Abstract
PURPOSE
To characterize the reactions of retinal glial cells (astrocytes and Müller cells) to retinal injury in mice that lack glial fibrillary acidic protein (GFAP) and vimentin (GFAP-/-Vim-/-) and to determine the role of glial cells in retinal detachment (RD)-induced photoreceptor degeneration.
METHODS
RD was induced by subretinal injection of sodium hyaluronate in adult wild-type (WT) and GFAP-/-Vim-/- mice. Astroglial reaction and subsequent monocyte recruitment were quantified by measuring extracellular signal-regulated kinase (Erk) and c-fos activation and the level of expression of chemokine monocyte chemoattractant protein (MCP)-1 and by counting monocytes/microglia in the detached retinas. Immunohistochemistry, immunoblotting, real-time quantitative polymerase chain reaction (PCR), and enzyme-linked immunosorbent assay (ELISA) were used. RD-induced photoreceptor degeneration was assessed by terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) and measurement of outer nuclear layer (ONL) thickness.
RESULTS
RD-induced reactive gliosis, characterized by GFAP and vimentin upregulation, Erk and c-fos activation, MCP-1 induction, and increased monocyte recruitment in WT mice. Absence of GFAP and vimentin effectively attenuated reactive responses of retinal glial cells and monocyte infiltration. As a result, detached retinas of GFAP-/-Vim-/- mice exhibited significantly reduced numbers of TUNEL-positive photoreceptor cells and increased ONL thickness compared with those of WT mice.
CONCLUSIONS
The absence of GFAP and vimentin attenuates RD-induced reactive gliosis and, subsequently, limits photoreceptor degeneration. Results of this study indicate that reactive retinal glial cells contribute critically to retinal damage induced by RD and provide a new avenue for limiting photoreceptor degeneration associated with RD and other retinal diseases or damage.
Virtually all forms of retinal injury or disease, including retinal trauma, choroidal neovascularization,1 retinal detachment (RD),2,3 glaucoma,4 and diabetic retinopathy,5 trigger reactive gliosis. In the retina, reactive gliosis is defined as characteristic changes in astrocyte and Müller cell morphology and as increased production of intermediate filament proteins, glial fibrillary acidic protein (GFAP), and vimentin.6 Astroglia play a central role in maintaining daily function and responding to all forms of pathologic conditions, such as trauma, ischemia, and neurodegenerative diseases. Accumulating evidence suggests that reactive astroglia either release neurotrophic factors to support cell survival7-9 or produce molecules that inhibit axon regeneration and repair to trigger neurocytotoxicity or secondary damage in nearby neurons and glial cells.10,11 Thus, they can be beneficial and harmful to the damaged neurons,12 and a better understanding of retinal glial reactions to injury and their contributions to disease development may facilitate the design of novel therapeutic strategies.
Mouse genetic technology provides a powerful tool for defining the specific roles played by reactive gliosis in the pathogenesis of retinal diseases. Reactive astroglial responses involve a multitude of extracellular and intracellular events. These include the upregulation of GFAP and vimentin, the activation of Erk13 and c-fos14 signaling pathways, the increased production of cytokines and chemokines15 such as tumor necrosis factor (TNF)-α and monocyte chemoattractant protein (MCP)-1, and the recruitment of monocytes/microglia to the injured area.16 Previously, we reported that mice deficient in two hallmark proteins of reactive astroglial cells, GFAP and vimentin, exhibit fewer morphologic changes in astroglia and less glial scarring after injury than mice without these deficiencies.17-19 In the retina, Müller cells, especially their end feet, are less resistant to severe stress.20 Therefore, GFAP-/-Vim-/- mice represent a highly interesting model for exploring the roles of reactive gliosis in the pathogenesis of retinal disease and injury. To date, little is known about how the absence of GFAP and vimentin affects the biochemical and intracellular events of reactive glial cells after disease or injury.
RD, referring to physical separation of the photoreceptor layer of the retina from the underlying retinal pigment epithelium, is the most common cause of blindness in young adults. RD develops in rhegmatogenous, tractional, and exudative RD, neovascular or age-related macular degeneration (AMD), and central serous chorioretinopathy, in which photoreceptors are highly vulnerable and undergo apoptosis.21-24 Neovascular AMD may be treated by photodynamic therapy or antivascular endothelial growth factor therapy directed at the abnormal permeability of neovascular vessels,25,26 but the only available treatment for rhegmatogenous RD is surgery. However, early and successful retinal reattachment surgery often fails to completely restore vision. Sometimes, even after successful reattachment surgery, a peripheral and parafoveal detachment may “crawl” to the fovea, resulting in depression of macular function, eventually causing secondary damage and visual dysfunction.27 Evidence suggests that widely spread glial cell remodeling and activation may play a role in collateral retinal damage associated with RD.3,28-32 Recently, we observed the induction (more than 100-fold within 1 hour) of MCP-1 by reactive Müller glia, which precedes other retinal glial changes30 and plays a key role in photoreceptor degeneration after RD in rats,30 likely resulting from the ability of MCP-1 to recruit neurotoxic phagocytotic monocytes to the injured area. We hypothesize that reactive retinal glia, which release MCP-1 and other cytokines and attract phagocytotic monocytes to the injured area, may be central to RD-associated secondary damage.
