, biochemical or molecular genetics paradigms, it is first necessary to determine if the peak exists. Furthermore, as noted above, even though ecdysteroid peaks have been demonstrated, these can represent large numbers of inactive steroid molecules (see Lafont et al., 2005) rather than the active molting hormone, 20E. Here, we have combined optimal culture and staging methods with differential radioimmunoassay (RIA) analysis and reverse-phase high performance liquid chromatography (RP-HPLC) to achieve a 20E titer during the third larval instar that should be of great value to investigators in the field of insect hormone action.
RESULTS
Larval growth curve and ecdysteroid titer
Because we suspected that insufficient developmental synchrony and staging precision contributed to the variable results among previous reports of third instar ecdysteroid titers, considerable effort was made to optimize Drosophila larval culture for these experiments. For example, pilot studies revealed that constant high humidity was essential for optimal larval health and developmental synchrony. Constant light is known to suppress circadian rhythmicity (Saunders et al., 2002; Taghert and Lee, 2005) and also allows for convenient "round-the-clock" access to all stages. This was absolutely necessary in order to accumulate large numbers of animals at 2- to 4-hour intervals for the HPLC/RIA compositional analyses.
Even among animals that are optimally grown, staging only once at the time of ecdysis from the second instar likely results in de-synchronization over the approximately two days until the time of puparium formation, which could result in missing low-amplitude peaks. Therefore, we took advantage of the well-characterized onset of salivary gland glue gene expression about midway through the third instar (Andres et al., 1993). Specifically, we used a single copy of a transgene expressing an SGS3:GFP fusion protein under the control of Sgs3 (Biyasheva et al., 2001) in an otherwise wild-type (Canton-S) background. Under our culture conditions, GFP in the salivary glands first became detectable through the body wall at 24 hr after ecdysis to the third instar, with a steady distal-to-proximal accumulation over the next 6 hr. The time of onset of GFP accumulation within distal salivary gland cells was about 4 hr earlier and less variable than that previously reported, probably because those larvae had been synchronized only at the time of hatching to the first instar (Biyasheva et al., 2001). Use of the GFP reporter allowed for a convenient visual re-screening at 24 hr for the initiation of glue protein synthesis, thus increasing the synchrony of larvae collected in the second half of the third instar. Prior to initiating this study, individuals of this genotype (w/+ or w/Y; P[Sgs3-GFP]2/+) were assayed for whole body ecdysteroids by RIA at the white puparium (WP) stage and found to have values very comparable to those of wild type (Canton-S) animals at the same stage (data not shown). As detailed below, we believe that these methods, when combined with efficient ecdysteroid extraction, chromatographic separation and differential radioimmunoassay-based characterization, resulted in an improved determination of 20E titers during the third instar.
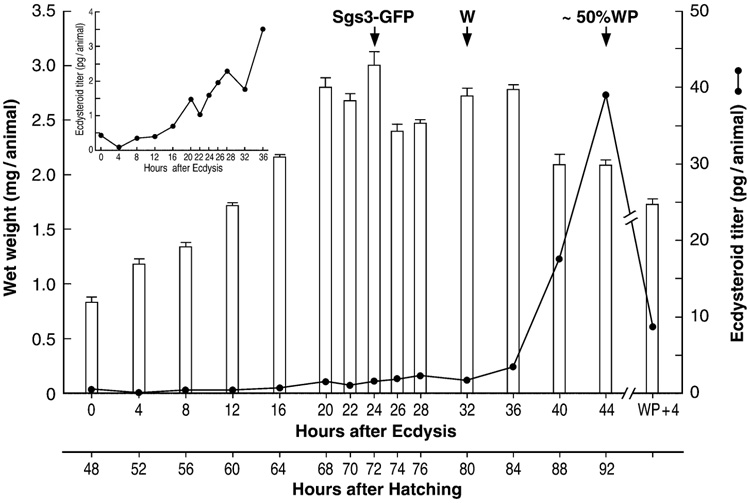
The average wet weight for each replicate sample of 30–40 larvae, collected every 2–4 hr during the third instar, and the resulting total ecdysteroid titer measured with the SHO3 antibody are shown in Fig. 1. The very small standard errors during the initial 24 hours of the growth curve (Figure 1) are indicative of both the precise synchrony and staging of the larvae and the optimum conditions in which they were cultured. Growth was very rapid during the first day, wherein the larval weight doubled almost every 12 hr (see Ashburner, 1989), achieving a maximum weight of about 3 mg/larva at 24 hr after ecdysis. At 24 hr, only those animals showing SGS3:GFP fusion protein expression in their distal salivary glands were selected. During the second half of the instar, the standard error of mean body weight remained low and almost 50% of the animals pupariated within a 0.5–1.0 hr period (100% in 4 hr), suggesting continued synchronous development. Under such conditions, these larvae began to leave the food and wander (W) at 32 hr, and by 44 hr almost 50% had completed white puparium formation and were designated as white puparia (WP44). All animals had completed puparium formation by 48 hr. The white puparium stage lasts only about 20–30 min, as subsequent tanning of the puparium signals formation of the prepupa. The WP+4 hr sample represents animals just prior to ecdysis to the pupa; apolysis, including head eversion, would occur about 8 hr thereafter.
Figure 1.
Larval growth curve and crude ecdysteroid titer. Shown are the mean wet weight (mg/animal +/− SE) and the whole body ecdysteroid titer in pg ecdysteroid/animal, as measured using the SHO3 antiserum, during the third larval instar. Sgs3-GFP denotes the time that the Sgs3-GFP fusion protein becomes significantly expressed in the larval salivary glands. (W), beginning of wandering; (~50% WP), about 50% of animals had formed white puparia (WP) by 44 hr after ecdysis. Their titer was identical to that of the larvae at 44 hr. (WP + 4), 4 hr after formation of a white puparia. Inset was included to clarify the small multiple titer elevations and peaks occurring at 8, 20 and 28 hr.
Following ecdysis from the second instar, the ecdysteroid titer measured by the SHO3 antiserum declined to a low baseline value of less than 0.2 pg E-equivalents/larva by 4 hr (Fig. 1). Beginning at 8 hr, and except for minor apparent reductions at 22 and 32 hr (Fig. 1 inset), the titer then began a slow monotonic increase, achieving a level of about 3.5 pg/larva by 36 hr. Over the next 8 hr, the titer increased rapidly, achieving a maximum of 40 pg/animal in both the larval (44) and white puparium (WP44) samples taken at 44 hr after ecdysis (and in additional WP samples), only to fall back even more dramatically in WP+4 pre-pupae. The titer measured with the H22 antibody was generally higher (data not shown; see Discussion).
RP-HPLC/RIA analysis
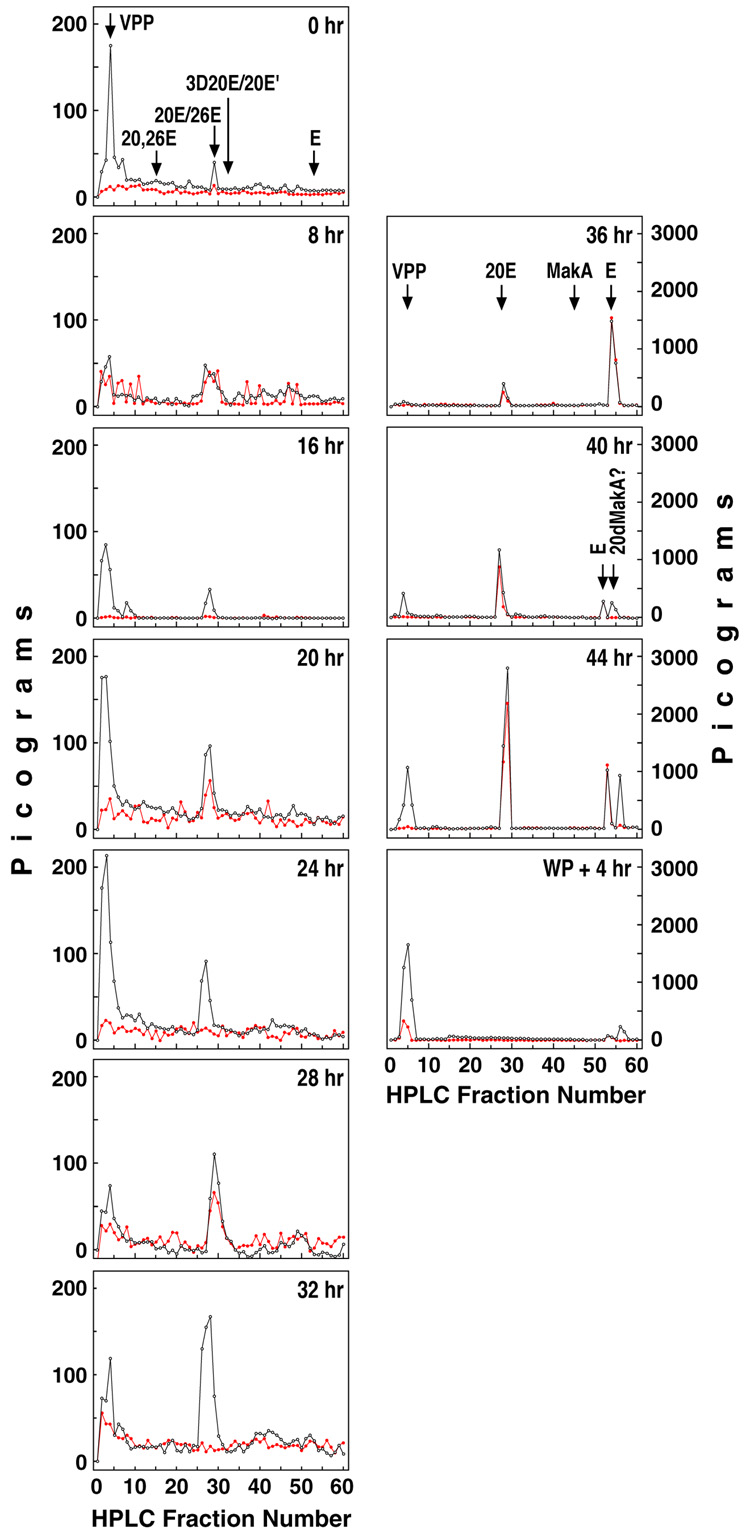
The above ecdysteroid titer, however, does not represent the molting hormone (20E) titer. Following the RP-HPLC and RIA analyses of ecdysteroids in the larval extracts, the changing profiles of the more-polar ecdysteroids present in animals at selected time points were discerned (Fig. 2). The results from the differential screening by H22 (black) and SHO3 (red) anti-ecdysteroid antibodies (Warren and Gilbert, 1986, 1988; Kiriishi et al., 1990), expressed in pg E equivalents, are graphed versus the HPLC fraction number. Only the initial portion of the chromatogram, up to 60 min elution time employing from 30% to 40 % methanol, is shown. This includes the very-polar products (VPP), composed mainly of ecdysteroid metabolic conjugates and ecdysonic acids, and those fractions containing free ecdysteroids (including 20E and E). Their elution positions as well as those of various ecdysteroid metabolite standards are indicated.
