Abstract
Methoxychlor (MXC) is an organochlorine pesticide with estrogenic, anti-estrogenic, and anti-androgenic properties. To investigate whether transient developmental exposure to MXC could cause adult ovarian dysfunction, we exposed Fischer rats to 20 μg/kg/day (low dose; environmentally relevant dose) or 100 mg/kg/day (high dose) MXC between 19 days post-coitum and postnatal day 7. Multiple reproductive parameters, serum hormone levels, and ovarian morphology and molecular markers were examined from prepubertal through adult stages. High dose MXC accelerated pubertal onset and first estrus, reduced litter size, and increased irregular cyclicity (P < 0.05). MXC reduced superovulatory response to exogenous gonadotropins in prepubertal females (P < 0.05). Rats exposed to high dose MXC had increasing irregular estrous cyclicity beginning at 4 months of age, with all animals showing abnormal cycles by 6 months. High dose MXC reduced serum progesterone, but increased luteinizing hormone (LH). Follicular composition analysis revealed an increase in the percentage of preantral and early antral follicles and a reduction in the percentage of corpora lutea in high dose MXC-treated ovaries (P < 0.05). Immunohistochemical staining and quantification of the staining intensity showed that estrogen receptor β was reduced by high dose MXC while anti-Mullerian hormone was upregulated by both low- and high dose MXC in preantral and early antral follicles (P < 0.05). High dose MXC significantly reduced LH receptor expression in large antral follicles (P < 0.01), and down-regulated cytochrome P450 side-chain cleavage. These results demonstrated that developmental MXC exposure results in reduced ovulation and fertility and premature aging, possibly by altering ovarian gene expression and folliculogenesis.
Keywords: ovary, development, folliculogenesis, endocrine disruptors, steroidogenesis, luteinizing hormone receptor, anti-Mullerian hormone, estrogen receptor-β
Introduction
The major functions of the adult ovary are steroidogenesis and ovulation, which are intricately related to the process of folliculogenesis. These functions are mediated by spatio-temporal expression of genes regulated primarily by the gonadotropins, follicle stimulating hormone (FSH) and luteinizing hormone (LH). FSH is required for follicular growth and LH is required for final follicular maturation and ovulation. The ovary gains gonadotropin responsiveness by acquiring FSH receptors and LH receptors (LHR) (Hirshfield, 1991; Richards, 2001). Under the influence of FSH, the expression of steroidogenic enzymes such as P450scc and P450aromatase are upregulated (Ronen-Fuhrmann et al., 1998; Logan et al., 2002). FSH also stimulates the expression of LHR (Zeleznik et al., 1974; Erickson et al., 1979; Segaloff et al., 1990; Chen et al., 2000; Saxena et al., 2004).
Luteinizing hormone elicits expression of steroidogenic enzymes primarily within the theca cells, which results in increased androgen production. Theca cell-derived androgens are converted into estradiol-17β (E2) by P450aromatase in the granulosa cells. Increasing serum E2 acts on the hypothalamus and pituitary to trigger a pre-ovulatory LH surge (Palermo, 2007), ensuring the final maturation of the follicles and ovulation. In contrast to the pattern of expression of the steroidogenic enzymes, anti-Mullerian hormone (AMH), a TGF-β family member, is expressed in growing pre-antral follicles, and its expression is reduced in antral and pre-ovulatory follicles (Themmen, 2005; Uzumcu et al., 2006). AMH inhibits the initial recruitment of primordial follicles into growing follicles (Durlinger et al., 1999). Therefore, AMH is considered to be a growth inhibitor that controls the rate of folliculogenesis (Themmen, 2005).
Ovarian follicles are established during early ovarian development. In rodents this occurs during late fetal and early postnatal life, and includes oocyte nest breakdown via germ cell apoptosis (Pepling and Spradling, 2001), follicular assembly, and the initial transition from the primordial to the primary follicle stage (Skinner, 2005). These early developmental processes predicate the size of the initial, limited number of follicles in the ovary, which in turn dictate the reproductive lifespan of a female. All of these processes are regulated by E2 (Kezele and Skinner, 2003; Chen et al., 2007) as well as local growth factors (Skinner, 2005). Environmental endocrine disruptors that mimic estrogenic compounds (e.g., diethylstilbestrol, DES; and genistein) cause detrimental effects on these early ovarian processes(Iguchi et al., 1990; Chen et al., 2007), and therefore can negatively impact adult reproductive health.
Environmental endocrine disruptors are synthetic or natural compounds that adversely affect the function of the endocrine system. Methoxychlor [1,1,1-trichloro-2,2-bis(4-methoxyphenyl)ethane; MXC] is considered to be a model endocrine disruptor (Cummings, 1997) that exerts estrogenic activities (Hall et al., 1997) and is likely to affect early ovarian development, folliculogenesis, and adult ovarian function. The major metabolites of MXC are 1,1,1-trichloro-2,2-bis(4-hydroxyphenyl)ethane (HPTE) and 1,1,1-trichloro-2-(4-hydroxyphenyl)-2-(4-methoxyphenyl) ethane (mono OH-MXC), each of which possesses estrogenic or anti-estrogenic activities depending on the estrogen receptor (ERα or ERβ) subtype with which they interact (Gaido et al., 1999; Gaido et al., 2000).
Both ERα and β are expressed in the ovary during development and adulthood. Gene deletion studies show that ERβ plays a more significant role locally in the ovary, while ERα regulates estrogen-mediated feedback within the hypothalamus and pituitary in mice (Woodruff and Mayo, 2005). Whether these findings in mice apply to rats is not exactly known. However, it is known that similar immunolocalization patterns of ERs are observed in mice (Jefferson et al., 2000) and rats (Sar and Welsch, 1999). ERα is primarily expressed in germinal epithelium, stromal cells, and theca cells in the rat ovary starting on postnatal day (PND) 1, and the intensity of expression increases with age (Sar and Welsch, 1999). ERβ is expressed in granulosa cells of growing follicles starting on PND 5 (Sar and Welsch, 1999). ERβ is essential for FSH-induced GC differentiation as well as for gaining responsiveness to LH in mice (Couse et al., 2005). Prior studies using MXC have shown that antral follicles are particular targets for its adverse actions (Borgeest et al., 2004; Miller et al., 2005) mediated via estrogen receptor pathways (Miller et al., 2006).
Long-term MXC exposure during the developmental periods affects ovarian function and fertility (Gray et al., 1989; Chapin et al., 1997). However, the effect of MXC exposure that is limited to the time of early ovarian development is not known. It has been shown that exposure to endocrine disruptors during early life affects not only development but also have long lasting adverse health consequences. For example, exposure of mice to DES between PND 1 and 5 resulted in altered gene expression and induced uterine pathologies (Newbold, 2004). Sheep that are exposed to bisphenol A (BPA) and MXC during in utero development show a delayed LH surge and a dampened LH surge magnitude during adult life (Savabieasfahani et al., 2006). Rats that were developmentally exposed to estrogenic compounds had increased incidence of prostate cancer (Ho et al., 2006). Early postnatal MXC exposure inhibits ovarian folliculogenesis and stimulates AMH production in prepubertal rats (Uzumcu et al., 2006). However, there are only limited studies investigating the consequences of transient endocrine disruptor exposure precisely targeting early ovarian development on adult ovarian function and reproductive viability.
Therefore, the objective of this study was to investigate the effect of transient MXC exposure during fetal and neonatal ovarian development [i.e., 19 days post coitum (dpc) to PND 7] on adult ovarian function and female reproductive parameters, including age of puberty and first estrus, regularity of the estrous cycles, litter size, superovulatory response and reproductive senescence. In addition, to explore potential mechanisms, we investigated morphological and molecular changes in the ovary that are associated with the effects of developmental MXC exposure on reproductive parameters.
Materials and Methods
Animals
Fischer (CDF) inbred rats were obtained from Charles River Laboratories (Wilmington, MA) to generate timed-pregnant females. The inbred Fischer strain was used since it has minimal polymorphism, which facilitates the detection of treatment effects. The animals were maintained in a room with controlled illumination (lights on 0700 h-2100 h), temperature (26–28 C), and humidity (30–70%). Rats were given free access to a soy-free scientific diet 5V01 rat chow (Lab Diet manufactured by PMI Nutrition International LLC, Brentwood, MO) and tap water ad libitum. The soy-free diet was given to reduce the quantity of phytoestrogens in the feed and to minimize background-level exposure to estrogenic compounds (Boettger-Tong et al., 1998). All procedures in the present study were carried out in accordance with the guidelines of the Rutgers University Animal Care and Facilities Committee.
