Abstract
Objective: We dynamically measured serum inhibin B and estradiol in the early stage of hormonal stimulation to predict the ovarian response in in vitro fertilization (IVF) treatment. Methods: A total of 57 patients (<40 years of age) who underwent the first cycle of long protocol IVF or introcytoplasmic sperm injection (ICSI) treatment were included. Serum inhibin B, estradiol, follicle stimulating hormone (FSH) and luteinizing hormone (LH) levels were measured four times: (1) on Day 3 of the menstrual cycle (basal); (2) on the day before the first administration of gonadotrophin (Gn) (Day 0); (3) on Day 1 of Gn therapy; and (4) on Day 5 of Gn therapy. Comparisons of these measurements with ovarian responses and pregnancy outcomes were made and analyzed statistically. Results: (1) On Day 1 and Day 5 of recombinant FSH (rFSH) stimulation, ovarian response, i.e., numbers of follicles, oocytes, fertilized oocytes, and embryos, had a positive correlation (r s=0.46~0.61, P=0.000) with raised inhibin B and estradiol concentrations, but a negative correlation (r s=−0.67~−0.38, P=0.000 or P<0.01) with total rFSH dose and total days of rFSH stimulation. (2) No significant variation (P>0.05) between the pregnant and non-pregnant groups on the basis of mean age or on all hormone concentrations at four times of the IVF cycle was observed. However, all the seven patients aged >35 years did not reach pregnancy. Conclusions: (1) Serum inhibin B and estradiol concentrations obtained shortly after Gn therapy may offer an accurate and early prediction of ovarian response; (2) Low levels of serum inhibin B and estradiol obtained shortly after Gn stimulation indicate the need for a longer period of Gn treatment and a higher daily dosage; (3) No obvious pregnancy difference among patients of age <35 years was found; however, IVF pregnancy outcome is significantly lower in women of age >35 years.
Keywords: Inhibin B, Estradiol, Ovarian response, In vitro fertilization (IVF), Pregnancy
INTRODUCTION
Since the advancement of in vitro fertilization-embryo transfer (IVF-ET) in assisted reproductive technologies (ART), controlled ovarian hyperstimulation (COH) has been widely utilized. However, women may react differently to the dosage of gonadotrophin (Gn), resulting in different ovarian responses and different IVF pregnancy outcomes. Poor ovarian response to Gn stimulation results in a small number of oocytes collected and thus a smaller number of embryos available for transfer, which therefore reduces the success rate of IVF (Bancsi et al., 2002). On the other hand, an excessive ovarian response to Gn stimulation increases the risk for ovarian hyperstimulation syndrome (OHSS) (Navot et al., 1992).
A patient’s ovarian response to Gn stimulation is mainly determined by her ovarian reserve, which comprises the quantity and functional capacity of follicles and germ cells. Pre-evaluation of ovarian reserve and prediction of ovarian response would provide a valuable means of assisting clinicians in selecting appropriate Gn dose for each patient. This customized prescription of Gn dosage will not only reduce the possibility of either poor or excessive ovarian responses but also provide better chance to reach optimal ovarian response and ultimately improve the outcome of IVF pregnancy.
Traditional criteria used to predict ovarian response to Gn stimulation include the patient’s age, baseline serum concentration of hormones such as follicle stimulating hormone (FSH), estradiol, FSH to luteinizing hormone (LH) ratio, and dynamic tests (such as the clomiphene citrate (CC) challenge test, the Gn-releasing hormone analogue (GnRHa) stimulation test, and the human menopausal Gn (HMG) stimulation test) (Navot et al., 1987; Scott et al., 1989; Scott and Hofmann, 1995; Toner et al., 1991a; Cahill et al., 1994; Licciardi et al., 1995; Mukherjee et al., 1996; Kim et al., 1997; Ravhon et al., 2000).
The effects of age, basal FSH and estradiol concentrations have been well documented (Scott et al., 1989; Scott and Hofmann, 1995; Toner et al., 1991b; Cahill et al., 1994; Licciardi et al., 1995; Mukherjee et al., 1996; Kim et al., 1997). However, the most important aspect of diminished ovarian reserve and associated decline in reproductive potential is that its onset is highly variable (Mosher and Pratt, 1990; 1991; Maroulis, 1991). This means that functional ovarian age as reflected by basal FSH and estradiol concentrations can be discordant with biological age (Scott et al., 1989; Scott and Hofmann, 1995; Toner et al., 1991b; Cahill et al., 1994). Patients who have normal FSH and estradiol levels or are younger than 35 years of age may still have a diminished ovarian reserve that leads to poor response to Gn stimulation (Farhi et al., 1997). They may have a decreased Day 3 serum inhibin B concentration despite the unchanged Day 3 serum FSH and estradiol concentrations (Seifer et al., 1997; 1999). Inhibin B was suggested as an early sensitive predictor of ovarian reserve (Seifer et al., 1997; 1999). With better understanding of the control of synthesis and secretion of the inhibins and their potential endocrine role in the menstrual cycle, attention has turned to the possibility of this family of peptides to provide a more direct index of ovarian reserve and an improved predictor of ART outcome (Hughes et al., 1990; Groome et al., 1994; 1996; Balasch et al., 1996; Lockwood et al., 1996; Seifer et al., 1997; 1999; Danforth et al., 1998; Corson et al., 1999; Hall et al., 1999; Peñarrubia et al., 2000; Laven and Fauser, 2004; Shanthi et al., 2004).
Inhibins are dimeric polypeptides produced by granulosa cells and composed of an α-subunit along with a βA-subunit (inhibin A) or a βB-subunit (inhibin B). Inhibin B concentrations rise across the luteal-follicular transition and peak in the mid-follicular phase, suggesting that they are secreted by the developing cohort of follicles, and may mark the number or quality of developing follicles at the baseline (Groome et al., 1996). In contrast, inhibin A does not begin to increase until just after the increase of estradiol in the late follicular and luteal phases, suggesting that it is secreted by the dominant follicle and may indicate follicle maturity (Groome et al., 1994; Hall et al., 1999).
Because of being produced by granulosa cells, inhibin B has been suggested as a direct biochemical marker of ovarian reserve and may prove to be a useful adjunct in discriminating patients who are likely to respond to exogenous ovarian stimulation (Balasch et al., 1996; Danforth et al., 1998; Hall et al., 1999; Seifer et al., 1999). Danforth et al.(1998) found that women with normal menstrual cycle in the ages of 46~52 years had lower Day 3 serum inhibin B levels than those in the ages of 39~45 years. Seifer et al.(1999) considered inhibin B as a direct indicator of ovarian reserve and FSH as an indirect indicator. They discovered that the decrease of ovarian reserve might be demonstrated by a decrease in basal inhibin B concentration before a rise in FSH concentration. However, other studies do not support such a claim (Hughes et al., 1990; Corson et al., 1999; Hall et al., 1999), and this discrepancy needs further clarification.