Figure 2.
RP-HPLC/RIA analysis of whole body extracts during the third larval instar in Drosophila. The ecdysteroid content, as measured by RIA using either the H22 (black) or SHO3 antisera (red) is shown for each HPLC fraction (1 ml) in selected samples as indicated (in hours after ecdysis to the third instar). (VPP), very polar products; (E), ecdysone; (20E), 20-hydroxyecdysone; (26E), 26-hydroxyecdysone; (20,26E), 20,26-dihydroxyecdysone; (3D20E), 3-dehydro-20-hydroxyecdysone; (20E'), 3-α-epi-20-hydroxyecdysone; (MakA), makisterone A; (20dMakA), 20-deoxymakisterone A (24-methylecdysone); 44, combined larval and white puparial (44WP) extracts; (WP + 4 hr), pre-pupae 4 hr after formation of white puparia.
Ecdysteroid identity was established by differential RIA analysis of the reverse-phase eluted fractions and comparison of the results to those of standards. As the entire larval extract was needed for these analyses, normal-phase chromatographic confirmation of peak identity would require an additional set of samples (see below). In the methanol system used for its greater injection load capacity, 20E co-elutes with 26-hydroxyecdysone (26E), an alternative hydroxylated metabolite of E, while the 20E metabolites 3-dehydro-20-hydroxyecdysone (3D20E) and 3-α-epi-20-hydroxyecdysone (20E') are only marginally resolved from 20E. Fortunately, 26E has very low affinity for the SHO3 antibody and 3D20E and 20E' (which do cross-react with the SHO3 antisera) were not observed (see below). Thus, the absolute titer of 20E can be elucidated after RP-HPLC using the RIA employing the SHO3 antibody. As shown in Figure 2, the level of specific SHO3 immunoreactivity at the retention time of 20E undergoes "rhythmic" fluctuations during most of the instar. It is very low at 0 hr, 16 hr, 24 hr and 32 hr, but is clearly elevated at 8 hr, 20 hr and 28 hr, prior to the final surge of 20E occurring at 44 hr (~50%WP), as measured in the pool of the larval (44) and white puparia (WP44) samples.
Curiously, little or no residual E was detected in these early pre-wandering feeding larvae, even though it is the secreted product of the ring gland and the immediate precursor to 20E. However, by 36 hr, just at the beginning of the large surge of ecdysteroid biosynthesis by the larval ring gland prior to pupariation (Parvy et al., 2005), there is considerable E present, i.e. 5-fold more than 20E (note axis change). Later, it may be accompanied by its often identified co-secretory product 20-deoxymakisterone A, based on elution profiles and antibody cross-reactivities (Redfern, 1984; Pak and Gilbert, 1987). However, no makisterone A (MakA) was detected in larvae at this or any time point, likely as a result of their primarily yeast-based diet (Redfern, 1986). The whole-body levels of VPP, primarily detected with the H22 antiserum, also vary greatly during the instar. As such, they are further evidence for extensive, primarily side-chain, ecdysteroid metabolism at these stages of development. Other, less-polar ecdysteroid compounds were also detected by one or both antisera in many of the chromatograms during this period of larval development (see Pak and Gilbert, 1987).
In order to identify 26E and specifically rule out significant levels of the closely migrating (in RP-methanol systems) 20E metabolites 3D20E and 20E’, additional chromatography on separate, large mixed-age populations of feeding (pre-wandering) third instar larvae was undertaken. Initial methanol-based chromatographic analysis of these additional larval samples was consistent with the data shown in Fig 2, i.e. a large peak was observed with the retention time of 20E. It had a much higher reactivity with the H22 antisera than with the SHO3 antisera (data not shown). This single peak was resolved by subsequent acetonitrile (ACN)-based RP-HPLC into two H22-reactive peaks (data not shown). Only the first peak was detected by the SHO3 antisera, at a level about equal to the H22 determination, and so consists only of 20E. In this alternative system (Sommé-Martin et al., 1981), no signs of the well-resolved 20E metabolites 3D20E or 20E’ appeared in replicate samples of over a thousand individuals. Finally, in subsequent normal-phase TLC analysis, the second less-polar compound migrated similarly to 26E (data not shown).
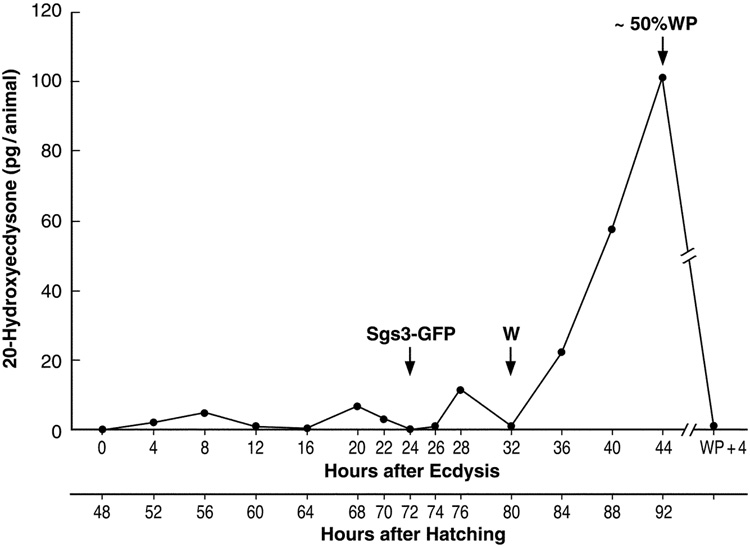
Larval whole-body 20E concentrations
Thus, using the SHO3-based RIA analysis of the RP-HPLC fractions (Fig. 2) corrected for SHO3's five-fold greater affinity for E than 20E (Kiriishi et al., 1990), we calculated the periodic elevation of whole-body 20E concentration during the third instar as shown in Figure 3. Three small peaks (from 5–15 pg/larva) of active molting hormone are observed at 8 hr, 20 hr and 28 hr. They are separated by periods where little or no 20E is detected, i.e. apparently only 26E is present. These multiple small peaks of 20E are then followed by the ~7-fold elevation in the 20E titer observed by 44 hr, at which time ~50% of the larvae had undergone metamorphosis to the white puparium stage (~50%WP). However, just 4 hr later (WP + 4), the 20E concentration had declined to the baseline level (< 1 pg/animal).
Figure 3.
Whole body titer of 20-hydroxyecdysone during the third larval instar in Drosophila. The concentration of 20E was determined using RIA with the SHO3 antisera following sample resolution by RP-HPLC. A correction for 20E cross-reactivity was made, i.e. a 5-fold increase relative to E. Sgs3-GFP denotes the time that the Sgs3-GFP fusion protein is first significantly expressed in the larval salivary glands. (W), beginning of wandering; (~50% WP), about 50% of animals had formed white puparia (WP) by 44 hr after ecdysis. Extracts of larvae and pupae at 44 hr were combined for this analysis. (WP + 4), pre-pupae 4 hr after formation of white puparia.