Treatments
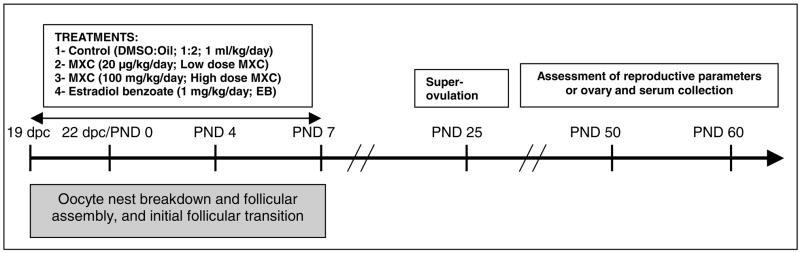
As shown in Figure 1, time-pregnant females received two different treatment dosages of MXC (Sigma), 20 μg/kg/day (low dose MXC) and 100 mg/kg/day (high dose MXC) in 1 ml/kg vehicle. Control animals only received vehicle (dimethylsulfoxide (DMSO):sesame oil; 1:2; control). An additional group of animals received 1 mg/kg/day of beta-estradiol-3-benzoate (EB) as a positive control of estrogenic action.
Fig. 1. Experimental design.
Timed-pregnant Fischer (CDF) rats were injected (ip) with daily 20 μg/kg (low dose MXC) or 100 mg/kg MXC (high dose MXC), 1 mg/kg EB (EB), or vehicle (DMSO:sesame oil; 1:2; Control) between 19 and 22 day post coitum (dpc) (sperm-positive vaginal smear day = 0 dpc). After birth, the female offspring were injected daily (sc) with the same treatments between postnatal day (PND) 0 and 7. In the first experiment, animals were monitored daily for the vaginal opening starting PND 25, for the first estrus using vaginal cytology, and for regularity of the first cycle. On the proestrus day of the 3rd cycle, animals in each treatment group were divided into two groups. In one group, ovaries were collected for histology and immunohistochemistry. Serum was collected for hormone measurement by radioimmunoassay (RIA). The other group was bred to assess pregnancy rate and litter size; and after birth, the dams’ cycles were followed until 12 months of age to determine any premature reproductive aging. In another experiment, a separate group of timed-pregnant females were treated, superovulated, and the number of oocytes released was determined as described in Materials and Methods. N= 5–9 from 3–5 litters in each experiments. Critical ovarian processes that occur during the treatment period are shown in a gray box below the time line.
The 20 μg/kg/day MXC is considered to be an environmentally relevant, low dose of MXC (Palanza et al., 2002; Laviola et al., 2005). The reported environmental levels of MXC range from 160 mg/liter in waters down-stream MXC-sprayed areas to 0.1 ng/kg/day, the FDA’s calculated average daily intake of MXC in adults (cited in (ATSDR, 2002)). The 100 mg/kg/day MXC represents the mid-low range of doses of MXC that was used in previous studies by this laboratory and others (Gray et al., 1989; Chapin et al., 1997; Uzumcu et al., 2006). In addition, in previous studies, 100–200 mg/kg/day MXC was shown to cause delayed and transgenerational epigenetic effects in males when treatment occurred during testis differentiation period (Anway et al., 2005). The rationale was to test whether a similar dose of MXC (100 mg) would cause a delayed effect in females if the treatment took place during early ovary development.
The tissue concentrations of MXC in rats that are exposed to 100 mg/kg day and 20 μg/kg day MXC should reach approximately 300 μM and 60 nM respectively. Approximately 23% of the MXC is metabolized into the more active metabolite HPTE (Kapoor et al., 1970), therefore it is reasonable to assume that serum HPTE levels can reach approximately 69 μM and 13.8 nM in rats in this study. Since the EC50 value for HPTE to ERα is 51 nM and the relative binding affinity of HPTE to ERβ is 5 times higher than that to ERα (Gaido et al., 1999), it is also reasonable to assume that HPTE and other major MXC metabolites of MXC (e.g., mono OH MXC), which have comparable affinities to ERβ and ERα (Gaido et al., 2000), are likely to bind and act on these receptors.
The rats were treated for 12 days between 19 dpc and PND 7. On 19 dpc, 3–5 dams were randomly assigned to each treatment group. In order to precisely control the dosage, we used parenteral routes. The treatment was administered to the dams via an ip injection. The day of birth was designated as PND 0. The litter size was adjusted to 8–10 offspring/dam. The female offspring were selected for postnatal exposure and treated via sc injection daily from PND 0–7 (Fig. 1). The first injection was within the first 24 h following birth.
Assessment of female reproductive function
Pubertal age was determined by observing the vaginal opening starting with PND 25. Estrous cyclicity was determined by performing vaginal smears starting with day of puberty. The first estrus was determined by the presence of keratinized vaginal epithelial cells in the vaginal smear. Individual females were placed with an untreated male overnight on the afternoon of their third proestrus day. A sperm-positive vaginal smear the next morning was considered a successful mating. Females displaying a vaginal cytology with considerable leukocyte presence up to and including the 7th day after mating were considered pregnant, and the pregnancy was subsequently confirmed with a live delivery of the litter. In our rat colony, delivery normally occurs on 22 dpc (sperm-positive vaginal sample day = 0). The litter size, sex ratio, anogenital distance, and gross anatomy of the neonates were examined on the day of birth (PND 0). The females failing to show sperm-positive vaginal smear after the initial mating attempt were mated until displaying a diestrus pattern of vaginal cytology (see below). The mating procedure was repeated up to three times (if the previous attempts were unsuccessful) to determine the pregnancy rate.
Prepubertal superovulation study
A separate set of animals were treated the same as described in Treatments (Fig. 1). All rats then received a single ip injection of 7.5 IU pregnant mare serum gonadotropin (PMSG; Sigma) between 1500 h and1600 h on PND 24–26, followed 48 h later with 15 IU human chorionic gonadotropin (hCG; Sigma). In a pilot study, it was determined that these doses of PMSG and hCG in combination cause a submaximal number of ovulations. The animals were sacrificed 16–20 h after the hCG injection. The oocytes from both ovaries were counted after removal of cumulus cells with incubation in 100 μl Hank’s balanced salt solution (Invitrogen) containing 0.1 mg/ml hyaluronidase (Sigma) at 37 C for 5 min. The total number of oocytes from both ovaries were recorded and used as one replicate in analysis. The ovaries were weighed after being cleaned of connective tissues, oviduct, and bursa.
Serum and ovary collection
While some of the littermates were mated to determine the pregnancy rate and litter size, some were sacrificed on the day of third proestrus between PND 50 and 60. The blood was collected for hormone radioimmunoassay (RIA) and the ovaries were collected for histology and immunohistochemistry (IHC). The collections were conducted the morning of proestrus between 0800 h and 1200 h, or on the estrous day if the animals were showing persistent estrus. The blood from the inferior vena cava was collected, kept at 4°C for 4 h, and centrifuged at approximately 400 × g for 15 minutes for serum separation. The serum was stored at −80°C until RIA.
Hormone radioimmunoassay
Hormones were measured in 100 μL serum samples using commercially available radioimmunoassay (RIA) kits, according to the manufacture’s instructions. Concentrations of serum FSH and LH were determined using rat specific, double-antibody I125 radioimmunoassay kits (GE Healthcare, Piscataway, NJ). Serum progesterone (P4) and E2 was measured using Coat-A-Count RIA kits (Siemens Medical Solutions).
Ovarian histology
The ovaries were fixed in Bouin’s fixative at room temperature for 3 h, processed, embedded in paraffin, and sectioned at 5 μm. The number of follicles and corpora lutea (CL) were counted and classified in 6–7 hematoxylin and eosin stained serial sections encompassing 200 μm from the largest cross-sectional region through the center of the ovary and averaged as previously described (Uzumcu et al., 2006).
Immunohistochemistry
The IHC was conducted as previously described (Uzumcu et al., 2002). Briefly, sections were deparaffinized in Citrisolv (D-limonene, Fisher) and rehydrated in PBS for 10 min. Antigen retrieval was performed by microwaving slides in 0.01 M sodium citrate buffer (pH 6.0) for 15 min. The sections were blocked with 1% serum for 30 min and incubated with the primary antibodies at room temperature overnight in a humidified chamber. Primary antibodies included ERβ (Affinity, PA1-310B), StAR (gift from Dr. Doug Stocco, Texas Tech, Lubbock, TX), and others from Santa Cruz Biotechnology: AMH (sc-6886); P450scc (sc-18043); ERα (sc-542); and LHR (sc-25828). Biotinylated anti-rabbit or goat secondary antibodies (Santa Cruz) were added at 1:200 dilution and incubated for 60 min at room temperature. Detection was with streptavidin-FITC (Vector Laboratories). All sections were counterstained with ethidium homodimer-2 (EthD-2, Invitrogen; not shown). After three washes in PBS, slides were mounted in Prolong Gold Anti-fade reagent (Invitrogen). Negative controls sections were treated identical, except primary antibody was replaced with buffer solution. Sections were analyzed and imaged using a Nikon Eclipse E800 microscope with epifluorescent attachments and suitable filters for streptavidin-FITC (green) and EthD-2 detection (red). Images were acquired with a Nikon DXM1200F camera with ACT1 software (Version 2) at equal exposure levels. Minimal and equal adjustments, if any, for only brightness were made on all images with Adobe Photoshop CS and quantified.