In the present study, we analyzed the responses of retinal glial cells and monocyte/microglia to RD in GFAP-/-Vim-/- mice and their contributions to RD-induced photoreceptor damage. Our results show that the absence of two intermediate filament proteins attenuates the reactive responses of retinal glial cells and, as a result, limits RD-induced monocyte recruitment and photoreceptor cell loss. This result suggests a new therapeutic avenue for treating neural damage after RD or in other retinal and central nervous system (CNS) diseases and injuries.
MATERIALS AND METHODS
Animals and Induction of RD
Adult (12-16 weeks) male GFAP-/-Vim-/-17,18,33,34 and age-matched wild-type (WT) mice were used in this study. Both WT and GFAP-/- Vim-/- mice were of a C57BL6-129SV-129Ola-SF2J mixed genetic background. All experimental procedures and use of animals followed the protocol approved by the Animal Care and Use Committee of the Schepens Eye Research Institute and conformed to the standards set forth in the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. RD was induced in the right eyes, as previously reported, with minor modification.35 Briefly, mice were anesthetized by intraperitoneal injection of a mixture of 62.5 mg/kg ketamine and 12.5 mg/kg xylazine (both from Phoenix Pharmaceutical, St. Joseph, MO). After pupil dilation with 1% cyclopentolate and 2.5% phenylephrine hydrochloride (Akorn, Buffalo Grove, IL), a scleral puncture was made at the supranasal equator using a glass micropipette. Approximately 1 μL vitreous fluid was removed to reduce intraocular pressure. A glass micropipette was then inserted into the subretinal space, and 1 μL sodium hyaluronate (Healon GV; Pharmacia & Upjohn, Uppsala, Sweden) was injected. Mice that received scleral punctures without subretinal injections served as controls. Animals with conditions such as lens injury, vitreous hemorrhage, and eye infection were excluded from this study.
Immunohistochemistry
Immunohistochemistry (IHC) was performed as previously reported.35,36 Briefly, 10-μm retinal sections through the optic nerve head were prepared and subjected to reaction with primary antibodies against phospho-Erk (pErk, 1:200; Cell Signaling Technology, Beverly, MA), c-fos (1:200; Santa Cruz Biotechnology, Santa Cruz, CA), MCP-1 (1:100; Peprotech, Rocky Hill, NJ), GFPA (1:100; Sigma-Aldrich, St. Louis, MO), CD11b (1:200; Serotec, Raleigh, NC), or vimentin (1:200; Sigma-Aldrich). Retinal sections incubated with a buffer without the primary antibodies were used as negative controls. Fluorescein, rhodamine, or Cy3-conjugated secondary antibodies, including goat anti-mouse immunoglobulin G (IgG) and anti-rabbit IgG and anti-rat IgG conjugated to Alexa Fluor 488 (Molecular Probes, Eugene, OR), were used. Retinal sections were mounted with mounting media (H-1500, Vectashield; Vector Laboratories, Burlingame, CA) containing 4′,6′ diamidino-2-phenylindole (DAPI) to reveal nuclear structure. To count immunolabeled cells, photomicrographs of retinal sections were taken from the center of the detached retinal area using a microscope equipped with fluorescence illumination (DMRXA; Leica, Wetzlar, Germany) and were analyzed with computer software (OpenLab, version 2.2.5; Improvision, Inc., Lexington, MA). pErk+, c-fos+, and CD11b+ cells (original magnification, 200×) were counted in a masked fashion.
Western Blot Analysis
Western blot analysis was performed as previously described.36 In brief, the nitrocellulose membrane was incubated in a blocking buffer containing rabbit anti-pErk (1:1000; Cell Signaling Technology) overnight at 4°C. Chemiluminescence was detected with an alkaline phosphatase-conjugated anti-rabbit IgG (1:20,000; Promega, Madison, WI) and CDP-star substrate (Amersham Pharmacia Biotech, Buckinghamshire, UK). The membrane was then stripped and incubated in a blocking buffer containing rabbit anti-Erk (1:1000; Cell Signaling Technology) overnight at 4°C. After digital scanning, densities of each band were determined (Multi-Analyst software, version 1.0.2, Bio-Rad, Hercules, CA).
RNA Extraction and Reverse Transcription-Polymerase Chain Reaction
Total RNA extraction and RT-PCR were performed as previously reported.30 Briefly, 2 μg total retinal RNA was used in the RT reaction. First-strand cDNA was synthesized using a real-time PCR thermal cycler (ABI7700; Applied Biosystems, Foster City, CA). Real-time quantitative PCR was performed (TaqMan Universal PCR Master Mix kit; Applied Biosystems), as previously reported.30 DNA sequences of forward and reverse PCR primers for MCP-1 were 5′-ACTCACCTGCTGCTACTCATTCACC-3′ and 5′-CTACAGCTTCTTTGGGACACCTGCT-3′; for mMCP1 they were VIC-ATC CCA ATG AGT AGG CTG GAG AGC TAC AAG AGG ATC-TAMRA-3′. PCR products were resolved by agarose gel electrophoresis and confirmed by DNA sequencing. For relative comparison, the Ct values of real-time PCR results were analyzed using the ΔCt method according to the manufacturer's instructions. The amount of sample cDNA was normalized to the standard internal control obtained using primers for 18 ribosomal RNA.30
Enzyme-Linked Immunosorbent Assay
Protein extractions from the posterior lens capsule, vitreous, and neuroretina were collected 3 days after RD, and ELISA was performed as previously reported.30 Briefly, 100 μg total protein was used for mouse MCP-1 ELISA (Biosource, Camarillo, CA), which was performed according to the manufacturer's guidelines. Absorbance at a wavelength of 450 nm was measured in 96-well plates with a spectrophotometer (SpectraMax 190; Molecular Devices; Sunnyvale, CA).