Normal baseline hormonal values, such as inhibin B, estradiol and FSH, do not guarantee that an endocrine system is functioning normally. In fact, patients with baseline values in the normal range may still have a diminished ovarian reserve (Farhi et al., 1997; Kim, 1998; Fábregues et al., 2000; Ranieri et al., 2001). Thus, a number of provocative tests have been devised to indirectly assess ovarian reserve and identify patients who might not be defined by basal hormone screening alone (Navot et al., 1987; Scott et al., 1989; Hofmann et al., 1998; Ravhon et al., 2000). However, whether these indirect provocative tests are more informative of ovarian reserve than basal hormone remains controversial (Galtier-Dereure et al., 1996; Ravhon et al., 2000). Furthermore, neither basal hormonal measurements nor those dynamic tests are capable of providing direct manifestation concerning the responsiveness of the ovaries to the exogenous Gn used in ovarian stimulation for ART (Roest et al., 1996; Farhi et al., 1997; Peñarrubia et al., 2000).
Recently, Phelps et al.(1998) found that estradiol concentration obtained on Day 4 of Gn therapy was highly predictive of a successful ovulation induction and pregnancy outcome in IVF cycles. Neither observation of any variation of inhibin B nor measurement of baseline hormone values was mentioned in their study. This was explained on the basis that estradiol concentration obtained early after the initiation of ovarian stimulation directly reflects follicular activity and ovarian responsiveness to the ongoing regimen of Gn stimulation. Further studies are required to investigate whether in GnRHa-recombinant FSH (rFSH) treatment cycles there is any correlation between the increase in inhibin B concentration and the ovarian response and the IVF outcome. Since inhibin B may regulate the production of estradiol through increasing the level of substrates of estradiol, it would be of interest to delineate whether inhibin B is more sensitive than estradiol in predicting ovarian response to Gn stimulation (Porchet et al., 1994).
This study confirmed and expanded the findings from previous studies (Phelps et al., 1998; Dzik et al., 2000; Ravhon et al., 2000). A total of 57 patents undergoing their first cycle of long protocol IVF or introcytoplasmic sperm injection (ICSI) treatment were studied, and serum inhibin B, estradiol, FSH, LH levels were detected four times in the IVF cycle: on Day 3 of the menstrual cycle, before the first administration of Gn (Day 0), on Day 1 after Gn therapy, and on Day 5 after Gn therapy. This study was designed to identify prospectively the values of basal and dynamic measurements of inhibin B, estradiol, FSH, and LH following Gn therapy for IVF cycles in predicting ovarian response to Gn stimulation and IVF pregnancy outcome. Comparisons of these basal and dynamic measurements with different ovarian responses enabled us to customize the dosage at the early stage of Gn and GnRHa treatment to improve the IVF outcome.
MATERIALS AND METHODS
Patients
After approval from Hospital Ethics Committee and consents from all participants, the study was commenced in January 2004 and concluded in September 2004. Fifty-seven patients were selected from the regular patients admitted at the Reproductive Medicine Department, Women’s Hospital, School of Medicine, Zhejiang University, Hangzhou, China, for their first cycle of IVF/ICSI treatment. All patients accepted had regular menstrual cycles, as well as normal serum prolactin (PRL) level and without hormone treatment within 3 months. The patients’ ages ranged from 24 to 39 years with a mean age of (30.8±3.2) years (mean±SD). Thirty-two patients belonged to tubal infertility, 19 to male infertility, and 6 to both tubal and male infertilities. Twenty-five patients were of primary infertility, and 32 of secondary infertility. Twenty-seven patients underwent IVF, and 30 underwent ICSI.
Sample collection
In the morning, 8:00 a.m. to 10:00 a.m., on Day 3 of patient’s menstrual cycle, 3~5 ml venous blood sample was taken to be assayed. Concentrations of inhibin B, estradiol, FSH, LH, and PRL were measured. Ovarian stimulation was started using rFSH (Gonal-F®; Serono, Switzerland) under pituitary suppression with the daily subcutaneous injection of 0.05~0.10 mg of triptorelin acetate (Decapeptyl®; Ferring, Germany) in the midluteal phase, i.e., after basal temperature had risen for 6~7 d and the serum progesterone concentration was greater than 36 nmol/L. Starting on Day 3 of the next menstrual cycle, rFSH was injected daily with a dosage of 225~375 U/d for the first 2 d, and 150~300 U/d thereafter. More blood samples were collected and assayed for inhibin B, estradiol, FSH and LH in the mornings of Day 0 (before injection of rFSH), on Day 1 and Day 5 of rFSH injection, respectively. On Day 8 of the menstrual cycle (after 5 d of rFSH injection), patients took the first ultrasonographic scan and repeated the scan every 2 or 3 d as necessary. The dosage of rFSH was adjusted from Day 5 of stimulation according to the ovarian response. When at least 1 follicle ≥18 mm in diameter or at least 3 or more follicles ≥15 mm in diameter appeared, we stopped the injections of rFSH and triptorelin acetate for 36 h and then injected human chorionic gonadotrophin (HCG) (10 000 U) (Profasi®; Serono, Switzerland). Oocyte aspiration was performed with vaginal ultrasonography 34~36 h after HCG administration, and embryo (n≤4) transfer (ET) was done 2 to 3 d later. Then we started the injection of 80 mg progesterone daily. Serum HCG level was measured 2 weeks later, and if serum HCG level was more than or equal to normal level, we performed ultrasonography to detect the pulse of fetus to confirm pregnancy.
Hormone assay
According to the manufacturer’s protocol, inhibin B was analyzed in duplicate using a double-antibody enzyme-linked immunosorbent assay (ELISA; Serotec, Kidlington, Oxford, Oxon, UK). Estradiol, FSH and LH were measured using the microparticle enzyme immuno-assay by the ECL2012 system (Rocer).
Statistical analysis
Data were analyzed using SPSS 10.0 for Windows. Pearson’s correlation analysis or Spearman’s correlation analysis was performed to assess the correlation between different variables and ovarian response and the correlation between one variable and another as appropriate. Independent dual-sample test or Pearson’s square test was performed to assess the correlation between various variables and pregnancy outcome as appropriate. One-way analysis of variance (ANOVA) test or Kruskal-Wallis (K-W) test was used to analyze the difference of variables in poor, normal, and OHSS ovarian responses with changes in ovarian response as appropriate. Mann-Whitney (M-W) test was performed to analyze the difference between ovarian response and pregnancy outcome.