Expression of CYP306A1 (Phantom) and CYP302A1 (Disembodied) during the third instar
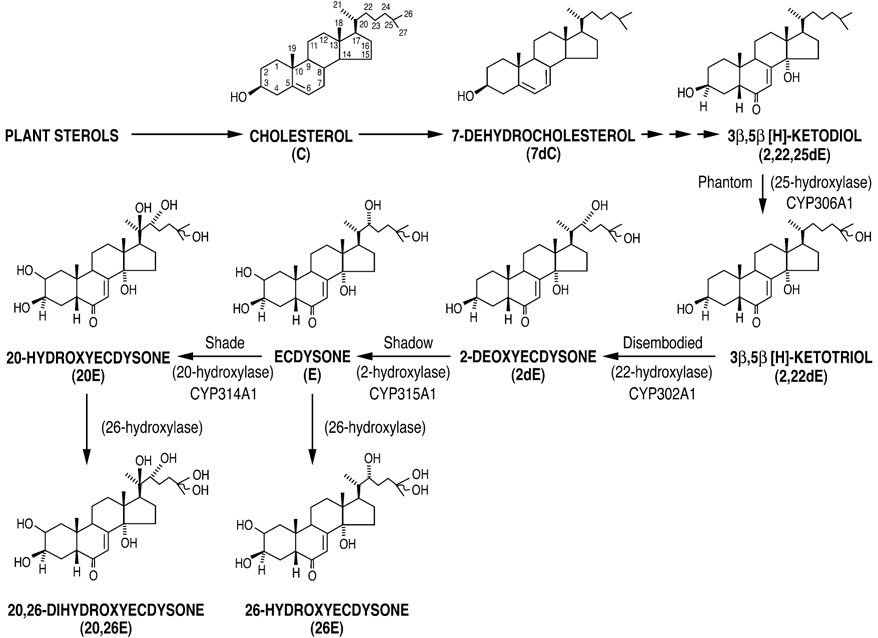
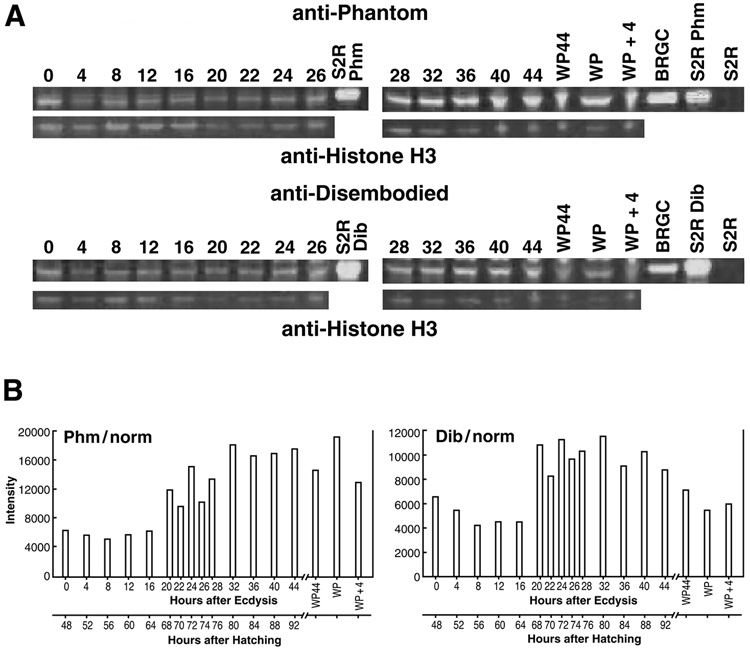
At least four cytochrome P450 (CYP) enzymes are presently known to be involved in the biosynthesis of E and 20E in Drosophila (Fig. 4) (Warren et al., 2002, 2004; Petryk et al., 2003; Niwa et al., 2004). Quantitative Western blot analysis, performed on the insoluble protein remaining after ecdysteroid extraction (see Methods), reveals significant expression throughout the third instar of both Phantom (Phm, the terminal 25-hydroxylase) and Disembodied (Dib, the subsequent 22-hydroxylase) (Fig. 5A), along with that of histone-H3 (similar data for tubulin E7 not shown). Titers of Phm and Dib, normalized to the expression of both histone H3 and tubulin E7, are present from the beginning of the instar and remain low and relatively stable for about the first half of the instar (Fig. 5B). Later, following some initial fluctuation resulting from much lower levels of both normalization proteins beginning at 20 hr, expression of these two cytochrome P450 proteins attain maxima about the time the larvae begin to wander (32 hr). They generally remain at least 2-fold elevated through puparium formation, corresponding to the period of maximal E biosynthesis and secretion by the brain-ring gland complex in vitro (Parvy et al., 2005) and maximal whole body 20E titer (Fig. 2 and Fig. 3). The raw immuno-expression levels of Phm and Dib (Fig. 5A), normalized at gel loading to the wet weight of the extracted whole body tissues, are nevertheless very similar to histogram Fig. 5B, i.e. low during the first half of the instar and 2-fold higher towards the end.
Figure 4.
Biosynthesis and metabolism of E and 20E. Four cytochrome P450 (CYP) enzymes have thus far been identified as catalyzing the last four hydroxylation reactions in Drosophila ecdysteroidogenesis. Also shown are 26E and 20,26E, the products of an inactivation reaction, i.e. ecdysteroid 26-hydroxylation, catalyzed by an as yet uncharacterized CYP enzyme.
Figure 5.
Profiles of Dib and Phm protein expression. Protein samples where prepared as described in Methods, separated on 4–12% Bis-Tris NuPAGE gels and blotted onto PDVF membranes. (A). Shown are representative blots probed for the biosynthetic enzymes Phm (top set, anti-Phantom) or Dib (bottom set, anti-Disembodied). Below each are shown the same blots probed for the protein loading control histone H3 (tubulin E7 not shown). Times for larvae are in hours after ecdysis to the third instar. WP44, animals at 44 hr that had developed into white puparia; (WP), additional samples of white puparia; WP + 4, pre-pupae 4 hrs after white puparium formation. (B). Protein levels where quantified using Odyssey software (Licor). The average of two measurements each for tubulin E7 and histone H3 were used to derive a normalized average value for total protein loaded per lane. Two measurements each for Phm and Dib were used to give an average amount for each. This average Phm or Dib value was divided by the normalized averaged value for total protein to yield the intensity numbers shown in the graphs (y-axis). Two blots where run for each sample and an average value was obtained for each time point. BRGC, brain ring gland complex; S2R, protein extracted from transfected S2R cells expressing either Phm or Dib or else an un-transfected S2R control.
DISCUSSION
While the precise synchronization of the larvae during the third instar was very important to the success of this study, a second critical factor involved the use of sensitive ecdysteroid-specific radioimmunoassays to quantify whole body concentrations of the active molting hormone, 20E. Since the ecdysteroid RIA was developed over 30 years ago (Borst and O’Connor, 1972), it has been applied many times to the investigation of “molting hormone” titers during Drosophila development (Richards, 1981; Andres et al., 1993). This has been especially true for the critical third (final) larval instar when the insect must be reprogrammed so that it can undergo metamorphosis to the pupa and then the adult in response to a large surge in 20E, a phenomenon studied mainly in the Lepidoptera (Bollenbacher et al., 1975; Riddiford, 1976, 1995). The requisite involvement of ecdysteroid signaling in these complex processes is central to present theories of arthropod development (Ashburner et al., 1974; Yao et al., 1993; Buszczak and Segraves, 1998; Bialeccki et al., 2002; Lafont et al., 2005; Henrich, 2005). However, the RIA, particularly when applied directly to whole body extracts, yields only a rough estimate of the molting hormone titer, as each ecdysteroid component present gives a variable response (cross-reactivity) in the assay depending on affinity characteristics of the antibody used. That is, since only 20E is presently thought to be the principal, or perhaps only, molting hormone, such data are approximate at best. This is important because developmental studies in Drosophila that rely on the application of 20E either in vitro or in vivo can only be interpreted accurately when physiological rather than pharmacological quantities are used.
Nevertheless, past ecdysteroid titer data indicate to varying degrees the existence of one or more discrete small “peaks” of putative molting hormone activity prior to the final surge signaling metamorphosis to the pupa (see Richards, 1981; Berreur et al., 1984; Schwartz et al., 1984; Deak et al., 1988; Parvy et al., 2005; see Andres et al., 1993). These would perhaps be analogous to the so-called “commitment” peak(s) observed during the last larval stage of Lepidoptera (Bollenbacher et al., 1975; Riddiford, 1976; Wolfgang and Riddiford, 1986; Parlak et al., 1992; Dai et al., 1995). In moths, these transient increases in ecdysteroid titer have long been thought to initiate the re-programming of the organism for the subsequent larval-pupal metamorphic molt (see Riddiford, 1995). The elucidation of a “commitment peak" in the Drosophila literature apparently depends on the anti-ecdysteroid antibody chosen for the analysis and the effort expended to assure precise staging of the animals, but physiological analyses analogous to those done on Lepidoptera have not been reported.