Quantification of Photoreceptor Cell Loss
We quantified photoreceptor cell loss using two different methods: measuring the ONL thickness in hematoxylin and eosin-stained retinal sections 7 days after RD35 and counting cells positive for TdT-dUTP terminal nick-end labeling (TUNEL) 3 days after RD. As previously reported,30 the center of the detached retinal area was photographed, and the area of ONL was measured with laboratory software (OpenLab; Improvision). TUNEL was performed using a detection kit (ApopTag Fluorescein In Situ Apoptosis; S7110; Chemicon International, Temecula, CA). TUNEL-positive cells were counted in a masked fashion, and areas of the detached portions of the retinas or control retinal sections were measured. The number of TUNEL-positive cells was expressed as cells per unit of detached retinal areas.
Statistical Analysis
Statistical significance of data obtained from immunoblot, RT-PCR, ELISA, cell counting, and measurement of ONL thickness was determined by unpaired Student's t-test with the use of software for Macintosh computer (StatView 4.11J; Abacus Concepts, Berkeley, CA). Significance level was set at P < 0.05. All values were expressed as the mean ± SD without citation.
RESULTS
Induction of Reactive Gliosis after RD
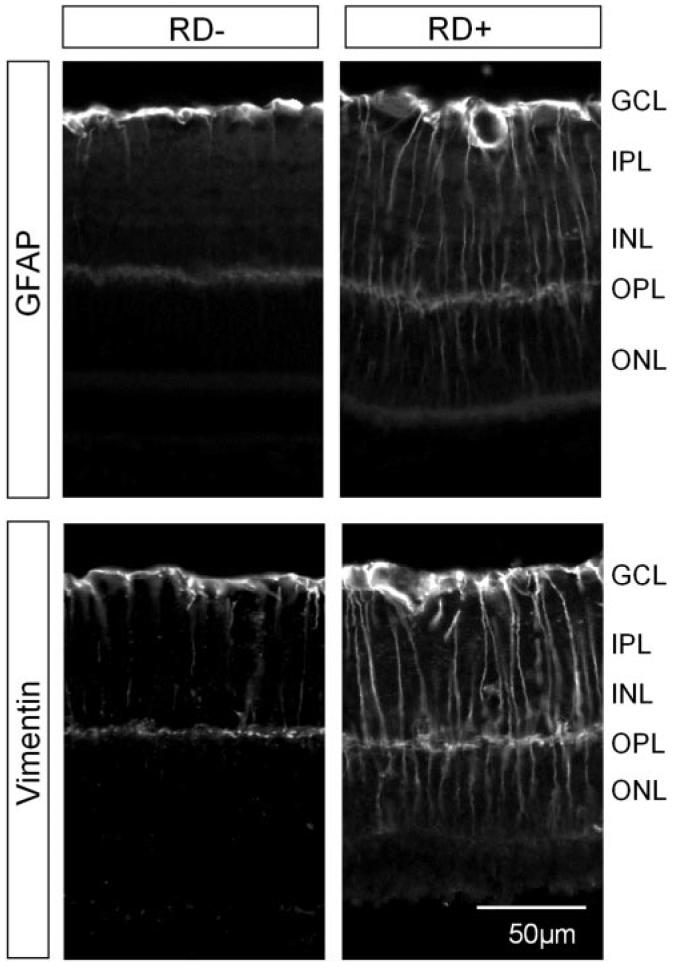
To confirm that RD induces astrocyte and Müller cell responses and reactive gliosis in the mouse retina, we compared the immunoreactivity of GFAP and vimentin before and after the induction of RD in adult WT mice. IHC was performed on retinal sections obtained from the control (sham-operated) group or from the mice with RD. In the controls, GFAP immunoreactivity was limited to the inner margin of the retina, colocalizing with astrocytes, whereas vimentin was detected in Müller cell processes spanning throughout the entire retina (Fig. 1). Three days after RD, intensive GFAP immunoreactivity was detected in Müller cell bodies and their processes. Marked upregulation of vimentin expression was also observed in Müller cell processes (Fig. 1). Data suggest the induction of retinal reactive gliosis after RD.
FIGURE 1.
Upregulation of GFAP and vimentin in astroglial cells after RD. Representative photographs of immunodetection of GFAP and vimentin in retinal sections of the control (RD-) and detached (RD+) retinas 3 days after surgery show increased expression of GFAP and vimentin in the retina with RD. GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer.
To further confirm that RD induces reactive gliosis, we investigated Erk and c-fos activation/phosphorylation in astrocytes and Müller cells before and after RD. IHC for pErk and c-fos were performed on retinal sections 3 days after RD. In control mice, moderate immunoreactivity of pErk and c-fos (Fig. 2A) was detected in the inner plexiform layer (IPL) and outer plexiform layer (OPL) but not in other retinal layers. No signal was detected in the inner nuclear layer (INL) of control mice, where Müller cell bodies are located. Three days after RD, intensive staining of pErk and c-fos (Fig. 2A) was detected in the ganglion cell layer (GCL) and INL of the detached retinas, colocalized with immunostaining for astrocyte and Müller cell markers (data not shown). Consistent with a previous report,29 we noted that immunostaining for pErk was associated with cell bodies and cellular processes of reactive astrocytes and Müller cells, whereas c-fos immunostaining was limited to their nuclei (Fig. 2A). This induction of pErk and c-fos in reactive Müller cells was observed only in the detached retinal areas but not in adjacent undetached retinal areas. Results demonstrated that RD triggers reactive gliosis in astrocytes and Müller glia in mice.