RESULTS
All the 57 patients reached oocyte aspiration, of which 53 reached ET (3 ET failures due to vaginal bleeding and 1 failure due to fever). A total number of 162 embryos were transferred. Twenty-one women became pregnant with 3 cases of triplets and 2 cases of twins. The clinical pregnancy rate per ET was 39.6% (21/53), clinical pregnancy rate per cycle was 36.8% (21/57), and implantation rate was 17.3% (28/162). Five patients had poor response (criterion for poor response is oocytes ≤4), 5 patients developed OHSS, and other 47 patients had normal ovarian response.
Table 1 shows patient’s characteristics, ovarian response, and dose of rFSH used. Mean numbers of follicles and eggs were 15.1±6.6 and 13.7±6.4, respectively. Median number of fertilized eggs, embryos, and transferred embryos were 9.0 (1~25), 8.0 (1~25) and 3.0 (1~4), respectively. Median days of rFSH stimulation, ampoules of rFSH and infertility years were 11.0 (8~23), 30.0 (20~82) and 5.0 (1~14), respectively.
Table 1.
Patient characteristics, dose of rFSH used, and ovarian response
| Mean±SD | M (Qr)* | Range | |
| Age (years) | 30.8±3.2 | 24~39 | |
| Follicles | 15.1±6.6 | 4~31 | |
| Eggs | 13.7±6.4 | 3~31 | |
| Fertilized eggs | 9.0 (7.5) | 1~25 | |
| Embryos | 8.0 (7.0) | 1~25 | |
| Transferred embryos | 3.0 (0.0) | 1~4 | |
| Days of rFSH stimulation | 11.0 (2.0) | 8~23 | |
| Ampoules of rFSH | 30.0 (11.0) | 20~82 | |
| Infertility years | 5.0 (4.0) | 1~14 |
M (Qr): median (grade)
Table 2 shows median values of hormone concentrations at four different times of IVF cycle. Data given in the table were presented in median and grade. Inhibin B and estradiol concentrations rose across the increase of days of rFSH stimulation.
Table 2.
Hormone concentrations at four times of IVF cycle
| Inhibin B (pg/ml) | Estradiol (pmol/L) | FSH (U/L) | LH (U/L) | |
| Basal | 36.5 (41.3) | 127.4 (89.0) | 6.9 (1.8) | 3.7 (1.6) |
| Day 0 | 16.7 (29.0) | 66.3 (44.7) | 3.2 (2.2) | 2.0 (1.8) |
| Day 1 | 53.4 (44.9) | 157 (130.8) | 8.3 (3.0) | 2.0 (1.6) |
| Day 5 | 696.2 (875.9) | 1743.3 (2630.2) | 8.8 (3.5) | 1.5 (0.7) |
Data are expressed as median (grade)
Correlations between hormone concentrations and age, ovarian response, dose of rFSH used
On Days 1 and 5 of rFSH stimulation, inhibin B and estradiol concentrations had a positive correlation with ovarian response (numbers of follicles, oocytes, fertilized oocytes and embryos) (r s=0.46~0.61, P=0.000) (Table 3), but a negative correlation with total rFSH dose and days of rFSH administrated (r s=−0.67~−0.38, P=0.000 or P<0.01). Inhibin B concentrations on Days 1 and 5 and estradiol concentration on Day 5 had a negative correlation with age (r s=−0.38~−0.28, P<0.01). However, there was no correlation between age and estradiol concentration on Day 1 (r s=−0.20, P>0.05). This clearly indicated that higher total Gn dose and more days of Gn stimulation were needed in the case of lower serum inhibin B and estradiol concentrations shortly after Gn therapy.
Table 3.
Spearman’s correlation analysis for the correlations between hormone concentrations and age, ovarian response, and dose of rFSH used
| Inhibin B on Day 1 |
Inhibin B on Day 5 |
Estradiol on Day 1 |
Estradiol on Day 5 |
|||||
| rs | P | rs | P | rs | P | rs | P | |
| Age | −0.37 | 0.008 | −0.38 | 0.004 | −0.20 | 0.16 | −0.28 | 0.040 |
| Follicles | 0.57 | 0.000 | 0.60 | 0.000 | 0.50 | 0.000 | 0.61 | 0.000 |
| Eggs | 0.57 | 0.000 | 0.61 | 0.000 | 0.47 | 0.000 | 0.58 | 0.000 |
| Fertilized eggs | 0.54 | 0.000 | 0.55 | 0.000 | 0.51 | 0.000 | 0.48 | 0.000 |
| Embryos | 0.57 | 0.000 | 0.55 | 0.000 | 0.52 | 0.000 | 0.46 | 0.000 |
| Total rFSH dose | −0.59 | 0.000 | −0.38 | 0.000 | −0.53 | 0.000 | −0.67 | 0.000 |
| Days of rFSH stimulation | −0.47 | 0.001 | −0.65 | 0.000 | −0.45 | 0.001 | −0.52 | 0.000 |
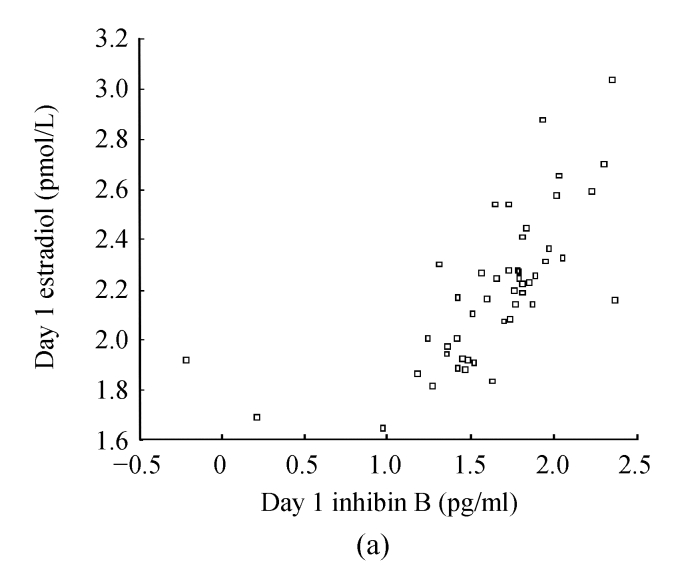
Basal hormone, Inhibin B, estradiol, FSH, and LH levels before rFSH stimulation had no correlation with age, ovarian response and dose of rFSH used (P<0.05). There was no correlation among basal inhibin B and estradiol concentrations and those on Day 1 and Day 5 of rFSH stimulation (r s=−0.22~−0.17, P>0.05). Negative correlation existed between age and the numbers of follicles and oocytes (r s=−0.30~−0.27, P<0.05). Inhibin B concentration had a positive correlation with estradiol concentration after rFSH stimulation (r s=0.62~0.79, P=0.000) (Fig.1).