The staging of Drosophila involves one or more synchronizations at egg laying, hatching, subsequent larval-larval ecdysis, wandering behavior or observable morphological events such as salivary gland glue synthesis, storage and secretion, spiracle eversion, pupariation, head eversion and eye pigmentation. Even with well staged animals, the crude ecdysteroid titers measured during the third larval instar in this study (Figure 1) are similar to previous data, in that fluctuations are noticeable, but in many cases equivocal (see Parvy et al., 2005). The use of antibody preparations with a variable spectrum of ecdysteroid affinities is one reason for the problem, since in addition to E and 20E, they can detect many, but not necessarily all, of the prominent ecdysteroid metabolites whose formation was noted following the administration of labeled ecdysteroid to third instar larvae (e.g. Somme-Martin et al. 1988). This is true not only of the H22 and SHO3 antibodies, but also of the other antibody preparations used previously (see Richards, 1981; Berreur et al., 1979, 1984; Schwartz et al., 1984). Nevertheless, both the H22 and SHO3 antisera exhibit similar affinity to 20E. This was observed in the almost identical large peaks of ecdysteroid detected by both the H22 and SHO3 antibodies at the retention time of 20E in the 36 hr, 40 hr and 44 hr RP-HPLC (methanol) chromatograms (Fig. 3). At these late stages of third instar development, i.e. just prior to pupation, ecdysteroids in larvae and pre-pupae are known to be primarily free 20E and E, along with both more polar and less polar metabolites (Belinski-Deutsch et al., 1983; Berreur et al., 1984). This differential response involving two antisera is analogous to that found during our identification of the true product of the prothoracic glands of many insects. Long thought to be E, it was only after the proper antiserum was chosen for the in vitro assay that it was subsequently identified by similar RP-HPLC/RIA to be primarily 3-dehydroecdysone (Warren et al., 1988).
While the HPLC/RIA data indicate three discrete peaks of 20E in whole larvae occurring at 8, 20, and 28 hr after ecdysis (Fig. 3), it also suggests the presence of three similarly discrete peaks of 26E at 16, 24 and 32 hr. Both 26E and its ultimate oxidized product ecdysonic acid are present throughout the development of many insects, including dipterans (Koolman, 1980; Lafont et al., 1983; Moribayashi et al., 1985; Warren and Gilbert, 1986; Warren et al., 1986; Pak and Gilbert, 1987; Whisenton et al., 1989; Lafont et al., 2005). Based on the additional analysis of separate populations of pre-wandering third instar larvae, this appears to also be the case in Drosophila. However, in contrast to the findings of Berreur et al., (1984), little if any free E was detected in any of these early third instar extracts. Yet these repetitive, discrete elevations of 20E and 26E are the likely result of the increased secretion of E by the prothoracic gland cells of the ring gland, followed by its very rapid and efficient conversion to 20E by peripheral 20-hydroxylase (ecdysone 20-monooxygenase) activity (E20MO) (see Petryk et al., 2003) and/or to 26E by tissue 26-hydroxylase activity (Kayser et al., 1997; Williams et al., 2000) (Fig. 4). What is not clear is whether E synthesis during this period is relatively constant (Redfern, 1983; Berreur et al., 1984; Dai and Gilbert, 1991; Parvy et al., 2005) or intermittent and possibly rhythmic, as reported in Bombyx during the first half of the last larval instar (Kiguchi et al., 1985; Shirai et al., 1993; Dai et al., 1995).
To address this issue, we studied the expression of Phm and Dib, two cytochrome P450 enzymes specific to the prothoracic gland cells of the larval ring gland, that are required for E biosynthesis (Warren et al., 2002, 2004; Niwa et al., 2004). By Western blot analysis, the significant, although low, expression of both proteins in these same early third instar larval samples is indicative of a rather constant capacity to synthesize E during this period (Fig. 5). The only clear upward trend occurs in the mid to latter half of the instar, i.e. just prior to and during the period of maximal whole body 20E concentration (Fig. 3) and rate of secretion of E from the brain-ring gland complex (Parvy et al., 2005). Overall, these data are consistent with prior semi-quantitative RT-PCR and immunohistochemical data, indicating relatively constant phm and dib expression during the early third instar, although the expression of both phm and dib does appear later in the instar to be under the control of the nuclear receptor βFtz-F1 in the ring gland (Parvy et al., 2005). Only the analysis of specific Phm and Dib enzymatic activities during this period, along with that of the 2-hydroxylase, Shadow (Warren et al., 2002) and the 20-hydroxylase, Shade (Petryk et al., 2003), will clarify the importance of CYP enzyme regulation to the changing titers of active molting hormone during Drosophila ecdysteroidogenesis (Fig. 4).
Nevertheless, during most of Drosophila development, i.e. during mid-embryogenesis and at the larval-larval and larval-pupal ecdyses, the timing of E biosynthesis does appear to have great importance in determining the 20E titer, relative to the activity of the 20-hydroxylase. That is, there always seems to be sufficient tissue 20-hydroxylase activity to quickly and efficiently convert the majority of the secreted E into 20E (Mitchell and Smith, 1988). This is usually reflected in an elevated 20E/E ratio, as observed in the time points after 36 hr (Fig. 2). This relationship remains true even in transgenic animals expressing the 20-hydroxylase. That is, when the specific P450 enzyme catalyzing this ecdysteroid activation reaction (CYP315A1, Shade) is expressed ectopically in embryonic, larval and pupal tissues that normally do not exhibit this activity, normal development to the adult is observed (Petryk et al., 2003). However, ovarian development stops at stage 8–9 and these animals are sterile unless Shade is expressed within the ovary, the site of adult ecdysteroid biosynthesis (Warren et al., 1996).
Thus, at 8, 20 and 28 hr, the level of tissue 20-hydroxylase appears sufficient to efficiently convert all newly synthesized E into 20E. However, at other times, i.e. 16, 24 and 32 hr, little 20-hydroxylase activity is apparent. Instead, during these periods of low or no Shade activity, alternative metabolism of E to 26E would appear to be most prominent. The changing titer of 20E during this period would then be the result of the intermittent expression and/or modulation of the activity of the 20-hydroxylase (Mitchell and Smith, 1988), similar to that observed in other Diptera (Darvas et al., 1993), rather than to the intermittent biosynthesis of E.
Likewise, changes in the 26E titer would be the result of changes in the expression and/or activity of the 26-hydroxylase, another P450 enzyme catalyzing the hydroxylation of both E and 20E at the C26 position to 26E and 20,26E, respectively (see Lafont et al., 2005) (Fig. 4). Such biologically inactive side-chain hydroxylated metabolites can then undergo sequential oxidation to the polar acids or be conjugated to polar acids to form very polar products (VPP). Indeed, increases in the titer of these VPP seem to be temporally related to both the reduction of 20E and the increase in 26E levels, i.e. at 0 hr following the ecdysteroid peak occurring near the end of the second instar (Maroy et al., 1980), and at 16 hr, 24 hr, 32 hr and after 44 hr (Fig. 2). The factors that ultimately control the expression and activity of these two important enzymes remain unclear. However, it has been demonstrated in various insect species both in vivo and in vitro that endogenous or exogenous E can act to up-regulate 20-hydroxylase activity (Warren and Gilbert, 1986; see Lafont et al., 2005), as apparently is occurring between 36 and 40 hr (Fig. 2). In turn, endogenous or exogenous 20E can up-regulate 26-hydroxylase activity (Warren and Gilbert, 1986; Williams et al., 1997; Kayser et al., 1997). In addition to direct hanges in P450 expression levels, the intrinsic activity of both of these enzymes may be directly modulated by reversible phosphorylation (Hoggard et al., 1989; Williams et al., 2000).
Apart from the question of how the organism coordinates the intermittent biosynthesis of 20E during the third larval instar of Drosophila, our data provide a framework for investigating the physiological functions of these periodic elevations of molting hormone for post-embryonic development. From the molecular perspective, we provide strong correlative evidence that large-scale changes in transcript profiles do reflect hormonal regulation (Sullivan and Thummel, 2003). During the third instar, Andres et al. (1993) observed four periods of coordinated changes in expression of ecdysteroid-regulated genes. Allowing for differences in developmental timing, probably related to differences in culture and staging methods, one can comfortably overlay their four transition times with our four 20E peaks. Particularly striking is the ~8-hour interval between their second and third transitions, a remarkable match to the interval between our second and third peaks at 20 and 28 hours, respectively. As a specific case, consider the glue protein genes whose products are secreted by the salivary glands prior to pupariation and allow the puparium to adhere to its substrate during metamorphosis. Expression of this gene family is coordinately activated in the mid-third instar (Andres et al., 1993) and has long been thought to be induced by 20E (Hansson and Lambertsson, 1983, 1989). In this study, initial accumulation of SGS3:GFP fusion protein, beginning at 24 hr, occurs shortly after the second small peak of 20E at 20 hr. Given the time required for GFP fusion protein translation and accumulation in Drosophila cells (Hazelrigg et al., 1998), our observations are consistent with direct transcriptional induction of Sgs3 by the 20-hr peak of 20E.