Quantification of immunohistochemical staining intensity
Mean staining intensity per unit area of selected structures or the entire section for each marker was determined using Image J software (NIH, http://rsb.info.nih.gov/ij/). The polygonal selection tool was used to select the respective structures as described below. For ERβ (n = 10) and AMH (n = 15), the mean staining intensity was determined for granulosa cells of each follicle, excluding oocyte, antral space, and theca layer in randomly selected preantral or early antral stage follicles. For LHR, the mean staining intensity per unit area for the theca layer surrounding randomly selected large antral follicles (n = 12) was determined. For P450scc, two separate analyses were made. The mean staining intensity (a) for the entire ovarian sections (n = 3) and (b) for individual CL (n = 5) were determined. All images were in RGB mode and results were calculated using brightness values.
The IHC was used as a semi-quantitative method in this study because the ovary is a heterogeneous tissue, with different expression patterns and structures, which may also be affected by MXC differentially. Thus, IHC, combined with quantification of staining intensity allows us to more accurately determine these differences at protein level, details of which may be lost by using methods that require homogenization.
Determination of reproductive aging
After delivery, the estrous cyclicity of the dams was followed by vaginal cytology for the subsequent 12 months, for 12 days each month, to determine reproductive aging. The estrous cyclicity of the females that failed to achieve pregnancy was followed in the same manner as described above. The classification of pattern of vaginal cytology was as follows: proestrus — nucleated, mostly round epithelial cells and no leucocytes; estrus — large numbers of irregularly shaped keratinized epithelial cells, that lost cell nucleus; and diestrus — reduced numbers of epithelial cells and the presence of leukocytes. The cycles were categorized into normal, persistent estrus (PE), persistent diestrus (PD), or prolonged estrous cycle (Prolonged) as previously described (Franczak et al., 2006). Briefly, normal cycle: interestrus interval of 4–5 days; persistent estrus or persistent diestrus: 5 or more consecutive days of estrous or diestrus pattern of vaginal cells, respectively. Prolonged estrous cycles: interestrus intervals were greater than 5 days, and persistent estrus or persistent diestrus was not observed.
Statistical analysis
Age at puberty and first estrus, litter size, pregnancy rate, serum hormone levels, number of ovulation and ovarian weight in response to gonadotropins, intensity of IHC staining and regularity of estrous cycles were analyzed using GraphPad Prism (GraphPad Software, San Diego, CA). The values were expressed as the mean ± SEM. Statistical analysis was performed using a one-way ANOVA (Fig. 2 A, B, and D; Fig. 3; Fig. 5 E, J, and O; and ovarian weights) or two-way ANOVA (Fig. 4 E and F) followed by Dunnett’s Multiple Comparison test, Chi-square analysis (Fig. 2 C, Fig. 6, and pregnancy rate) or paired t test (Fig 5 T). All experiments were repeated 3–5 times (i.e. 3–5 litters), using at least 1–4 animals for each treatment in each experimental repeat. Therefore, 5–9 animals were used for each experimental group. A statistically significant difference was confirmed at P < 0.05.
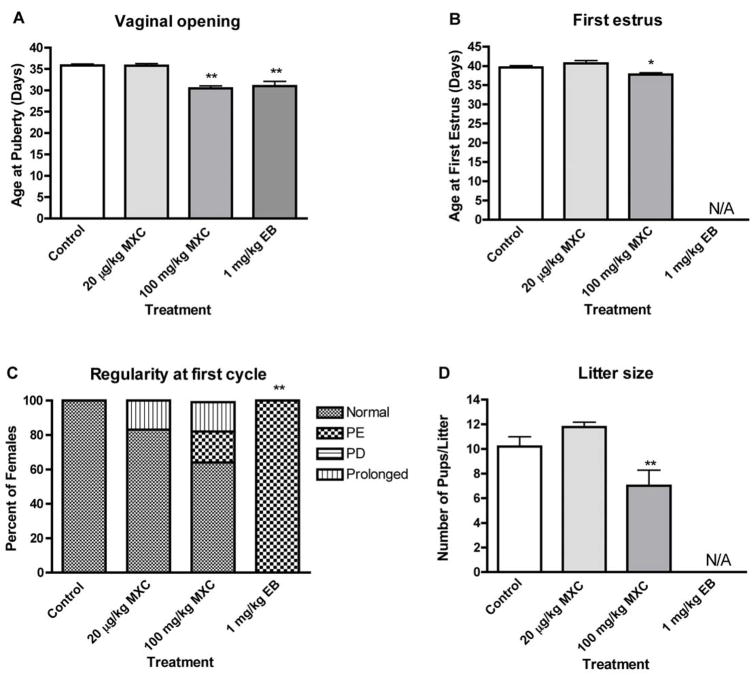
Fig. 2. Effects of developmental methoxychlor exposure on reproductive parameters in adult female rats.
The timed-pregnant females were treated as described in Figure 1. Reproductive parameters including age of vaginal opening (age at puberty; A) and first estrus (B), the regularity of the first estrous cycle (C), and litter size (D) were evaluated as described in Materials and Methods. Normal = normal estrous cycle; PE = persistent estrus; PD = persistent diestrus; Prolonged = prolonged estrous cycle; and N/A = no cycle or successful pregnancy was established. *P < 0.05, **P < 0.01.
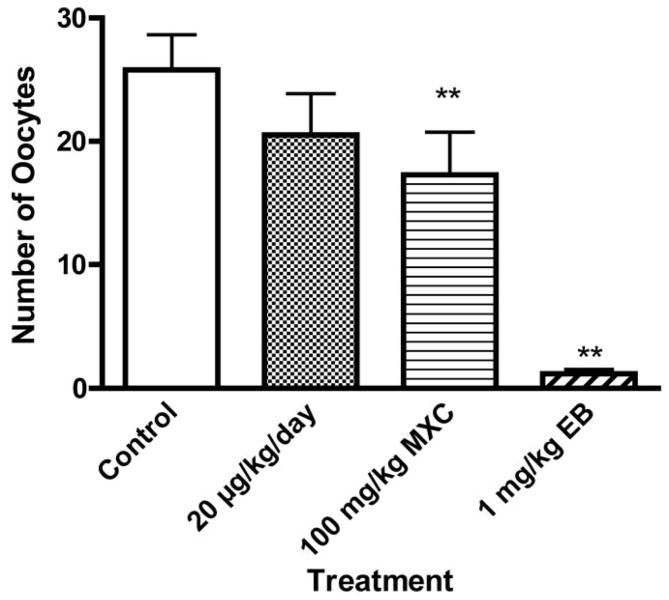
Figure 3. Effect of developmental exposure to methoxychlor on number of ovulations in response to exogenous gonadotropin stimulation in prepubertal rats.
The timed-pregnant females were treated as described in Figure 1. The number of oocytes released in response to 7.5 IU PMSG and 15 IU hCG was determined as described in Materials and Methods. **P < 0.01. N = 5–9
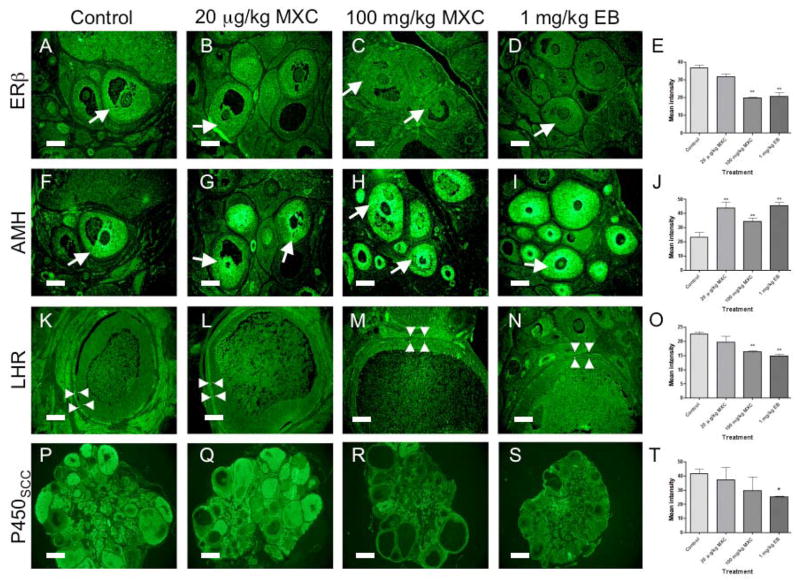
Figure 5. The effect of developmental MXC exposure on immunolocalization of ovarian markers in adult rats.