Fig. 1.
Correlations between inhibin B and estradiol concentrations on (a) Day 1 and (b) Day 5. There were positive correlations between inhibin B concentrations and estradiol concentrations on Day 1 (r s=0.62, P=0.000) and Day 5 (r s=0.79, P=0.000) after rFSH stimulation
Correlations between all variables and pregnancy outcome
Table 4 shows mean values and standard deviation (SD) of age, follicles, eggs, and the independent samples test for the ampoules of rFSH, days of rFSH stimulation, fertilized eggs, embryos, transferred embryos, and infertility years in pregnancy group (n=21) and non-pregnancy group (n=36).
Table 4.
Independent sample tests for age, follicles, eggs, ampoules of rFSH, days of rFSH stimulation, fertilized eggs, embryos, transferred embryos and infertility years in pregnancy and non-pregnancy groups
| Group | Age (year) | Follicles | Eggs | Ampoules of rFSH | Days of rFSH stimulation | Fertilized eggs | Embryos | Transferred embryos | Infertility years |
| Pregnancy (n=21) | 30.6±2.4 | 15.0±7.3 | 13.4±6.7 | 30 (11) | 11 (2.5) | 8.0 (8.5) | 9.5 (8.0) | 3.0 (0.5) | 5.0 (4.5) |
| Non-pregnancy (n=36) | 30.6±3.7 | 15.6±6.1 | 14.3±6.3 | 31 (11) | 11 (2.0) | 9.5 (8.2) | 9.0 (7.5) | 3.0 (0.0) | 4.5 (3.8) |
| P | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 |
Data are expressed as mean±SD or median (grade)
Table 5 shows independent samples test for hormone concentrations at four different times of IVF cycle in pregnancy group (n=21) and non-pregnancy group (n=36).
Table 5.
Independent sample tests for the hormone concentrations at four times of IVF cycle in pregnancy and non-pregnancy groups
| Group | Inhibin B (pg/ml) |
Estradiol (pmol/L) |
Basal FSH (U/L) | Basal LH (U/L) | ||||||
| Basal | Day 0 | Day 1 | Day 5 | Basal | Day 0 | Day 1 | Day 5 | |||
| Pregnancy (n=21) | 26.4 (18.1) | 8.8 (34.2) | 46.2 (39.7) | 603.3 (837.2) | 114.5 (93.1) | 72.9 (47.4) | 157.5 (140.4) | 1562.5 (3254.5) | 7.0 (1.8) | 3.7 (1.5) |
| Non-pregnancy (n=36) | 38.5 (45.7) | 18.6 (27.7) | 57.2 (65.3) | 762.5 (1059.3) | 133.9 (94.2) | 66.2 (39.8) | 174.9 (155.1) | 2133 (2533) | 6.9 (1.9) | 3.8 (2.1) |
| P | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 |
Data are expressed as median (grade)
There was no significant difference between pregnancy and non-pregnancy groups in relation to different variables (P>0.05). In this study, all the 7 patients of age >35 years failed to become pregnant.
Correlations between pregnancy outcome and cause of infertility, sort of infertility, and IVF manner
Pearson’s square tests as shown in Table 6 show that pregnancy outcome had no correlation with cause of infertility, sort of infertility or IVF manner (P>0.05).
Table 6.
Pearson’s square test for the correlations between pregnancy outcome and cause of infertility, sort of infertility, or IVF manner
| χ2 | df | P | |
| Cause of infertility | 0.53 | 2 | 0.77 |
| Sort of infertility | 0.50 | 1 | 0.70 |
| IVF manner | 0.91 | 1 | 0.34 |
Comparisons of variables in poor, normal, and OHSS ovarian responses with pregnancy outcome
For the benefit of easier and more direct comparisons of ovarian responses to different variables, we categorized the results into three different groups: poor response (5 patients whose oocytes were ≤4), normal response (47 cases), and OHSS (5 patients who developed OHSS).
Comparisons of variables among the three groups are shown in Table 7 (results of one-way ANOVA test) and Table 8 (results of K-W test).
Table 7.
One-way ANOVA test for the difference of variables in the three groups with different ovarian response
| Group | Age (year) | Follicles | Eggs | Fertilized eggs | Embryos | Days of rFSH stimulation | Ampoules of rFSH |
| Poor response (n=5) | 33.4±3.0 | 4.0±0.0 | 4.0±0.0 | 3.2±1.2 | 3.0±1.3 | 50.2±20.9 | 15.0±5.1 |
| Normal response (n=47) | 30.5±3.3 | 15.4±5.7 | 13.9±5.5 | 9.5±5.1 | 9.0±4.8 | 30.2±6.8 | 11.0±1.6 |
| OHSS (n=5) | 30.6±1.3 | 22.6±5.1 | 21.2±6.7 | 14.8±4.6 | 14.2±5.0 | 30.4±5.7 | 11.0±1.5 |
| P | 0.16 | 0.000 | 0.000 | 0.001 | 0.002 | 0.000 | 0.001 |
Data are expressed as mean±SD
Table 8.
K-W test for the difference of variables in the three groups with different ovarian response
| Group | Inhibin B concentration (pg/ml) |
Estradiol concentration (pmol/L) |
||||||
| C5 | C5−Cb | C5−C0 | C5−C1 | C5 | C5−Cb | C5−C0 | C5−C1 | |
| Poor response (n=5) | 164.7 (155.4) | 132.2 (83.1) | 153.6 (98.0) | 180.3 (198.4) | 303.2 (1241.2) | 91.2 (144.7) | 202.0 (566.9) | 137.0 (1358.9) |
| Normal response (n=47) | 696.2 (907.9) | 659.7 (980.2) | 640.2 (1000.2) | 647.9 (917.9) | 1709.5 (2252.0) | 1582.1 (1934.5) | 1920.0 (25.2.4) | 1619.0 (2278.4) |
| OHSS (n=5) | 1263.5 (792.9) | 1237.1 (642.3) | 1358.7 (743.5) | 1027.3 (614.9) | 4261.0 (1689.1) | 4129.6 (1070.3) | 3586.0 (2072.1) | 3359.2 (1899.5) |
| P | 0.001 | 0.001 | 0.007 | 0.006 | 0.002 | 0.001 | 0.005 | 0.009 |
C 5: concentration on Day 5; C b: basal concentration; C 0: concentration on Day 0; C 1: concentration on Day 1
Age was not an influential factor among the three different groups (P>0.05).