FIGURE 2.
Absence of GFAP and vimentin suppresses RD-induced Erk and c-fos activation in retinal glial cells. (A) Representative photographs of retinal sections that reveal immunoreactivity of pErk or c-fos in retinal sections of WT mice and GFAP-/-Vim-/- mice 3 days after sham operations (RD-) or RD(RD+). Arrowheads: pErk+ or c-fos+ astrocytes in the GCL. Arrows: pErk+ or c-fos+ Müller cells in the INL. Quantification of pErk+ (B) and c-fos+ (C) cells in the INL. After RD, the number of pErk+ cells in retinal sections of WT mice was significantly higher than in GFAP-/-Vim-/- mice.
Reduced Retinal Glial Responses to RD in GFAP-/-Vim-/- Mice
We previously reported that genetic ablation of GFAP and vimentin from astroglia led to reduced astrogliosis after retinal injury.17 To determine whether astroglial cells of GFAP-/-Vim-/- mice also display impaired reactive gliosis after RD, we studied pErk and c-fos expression before and after RD in these mice. As expected, moderate labeling of pErk and c-fos (Fig. 2) was detected in the INL of sham-operated GFAP-/-Vim-/- mice. Even at 3 days after RD, few pErk+ and c-fos+ cells were observed in the retinas of GFAP-/-Vim-/- mice (Fig. 2), contrary to that seen in the WT retinas. Cell counts in the INL of WT mice indicated a 14-fold increase in the number of pErk+ cells or a 6.7-fold increase in the number of c-fos+ cells after stimulation by RD compared with sham-operated controls (Fig. 2B). The absence of GFAP and vimentin in retinal glial cells significantly decreased pErk+ and c-fos induction. The number of pErk+ cells in GFAP-/-Vim-/- retinas was only 29.5% of that in WT retinas, and the number of c-fos+ cells was only 23.3%, indicating suppressed reactive gliosis in retinas of GFAP-/-Vim-/- mice (Fig. 2). This result was corroborated through immunoblotting. We found that RD stimulated Erk phosphorylation in WT, but not in GFAP-/-Vim-/-, mice without affecting the levels of Erk expression (Fig. 3A). Three days after RD, a significant increase in the ratio of pErk versus total Erk was detected in WT mice but not in GFAP-/-Vim-/- mice (Fig. 3B). Together these data demonstrate that the deletion of GFAP and vimentin suppresses retinal glial activation and reactive gliosis associated with RD.
FIGURE 3.
Western blot analysis of Erk phosphorylation after RD. (A) Representative photograph of immunoblots reacted with anti-pErk (pErk) and anti-total Erk (Erk) antibodies 3 days after mice underwent sham operation or RD. (B) Quantification of the relative ratio of pErk to total Erk in WT retinas (gray) and in GFAP-/-Vim-/- retinas (black, G-/-V-/-). pErk was significantly induced after RD in WT but not in GFAP-/-Vim-/- mice.
Absence of GFAP and Vimentin in Müller Cell Production of MCP-1 after RD
Previously, we showed that reactive Müller cells release MCP-1, which contributes critically to RD-induced photoreceptor degeneration.30 Therefore, we asked whether the absence of GFAP and vimentin attenuates cytokine induction, particularly of MCP-1, after RD. Patterns and levels of expression of MCP-1 mRNA and protein were determined by IHC, RT-PCR, and ELISA. An increase in MCP-1 immunoreactivity was detected in the INL (Fig. 4A, arrows), colocalizing with retinal glial cell marker glutamine synthetase in WT mice (Fig. 4B), 3 days after RD. In contrast, in GFAP-/-Vim-/- mice, only weak labeling of MCP-1 was detected after RD (Fig. 4A). In agreement with the IHC results, ELISA results suggested the induction of MCP-1 protein expression in retinas of WT, but not of GFAP-/-Vim-/-, mice after RD (Fig. 4B). The level of MCP-1 protein expression in retinas of GFAP-/-Vim-/- mice was 4.6-fold lower than that measured in the retinas of WT mice after RD. Similarly, RT-PCR results supported the suppression of MCP-1 expression in the retinas of GFAP-/-Vim-/- mice after RD. In WT mice, the MCP-1 mRNA level detected in the detached retinas increased to 59-fold more than that of sham-operated WT controls; however, in the retinas of GFAP-/- Vim-/- mice, the MCP-1 mRNA level in the detached retinas increased to only 10-fold more than that of sham-operated GFAP-/-Vim-/- mice (Fig. 4C). Thus, it is shown that genetic ablation of GFAP and vimentin suppresses the induction of MCP-1 after RD.
FIGURE 4.
MCP-1 induction in WT but not GFAP-/-Vim-/- retinas after RD. (A) Representative photographs of anti-MCP-1 staining in WT and GFAP-/-Vim-/- mice, and triple labeling of a WT retina with anti-MCP-1, anti- glutamine synthetase (GS) and DAPI 3 days after sham operations (RD-)or RD (RD+). Arrows: MCP-1+ cells in the INL. (B) Quantification of MCP-1 protein expression by ELISA in retinas with (RD+) or without RD (RD-). (C) Quantification of MCP-1 mRNA levels by RT-PCR in retinal tissues with RD in WT (WT) or GFAP-/- Vim-/- mice (G-/-V-/-). Expression of MCP-1 was significantly increased in the retinas of WT but not of GFAP-/-Vim-/- mice 3 days after RD.