The timed-pregnant females were treated as described in Figure 1. The ovaries were collected between PND 50 and 60. The immunohistochemistry was performed as described in Material and Methods. Staining intensity with streptavidin-FITC (green) indicates the immunoreactivity for estrogen receptor β (ERβ; A–D); anti-Mullerian hormone (AMH; F–I); luteinizing hormone receptor (LHR; K–N); and cytochrome P450 side-chain cleavage (P450scc; P–S). Images were assembled in Adobe Photoshop and minimal and equal adjustments for brightness were made. The quantification of staining intensities was performed using Image J software for each marker (ERβ, E; AMH, J; LHR, O; and P450scc, T). In the control ovary (A), the immunoreactivity for ERβ was found in granulosa cells in antral follicles (arrow). The immunoreactivity for ERβ in ovaries treated with 20 μg/kg MXC was similar (B; arrow) to that in control ovaries. The 100 mg/kg MXC (C) or 1 mg/kg EB (D) treatment greatly diminished or completely eliminated immunoreactivity for ERβ (arrows). Quantification showed a significant reduction in the staining intensity of ERβ in high dose MXC and EB-treated ovaries (P < 0.01, E). AMH was immunolocalized to granulosa cells of early antral follicles in control ovaries (F; arrow). Low dose MXC (G), high dose MXC (H), and EB (I) treatment intensified AMH immunoreactivity (arrows), which was supported by quantification results (P < 0.01; J). The LHR immunoreactivity was strong in theca cells (arrowheads) surrounding large antral follicles and interstitium of control ovaries (K). LHR immunoreactivity was not altered in 20 μg/kg MXC-treated ovaries (L), but was reduced or eliminated in theca cells of large antral follicles (arrowheads) in ovaries treated with 100 mg/kg MXC (M) or 1 mg/kg EB (N). The quantification showed a significant reduction in LHR intensity in high dose MXC and EB-treated ovaries (P < 0.01; O). P450scc immunoreactivity was present in the theca cells, interstitium, and CL of control ovaries (P). P450scc immunoreactivity in the theca and interstitium of 20 μg/kg MXC was slightly reduced (Q). In 100 mg/kg MXC group, some ovaries showed reduced levels of P450scc immunoreactivity (R) while others showed normal levels (not shown), but the intensity was not statistically different (T). EB treatment down-regulated P450scc immunoreactivity (S), which was significant (P < 0.05; T). Scale bars show 100 μm (A–N) and 500 μm (P–S). * P < 0.05; ** P < 0.01. The results represents two separate IHC using 2 sections for at least 3 separate animals for each treatment group.
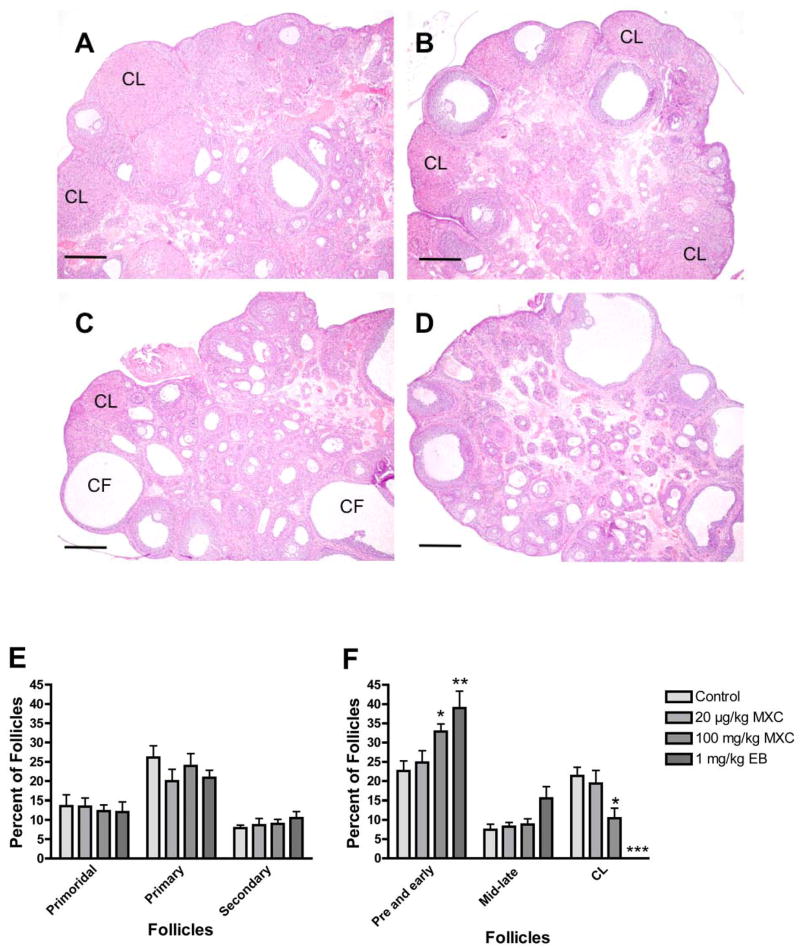
Figure 4. Effect of developmental methoxychlor exposure on the morphology and follicular composition in adult rat ovaries.
The timed-pregnant females were treated as described in Figure 1. Ovaries were collected between PND 50 and 60. Histology (A–D) and follicle counts and composition analysis (E and F) were performed as described in Materials and Methods. Control ovaries contained follicles at all developmental stages as well as corpora lutea (CL; A), and 20 μg/kg MXC did not change the ovarian size and morphology (B). However, ovaries treated with 100 mg/kg MXC were smaller and contained more small antral follicles, but fewer corpora lutea (CL; C). Large antral follicles were present, but some were cystic (CF). Ovaries treated with EB were smaller and had more small antral follicles but no CL (D). Scale bars show 500 μm. Follicular classification and percentage of follicles in each stage to the total follicle number/section was determined as described in Materials and Methods (E and F). *P < 0.05; **P < 0.01; ***P < 0.001.
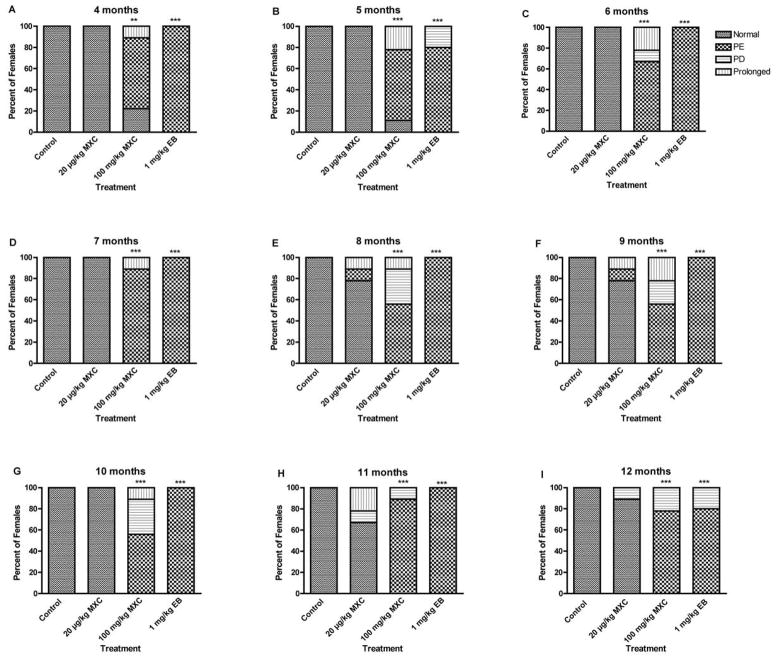
Figure 6. Effect of developmental MXC exposure on reproductive aging and age-related disruption in estrous cyclicity.
The timed-pregnant females were treated as described in Figure 1. Estrous cyclicity of offspring was monitored between 4 and 12 months for 12 days each month and classified as described in Materials and Methods. Normal = normal estrous cycle, PE = persistent estrus, PD = persistent diestrus, and Prolonged = prolonged estrous cycle. **P < 0.01; ***P < 0.001. N= 5–9.
Results
Developmental MXC exposure altered reproductive parameters during adulthood
Age of puberty and the first estrus
High dose MXC treatment significantly reduced the age of puberty, as determined by vaginal opening (P < 0.01; Fig. 2A). While vaginal opening day for control animals was 35.8 ± 0.3, females treated with high dose MXC showed vaginal opening on day 30.5 ± 0.6. The vaginal opening day for animals treated with EB was 31.0 ± 1.1, which was significantly different from that of controls (P < 0.01). In addition, age of first estrous cycle (Fig. 2B) was significantly accelerated (P < 0.05) for females that were treated with high dose MXC (37.8 ± 0.5) as compared to the control (39.6 ± 0.5). In animals that were exposed to EB, no estrous cycle was observed.
Regularity of the first estrous cycle
All the control animals showed regular cycles (Fig. 2C). High dose MXC treatment resulted in irregular cyclicity in 35% of the animals [P > 0.05; 17.5% prolonged and 17.5% persistent estrus (PE)], while 17.5% of the low dose MXC-treated animals showed prolonged estrous cycle (P > 0.05). All the EB-treated animals showed PE (P< 0.01; Fig. 2C).