The numbers of follicles, oocytes, fertilized oocytes and embryos were significantly different (P<0.01) among the three different groups. The total rFSH dose and days of rFSH stimulation in the poor ovarian response group were higher than those in the normal ovarian response and the OHSS groups (P<0.01).
There was no significant difference in the levels of basal inhibin B, pre-rFSH inhibin B (Day 0) and post-rFSH inhibin B (Day 1) among the three groups (P>0.05). However, inhibin B concentration on Day 5 of rFSH stimulation was significantly different among the three groups (P<0.01).
There was no significant difference (P>0.05) in the levels of basal estradiol, estradiol on Day 0 and Day 1 of rFSH stimulation among the three groups (P>0.05), but estradiol levels showed a significant difference on Day 5 of rFSH stimulation among the three groups (P<0.05).
FSH and LH concentrations at four different times of IVF cycle were very similar among the three groups (P>0.05).
Table 9 shows comparison of pregnancy outcome among the three groups. The pregnancy outcome was found similar by M-W test (U=296, P>0.05).
Table 9.
Pregnancy outcome among the three groups
| Group | Pregnancy | Non-pregnancy | Total |
| Poor response (n=5) | 2 | 2 | 4 |
| Normal response (n=47) | 15 | 29 | 44 |
| OHSS (n=5) | 4 | 1 | 5 |
| Total | 21 | 32 | 53 |
DISCUSSION
Although there are many factors that affect the extent of COH and the result of IVF-ET, it is generally accepted that the ovarian response to the Gn stimulation plays a key role in an IVF outcome. In the present study, we demonstrated the predictive values of various ovarian reserve indicators that affect ovarian response as well as IVF pregnancy outcome. An accurate prediction of ovarian response could provide a valuable means in prescribing appropriate Gn and GnRHa dosage in the early days of treatment for a patient. This timely dosage adjustment may avoid the poor ovarian response and the risk of OHSS, while IVF-ET outcome was improved.
Significance of inhibin B measurement after Gn stimulation in predicting the ovarian response and IVF pregnancy outcome
Dzik et al.(2000) found that the mean baseline inhibin B concentrations and the inhibin B concentration 24 h after exogenous follicle-stimulating hormone ovarian reserve test (EFORT) were significantly higher in good responders than that in poor responders. Thus, they concluded that inhibin B concentration 24 h after EFFORT may provide a method for predicting ovarian response to hyperstimulation in a subsequent IVF cycle. Ravhon et al.(2000) reported that ovarian response was defined in two ways: the number of oocytes/total rFSH dose and square root (the number of follicles/total rFSH dose). The following measurements were highly correlated with the ovarian response to stimulation: increase in estradiol (Day 3 estradiol concentration−Day 2 estradiol concentration) (r s=0.68, P<0.0001) and sum of inhibin B (Day 3 estradiol concentration+Day 2 inhibin B concentration) (r s=0.60, P<0.0001). Age and basal concentrations of FSH and inhibin B were inferior to the preceding measurements in terms of correlation with the ovarian response. Although it was concluded that dynamic measurements of inhibin B and estradiol following single administration of buserelin acetate were highly correlated with the ovarian response to stimulation for IVF treatment, the correlations between different variables and the pregnancy outcome remain unknown. Moreover, those reports did not provide direct information regarding the responsiveness of the ovaries to the exogenous Gn used in ovarian stimulation for ART. In fact, other studies proposed that the clinical response to Gn treatment would be a more direct way to assess ovarian reserve and a better predictor of fertility potential (Roest et al., 1996; Farhi et al., 1997; Peñarrubia et al., 2000). Phelps et al.(1998) found that the estradiol concentration measured on Day 4 of Gn therapy was highly predictive of successful ovulation induction and pregnancy outcome in GnRHa-rFSH cycles.
This study confirmed previous studies and expanded some of their findings (Phelps et al., 1998; Ravhon et al., 2000; Dzik et al., 2000; Urbancsek et al., 2005b). We performed dynamic assays of basal inhibin B, estradiol, FSH, and LH. These measurements were taken on Day 3 of the menstrual cycle, and at three different times in the rFSH stimulation cycle: before injection of rFSH (Day 0), Day 1 and Day 5 after injection of rFSH. These data were subjected to statistical analyses for correlations between ovarian responses and different sets of variables.
Our findings
Our results suggest that serum inhibin B and estradiol concentrations obtained shortly after Gn therapy may offer an accurate and early prediction of ovarian response. Low levels of inhibin B and estradiol may indicate the possibility of low ovarian response; conversely, high levels of inhibin B and estradiol obtained on Day 5 indicate the occurrence of OHSS (Table 9). With early detection of low ovarian response or OHSS, a timely adjustment of dosage of rFSH and GnRHa can be considered in order to correct the physical conditions and to improve the ovarian response.
A negative correlation between age and inhibin B existed on Day 1 after injection of rFSH, indicating that the older the age, the lower the inhibin B level after Gn stimulation. However, there was no correlation between age and estradiol level on Day 1 after injection of rFSH, indicating that inhibin B concentration may be a more sensitive and earlier index of ovarian response than serum estradiol concentration and age.
The concentrations of inhibin B and estradiol had a higher correlation coefficient (r s=0.47~0.61, P=0.000) with the numbers of follicles and oocytes than that with age (r s=−0.27~−0.30, P<0.05), indicating that the inhibin B and estradiol levels in the early days can better predict the ovarian response to Gn stimulation than age.
We can conclude that the basal inhibin B and estradiol concentrations could not predict the change of inhibin B and estradiol after Gn stimulation. This may be due to the patients’ relatively young ages (mean value of (30.8±3.2) years).
We can also conclude that it is less valuable to use concentrations of inhibin B, estradiol, FSH and LH on the early days after Gn stimulation as a predictive marker of IVF pregnancy outcome for younger women. These results agree with those of Hall et al.(1999) and Urbancsek et al.(2005a), but contradict with that reported by Seifer et al.(1997). Indeed, it is highly possible that uterine receptivity and a perfect dialogue between good-quality embryos and receptive endometrium may play a more important role in an IVF pregnancy outcome.