Suppression of Monocyte Infiltration after RD in GFAP-/-Vim-/- Mice
Reactive retinal glial cells produce cytokines and chemokines that recruit monocytes to the site of injury37,38 or to detached retinal areas after RD.31 To investigate whether ablation of GFAP and vimentin influences monocyte recruitment after RD, we counted infiltrated monocytes in retinas of WT and GFAP-/-Vim-/- mice. Anti-CD11b was used to identify bone marrow-derived monocytes. In the sham-operated retinas, CD11b+ cells were detected in the inner retina, especially the IPL, but they were rarely seen in the subretinal space (SRS). Similar numbers of CD11b+ cells were detected in the nondetached retinas of WT and GFAP-/-Vim-/- mice (Fig. 5C). Three days after RD in WT mice, the number of CD11b+ monocytes was significantly increased in the OPL (Fig. 5A); most of them attached to the surfaces of photoreceptor outer segments in the SRS. In GFAP-/-Vim-/- mice, however, we did not notice any increase in the number of infiltrated monocytes after RD compared with the sham-operated controls (Figs. 5B-D). These results indicate that the ablation of GFAP and vimentin attenuates retinal glial cell responses and monocyte infiltration after RD, suggesting a key role for reactive retinal glial cells in recruiting bone marrow-derived monocytes to the injured areas—likely through the production of chemokines such as MCP-1.
FIGURE 5.
Monocyte recruitment into the detached retinas of WT but not of GFAP-/-Vim-/- mice. IHC of anti- CD11b, a monocyte marker, in retinal sections of WT (A) and GFAP-/- Vim-/- (B) mice 3 days after RD. CD11b+ cells were detected in the OPL (arrows) and SRS (arrowheads) of WT mice. Quantification of CD11b+ cells in the OPL (C) and SRS (D) with or without RD. The number of CD11b+ cells was significantly reduced in GFAP-/-Vim-/- retinas after RD.
Reduced Photoreceptor Degeneration after RD in GFAP-/-Vim-/- Mice
A critical question is whether the suppression of retinal glial cell and monocyte responses to neuron damage in GFAP-/-Vim-/- mice results in a beneficial or detrimental effect on the retina. To address this question, we compared photoreceptor cell viability in WT and GFAP-/-Vim-/- mice. RD induces photoreceptor cell apoptosis that peaks on day 3.21,35,39 By 7 days after RD, a significant decrease in ONL thickness can be detected in WT mice.35 In this study, we detected numerous TUNEL-positive apoptotic photoreceptor cells in the detached retinas of WT mice 3 days after RD (Fig. 6A). In contrast, few TUNEL-positive cells were found in the detached retinas of GFAP-/-Vim-/- mice (Fig. 6B). Significantly fewer TUNEL-positive cells were counted in the detached retinas of GFAP-/-Vim-/- mice than of WT mice. Moreover, 7 days after RD, WT retinas revealed a 55% reduction in ONL thickness compared with that of sham-operated control retinas, whereas ONL thickness of GFAP-/-Vim-/- mice remained unchanged before and after RD (Fig. 7). These data strongly suggest that reactive astroglial cells play an active role in RD-associated photoreceptor cell apoptosis or degeneration.
FIGURE 6.
RD-induced photoreceptor apoptosis in WT and GFAP-/-Vim-/- mice. TUNEL assay in the detached retinal areas of WT (A) and GFAP-/- Vim-/- (B) mice 3 days after RD. (C) Quantification of TUNEL-positive cells. Note that the number of TUNEL-positive cells is significantly lower in the retinas of GFAP-/-Vim-/- mice than in those of WT mice.
FIGURE 7.
Absence of GFAP and vimentin genes prevents RD-induced photoreceptor degeneration. (A) Hematoxylin and eosin-stained retinal sections taken from WT and GFAP-/-Vim-/- mice 7 days after sham operation (RD-) or RD (RD+). (B) Quantification of ONL thickness. Note that the decrease in ONL thickness after RD is significantly reduced in retinas of GFAP-/-Vim-/- mice than in those of WT mice.
DISCUSSION
Although reactive gliosis has been associated with all forms of retinal and CNS disorders in humans, the functional significance of this glial response to injury remains elusive. In the present study, we used RD as a model to investigate the reactions of retinal glial cells in GFAP-/-Vim-/- mice and their impact on neuron survival. Our results showed that the absence of two intermediate filament proteins in astroglia, GFAP and vimentin, largely attenuates retinal glial activation and monocyte recruitment after retinal injury and promotes photoreceptor cell survival after RD. In a companion study, we also demonstrated that glial scar formation in the SRS is greatly reduced in mutant mice (Verardo MR, et al. IOVS 2005; 46: ARVO E-Abstract 2443). These studies provide the first strong evidence supporting a central role for reactive retinal glial cells in the pathogenesis of RD and retinal neuron damage.