Pregnancy rate and litter size
In high dose MXC-treated animals, the pregnancy rate decreased and 2 out of 7 females failed to become pregnant, although this decrease was not statistically significant (P > 0.05). In contrast, all the control (6 out of 6) and low dose MXC-treated females (9 out of 9) became pregnant. When treated with 1 mg/kg EB, 5 out of 5 females failed to conceive and/or maintain a pregnancy. The litter size of the dams treated with high dose MXC was significantly decreased (P < 0.01; Fig. 2D). Exposure to MXC did not cause any significant change in sex ratio or anogenital distance as compared to control animals at birth (not shown).
Developmental MXC exposure reduced ovulation in response to exogenous gonadotropins
To determine whether reduced pregnancy rate and litter size in MXC-exposed females were due to reduced ovulation in response to gonadotropins in the ovary, we challenged the rats with exogenous gonadotropins (i.e., PMSG and hCG) and determined the number of oocytes ovulated. The number of oocytes was decreased (P < 0.01) in high dose MXC-treated females (15.7 ± 3.5) as compared to the control females (25.8 ± 2.8; Fig. 3). The EB-exposed animal had greatly diminished ovulation in response to exogenous gonadotropins, and these females ovulated only 1.2 ± 0.4 oocytes/animal. Although the low dose MXC exposure reduced the number of oocytes released to 20.5 ± 3.7, the results were not significantly different from that of the control (P > 0.05). Although ovarian weight (g) was lower in low dose MXC (0.027 ± 0.003), high dose MXC (0.026 ± 0.002), and EB (0.024 ± 0.001) treated groups as compared to controls (0.029 ± 0.003), the changes were not statistically significant.
Developmental MXC exposure elevated serum LH levels while tended to reduce progesterone levels
Serum was collected from females on proestrus and P4, E2, FSH, and LH levels were measured (Table 1). Low dose MXC stimulated E2 (P < 0.01), but did not affect P4, LH, or FSH. High dose MXC tended to reduce P4, (P = 0.07), stimulated LH (P < 0.05), but had no affect on E2 or FSH. The EB-treated animals had reduced E2 (P < 0.01), but increased FSH levels (P < 0.05). EB also tended to have reduced levels of P4, (P = 0.07), and LH (P = 0.13)
Table 1.
The effect of fetal and neonatal methoxychlor exposure on serum hormone levels between postnatal day (PND) 50 and 60.1
| Treatment groups | Estradiol (Mean ± SEM; pg/ml) | Progesterone (Mean ± SEM; ng/ml) | FSH (Mean ± SEM; ng/ml) | LH (Mean ± SEM; ng/ml) |
|---|---|---|---|---|
| Control | 382 ± 12.6 | 229.9 ± 68.4 | 4.35 ± 0.34 | 1.30 ± 0.24 |
| 20 μg/kg/day MXC | 503 ± 2.0* | 202.9 ± 8.68 | 5.39 ± 1.29 | 1.60 ± 0.35 |
| 100 mg/kg/day MXC | 311 ± 55.2 | 70.5 ± 9.22 | 3.99 ± 0.24 | 2.04 ± 0.20* |
| 1 mg/kg/day EB | 257.0 ± 26.6** | 100.4 ± 27.22 | 6.01 ± 0.40* | 0.67 ± 0.23 |
Blood was collected on the morning of proestrus day except EB group, where all animals showed persistent estrus.
P < 0.01;
P < 0.05; n =3–6.
Developmental MXC exposure altered ovarian follicular composition
To investigate the morphological basis of the alterations in reproductive parameters, we examined ovarian histology between PND 50 and 60. Control ovaries contained follicles at all developmental stages as well as CL (Fig. 4A). The size of the ovary did not change in low dose MXC-treated animals, and their ovaries contained follicles at stages of the development and some CL, similar to control ovaries (Fig. 4B). High dose MXC-treated ovaries appeared smaller in size as compared to control ovaries and contained more antral follicles and very few CL (Fig. 4C). The ovaries of the EB-treated animals were smaller, contained numerous antral follicles, but had no CL (Fig. 4D).
Examination of follicular composition demonstrated that there were no significant changes in the percentage of the primordial, primary, and secondary follicles in the ovaries (Fig. 4E). The percentage of preantral and early antral follicles increased in high dose MXC-treated ovaries (P < 0.05) while the mid- and late-antral follicle number remained unchanged (P > 0.05). There was a significant reduction in the percentage of CL due to high dose MXC treatment (P < 0.05; Fig. 4F). Follicular composition in low dose MXC-treated ovaries was similar to that of the control ovaries. EB-treated ovaries had a significantly higher number of preantral and early antral follicles (P < 0.01) but no CL.
Developmental MXC exposure affected immunolocalization of ovarian markers in adult ovary
We examined immunolocalization and relative levels of ERα, ERβ, AMH, LHR, P450scc, and StAR.
Estrogen receptor (ER)α and ERβ
ERα immunoreactivity was localized in theca and interstitial cells as previously reported (Sar and Welsch, 1999), and its level was not significantly altered by MXC exposure. However, EB exposure lead to upregulation or ectopic expression of ERα in granulosa cells (not shown). In contrast, ERβ immunoreactivity was altered by both high dose MXC and EB (Fig. 5C and D). In control ovaries, ERβ immunoreactivity was observed in granulosa cells at all stages of growing follicles (Fig. 5A) and was not altered by low dose MXC (Fig. 5B). The treatment with high dose MXC nearly eliminated the immunoreactivity for ERβ in granulosa cells of antral follicles (Fig. 5C) while ERβ immunoreactivity in earlier stage follicles did not change (not shown). The ERβ immunoreactivity was absent in all stages of follicles in EB-treated ovaries (Fig. 5D). Quantification with Image J software showed that the staining intensities for ERβ in high dose MXC and EB-treated ovaries were significantly reduced (P < 0.01; Fig 5E).
Anti-Mullerian hormone
We examined AMH level in the ovary after MXC exposure because AMH is a negative regulator of follicle growth and differentiation (Visser and Themmen, 2005). The AMH immunoreactivity was abundant in growing preantral follicles and reduced in antral follicles of control ovaries (Fig. 5F). In contrast, the AMH immunoreactivity in both low and high dose MXC-treated ovaries was stronger in all stages of follicles including early antral follicles (Fig. 5G and H). AMH expression was upregulated by high dose MXC in the same antral follicle stages; wherein ERβ expression was reduced in high dose MXC-treated ovaries (Fig. 5C). The quantification showed that the staining intensity for AMH in low- and high-MXC-treated ovaries significantly upregulated (P < 0.01; Fig. 5 J). EB strongly stimulated AMH (Fig. 5I) as evidenced by significantly increased staining intensity (P < 0.01; Fig. 5J).
Luteinizing hormone receptor
To determine a potential role of alterations in LHR expression in reduced ovulatory response in high dose MXC-treated animals (Fig. 3) we examined LHR levels in the ovary using IHC followed by quantification. The theca cells surrounding large antral follicles as well as interstitial cells in control ovaries showed strong LHR immunoreactivity (Fig. 5K). Low dose MXC exposure did not affect the LHR immunoreactivity (Fig. 5L). In contrast, high dose MXC-treated ovaries had very little LHR immunoreactivity in theca cells surrounding large antral follicles and in the interstitial cells (Fig. 5M). EB exposure completely eliminated LHR immunoreactivity in the theca cells and in the interstitium (Fig. 5N). When we quantified the staining intensity of the LHR in the theca cells surrounding the antral follicles, the staining intensity in ovaries treated with high dose MXC and EB was significantly reduced (P < 0.01; Fig 5O).
P450 side-chain cleavage (P450scc)
P450scc plays a critical role in steroidogenesis. The immunoreactivity for P450scc was primarily in theca and interstitial cells and CL in control ovaries (Fig. 5P). The 450scc immunoreactivity in low does MXC was slightly reduced (Fig. 5Q), but the difference was not significantly different from control (P > 0.05; Fig. 5T). The P450scc immunoreactivity in high dose MXC-treated ovaries was variable: while some ovaries had low levels of P450scc (Fig. 5R), other had normal levels (not shown), but the difference was not statistically significant (P > 0.05; Fig. 5T). When the staining intensity in CL was quantified separately, it was significantly reduced in high dose MXC-treated ovaries (P < 0.01), but not in low dose MXC-treated ovaries (not shown). The P450scc immunoreactivity in EB-treated ovaries was low in theca and interstitial cells (Fig. 5S) and the quantification showed that the staining intensity was significantly reduced (P < 0.05). EB-treated ovaries rarely had CL. StAR expression was unaffected by the MXC treatment but down-regulated by EB treatment (not shown). All negative control sections showed no immunoreactivity (not shown).
Developmental MXC exposure led to early reproductive aging
To assess any potential premature reproductive aging in MXC-treated animals, the estrous cycles of the rats were followed for 12 days every month until 12 months of age using vaginal cytology (Fig. 6A–I).