Effects of basal hormone in predictions of ovarian response and IVF pregnancy outcome
This study shows that basal inhibin B, estradiol, FSH, and LH do not correlate with the numbers of follicles, oocytes, and IVF pregnancy. These results agree with previous reports (Hughes et al., 1990; Rotsztejn and Asch, 1991; Corson et al., 1999; Hall et al., 1999; Frattarelli et al., 2000; Ravhon et al., 2000). It is understandable that the basal inhibin B (36.5 ng/ml), estradiol (127.4 pmol/L) or FSH (6.9 U/L) levels had no correlation with ovarian response and pregnancy outcome, because patients’ ages in this study are relatively young [(30.8±3.2) years] and their basal hormone levels are in the normal range. There were 5 patients of low ovarian response and 5 patients developed OHSS, which confirmed that there is an uncertainty between basal hormone level and ovarian response; the normal baseline hormone levels cannot guarantee normal ovarian response to Gn stimulation.
Effects of age on predictions of ovarian response and pregnancy outcome
Our results show that pregnancy outcome may be significantly lower in women of age >35 years, but the pregnancy outcome varies little for those of age <35 years. Our results agree with previous reports (Spandorfer et al., 2000; Watt et al., 2000). Watt et al. (2000) reported that the ovarian response to Gn stimulation decreased with age as demonstrated by lower estradiol concentrations and that patients needed larger doses of Gn for stimulation. They also reported that age had a negative correlation with the numbers of follicles, oocytes, and pregnancy rate. Spandorfer et al.(2000) found that the implantation rate did not change in women of age <35 years, but it reduced by 2.77% per year in those of age ≥35 years. The implantation rate was 36.8% in women of age <33 and only 2.3% in those of age >44.
Correlation between ovarian response and pregnancy outcome
The success of IVF pregnancy depends on several factors such as patient’s age, embryo quality, uterine receptivity, and a perfect dialogue between good-quality embryos and receptive endometrium. However, the correlation between ovarian response and IVF pregnancy outcome remains controversial (Toner et al., 1991a; Simon et al., 1995; 1998; Pellicer et al., 1996; Sharara and McClamrock, 1999; Kably et al., 2000; Bancsi et al., 2002).
Previous reports indicated that low estradiol concentration may induce the decline in oocytes number and embryos quality which may lead to low fertilization rate and low pregnancy rate (Kably et al., 2000). Different ovarian responses were considered to contribute to different IVF pregnancy rates (Kably et al., 2000; Bancsi et al., 2002). Poor ovarian responders had significantly lower clinical and ongoing pregnancy rates than did normal responders (Bancsi et al., 2002). In contrast, Toner et al.(1991a) retrospectively reported that implantation and pregnancy rates were not different among the women retrieved 1~5, 6~10, or >10 oocytes, despite a decrease in the numbers of retrieved oocytes and embryos in low responders. Low peak estradiol concentration may be in favor of embryos implantation. Although high responders had more retrieved oocytes and good-quality embryos, their pregnancy rates were similar to low and normal responders’ (Simon et al., 1998; Sharara and McClamrock, 1999). High estradiol concentration and altered estradiol/progesterone ratios, which also are associated with the impairment of endometrial receptivity, may be the main factors affecting endometrial receptivity in high responders (Simon et al., 1995; Pellicer et al., 1996).
Our results show that although there were significant differences in the numbers of follicles, oocytes, fertilized oocytes, and embryos among poor, normal, and OHSS groups, the cycle pregnancy rates were not different. Our results agree with previous reports (Toner et al., 1991a; Simon et al., 1995; 1998; Pellicer et al., 1996; Sharara and McClamrock, 1999). However, for the poor responder, the number of embryos obtained in the IVF cycle is only enough to have one chance of ET. An early, accurate identification of patients who tend to have poor response would help clinicians to make a prompt modification on the dosage of Gn and GnRHa for stimulation, so that ovarian response can be improved and more retrieved oocytes and better embryos can be gained. As a result, women have more chances to accept frozen-thawed ET. As to the OHSS patients, an appropriate dose of Gn and GnRHa will probably reduce or avoid the occurrence of OHSS.
We, therefore, conclude that serum inhibin B and estradiol levels obtained shortly after Gn therapy offer an early and accurate prediction of ovarian response to Gn stimulation. They are superior indicators compared with age or basal hormonal levels in predicting ovarian response. Serum inhibin B and estradiol levels obtained shortly after Gn therapy predict poor ovarian response and OHSS. A higher total Gn dose and longer COH cycle would be warranted for the case of poor ovarian response. In predicting ovarian response of women in different ages, serum inhibin B may be a more sensitive index than serum estradiol. Normal basal hormonal levels could not guarantee normal ovarian response to Gn stimulation, and possess little predictive value for IVF pregnancy outcome in younger women. Age has negative correlation with the numbers of follicles and oocytes, and pregnancy outcome may be significantly lower in women of age >35 years, but the pregnancy outcome varies little for those of age <35 years. Cycle pregnancy rate does not correlate with ovarian response.