Although RD-induced glial responses have been better characterized in cats and rats than in mice,29 in the present study, we report similar retinal glial responses in a mouse model of RD. Interestingly, the absence of GFAP and vimentin attenuated the reactions of retinal glial cells to injury, as evidenced by the attenuated activation of Erk and c-fos and the reduced production of MCP-1 in the detached retinas of GFAP-/-Vim-/- mice. Counts of pErk+ retinal glial cells in the detached retinas revealed a greater than 10-fold reduction in the number of pErk+ cells in GFAP-/-Vim-/- retinas than in WT retinas. This result was further supported by Western blot analysis. It should be noted that IHC and cell counts revealed greater pErk induction after RD than did Western blot because IHC enabled selective exclusion of background pErk labeling, whereas Western blot analysis detected a base level of pErk expression in the uninjured retina that offset the changes in pErk levels induced after RD. Together, these data show that the absence of GFAP and vimentin blocks RD-induced activation of intracellular signals, Erk, and c-fos in reactive retinal glial cells.
Connections between the function of astroglial intermediate filaments and the activation of intracellular signals remain poorly understood. It is possible that the absence of intermediate filament proteins in astrocytes and Müller cells disables subcellular translocation and phosphorylation of Erk and, as a result, blocks the signaling event that induces glial activation and the transcription programs regulating cytokine production. It has been shown that vimentin can directly interact with Erk and can transport pErk to the nuclei40 and that the activation of Erk in reactive retinal glial cells stimulates MCP-1 expression after an injury,41 leading to reduced monocyte recruitment to the OPL and SRS. In a parallel study, we discovered that RD-induced monocyte recruitment is abolished in MCP-1- deficient mice,42 supporting the idea that MCP-1 induction is central to monocyte infiltration after RD. A recent study by another group shows that vimentin deficiency affects adhesion and transcellular migration of leukocytes through endothelial cells.43 Thus, we cannot rule out the possibility that the absence of GFAP and vimentin may change cellular properties of other cell types that influence monocyte recruitment after RD in GFAP-/-Vim-/- mice.
The most significant finding in the present study is that mice deficient in GFAP and vimentin exhibit greatly reduced photo-receptor degeneration after RD. It has already been shown that the activation of Erk and c-fos can be detected within 15 minutes of RD in reactive retinal glial cells, preceding the induction of MCP-1, which begins approximately 1 hour after RD.30 Monocyte recruitment and photoreceptor degeneration,35,39 which peak 3 days after RD, are the later events after RD. These data suggest that RD triggers Erk and c-Fos activation in reactive retinal glial cells, which produce MCP-1 to attract monocytes to the vicinity of the injured retina44 and to generate neurotoxicity. Several studies report that MCP-1 is a key regulator of RD-induced neural damage. Subretinal administration of MCP-1 alone has been found to be toxic to photoreceptor cells under the RD.42 Consistent with this finding, our study reports that retinal glial cells in GFAP-/-Vim-/- mice fail to respond to retinal injury or to express MCP-1 after RD, leading to much improved photoreceptor cell survival. However, the absence of GFAP and vimentin suppressed only 70% of RD-induced photoreceptor apoptosis (Fig. 6) in comparison with WT mice. A possible explanation is that multiple mechanisms are involved in RD-induced photoreceptor cell loss. The absence of GFAP and vimentin does not completely abolish retinal glial cell responses to injury. Interestingly, quantitative analyses of MCP-1 induction, monocyte recruitment, and photoreceptor degeneration revealed similar levels of reduction (approximately 70%) in each process in GFAP-/-Vim-/- mice compared with that in WT mice. In any case, the results demonstrate a crucial role for reactive gliosis in retinal neuron damage after RD.
In summary, this study shows that the induction of reactive gliosis and retinal glial cell activation is an important step in the pathogenesis of RD and that attenuation of this process may have therapeutic benefits for patients with RD. Activation of retinal glial cells has long been observed in patients with glaucoma,4 diabetic retinopathy,5 and other retinal and CNS diseases and may contribute to the pathogenesis of these diseases. These findings may thus open new therapeutic avenues for treating retinal and CNS degeneration and disorders.
Acknowledgments
Supported by National Eye Institute Grant EY012983; Massachusetts Lion's Eye Research Fund; Department of Defense Grant W81XWH-04-1-0892; Sybil B. Harrington Scholar Award (DFC); Alcon Research Award (JWM); Bausch & Lomb Vitreoretinal Fellowship (TN); National Eye Institute Grant EY00888 (SKF); Swedish Research Council Grant 11548; Swedish Stroke Association; Swedish Society for Medicine (MP); Hjärnfonden; Swedish Society for Medical Research (UW); National Eye Institute Core Grant EY014104 (Massachusetts Eye and Ear Infirmary); and Core Grant EY003790 (Schepens Eye Research Institute).
Footnotes
Disclosure: T. Nakazawa, None; M. Takeda, None; G.P. Lewis, None; K.-S. Cho, None; J. Jiao, None; U. Wilhelmsson, None; S.K. Fisher, None; M. Pekny, None; D.F. Chen, None; J.W. Miller, None
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be marked “advertisement” in accordance with 18 U.S.C. §1734 solely to indicate this fact.