All control animals showed normal estrous cycles through 4 to 12 months (Fig. 6A–I). Exposure to high dose MXC induced abnormal cyclicity starting at 4 months and 78% of animals exhibited abnormal cyclicity at 4 months (67% PE and 11% prolonged; Fig. 6A) and 89% abnormal cyclicity at 5 months (22% prolonged and 67% PE; Fig. 6B; P < 0.05). All high dose MXC-treated animals showed irregular cycles between 6 and 12 months of age (Fig. 6 C–I), during which time most of the animals showed PE, although some showed PD or prolonged cycles. All low dose MXC exposed animals showed normal estrous cycles between 4 and 7 months (Fig. 6A – D). However, estrous cyclicity of the animals treated with low dose MXC displayed some degree of irregular cyclicity (11% – 33%) between months 8 and 12 (Fig. 6E – I), but these were not statistically significant (P > 0.05). All the EB-treated animals showed abnormal cycles, primarily PE, between 4 and 12 months of age (Fig. 6A – I).
Discussion
The current study investigated the effects of transient exposure to environmentally relevant low and high doses of MXC during fetal and neonatal ovarian development periods on female reproductive parameters, superovulatory response, serum hormone levels, and morphology and levels of key markers in the ovary. Several aspects of this study are new: (i) testing an environmentally relevant low dose of MXC in the ovary, (ii) limiting the treatment to the time of early ovarian development, (iii) examining the direct effects of MXC in the ovary by a superovulation study, and (iv) examining the effects of MXC on key ovarian markers. High dose MXC accelerated entry into puberty and first estrus, decreased female fertility, and increased irregular cyclicity and premature reproductive aging. In addition, high dose MXC reduced superovulatory response, increased serum LH levels, and altered follicular composition and CL numbers as well as levels of several key ovarian markers in the ovary.
Previous studies (Gray et al., 1989; Chapin et al., 1997) in rats using similar doses of MXC for longer exposure periods resulted in early vaginal opening and first estrus, irregular estrous cycles, reduced pregnancy rate and litter size, and early cessation of estrous cycles. In the present study MXC treatment was limited to a shorter time period during development, but produced similar abnormalities. These results show that MXC exposure during 19 dpc-PND 7 results in reproductive abnormalities during adulthood and highlight the vulnerability of biological processes to environmental insults during development.
The reduced pregnancy rate and litter size observed in this study can be due to the effects of MXC in the hypothalamus, pituitary, uterus, and/or oviduct as well as in the ovary. Developmental exposure to estrogenic compounds can affect the hypothalamus and pituitary (Gorski, 1963), uterus, and oviduct (Block et al., 2000). Furthermore, exposure to estrogenic compounds including E2 during early ovarian development directly affects the ovary as shown in vitro (Kezele and Skinner, 2003; Chen et al., 2007). In addition, MXC (or its metabolites) inhibit antral follicle growth and induce atresia in culture (Miller et al., 2005; Gupta et al., 2006a; Miller et al., 2006). The MXC metabolite HPTE inhibits steroidogenesis and stimulates AMH production in rat granulosa cells in vitro (Uzumcu et al., 2006; Zachow and Uzumcu, 2006). Therefore, it is possible that MXC during 19 dpc-PND 7 affects the ovary directly, and thus leads to adult reproductive dysfunction. The present study tested this hypothesis in vivo using a superovulation protocol. Importantly, the number of oocytes ovulated following treatment with PMSG and hCG was significantly reduced. These results suggest that MXC exposure resulted in deficiencies within the ovary that, in turn, lead to the inability of the ovary to respond to gonadotropins effectively. Studies with mice lacking ERα have shown that ovaries in aged animals cannot respond to exogenous gonadotropins while ovaries are responsive in prepubertal animals (Couse et al., 1999). These studies suggest that ovarian pathology in animals lacking ERα is acquired after puberty, perhaps due to exposure to irregular gonadotropin levels stemming from a disrupted estrogen-mediated feedback mechanism within the hypothalamus-pituitary-ovarian axis (Couse et al., 1999). In our studies, ovarian responsiveness to exogenous gonadotropins was already suboptimal in prepubertal animals, when the superovulation studies were conducted, prior to the establishment of hypothalamic-pituitary-ovarian axis. Thus, it is likely that developmental MXC exposure directly affects the ovary, arguably at the level of gonadotropin sensitivity (e.g., LHR levels).
The IHC results showed that high dose MXC alters production of several key ovarian markers, including ERβ, AMH, LHR, and P450scc that are critical for ovarian function. ERβ was down-regulated in the granulosa cells of antral follicles. In contrast, AMH was upregulated in the granulosa cells of the same stage of antral follicles where ERβ was down-regulated by high dose MXC. In previous studies with mice, ERβ has been shown to be critical for FSH-induced granulosa cell differentiation and LH responsiveness (Krege et al., 1998). More recent studies have shown that the level of LHR is reduced in ERβ knockout mice (Couse et al., 2005). Previous investigations have also shown that AMH inhibits FSH-induced follicle selection (di Clemente et al., 1994; Durlinger et al., 2001) and reduces follicular LHR expression (di Clemente et al., 1994; Josso et al., 1998). It is possible that upregulation of AMH and down-regulation of ERβ in preantral and early antral follicles lead to an inhibition of FSH-regulated follicular selection and growth in these follicles. A significant accumulation in the numbers of preantral and early antral follicles without a corresponding increase in large antral follicle numbers in high dose MXC-treated ovaries indicates deficiencies in follicular growth, due to a possible reduction in FSHR responsiveness in the ovary. While serum FSH levels were unchanged, FSHR levels were not assessed due to the lack of a robust antibody for use in IHC. However, LHR protein levels in the theca and interstitial cells surrounding large antral follicles were significantly down-regulated. Despite an increase in serum LH levels, which is in agreement with the previous observation (Chapin et al., 1997), the reduction in LHR may explain the decrease in ovulation and the number of CL and the increase in cystic follicles. A similar reduction in CL numbers in rats exposed to 50–150 mg/kg/day MXC between 14 dpc and PND 42 has also been reported (Chapin et al., 1997).
Another important ovarian marker, the steroidogenic enzyme P450scc was suppressed in CL by high dose of MXC, while StAR was unchanged (not shown). A previous study by Ikeda et al., has shown that EB treatment between PND 1 and 5 reduced both P450scc and StAR on PND 6 and 14 (Ikeda et al., 2001). In that study, the expression of P450scc recovered by PND 21 in rats but StAR remained down-regulated. These studies suggest that MXC acts differently from EB. Indeed, EB down-regulated StAR production in the ovary in the present study (not shown), which is in agreement with the results of Ikeda et al (Ikeda et al., 2001). Furthermore, our previous in vitro study has shown that HPTE inhibits FSH-stimulated P450scc mRNA while StAR mRNA remains unchanged in immature rat granulosa cells (Zachow and Uzumcu, 2006), supporting our current results. In general, the IHC results demonstrate that MXC stimulates negative regulators of the folliculogenesis (e.g., AMH) while inhibiting positive regulators of (e.g., ERβ). The concomitant elevation of AMH and reduction of ERβ is also associated with a reduction in critical markers of follicular maturation (LHR and P450scc) in the ovary. Although not tested in the present study, previously proposed mechanisms of actions for MXC, such as oxidative stress (Gupta et al., 2006b) and apoptotic pathways (Miller et al., 2005), may play roles in the effects of MXC in ovarian dysfunction.
Finally, we examined the developmental effects of MXC on potential early reproductive aging. Fetal and neonatal MXC exposure resulted in irregular cyclicity and reproductive aging. The high dose MXC exposure resulted in irregular cyclicity in some rats starting with the first estrous cycle, but most of the rats were cycling initially. However, the adverse effect rapidly increased, and by 6 months of age, no normally cycling rats remained. The irregular cyclicity progressively worsened, and by 12 months all of the MXC-treated animals showed PE or PD. Similar effects were also observed in mice that were treated with MXC (6.8–135 mg/kg/day) during the first two weeks of life (Eroschenko et al., 1995). The MXC-treated mice showed prolonged cycles at highest dose of MXC starting by 3 months. Mice treated with all doses of MXC showed PE at 6 months and PE or PD at 12 months of age. These data show that MXC exposure results in a progressive deterioration of the reproductive system anywhere within the hypothalamic-pituitary-ovarian axis. This is in contrast to EB treatment, which resulted in irregular cycle from the beginning. In addition, although it was not statistically significant, animals given the low dose MXC showed irregular cyclicity at the initial cycle, and again starting at 8 months of age. Because this dose is considered to be environmentally relevant (Palanza et al., 2002; Laviola et al., 2005) and it also significantly affects some additional parameters (e.g., serum E2 and ovarian AMH levels), these observations require closer attention. Furthermore, the females in this study were exposed to low dose MXC only transiently during their development. It is likely that the effects would be more profound if the exposure occurred for a longer period of time as has been reported in rats given long-term low-dose dioxin regimens (Franczak et al., 2006). Moreover, fetal and neonatal exposure to the low does MXC can immediately alter follicular composition and AMH and ER immunolocalization in PND 7 ovaries (Mahakali Zama et al., submitted). Therefore, to fully understand the environmental risks of the MXC, it is important to perform a dose response studies with longer treatment periods in this low dose range in future.