In order to determine whether and which of the three small peaks of 20E corresponds to a commitment peak, it will be helpful to develop assays that reveal changes in developmental fate at cellular, tissue or organismal levels that are candidates for being under 20E regulation. A number of important cellular events are known to occur during the third instar, although the timing is uncertain for most of them (Weeks and Truman, 1986). Experiments with 20E-deficient mutants and hormone manipulations indicate the 20E induces the conversion of Golgi clusters to stacks of cisternae in imaginal discs midway through the third instar (Kondylis et al., 2001). Similarly, ecdysone receptor signaling has been implicated in PI3K-regulated programmed autophagy in the mid-third instar fat body (Rusten et al., 2004). Finally, at the level of the whole animal, consider wandering (Denlinger and Zdárek, 1994), which represents a cessation of feeding and a switch in phototropism as well as altered social behavior (Sawin-McCormack et al., 1995; Wu et al., 2003). In our study, larvae wandered at 32 hours after ecdysis, several hours after the third 20E peak. The 20E titer profile presented here sets the stage for further studies using precisely timed third-instar larvae in order to uncover other important correlates of 20E levels and, most important, to test for their hormonal regulation.
EXPERIMENTAL PROCEDURES
Chemicals and ecdysteroid standards
Common chemicals and HPLC-grade solvents were obtained through Fisher. E and 20E were purchased from Sigma, tracer [3H]-E (60 Ci/mmol) from NEN, and makisterone A (MakA) from Simes (Italy). The ecdysteroid metabolites, 26-hydroxyecdysone (26E) and 20,26-dihydroxyecdyone (20,26E), were purified from day one and three Manduca sexta embryos, respectively (Warren et al., 1986), while 20-deoxymakisterone A (20dMakA, 24-methylE) was synthesized in vitro by brain-ring gland complexes dissected from wandering third instar Drosophila larvae raised on a cornmeal-based diet (Pak and Gilbert, 1987). Both the 3-dehydro metabolites 3-dehydroecdysone (3DE) and 3-dehydro-20-hydroxyecdysone (3D20E), and their 3-α-epimeric derivatives, 3-α-epi-ecdysone (E') and 3-α-epi-20-hydroxyecdysone (20E’), were obtained by chemical oxidation and reduction of the parent ecdysteroid (Spindler, et al., 1977; Dinan and Rees, 1978). Rabbit anti-Phantom (Phm) peptide antibodies (Warren et al., 2004) and rabbit anti-Disembodied (Dib) antibodies, similarly made from an N-terminal Dib peptide (Warren et al., 2002), were obtained from FabGennix International (Shreveport, LA).
Drosophila strains and crosses
Animals were reared at the University of Arizona on standard corn flour/yeast/agar medium (Elgin and Miller, 1978). The larvae employed were the offspring of Canton-S males mated to virgin females from a homozygous stock of w; P[Sgs3-GFP]2, generated by A. Andres and obtained from the Bloomington Stock Center. This transgene is a second-chromosome insert in which the 5' regulatory sequences of salivary gland secretion protein 3 (Sgs3) drive the expression of a protein consisting of the amino-terminal half of the glue protein SGS3 fused to enhanced GFP (Biyasheva et al., 2001). The developmental expression pattern of this transgene is identical to that of endogenous Sgs3 and the fusion protein is secreted into the glue. Thus, this construct provides a convenient means of staging and synchronizing larvae during the second half of the third instar (Biyasheva et al., 2001). Crosses were set up in 1-liter cages with a minimum of 100 males and 100 females. A dime-sized blob of yeast paste, made fresh daily from active granulated Baker's yeast (Red Star) with enough water to yield the consistency of peanut butter, was provided to the adult files on a 60-mm plastic "egg-laying plate" at least once per day. Egg-laying plates contained 3% BactoAgar (Difco), 5% sucrose, and 0.5% acid mix containing 9:1 propionic acid:phosphoric acid (Spectrum, Baker).
Collection of third instar larvae
Since the major aim of this work was to correlate ecdysteroid titers with precise stages of development, the details of how the larvae were raised and staged are presented. From the time that the adult flies were placed in the mating cages, all subsequent steps took place in a walk-in environment chamber (Norlake), at 25°C, 70% relative humidity and constant light. The room was equipped with a Leica MZ12 stereomicroscope having a light source and filters to detect GFP fluorescence. Egg collections were made for two hours and the egg-laying plates were maintained in "humid chambers" (rectangular Rubbermaid containers with a floor of wet paper towels and one corner of the lid open to allow air circulation). At 20–22 hr after egg laying, newly hatched first instar larvae were collected with a microspatula (VWR) and transferred individually to a small blob of fresh yeast paste on a "larval feeding plate. " Larval feeding plates were 35-mm plastic Petri dishes with 0.4% agar (Form A, Moorehead & Co., Van Nuys, CA), 5% sucrose, 5% yeast extract (Difco), 2% nutritional yeast (Red Star) and 0.5% acid mix (see above). No more than 100 first instar larvae were placed on a single larval feeding plate. The lids were discarded and the feeding plates were maintained in a separate humid chamber.
To collect newly ecdysed third instar larvae, larvae were flushed out of their feeding plates with water at ~44 hours after hatching into an empty 60-mm Petri dish. Food debris and all but a thin layer of water were removed. All third instar larvae and all late second instar larvae with double vertical plates, indicating the appearance of a full set of new mouthparts (Park et al., 2002) were discarded. The remaining second instar larvae were left undisturbed for ~30 min, at which time newly molted third instar larvae were individually removed with No. 5 Dumont forceps or a red sable paintbrush and carefully transferred to a fresh feeding plate with no yeast paste. An additional collection of newly ecdysed third instar larvae was usually made about 30 min later. For any given third instar feeding plate, the collection time did not exceed 10 min and no more than 40 larvae were transferred to a single plate. Each third instar feeding plate was uncovered and placed in a covered 100-mm plastic Petri dish inside another humid chamber.
All third instar larvae were re-synchronized at 24 hr after ecdysis. Individual animals were removed from the food with a fine brush and checked for the presence of salivary gland GFP (SGS3:GFP fusion protein) by fluorescence stereomicroscopy. Larvae whose glands were 1/4- to 1/3-full of GFP were either collected for the 24-hr time point or transferred for further development to a fresh 35-mm feeding plate that contained 0.05% bromophenol blue (Sigma), which allows for subsequent staging during the wandering phase (Maroni and Stamey, 1983; Andres and Thummel, 1994). Larvae handled in this manner have >98% survival to adult eclosion. Tests were also done to confirm that handling did not delay the progression of GFP fluorescence along the length of the salivary glands. Larvae whose salivary gland GFP pattern did not meet selection criteria were discarded.
Batches of staged third instar larvae (30–40 animals) were collected until the total weight for each time point was between 400–700 mg. (Previous efforts to analyze a timed series of samples of ~100 mg each had yielded insufficient material.) Time points of collection (number of animals) were: 0 hr (535), 4 hr (350), 8 hr (311), 12 hr (293), 16 hr (255), 20 hr (215), 22 hr (205), 24 hr (240), 26 hr (195), 28 hr (199), 32 hr (158), 36 hr (145), 40 hr (185), and 44 hr (see below). At 32 hr, larvae initiated wandering and began to leave the food with their guts completely blue. Over the next 8 hr, the blue food remaining in the guts of these wandering larvae diminished, such that by 40 hr, only minimal residual blue remained at the tip of the gut. At 44 hr, two types of animal collections were made. By then, about one-half (169) of the animals were white puparia (WP). The other half (179) were still wandering larvae showing no blue dye in their evacuated guts. Nevertheless, these two samples yielded very similar titer data and so were combined for subsequent RP-HPLC/RIA analysis. By 48 hr after ecdysis, all animals had formed white puparia. Also collected were pre-pupae 4 hr after puparium formation (150; WP+4). Animals of the same time point were rinsed to remove food debris, carefully blotted to remove excess water and then transferred with forceps or brush to a 1.5-ml microcentrifuge tube that was weighed before and after. Each tube was flash-frozen in liquid nitrogen and stored at −80°C or below until sent under dry ice to the University of North Carolina to be analyzed. Control experiments verified that neither use of bromophenol blue in the feeding plates nor storage at −80°C for several months affected the total ecdysteroid content or the 20E titer of additional samples white prepupae (WP).