References
- 1.Balasch J, Creus M, Fábregues F, Carmona F, Casamitjana R, Ascaso C, Vanrell JA. Inhibin, follicle-stimulating hormone, and age as predictors of ovarian response in in vitro fertilization cycles stimulated with gonadotropin-releasing hormone agonist-gonadotropin treatment. Am J Obstet Gynecol. 1996;175(5):1226–1230. doi: 10.1016/S0002-9378(96)70032-7. [DOI] [PubMed] [Google Scholar]
- 2.Bancsi LF, Broekmans FJ, Eijkemans MJ, Jong F, Habbema JD, Velde ER. Predictors of poor ovarian response in vitro fertilization: a prospective study comparing basal markers of ovarian reserve. Fertil Steril. 2002;77(2):328–336. doi: 10.1016/S0015-0282(01)02983-1. [DOI] [PubMed] [Google Scholar]
- 3.Cahill DJ, Prosser CJ, Wardle PG, Ford WC, Hull MG. Relative influence of serum follicle stimulating hormone, age and other factors on ovarian response to gonadotrophin stimulation. Br J Obstet. 1994;101(11):999–1002. doi: 10.1111/j.1471-0528.1994.tb13047.x. [DOI] [PubMed] [Google Scholar]
- 4.Corson SL, Gutmann J, Batzer FR, Wallace H, Klein N, Soules MR. Inhibin B as a test of ovarian reserve for infertile woman. Hum Reprod. 1999;14(11):2818–2824. doi: 10.1093/humrep/14.11.2818. [DOI] [PubMed] [Google Scholar]
- 5.Danforth DR, Arbogast LK, Mrouch J, Kim MH, Kennard EA, Seifer DB, Friendman CI. Dimeric inhibin: a direct marker of ovarian aging. Fertil Steril. 1998;70(1):119–124. doi: 10.1016/S0015-0282(98)00127-7. [DOI] [PubMed] [Google Scholar]
- 6.Dzik A, Lambert MG, Lzzo VM, Soares JB, Pinotti JA, Seifer DB. Ihibin B response to EFORT is associated with the outcome of oocyte retrieval in the subsequent in vitro fertilization cycle. Fertil Steril. 2000;74(6):1114–1117. doi: 10.1016/S0015-0282(00)01627-7. [DOI] [PubMed] [Google Scholar]
- 7.Fábregues F, Balasch J, Creus M, Carmona F, Puerto B, Quinto L, Casamitjana R, Vanrell JA. Ovarian reserve test with human menopausal gonadotropin as a predictor of in vitro fertilization outcome. J Assist Reprod Genet. 2000;17(1):13–19. doi: 10.1023/A:1009441812247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farhi J, Homburg R, Ferber A, Orvieto R, Ben-Rafael Z. Non-response to ovarian stimulation in normogonadotrophic, normogonadal women: a clinical sign of impending onset of ovarian failure pre-empting the rise in the basal follicle stimulating hormone levels. Hum Reprod. 1997;12(2):241–243. doi: 10.1093/humrep/12.2.241. [DOI] [PubMed] [Google Scholar]
- 9.Frattarelli JL, Biergh PA, Drews MR, Drews MR, Sharara FI, Scott JR. Evaluation of basal estradiol levels in assisted reproductive technology cycles. Fertil Steril. 2000;74(3):518–524. doi: 10.1016/S0015-0282(00)00693-2. [DOI] [PubMed] [Google Scholar]
- 10.Galtier-Dereure F, de Bouard V, Picot MC, Vergnes C, Humeaus C, Bringer J, Hédon B. Ovarian reserve test with the Gn-releasing hormone agonist buserelin: correlation with in-vitro fertilization outcome. Hum Reprod. 1996;11(7):1393–1398. doi: 10.1093/oxfordjournals.humrep.a019406. [DOI] [PubMed] [Google Scholar]
- 11.Groome NP, Illingworth PJ, O′Brien M, Cooke I, Ganesan TS, Baird DT, McNeilly AS. Detection of dimeric inhibin throughout the human menstrual cycle by two-site enzyme immunoassay. Clin Endocrinol (Oxf) 1994;40(6):717–723. doi: 10.1111/j.1365-2265.1994.tb02504.x. [DOI] [PubMed] [Google Scholar]
- 12.Groome NP, Illingworth PJ, O′Brien M, Pai R, Rodger FE, Mather JP, McNeilly AS. Measurement of dimeric inhibin-B throughout the human menstrual cycle. J Clin Endocrinol Metab. 1996;81(4):1401–1405. doi: 10.1210/jc.81.4.1401. [DOI] [PubMed] [Google Scholar]
- 13.Hall JE, Welt CK, Cramer DW. Inhibin A and inhibin B reflect ovarian function in assisted reproduction but are less useful at predicting outcome. Hum Reprod. 1999;14(2):409–415. doi: 10.1093/humrep/14.2.409. [DOI] [PubMed] [Google Scholar]
- 14.Hofmann GE, Danforth DR, Seifer DB. Inhibin-B: the physiological basis of the clomiphene citrate challenge test for ovarian reserve screening. Fertil Steril. 1998;69(3):474–479. doi: 10.1016/S0015-0282(97)00531-1. [DOI] [PubMed] [Google Scholar]
- 15.Hughes EG, Robertson DM, Handelsman DJ, Hayward S, Healy DL, de Kretser DM. Inhibin and estradiol response to ovarian hyperstimulation: effects of age and predictive value for in vitro fertilization outcome. J Clin Endocrinol Metab. 1990;70(2):358–364. doi: 10.1210/jcem-70-2-358. [DOI] [PubMed] [Google Scholar]
- 16.Kably AA, Barron VJ, Tapia LRC, Krivitsky SK. Effect of blood concentrations of preovulatory estradiol on the quality of eggs and pre-embryos in patients treated with fertilization in vitro. Ginecol Obstet Mex. 2000;68(11):435–441. [PubMed] [Google Scholar]
- 17.Kim MR. Screening for ovarian reserve. Assist Reprod Rev. 1998;8(1):17–22. [Google Scholar]
- 18.Kim YK, Wasser SK, Fujimoto VY, Klein NA, Moore DE, Soules MR. Utility of follicle stimulating hormone (FSH), luteinizing hormone ratio (LH), estradiol and FSH: LH ratio in predicting reproductive age in normal women. Hum Reprod. 1997;12(6):1152–1155. doi: 10.1093/humrep/12.6.1152. [DOI] [PubMed] [Google Scholar]
- 19.Laven JS, Fauser BC. Inhibins and adult ovarian function. Mol Cell Endocrinol. 2004;225(1-2):37–44. doi: 10.1016/j.mce.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 20.Licciardi FL, Liu HC, Rosenwaks Z. Day 3 estradiol serum concentration as prognostic data of ovarian stimulating response and pregnancy outcome in patients undergoing in vitro fertilization. Fertil Steril. 1995;64(5):991–994. doi: 10.1016/s0015-0282(16)57916-3. [DOI] [PubMed] [Google Scholar]
- 21.Lockwood GM, Muttukrishna S, Groome NP, Knight PG, Ledger WL. Circulating inhibins and activin A during GnRH-analogue down-regulation and ovarian hyperstimulation with recombinant FSH for in-vitro fertilization-embryo transfer. Clin Endocrinol. 1996;45(6):741–748. doi: 10.1046/j.1365-2265.1996.8510861.x. [DOI] [PubMed] [Google Scholar]