References
- 1.Caicedo A, Espinosa-Heidmann DG, Pina Y, Hernandez EP, Cousins SW. Blood-derived macrophages infiltrate the retina and activate Muller glial cells under experimental choroidal neovascularization. Exp Eye Res. 2005;81:38–47. doi: 10.1016/j.exer.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 2.Lewis GP, Matsumoto B, Fisher SK. Changes in the organization and expression of cytoskeletal proteins during retinal degeneration induced by retinal detachment. Invest Ophthalmol Vis Sci. 1995;36:2404–2416. [PubMed] [Google Scholar]
- 3.Sethi CS, Lewis GP, Fisher SK, et al. Glial remodeling and neural plasticity in human retinal detachment with proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci. 2005;46:329–342. doi: 10.1167/iovs.03-0518. [DOI] [PubMed] [Google Scholar]
- 4.Wang L, Cioffi GA, Cull G, Dong J, Fortune B. Immunohistologic evidence for retinal glial cell changes in human glaucoma. Invest Ophthalmol Vis Sci. 2002;43:1088–1094. [PubMed] [Google Scholar]
- 5.Mizutani M, Gerhardinger C, Lorenzi M. Muller cell changes in human diabetic retinopathy. Diabetes. 1998;47:445–449. doi: 10.2337/diabetes.47.3.445. [DOI] [PubMed] [Google Scholar]
- 6.Lewis GP, Fisher SK. Up-regulation of glial fibrillary acidic protein in response to retinal injury: its potential role in glial remodeling and a comparison to vimentin expression. Int Rev Cytol. 2003;230:263–290. doi: 10.1016/s0074-7696(03)30005-1. [DOI] [PubMed] [Google Scholar]
- 7.Srebro Z, Dziobek K. Neuroprotection: the role of neuroglia. Folia Med Cracov. 2001;42:113–121. [PubMed] [Google Scholar]
- 8.Anderson MF, Blomstrand F, Blomstrand C, Eriksson PS, Nilsson M. Astrocytes and stroke: networking for survival? Neurochem Res. 2003;28:293–305. doi: 10.1023/a:1022385402197. [DOI] [PubMed] [Google Scholar]
- 9.Takuma K, Baba A, Matsuda T. Astrocyte apoptosis: implications for neuroprotection. Prog Neurobiol. 2004;72:111–127. doi: 10.1016/j.pneurobio.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Sandvig A, Berry M, Barrett LB, Butt A, Logan A. Myelin-, reactive glia-, and scar-derived CNS axon growth inhibitors: expression, receptor signaling, and correlation with axon regeneration. Glia. 2004;46:225–251. doi: 10.1002/glia.10315. [DOI] [PubMed] [Google Scholar]
- 11.Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- 12.Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia. 2005;50:427–434. doi: 10.1002/glia.20207. [DOI] [PubMed] [Google Scholar]
- 13.Neary JT, Kang Y, Shi YF. Signaling from nucleotide receptors to protein kinase cascades in astrocytes. Neurochem Res. 2004;29:2037–2042. doi: 10.1007/s11064-004-6876-y. [DOI] [PubMed] [Google Scholar]
- 14.Butler TL, Pennypacker KR. Temporal and regional expression of Fos-related proteins in response to ischemic injury. Brain Res Bull. 2004;63:65–73. doi: 10.1016/j.brainresbull.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 15.Little AR, Benkovic SA, Miller DB, O'Callaghan JP. Chemically induced neuronal damage and gliosis: enhanced expression of the proinflammatory chemokine, monocyte chemoattractant protein (MCP)-1, without a corresponding increase in proinflammatory cytokines(1) Neuroscience. 2002;115:307–320. doi: 10.1016/s0306-4522(02)00359-7. [DOI] [PubMed] [Google Scholar]
- 16.Izikson L, Klein RS, Luster AD, Weiner HL. Targeting monocyte recruitment in CNS autoimmune disease. Clin Immunol. 2002;103:125–131. doi: 10.1006/clim.2001.5167. [DOI] [PubMed] [Google Scholar]
- 17.Kinouchi R, Takeda M, Yang L, et al. Robust neural integration from retinal transplants in mice deficient in GFAP and vimentin. Nat Neurosci. 2003;6:8636–8688. doi: 10.1038/nn1088. [DOI] [PubMed] [Google Scholar]
- 18.Pekny M, Johansson CB, Eliasson C, et al. Abnormal reaction to central nervous system injury in mice lacking glial fibrillary acidic protein and vimentin. J Cell Biol. 1999;145:503–514. doi: 10.1083/jcb.145.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilhelmsson U, Li L, Pekna M, et al. Absence of glial fibrillary acidic protein and vimentin prevents hypertrophy of astrocytic processes and improves post-traumatic regeneration. J Neurosci. 2004;24:5016–5021. doi: 10.1523/JNEUROSCI.0820-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lundkvist A, Reichenbach A, Betsholtz C, Carmeliet P, Wolburg H, Pekny M. Under stress, the absence of intermediate filaments from Muller cells in the retina has structural and functional consequences. J Cell Sci. 2004;117:3481–3488. doi: 10.1242/jcs.01221. [DOI] [PubMed] [Google Scholar]
- 21.Arroyo JG, Yang L, Bula D, Chen DF. Photoreceptor apoptosis in human retinal detachment. Am J Ophthalmol. 2005;139:605–610. doi: 10.1016/j.ajo.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 22.Chang CJ, Lai WW, Edward DP, Tso MO. Apoptotic photoreceptor cell death after traumatic retinal detachment in humans. Arch Ophthalmol. 1995;113:880–886. doi: 10.1001/archopht.1995.01100070054025. [DOI] [PubMed] [Google Scholar]