Sample preparation and ecdysteroid titer determination
Methanol (1 ml) was added to the individual samples of frozen staged third instar larvae and each was extensively homogenized by hand with a close-fitting plastic pestle at room temperature. After several hours of repeated vigorous vortexing at 6°C, the mixtures were centrifuged at high speed and the solvent decanted. The residues were then repeatedly re-extracted with both methanol (2 × 0.5 ml) and ethanol (1 × 0.5 ml) by frequent vortexing over the following two days, while kept at 6°C. All solvent extracts for each vial of 30–40 larvae were then pooled (2.5 ml total). Extract pools and extracted tissue protein residues (kept under excess ethanol) were stored at −80°C. For an initial direct ecdysteroid analysis by RIA (Warren and Gilbert, 1988), replicate 125 µl aliquots (1/20 total) from each pooled replicate extract for any given time point were combined and subsequently evaporated (Speed-Vac, Savant) into individual glass tubes (6 × 50 mm). These preliminary whole body RIA titers employed the H22 and SHO3 polyclonal rabbit antibodies. The former antiserum was elicited with an E-based thyroglobulin immunogen derivatized via the side-chain C22 hydroxyl group and it detects (binds to) not only E and 20E (the E/20E cross-reactivity for this H22 bleed is 5), but also their respective side-chain conjugated and/or oxidized metabolites 26E and 20,26E and derived C26 ecdysonic acids (Warren et al., 1986). However, it does not detect the A-ring oxidation, epimerization or conjugation products of E or 20E such as 3DE or 3D20E, E' or 20E' or their polar phosphate esters (Warren and Gilbert, 1986; Warren et al., 1988). The SHO3 antiserum (a gift of Dr. Sho Sakurai, Kanazawa University) was the result of E-derivatization at the opposite end of the steroid molecule (via the C3 hydroxyl function) to thyroglobulin and so detects mainly E, 20E (E/20E cross-reactivity also about 5) and their A-ring oxidized, epimerized or conjugated metabolites, but not side-chain modified products (Kiriishi et al., 1990). As such, it compliments detection by the H22 antibody. [See Lafont et al. (2005) for a discussion of the chemistry of the known ecdysteroids]
Developmental ecdysteroid profile
To determine the concentration of 20E in alcohol extracts of the staged animals, the ecdysteroid composition at each time point was determined by RP-HPLC employing differential RIA analysis (Warren et al., 1986; 1988). The remaining replicate sample extracts for each time point were pooled, evaporated under low pressure and the residues taken up in the initial RP-HPLC solvent (1.5 ml of 30% methanol) containing Tween-20 (0.001%). Ecdysteroid components varying over a wide polarity range were resolved on a 4 µm Novapak-C18 column (3.9 × 300 mm; Waters) at a solvent flow rate of 1 ml/min. After an initial 30 min isocratic elution, a long, slow gradient to a 100% methanol endpoint was employed over two hours. Eluted fractions (1 ml) were divided and evaporated into RIA tubes for analysis using the H22 and SHO3 antisera.
Additional, more-intensive chromatographic studies were made of the whole body ecdysteroid composition in a mixed population of pre-wandering third instar larvae. Feeding larvae were obtained by floatation (0.5M sucrose) from un-crowded daily cultures of Canton-S animals. Bottles with only a few wandering animals were selected and only third instar larvae were found to be present in the food. The ecdysteroid content of these animals was initially analyzed as described above. Fractions eluting between 20E and E were then pooled, evaporated and re-chromatographed employing the same RP-HPLC column, but with an acetonitrile (ACN)-water system (10–25% ACN over 1 hr) that resolves 26E from 20E and better resolves the 20E-metabolites 3-dehydro-20E (3D20E) and 3-α-epi-20E (20E') from 20E (Sommé-Martin et al., 1981). As before, the differential RIA analysis of eluted fractions identified ecdysteroids which were then subjected to normal-phase, thin-layer chromatography (TLC) to confirm their identity (Warren and Gilbert, 1986).
Quantitative Western blots
The above alcohol extracted larval sample protein residues were sent to the University of Minnesota under dry ice. They were ground in cold PBS plus 4 × SDS sample buffer (250 mm Tris HCl, pH 6.8, 8% SDS, 40% glycerol, 0.02% bromophenol blue, 20% β-mercaptoethanol) (3:1). The final volume in microliters was 5 times the wet larval weight in micrograms. The samples were boiled 5 minutes and centrifuged at 14,000 rpm for 10 min at room temperature. The supernatants were used to make dilutions (1:3) in 1X sample buffer and stored at −80 degrees. Twenty wild type brain-ring gland complexes were treated the same, yielding 100 µl in 1X sample buffer. S2R protein samples were made by transfection of Schneider S2R cells with phantom or disembodied cDNA in pBRAcPA, using DDAB as the transfection reagent (Warren et al., 2002, 2004). After 5 days, the cells were collected, suspended in 1X sample buffer, boiled 5 min, and centrifuged at 14,000 rpm for 10 minutes. These supernatants and those from control non-transfected S2R cells were diluted in 1X sample buffer for gel loading (1:25).
All samples (24 µl were loaded onto each lane) were subjected to electrophoresis on 4–12% Bis-Tris gels (NuPAGE) and blotted onto PVDF using the Invitrogen protocol. Primary antibody reactions were at 4°C overnight, i.e. 1:500 of rabbit anti-Phm peptide antibodies (Warren et al., 2004) or rabbit anti-Dib peptide antibodies, 1:1000 rabbit anti-histone H3 (Upstate), or 1:25 mouse anti-tubulin E7 (Hybridoma Bank). Secondary antibody reactions were at room temperature for one hour (1:10K goat anti-mouse IRDye 700 or 800 or goat anti-rabbit IRDye 700 or 800). All washes were as recommended by Li-Cor Biosciences (Western Blotting Methods). Membranes were scanned using the Odyssey Infrared Imaging System. Protein bands were quantified using the Odyssey software. A number representing integrated intensity of the bands was used to derive the relative amounts.
ACKNOWLEDGEMENTS
We would like to thank Susan Whitfield for graphics, Jonathan Kahler for technical assistance, Carlos Michel for making fly food and John Ewer for advice on staging larvae. LLR particularly appreciates the larval culture and staging efforts of Macarena Busto and Laura Tank. Carl Thummel and Lucy Cherbas provided helpful discussions and encouragement throughout this project. This research was supported by grant IBN0130825 from the National Science Foundation (to LIG and JTW) and HD38363 from the National Institutes of Health (to LLR). M.B.O is an Investigator of the Howard Hughes Medical Institute.
Abbreviations
- BRGC
brain ring gland complex
- CYP
cytochrome P450
- 3D20E
3-dehydro-20-hydroxyecdysone
- 3DE
3-dehydroecdysone
- 20dMakA
20-deoxymakisterone A
- 20,26E
20,26-dihydroxyecdysone
- dib
disembodied
- E
ecdysone
- E'
3-α-epi-ecdysone
- 20E'
3-α-epi-20-hydroxyecdysone
- GFP
green fluorescent protein
- 20E
20-hydroxyecdysone
- 26E
26-hydroxyecdysone
- MakA
makisterone A
- phm
phantom
- RP-HPLC
reverse-phase HPLC
- Sgs3
salivary gland glue protein 3
- shd
shade
- TLC
thin-layer chromatography
- VPP
very polar products
REFERENCES
- Andres AJ, Fletcher JC, Karim FD, Thummel CS. Molecular analysis of the initiation of insect metamorphosis: a comparative study of Drosophila ecdysteroid-regulated transcription. Dev Biol. 1993;160:388–404. doi: 10.1006/dbio.1993.1315. [DOI] [PubMed] [Google Scholar]
- Andres AJ, Thummel CS. Methods for quantitative analysis of transcription in larvae and prepupae. In: Goldstein LSB, Fyrberg EA, editors. Drosophila melanogaster: Practical uses in Cell and Molecular Biology. San Diego: Academic Press; 1994. pp. 565–573. [DOI] [PubMed] [Google Scholar]