In conclusion, this study shows that transient exposure to MXC during development results in molecular and morphological alterations in the ovary, possibly leading to multiple reproductive abnormalities, including accelerated puberty, irregular reproductive cyclicity, and early reproductive senescence. The data suggest that the dysfunction is associated with a reduced responsiveness to gonadotropins, which is quite possibly mediated by a reduction in ERβ and upregulation in AMH levels. This leads to a progressive decline in female fertility. Overall, this study suggests that developmental MXC exposure directly affect the ovary, leading to adult ovarian dysfunction and female infertility.
Acknowledgments
The authors wish to thank Dr. Kathy Manger for her assistance in the preparation of this manuscript; Rebekah Nam and Michael Esmail for their help in caring for the rats; Dr. Rob Zachow for critically reading the manuscript; Kathleen Roberts, Rutgers Molecular Pathology Facility Core, for her help with histology. 1Current address for AnnMarie Armenti is Dept. Medicine, Stony Brook University, Stony Brook, NY 11794. 2Current address for Lisa Passantino is Bracco Research USA, Princeton, NJ 08540.
This study is supported by grant ES013854 and Molecular Pathology Facility Core is supported by grant ES05022 from the National Institute of Health.
Footnotes
The authors have nothing to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 2005;308:1466–1469. doi: 10.1126/science.1108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATSDR. Toxicological profile for methoxychlor. U.S. Department of Health and Human Services, Public Health Service; Atlanta, GA: 2002. [Google Scholar]
- Block K, Kardana A, Igarashi P, Taylor HS. In utero diethylstilbestrol (DES) exposure alters Hox gene expression in the developing mullerian system. Faseb J. 2000;14:1101–1108. doi: 10.1096/fasebj.14.9.1101. [DOI] [PubMed] [Google Scholar]
- Boettger-Tong H, Murthy L, Chiappetta C, Kirkland JL, Goodwin B, Adlercreutz H, Stancel GM, Makela S. A case of a laboratory animal feed with high estrogenic activity and its impact on in vivo responses to exogenously administered estrogens. Environ Health Perspect. 1998;106:369–373. doi: 10.1289/ehp.98106369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgeest C, Miller KP, Gupta R, Greenfeld C, Hruska KS, Hoyer P, Flaws JA. Methoxychlor-induced atresia in the mouse involves Bcl-2 family members, but not gonadotropins or estradiol. Biol Reprod. 2004;70:1828–1835. doi: 10.1095/biolreprod.103.022889. [DOI] [PubMed] [Google Scholar]
- Chapin RE, Harris MW, Davis BJ, Ward SM, Wilson RE, Mauney MA, Lockhart AC, Smialowicz RJ, Moser VC, Burka LT, Collins BJ. The effects of perinatal/juvenile methoxychlor exposure on adult rat nervous, immune, and reproductive system function. Fundam Appl Toxicol. 1997;40:138–157. doi: 10.1006/faat.1997.2381. [DOI] [PubMed] [Google Scholar]
- Chen S, Liu X, Segaloff DL. A novel cyclic adenosine 3′,5′-monophosphate-responsive element involved in the transcriptional regulation of the lutropin receptor gene in granulosa cells. Mol Endocrinol. 2000;14:1498–1508. doi: 10.1210/mend.14.9.0514. [DOI] [PubMed] [Google Scholar]
- Chen Y, Jefferson WN, Newbold RR, Padilla-Banks E, Pepling ME. Estradiol, progesterone, and genistein inhibit oocyte nest breakdown and primordial follicle assembly in the neonatal mouse ovary in vitro and in vivo. Endocrinology. 2007;148:3580–3590. doi: 10.1210/en.2007-0088. [DOI] [PubMed] [Google Scholar]
- Couse JF, Bunch DO, Lindzey J, Schomberg DW, Korach KS. Prevention of the polycystic ovarian phenotype and characterization of ovulatory capacity in the estrogen receptor-alpha knockout mouse. Endocrinology. 1999;140:5855–5865. doi: 10.1210/endo.140.12.7222. [DOI] [PubMed] [Google Scholar]
- Couse JF, Yates MM, Deroo BJ, Korach KS. Estrogen receptor-beta is critical to granulosa cell differentiation and the ovulatory response to gonadotropins. Endocrinology. 2005;146:3247–3262. doi: 10.1210/en.2005-0213. [DOI] [PubMed] [Google Scholar]
- Cummings AM. Methoxychlor as a model for environmental estrogens. Crit Rev Toxicol. 1997;27:367–379. doi: 10.3109/10408449709089899. [DOI] [PubMed] [Google Scholar]
- di Clemente N, Goxe B, Rémy JJ, Cate RL, Josso N, Vigier B, Salesse R. Inhibitory effect of AMH upon the expression of aromatase activity and LH receptors by cultured granulosa cells of rat and porcine immature ovaries. Endocrine. 1994;2:553–558. [Google Scholar]
- Durlinger AL, Gruijters MJ, Kramer P, Karels B, Kumar TR, Matzuk MM, Rose UM, de Jong FH, Uilenbroek JT, Grootegoed JA, Themmen AP. Anti-Mullerian hormone attenuates the effects of FSH on follicle development in the mouse ovary. Endocrinology. 2001;142:4891–4899. doi: 10.1210/endo.142.11.8486. [DOI] [PubMed] [Google Scholar]
- Durlinger AL, Kramer P, Karels B, de Jong FH, Uilenbroek JT, Grootegoed JA, Themmen AP. Control of primordial follicle recruitment by anti-Mullerian hormone in the mouse ovary. Endocrinology. 1999;140:5789–5796. doi: 10.1210/endo.140.12.7204. [DOI] [PubMed] [Google Scholar]
- Erickson GF, Wang C, Hsueh AJ. FSH induction of functional LH receptors in granulosa cells cultured in a chemically defined medium. Nature. 1979;279:336–338. doi: 10.1038/279336a0. [DOI] [PubMed] [Google Scholar]
- Eroschenko VP, Abuel-Atta AA, Grober MS. Neonatal exposures to technical methoxychlor alters ovaries in adult mice. Reprod Toxicol. 1995;9:379–387. doi: 10.1016/0890-6238(95)00025-6. [DOI] [PubMed] [Google Scholar]
- Franczak A, Nynca A, Valdez KE, Mizinga KM, Petroff BK. Effects of acute and chronic exposure to the aryl hydrocarbon receptor agonist 2,3,7,8-tetrachlorodibenzo-p-dioxin on the transition to reproductive senescence in female Sprague-Dawley rats. Biol Reprod. 2006;74:125–130. doi: 10.1095/biolreprod.105.044396. [DOI] [PubMed] [Google Scholar]
- Gaido KW, Leonard LS, Maness SC, Hall JM, McDonnell DP, Saville B, Safe S. Differential interaction of the methoxychlor metabolite 2,2-bis-(p-hydroxyphenyl)-1,1,1-trichloroethane with estrogen receptors alpha and beta. Endocrinology. 1999;140:5746–5753. doi: 10.1210/endo.140.12.7191. [DOI] [PubMed] [Google Scholar]
- Gaido KW, Maness SC, McDonnell DP, Dehal SS, Kupfer D, Safe S. Interaction of methoxychlor and related compounds with estrogen receptor alpha and beta, and androgen receptor: structure-activity studies. Mol Pharmacol. 2000;58:852–858. [PubMed] [Google Scholar]
- Gorski RA. Modification of ovulatory mechanisms by postnatal administration of estrogen to the rat. Am J Physiol. 1963;205:842–844. doi: 10.1152/ajplegacy.1963.205.5.842. [DOI] [PubMed] [Google Scholar]
- Gray LE, Jr, Ostby J, Ferrell J, Rehnberg G, Linder R, Cooper R, Goldman J, Slott V, Laskey J. A dose-response analysis of methoxychlor-induced alterations of reproductive development and function in the rat. Fundam Appl Toxicol. 1989;12:92–108. doi: 10.1016/0272-0590(89)90065-1. [DOI] [PubMed] [Google Scholar]
- Gupta RK, Miller KP, Babus JK, Flaws JA. Methoxychlor Inhibits Growth and Induces Atresia of Antral Follicles Through an Oxidative Stress Pathway. Toxicol Sci. 2006a doi: 10.1093/toxsci/kfl052. [DOI] [PubMed] [Google Scholar]