- 23.Cook B, Lewis GP, Fisher SK, Adler R. Apoptotic photoreceptor degeneration in experimental retinal detachment. Invest Ophthalmol Vis Sci. 1995;36:990–996. [PubMed] [Google Scholar]
- 24.Luthert PJ, Chong NH. Photoreceptor rescue. Eye. 1998;12:591–596. doi: 10.1038/eye.1998.149. [DOI] [PubMed] [Google Scholar]
- 25.Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–1444. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 26.Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 27.Francke M, Faude F, Pannicke T, et al. Glial cell-mediated spread of retinal degeneration during detachment: a hypothesis based upon studies in rabbits. Vision Res. 2005;45:2256–2267. doi: 10.1016/j.visres.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 28.Fisher SK, Lewis GP. Muller cell and neuronal remodeling in retinal detachment and reattachment and their potential consequences for visual recovery: a review and reconsideration of recent data. Vision Res. 2003;43:887–897. doi: 10.1016/s0042-6989(02)00680-6. [DOI] [PubMed] [Google Scholar]
- 29.Geller SF, Lewis GP, Fisher SK. FGFR1, signaling, and AP-1 expression after retinal detachment: reactive Muller and RPE cells. Invest Ophthalmol Vis Sci. 2001;42:1363–1369. [PubMed] [Google Scholar]
- 30.Nakazawa T, Matsubara A, Noda K, et al. Characterization of cytokine responses to retinal detachment in rats. Mol Vis. 2006;12:867–878. [PubMed] [Google Scholar]
- 31.Hisatomi T, Sakamoto T, Sonoda KH, et al. Clearance of apoptotic photoreceptors: elimination of apoptotic debris into the subretinal space and macrophage-mediated phagocytosis via phosphatidylserine receptor and integrin alphavbeta3. Am J Pathol. 2003;162:1869–1879. doi: 10.1016/s0002-9440(10)64321-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lewis GP, Sethi CS, Carter KM, Charteris DG, Fisher SK. Microglial cell activation following retinal detachment: a comparison between species. Mol Vis. 2005;11:491–500. [PubMed] [Google Scholar]
- 33.Pekny M, Leveen P, Pekna M, et al. Mice lacking glial fibrillary acidic protein display astrocytes devoid of intermediate filaments but develop and reproduce normally. EMBO J. 1995;14:1590–1598. doi: 10.1002/j.1460-2075.1995.tb07147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Colucci-Guyon E, Portier MM, Dunia I, Paulin D, Pournin S, Babinet C. Mice lacking vimentin develop and reproduce without an obvious phenotype. Cell. 1994;79:679–694. doi: 10.1016/0092-8674(94)90553-3. [DOI] [PubMed] [Google Scholar]
- 35.Yang L, Bula D, Arroyo JG, Chen DF. Preventing retinal detachment-associated photoreceptor cell loss in Bax-deficient mice. Invest Ophthalmol Vis Sci. 2004;45:648–654. doi: 10.1167/iovs.03-0827. [DOI] [PubMed] [Google Scholar]
- 36.Nakazawa T, Tamai M, Mori N. Brain-derived neurotrophic factor prevents axotomized retinal ganglion cell death through MAPK and PI3K signaling pathways. Invest Ophthalmol Vis Sci. 2002;43:3319–3326. [PubMed] [Google Scholar]
- 37.Ridet JL, Malhotra SK, Privat A, Gage FH. Reactive astrocytes: cellular and molecular cues to biological function. Trends Neurosci. 1997;20:570–577. doi: 10.1016/s0166-2236(97)01139-9. [DOI] [PubMed] [Google Scholar]
- 38.Stoll G, Jander S, Schroeter M. Inflammation and glial responses in ischemic brain lesions. Prog Neurobiol. 1998;56:149–171. doi: 10.1016/s0301-0082(98)00034-3. [DOI] [PubMed] [Google Scholar]
- 39.Hisatomi T, Sakamoto T, Goto Y, et al. Critical role of photoreceptor apoptosis in functional damage after retinal detachment. Curr Eye Res. 2002;24:161–172. doi: 10.1076/ceyr.24.3.161.8305. [DOI] [PubMed] [Google Scholar]
- 40.Helfand BT, Chou YH, Shumaker DK, Goldman RD. Intermediate filament proteins participate in signal transduction. Trends Cell Biol. 2005;15:568–570. doi: 10.1016/j.tcb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 41.D'Aversa TG, Yu KO, Berman JW. Expression of chemokines by human fetal microglia after treatment with the human immunodeficiency virus type 1 protein Tat. J Neurovirol. 2004;10:86–97. doi: 10.1080/13550280490279807. [DOI] [PubMed] [Google Scholar]
- 42.Nakazawa T, Hisatomi T, Nakazawa C, et al. Monocyte chemoattractant protein 1 mediates retinal detachment-induced photoreceptor apoptosis. Proc Natl Acad Sci USA. 2007;104:2425–2430. doi: 10.1073/pnas.0608167104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nieminen M, Henttinen T, Merinen M, Marttila-Ichihara F, Eriksson JE, Jalkanen S. Vimentin function in lymphocyte adhesion and transcellular migration. Nat Cell Biol. 2006;8:156–162. doi: 10.1038/ncb1355. [DOI] [PubMed] [Google Scholar]
- 44.Matsushima K, Larsen CG, DuBois GC, Oppenheim JJ. Purification and characterization of a novel monocyte chemotactic and activating factor produced by a human myelomonocytic cell line. J Exp Med. 1989;169:1485–1490. doi: 10.1084/jem.169.4.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]