- Ashburner M. Drosophila: A laboratory handbook. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1989. Developmental biology; pp. 139–298. [Google Scholar]
- Ashburner M, Chihara C, Meltzer P, Richards G. Temporal control of puffing activity in polytene chromosomes. Cold Spring Harbor Symp. Quant Biol. 1974;38:655–662. doi: 10.1101/sqb.1974.038.01.070. [DOI] [PubMed] [Google Scholar]
- Belinski-Deutsch S, Busson D, Lamour-Audit C, Porcheron P, Moriniere M, Berreur P. Relations between ecdysteroid levels and pupal development in the ecd-1 temperature-sensitive mutant of Drosophila melanogaster. J Insect Physiol. 1983;29:509–514. [Google Scholar]
- Berreur P, Porcheron P, Berreur-Bonnenfant J, Simpson P. Ecdysteroid levels and pupariation in Drosophila melanogaster. J Exp Zool. 1979;210:347–352. [Google Scholar]
- Berreur P, Porcheron P, Moriniere M, Berreur-Bonnenfant J, Belinski-Deutsch S, Busson D, Lamour-Audit C. Ecdysteroids during the third larval instar in 1(3)ecd-1ts, a temperature-sensitive mutant of Drosophila melanogaster. Gen Comp Endocrinol. 1984;54:76–84. doi: 10.1016/0016-6480(84)90201-6. [DOI] [PubMed] [Google Scholar]
- Bialecki M, Shilton A, Fichtenberg C, Segraves WA, Thummel CS. Loss of the ecdysteroid-inducible E75A orphan nuclear receptor uncouples molting from metamorphosis in Drosophila. Dev Cell. 2002;3:209–220. doi: 10.1016/s1534-5807(02)00204-6. [DOI] [PubMed] [Google Scholar]
- Biyasheva A, Do T-V, Lu Y, Vaskova M, Andres AJ. Glue secretion in the Drosophila salivary gland: a model for steroid-regulated exocytosis. Dev Biol. 2001;231:234–251. doi: 10.1006/dbio.2000.0126. [DOI] [PubMed] [Google Scholar]
- Bollenbacher WE, Vedeckis WV, Gilbert LI, D OCJ. Ecdysone titers and prothoracic gland activity during the larval-pupal development of Manduca sexta. Dev Biol. 1975;44:46–53. doi: 10.1016/0012-1606(75)90375-9. [DOI] [PubMed] [Google Scholar]
- Borst D, O'Connor JD. Arthropod molting hormone: Radioimmune assay. Science. 1972;178:418–419. doi: 10.1126/science.178.4059.418. [DOI] [PubMed] [Google Scholar]
- Buszczak M, Segraves WA. Drosophila metamorphosis: the only way is USP? Curr Biol. 1998;8:879–882. doi: 10.1016/s0960-9822(07)00550-7. [DOI] [PubMed] [Google Scholar]
- Dai J-d, Mizoguchi A, Satake SI, Ishizaki H, Gilbert LI. Developmental changes in the prothoracicotropic hormone content of the Bombyx mori brain - retrocerebral complex and hemolymph: analysis by immunogold electron microsopy, quantitative image analysis, and time-resolved fluoroimmunoassay. Dev Biol. 1995;171:212–223. doi: 10.1006/dbio.1995.1272. [DOI] [PubMed] [Google Scholar]
- Dai JD, Gilbert LI. Metamorphosis of the corpus allatum and degeneration of the prothoracic glands during the larval-pupal-adult transformation of Drosophila melanogaster: a cytophysiological analysis of the ring gland. Dev Biol. 1991;144:309–326. doi: 10.1016/0012-1606(91)90424-2. [DOI] [PubMed] [Google Scholar]
- Darvas B, Rees H, Hoggard N. Ecdysone 20-monooxygenese systems in flesh-flies (Diptera: Sarcophagidae), Neobellieria bullata and Parascophaga argyrostoma. Comp Biochem Physiol. 1993;105:765–773. [Google Scholar]
- Deak P, Zavorszky P, Maroy P. Moulting hormone regulates its receptor level in Drosophila melanogaster. Insect Biochem. 1988;18:847–852. [Google Scholar]
- Denlinger DL, Zdarek J. Metamorphosis behavior of flies. Annu. Rev. Entomol. 1994;39:243–266. doi: 10.1146/annurev.en.39.010194.001331. [DOI] [PubMed] [Google Scholar]
- Dinan L, Rees HH. Preparation of 3-epi-ecdysone and 3-epi-20-hydroxyecdysone. Steroids. 1978;32:629–638. doi: 10.1016/0039-128x(78)90073-9. [DOI] [PubMed] [Google Scholar]
- Elgin SR, Miller DW. Mass rearing of flies and mass production and harvesting of eggs. In: Ashburner M, Wright TRF, editors. The Genetics and Biology of Drosophila. New York: Academic Press; 1978. pp. 112–121. [Google Scholar]
- Hansson L, Lambertsson A. The role of su(f) gene function and ecdysterone in transcription of glue polypeptide mRNAs in Drosophila melanogaster. Mol Gen Genet. 1983;192:395–401. [Google Scholar]
- Hansson L, Lambertsson A. Steroid regulation of glue protein genes in Drosophila melanogaster. Hereditas. 1989;110:61–67. doi: 10.1111/j.1601-5223.1989.tb00418.x. [DOI] [PubMed] [Google Scholar]
- Hazelrigg T, Liu N, Hong Y, Wang S. GFP expression in Drosophila tissues: time requirements for formation of a fluorescent product. Dev Biol. 1998;199:245–249. doi: 10.1006/dbio.1998.8922. [DOI] [PubMed] [Google Scholar]
- Henrich V. The ecdysteroid receptor. In: Gilbert LI, Iatrou K, Gill S, editors. Comprehensive Molecular Insect Science. vol 3. Oxford: Elsevier; 2005. pp. 243–285. [Google Scholar]
- Hoggard N, Fisher M, Rees H. Possible role for covalent modification in the reversible activation of ecdysone 20-monooxgenase. Arch Insect Biochem Physiol. 1989;10:241–253. [Google Scholar]
- Kayser H, Winkler T, Spindler-Barth M. 26-hydroxylation of ecdysteroids is catalyzed by a typical cytochrome P450-dependent oxidase and related to ecdysteroid resistance in an insect cell line. Eur J Biochem. 1997;248:707–716. doi: 10.1111/j.1432-1033.1997.00707.x. [DOI] [PubMed] [Google Scholar]
- Kiguchi K, Agui N, Kawasaki H, Kobayashi H. Developmental time-table for the last larval and pharate pupal stages in the silkworm, Bombyx mori, with special reference to the correlation between the developmental events and hemolymph ecdysteroid levels. Rep Sericult Exp Stn. 1985;30:83–100. [Google Scholar]
- Kiriishi S, Rountree DB, Sakurai S, Gilbert LI. Prothoracic gland synthesis of 3-dehydroecdysone and its hemolymph 3β-reductase-mediated conversion to ecdysone in representative insects. Experientia. 1990;46:716–721. doi: 10.1007/BF01939944. [DOI] [PubMed] [Google Scholar]
- Kondylis V, Goulding SE, Dunne JC, Rabouille C. Biogenesis of Golgi stacks in imaginal discs of Drosophila melanogaster. Molecular Biology of the Cell. 2001;12:2308–2327. doi: 10.1091/mbc.12.8.2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolman J. Ecdysteroids in the blowfly, Calliphora vincina. In: Hoffmann JA, editor. Progress in Ecdysone Research. North-Holland: Elsevier; 1980. pp. 187–209. [Google Scholar]
- Lafont R, Blais C, Beydon P, Modde J, Enderle U, Koolman J. Conversion of ecdysone and 20-hydroxyecdysone into 26-OIC derivatives is a major pathway in larvae and pupae of species from three insect orders. Arch Insect Biochem Physiol. 1983;21:41–58. [Google Scholar]
- Lafont R, Dauphin-Villemant C, Warren JT, Rees H. Ecdysteroid chemistry and biochemistry. In: Gilbert LI, Iatrou K, Gill S, editors. Comprehensive Molecular Insect Science. vol 3. Oxford: Elsevier; 2005. pp. 125–195. [Google Scholar]
- Maroy P, Koczka K, Fekete E, Vargha J. Molting hormone titer of D. melanogaster larvae. Dros Info Serv. 1980;55:98–99. [Google Scholar]
- Maroni G, Stamey SC. Use of blue food to select synchronous, late third instar larvae. Dros Info Serv. 1983;59:142–143. [Google Scholar]
- Mitchell MJ, Smith SL. Ecdysone 20-monooxygenase activity throughout the life cycle of Drosophila melanogaster. Gen Comp Endocrinol. 1988;72:467–470. doi: 10.1016/0016-6480(88)90170-0. [DOI] [PubMed] [Google Scholar]
- Moribayashi A, Kurahashi H, Ohtaki T. Comparative studies on ecdysone metabolism between mature larvae and pharate pupae in the fleshfly, Sarcophaga peregrine. Arch Insect Biochem Physiol. 1985;2:237–250. [Google Scholar]
- Niwa R, Matsuda T, Yoshiyama T, Namiki T, Mita K, Fujimoto Y, Kataoka H. CYP306A1, a cytochrome P450 enzyme, is essential for ecdysteroid biosynthesis in the prothoracic glands of Bombyx and Drosophila. J Biol Chem. 2004;279:35942–35949. doi: 10.1074/jbc.M404514200. [DOI] [PubMed] [Google Scholar]
- Pak MD, Gilbert LI. A developmental analysis of ecdysteroids during the metamorphosis of Drosophila melanogaster. J Liquid Chromatogr. 1987;10:2591–2611. [Google Scholar]