- Gupta RK, Schuh RA, Fiskum G, Flaws JA. Methoxychlor causes mitochondrial dysfunction and oxidative damage in the mouse ovary. Toxicol Appl Pharmacol. 2006b doi: 10.1016/j.taap.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Hall DL, Payne LA, Putnam JM, Huet-Hudson YM. Effect of methoxychlor on implantation and embryo development in the mouse. Reprod Toxicol. 1997;11:703–708. doi: 10.1016/s0890-6238(97)00026-9. [DOI] [PubMed] [Google Scholar]
- Hirshfield AN. Development of follicles in the mammalian ovary. Int Rev Cytol. 1991;124:43–101. doi: 10.1016/s0074-7696(08)61524-7. [DOI] [PubMed] [Google Scholar]
- Ho SM, Tang WY, Belmonte de Frausto J, Prins GS. Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4. Cancer Res. 2006;66:5624–5632. doi: 10.1158/0008-5472.CAN-06-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iguchi T, Fukazawa Y, Uesugi Y, Takasugi N. Polyovular follicles in mouse ovaries exposed neonatally to diethylstilbestrol in vivo and in vitro. Biol Reprod. 1990;43:478–484. doi: 10.1095/biolreprod43.3.478. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Nagai A, Ikeda MA, Hayashi S. Neonatal estrogen exposure inhibits steroidogenesis in the developing rat ovary. Dev Dyn. 2001;221:443–453. doi: 10.1002/dvdy.1162. [DOI] [PubMed] [Google Scholar]
- Jefferson WN, Couse JF, Banks EP, Korach KS, Newbold RR. Expression of estrogen receptor beta is developmentally regulated in reproductive tissues of male and female mice. Biol Reprod. 2000;62:310–317. doi: 10.1095/biolreprod62.2.310. [DOI] [PubMed] [Google Scholar]
- Josso N, Racine C, di Clemente N, Rey R, Xavier F. The role of anti-Mullerian hormone in gonadal development. Mol Cell Endocrinol. 1998;145:3–7. doi: 10.1016/s0303-7207(98)00186-5. [DOI] [PubMed] [Google Scholar]
- Kapoor IP, Metcalf RL, Nystrom RF, Sangha GK. Comparative metabolism of methoxychlor, methiochlor, and DDT in mouse, insects, and in a model ecosystem. J Agric Food Chem. 1970;18:1145–1152. doi: 10.1021/jf60172a017. [DOI] [PubMed] [Google Scholar]
- Kezele P, Skinner MK. Regulation of ovarian primordial follicle assembly and development by estrogen and progesterone: endocrine model of follicle assembly. Endocrinology. 2003;144:3329–3337. doi: 10.1210/en.2002-0131. [DOI] [PubMed] [Google Scholar]
- Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci U S A. 1998;95:15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviola G, Gioiosa L, Adriani W, Palanza P. D-amphetamine-related reinforcing effects are reduced in mice exposed prenatally to estrogenic endocrine disruptors. Brain Res Bull. 2005;65:235–240. doi: 10.1016/j.brainresbull.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Logan KA, Juengel JL, McNatty KP. Onset of steroidogenic enzyme gene expression during ovarian follicular development in sheep. Biol Reprod. 2002;66:906–916. doi: 10.1095/biolreprod66.4.906. [DOI] [PubMed] [Google Scholar]
- Miller KP, Gupta RK, Flaws JA. Methoxychlor metabolites may cause ovarian toxicity through estrogen-regulated pathways. Toxicol Sci. 2006;93:180–188. doi: 10.1093/toxsci/kfl034. [DOI] [PubMed] [Google Scholar]
- Miller KP, Gupta RK, Greenfeld CR, Babus JK, Flaws JA. Methoxychlor Directly Affects Ovarian Antral Follicle Growth and Atresia through Bcl-2- and Bax-Mediated Pathways. Toxicol Sci. 2005;88:213–221. doi: 10.1093/toxsci/kfi276. [DOI] [PubMed] [Google Scholar]
- Newbold RR. Lessons learned from perinatal exposure to diethylstilbestrol. Toxicol Appl Pharmacol. 2004;199:142–150. doi: 10.1016/j.taap.2003.11.033. [DOI] [PubMed] [Google Scholar]
- Palanza P, Morellini F, Parmigiani S, vom Saal FS. Ethological methods to study the effects of maternal exposure to estrogenic endocrine disrupters: a study with methoxychlor. Neurotoxicol Teratol. 2002;24:55–69. doi: 10.1016/s0892-0362(01)00191-x. [DOI] [PubMed] [Google Scholar]
- Palermo R. Differential actions of FSH and LH during folliculogenesis. Reprod Biomed Online. 2007;15:326–337. doi: 10.1016/s1472-6483(10)60347-1. [DOI] [PubMed] [Google Scholar]
- Pepling ME, Spradling AC. Mouse ovarian germ cell cysts undergo programmed breakdown to form primordial follicles. Dev Biol. 2001;234:339–351. doi: 10.1006/dbio.2001.0269. [DOI] [PubMed] [Google Scholar]
- Richards JS. Perspective: the ovarian follicle--a perspective in 2001. Endocrinology. 2001;142:2184–2193. doi: 10.1210/endo.142.6.8223. [DOI] [PubMed] [Google Scholar]
- Ronen-Fuhrmann T, Timberg R, King SR, Hales KH, Hales DB, Stocco DM, Orly J. Spatio-temporal expression patterns of steroidogenic acute regulatory protein (StAR) during follicular development in the rat ovary. Endocrinology. 1998;139:303–315. doi: 10.1210/endo.139.1.5694. [DOI] [PubMed] [Google Scholar]
- Sar M, Welsch F. Differential expression of estrogen receptor-beta and estrogen receptor-alpha in the rat ovary. Endocrinology. 1999;140:963–971. doi: 10.1210/endo.140.2.6533. [DOI] [PubMed] [Google Scholar]
- Savabieasfahani M, Kannan K, Astapova O, Evans NP, Padmanabhan V. Developmental programming: differential effects of prenatal exposure to bisphenol-A or methoxychlor on reproductive function. Endocrinology. 2006;147:5956–5966. doi: 10.1210/en.2006-0805. [DOI] [PubMed] [Google Scholar]
- Saxena D, Safi R, Little-Ihrig L, Zeleznik AJ. Liver receptor homolog-1 stimulates the progesterone biosynthetic pathway during follicle-stimulating hormone-induced granulosa cell differentiation. Endocrinology. 2004;145:3821–3829. doi: 10.1210/en.2004-0423. [DOI] [PubMed] [Google Scholar]
- Segaloff DL, Wang HY, Richards JS. Hormonal regulation of luteinizing hormone/chorionic gonadotropin receptor mRNA in rat ovarian cells during follicular development and luteinization. Mol Endocrinol. 1990;4:1856–1865. doi: 10.1210/mend-4-12-1856. [DOI] [PubMed] [Google Scholar]
- Skinner MK. Regulation of primordial follicle assembly and development. Hum Reprod Update. 2005;11:461–471. doi: 10.1093/humupd/dmi020. [DOI] [PubMed] [Google Scholar]
- Themmen AP. Anti-Mullerian hormone: its role in follicular growth initiation and survival and as an ovarian reserve marker. J Natl Cancer Inst Monogr. 2005:18–21. doi: 10.1093/jncimonographs/lgi026. [DOI] [PubMed] [Google Scholar]
- Uzumcu M, Dirks KA, Skinner MK. Inhibition of platelet-derived growth factor actions in the embryonic testis influences normal cord development and morphology. Biol Reprod. 2002;66:745–753. doi: 10.1095/biolreprod66.3.745. [DOI] [PubMed] [Google Scholar]
- Uzumcu M, Kuhn PE, Marano JE, Armenti AE, Passantino L. Early postnatal methoxychlor exposure inhibits folliculogenesis and stimulates anti-Mullerian hormone production in the rat ovary. J Endocrinol. 2006;191:549–558. doi: 10.1677/joe.1.06592. [DOI] [PubMed] [Google Scholar]
- Visser JA, Themmen AP. Anti-Mullerian hormone and folliculogenesis. Mol Cell Endocrinol. 2005;234:81–86. doi: 10.1016/j.mce.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Woodruff TK, Mayo KE. To beta or not to beta: estrogen receptors and ovarian function. Endocrinology. 2005;146:3244–3246. doi: 10.1210/en.2005-0630. [DOI] [PubMed] [Google Scholar]
- Zachow R, Uzumcu M. The methoxychlor metabolite, 2,2-bis-(p-hydroxyphenyl)-1,1,1-trichloroethane, inhibits steroidogenesis in rat ovarian granulosa cells in vitro. Reprod Toxicol. 2006;22:659–665. doi: 10.1016/j.reprotox.2006.04.018. [DOI] [PubMed] [Google Scholar]
- Zeleznik AJ, Midgley AR, Jr, Reichert LE., Jr Granulosa cell maturation in the rat: increased binding of human chorionic gonadotropin following treatment with follicle-stimulating hormone in vivo. Endocrinology. 1974;95:818–825. doi: 10.1210/endo-95-3-818. [DOI] [PubMed] [Google Scholar]