- Park Y, Filippov V, Gill SS, Adams ME. Deletion of the ecdysis-triggering hormone gene leads to lethal ecdysis deficiency. Development. 2002;129:493–503. doi: 10.1242/dev.129.2.493. [DOI] [PubMed] [Google Scholar]
- Parlak O, Sakurai S, Kaya M, Ohtaki T. Content and possible role of ecdysteroids in the larval ovary of the silkworm, Bombyx mori. Invert Reprod Dev. 1992;21:1–6. [Google Scholar]
- Parvy JP, Blais C, Bernard F, Warren JT, Petryk A, Gilbert LI, O'Connor MB, Dauphin-Villemant C. A role for βFTZ-F1 in regulating ecdysteroid titers during postembryonic development in Drosophila melanogaster. Dev Biol. 2005;282:84–94. doi: 10.1016/j.ydbio.2005.02.028. [DOI] [PubMed] [Google Scholar]
- Petryk A, Warren JT, Marques G, Jarcho MP, Gilbert LI, Kahler J, Parvy JP, Li Y, Dauphin-Villemant C, O'Connor MB. Shade is the Drosophila P450 enzyme that mediates the hydroxylation of ecdysone to the steroid insect molting hormone 20-hydroxyecdysone. Proc Natl Acad Sci USA. 2003;100:13773–13778. doi: 10.1073/pnas.2336088100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redfern CPF. Ecdysteroid synthesis by the ring gland of Drosophila melanogaster during late larval pre pupal and pupal development. J Insect Physiol. 1983;29:65–72. [Google Scholar]
- Redfern CPF. Evidence for the presence of makisterone A in Drosophila larvae and the secretion of 20-deoxymakisterone A by the ring gland. Proc Natl Acad Sci USA. 1984;81:5643–5647. doi: 10.1073/pnas.81.18.5643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redfern CPF. Changes in patterns of ecdysteroid secretion by the ring gland of Drosophila in relation to the sterol composition of the diet. Experientia. 1986;42:307–309. [Google Scholar]
- Richards G. The radioimmunoassay of ecdysteroid titers in Drosophila melanogaster. Mol Cell Endocrinol. 1981;21:181–197. doi: 10.1016/0303-7207(81)90013-7. [DOI] [PubMed] [Google Scholar]
- Riddiford LM. Hormonal control of insect epidermal cell commitment in vitro. Nature. 1976;259:115–117. doi: 10.1038/259115a0. [DOI] [PubMed] [Google Scholar]
- Riddiford LM. Hormonal regulation of gene expression during lepidopteran development. In: Goldsmith M, Wilkins A, editors. Molecular Model Systems in the Lepidopteran. New York: Cambridge; 1995. pp. 305–316. [Google Scholar]
- Rusten TE, Lindmo K, Juhasz G, Sass M, Seglen PO, Brech A, Stenmark H. Programmed autophagy in the Drosophila fat body is induced by ecdysone through regulation of the PI3K pathway. Dev Cell. 2004;7:179–192. doi: 10.1016/j.devcel.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Saunders DS, Steel CGH, Vafopoulou X, Lewis RD. Insect Clocks. Amsterdam: Elsevier Science Publishing Company; 2002. 576 pp. [Google Scholar]
- Sawin-McCormack EP, Sokolowski MB, Campos AR. Characterization and genetic analysis of Drosophila melanogaster photobehavior during larval development. J Neurogenetics. 1995;10:119–135. doi: 10.3109/01677069509083459. [DOI] [PubMed] [Google Scholar]
- Schwartz MB, Imberski RB, Kelly TJ. Analysis of metamorphosis in Drosophila melanogaster: characterization of giant, an ecdysteroid-deficient mutant. Dev Biol. 1984;103:85–95. doi: 10.1016/0012-1606(84)90010-1. [DOI] [PubMed] [Google Scholar]
- Shirai Y, Aizono Y, Iwasaki T, Mori H, Sumida M, Matsubara F. Prothoracicotropic hormone is released five times in the 5th-larval instar of the silkworm, Bombyx mori. J Insect Physiol. 1993;39:83–88. [Google Scholar]
- Sommé-Martin G, Colardeau J, Lafont R. Metabolism and biosynthesis of ecdysteroids in the Drosophila development mutant ecd1. Insect Biochem. 1981;18:735–742. [Google Scholar]
- Spindler KD, Koolman J, Mosora F, Emmerich H. Catalytical oxidation of ecdysteroids to 3-dehydro products and their biological activities. J Insect Physiol. 1977;23:441–444. doi: 10.1016/0022-1910(77)90253-0. [DOI] [PubMed] [Google Scholar]
- Sullivan AA, Thummel CS. Temporal profiles of nuclear receptor gene expression reveal coordinate transcriptional responses during Drosophila development. Mol Endocrinol. 2003;17:2125–2137. doi: 10.1210/me.2002-0430. [DOI] [PubMed] [Google Scholar]
- Taghert PH, Lee Y. Tick-Talk: the cellular and molecular biology of Drosophila circadian rhythms. In: Gilbert LI, Iatrou K, Gill S, editors. Comprehensive Molecular Insect Science. vol 4. Oxford: Elsevier; 2005. pp. 357–394. [Google Scholar]
- Warren JT, Gilbert LI. Ecdysone metabolism and distribution during the pupal-adult development of Manduca sexta. Insect Biochem. 1986;16:65–82. [Google Scholar]
- Warren JT, Gilbert LI. Radioimmunoassay of ecdysteroids. In: Gilbert LI, Miller TA, editors. Immunological Techniques in Insect Biology. Berlin: Springer-Verlag; 1988. pp. 181–214. [Google Scholar]
- Warren JT, Steiner B, Dorn A, Pak M, Gilbert LI. Metabolism of ecdysteroids during the embryogenesis of Manduca sexta. J Liquid Chromatogr. 1986;9:1759–1782. [Google Scholar]
- Warren JT, Sakurai S, Rountree DB, Gilbert LI, Lee SS, Nakanishi K. Regulation of the ecdysteroid titer of Manduca sexta: reappraisal of the role of the prothoracic glands. Proc Natl Acad Sci USA. 1988;85:958–962. doi: 10.1073/pnas.85.3.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren JT, Bachmann JS, Dai JD, Gilbert LI. Differential incorporation of cholesterol and cholesterol derivatives into ecdysteroids by the larval ring glands and adult ovaries of Drosophila melanogaster: a putative explanation for the l(3)ecd1 mutation. Insect Biochem Mol Biol. 1996;26:931–943. doi: 10.1016/s0965-1748(96)00059-8. [DOI] [PubMed] [Google Scholar]
- Warren JT, Petryk A, Marques G, Jarcho M, Parvy J, Dauphin-Villemant C, O'Connor M, Gilbert LI. Molecular and biological characterization of two P450 enzymes in the ecdysteroidogenic pathway of Drosophila melanogaster. Proc Natl Acad Sci USA. 2002;99:11043–11048. doi: 10.1073/pnas.162375799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren JT, Petryk A, Marques G, Parvy JP, Shinoda T, Itoyama K, Kobayashi J, Jarcho M, Li Y, O'Connor MB, Dauphin-Villemant C, Gilbert LI. Phantom encodes the 25-hydroxylase of Drosophila melanogaster and Bombyx mori: a P450 enzyme critical in ecdysone biosynthesis. Insect Biochem Mol Biol. 2004;34:991–1010. doi: 10.1016/j.ibmb.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Weeks JC, Truman JW. Steroid control of neuron and muscle development during the metamorphosis of an insect. J Neurobiol. 1986;17:249–267. doi: 10.1002/neu.480170308. [DOI] [PubMed] [Google Scholar]
- Whisenton L, Warren JT, Manning M, Bollenbacher W. Ecdysteroid titres during pupal-adult development of Aedes aegypti: basis for a sexual dimorphism in the rate of development. J Insect Physiol. 1989;35:67–73. [Google Scholar]
- Williams D, Chen JMF, Rees H. Characterization of ecdysteroid 26-hydroxylase: an enzyme involved in molting hormone inactivation. Arch Biochem Biophys. 2000;376:389–398. doi: 10.1006/abbi.2000.1731. [DOI] [PubMed] [Google Scholar]
- Williams DR, Chen JH, Fisher MJ, Rees HH. Induction of enzymes involved in molting hormone (ecdysteroid) inactivation by ecdysteroids and an agonist, 1,2-dibenzoyl-1-tertbutylhydrazine (RH-5849) J Biol Chem. 1997;272:8427–8432. doi: 10.1074/jbc.272.13.8427. [DOI] [PubMed] [Google Scholar]
- Wolfgang WJ, Riddiford LM. Larval cuticular morphogenesis in the tobacco hornworm, Manduca sexta, and its hormonal regulation. Dev Biol. 1986;113:305–316. doi: 10.1016/0012-1606(86)90166-1. [DOI] [PubMed] [Google Scholar]
- Wu Q, Wen T, Lee G, Park JH, Cai HN, Shen P. Developmental control of foraging and social behavior by the Drosophila neuropeptide Y-like system. Neuron. 2003;39:147–161. doi: 10.1016/s0896-6273(03)00396-9. [DOI] [PubMed] [Google Scholar]
- Yao TP, Forman BM, Jiang Z, Cherbas L, Chen JD, McKeown M, Cherbas P, Evans RM. Functional ecdysone receptor is the product of EcR and Ultraspiracle genes. Nature. 1993;366:476–479. doi: 10.1038/366476a0. [DOI] [PubMed] [Google Scholar]