- 22.Maroulis GB. Effect of aging on fertility and pregnancy. Semin Reprod Med. 1991;9(3):165–175. doi: 10.1055/s-2007-1019407. [DOI] [Google Scholar]
- 23.Mosher WD, Pratt WF. Fecundity and Infertility in the United States, 1965-1988. Hyattsville, MD: National Center for Health Statistics; 1990. [Google Scholar]
- 24.Mosher WD, Pratt WF. Fecundity and infertility in the United States: incidence and trends. Fertil Steril. 1991;56(2):192–193. [PubMed] [Google Scholar]
- 25.Mukherjee T, Copperman AB, Lapinski R, Sandler B, Bustillo M, Grunfeld L. An elevated Day 3 follicle stimulating hormone: luteinizing hormone ratio (FSH/LH) in the presence of a normal Day 3 FSH predicts a poor response to controlled ovarian hyperstimulation. Fertil Steril. 1996;65(3):588–593. doi: 10.1016/s0015-0282(16)58159-x. [DOI] [PubMed] [Google Scholar]
- 26.Navot D, Rosenwaks Z, Margalioth EJ. Prognostic assessment of female fecundity. Lancet. 1987;330(8560):645–647. doi: 10.1016/S0140-6736(87)92439-1. [DOI] [PubMed] [Google Scholar]
- 27.Navot D, Bergh PA, Laufer N. Ovarian hyperstimulation syndrome in novel reproductive technologies: prevention and treatment. Fertil Steril. 1992;58(2):249–261. doi: 10.1016/s0015-0282(16)55188-7. [DOI] [PubMed] [Google Scholar]
- 28.Pellicer A, Valbuena D, Cano F, Remohi J, Simon C. Lower implantation rates in high responders: evidence for altered endocrine milieu during the preimplantation period. Fertil Steril. 1996;65(6):1190–1195. doi: 10.1016/s0015-0282(16)58337-x. [DOI] [PubMed] [Google Scholar]
- 29.Peñarrubia J, Balasch J, Fábregues F, Carmona F, Casamitjana R, Moreno V, Calafell JM, Vanrell1 JA. Day 5 inhibin B serum concentrations as predictors of assisted reproductive technology outcome in cycles stimulated with gonadotrophin-releasing hormone agonist-gonadotrophin treatment. Hum Reprod. 2000;15(7):1499–1504. doi: 10.1093/humrep/15.7.1499. [DOI] [PubMed] [Google Scholar]
- 30.Phelps JY, Levine AS, Hickman TN, Zacur HA, Wallach EE, Hinton EL. Day 4 estradiol levels predict pregnancy success in women undergoing controlled ovarian hyperstimulation for IVF. Fertil Steril. 1998;69(6):1015–1019. doi: 10.1016/S0015-0282(98)00059-4. [DOI] [PubMed] [Google Scholar]
- 31.Porchet HC, Cotonnec JYL, Loumaye E. Clinical pharmacology of recombinant human follicle-stimulating hormone. III. Pharmacokinetic-pharmacodynamic modelling after repeated subcutaneous administration. Fertil Steril. 1994;61(4):687–695. [PubMed] [Google Scholar]
- 32.Ranieri DM, Phophong P, Khadum I, Meo F, Davis C, Serhal P. Simultaneous evaluation of basal FSH and estradiol response to GnRH analogue (F-G-test) allows effective drug regimen selection for IVF. Hum Reprod. 2001;16(4):673–675. doi: 10.1093/humrep/16.4.673. [DOI] [PubMed] [Google Scholar]
- 33.Ravhon A, Lavery S, Michael S, Donaldson M, Margara R, Trew G, Winston R. Dynamic assays of inhibin B and estradiol following buserelin acetate administration as predictors of ovarian response in IVF. Hum Reprod. 2000;15(11):2297–2301. doi: 10.1093/humrep/15.11.2297. [DOI] [PubMed] [Google Scholar]
- 34.Roest J, van Heusden AM, Mous H, Zeilmaker GH, Verhoeff A. The ovarian response as a predictor for successful in vitro fertilization treatment after the age of 40 years. Fertil Steril. 1996;66(6):969–973. doi: 10.1016/s0015-0282(16)58691-9. [DOI] [PubMed] [Google Scholar]
- 35.Rotsztejn DA, Asch RH. Effect ofagingon assisted reproductive technologies (ART): experience from oocyte donation. Semin Reprod Med. 1991;9(3):272–279. doi: 10.1055/s-2007-1019419. [DOI] [Google Scholar]
- 36.Scott RT, Hofmann GE. Prognostic assessment of ovarian reserve. Fertil Steril. 1995;63(1):1–11. [PubMed] [Google Scholar]
- 37.Scott RT, Toner JP, Muasher SJ, Oehninger S, Robinson S, Rosenwaks Z. Follicle stimulating hormone levels on cycle Day 3 are predictive of in vitro fertilization outcome. Fertil Steril. 1989;51(4):651–654. doi: 10.1016/s0015-0282(16)60615-5. [DOI] [PubMed] [Google Scholar]
- 38.Seifer DB, Lambert-Messerlian G, Hogan JW, Gardiner AC, Blazar AS, Berk CA. Day 3 serum inhibin-B is predictive of assisted reproductive technologies outcome. Fertil Steril. 1997;67(1):110–114. doi: 10.1016/S0015-0282(97)81865-1. [DOI] [PubMed] [Google Scholar]
- 39.Seifer DB, Scott RT, Bergh PA, Abrogast LK, Friedman CI, Mack CK, Danforth DR. Women with declining ovarian reserve may demonstrate a decrease in Day 3 serum inhibin B before a rise in Day 3 follicle-stimulating hormone. Fertil Steril. 1999;72(1):63–65. doi: 10.1016/S0015-0282(99)00193-4. [DOI] [PubMed] [Google Scholar]
- 40.Shanthi MK, Harris S, Hugh MG, Muttukrishna S. Inhibin B and anti-Mullerian hormone: markers of ovarian response in IVF/ICSI patients? Int J Obstet Gynaecol. 2004;111(Suppl.):1248–1253. doi: 10.1111/j.1471-0528.2004.00452.x. [DOI] [PubMed] [Google Scholar]
- 41.Sharara FI, McClamrock HD. High estradiol levels and high oocyte yield are not detrimental to in vitro fertilization outcome. Fertil Steril. 1999;72(3):401–405. doi: 10.1016/S0015-0282(99)00293-9. [DOI] [PubMed] [Google Scholar]
- 42.Simon C, Cano F, Valbuena D, Remohi J, Pellicer A. Clinical evidence for a detrimental effect on uterine receptivity of high serum estradiol levels in high and normal responder patients. Hum Reprod. 1995;10(9):2432–2437. doi: 10.1093/oxfordjournals.humrep.a136313. [DOI] [PubMed] [Google Scholar]
- 43.Simon C, Garcia VJJ, Valbuena D, Painado JA, Moreno C, Remohí J, Pellicer A. Increasing uterine receptivity by decreasing estradiol levels during the preimplantation period in high responders with the use of a follicle-stimulating hormone step-down regimen. Fertil Steril. 1998;70(2):234–239. doi: 10.1016/S0015-0282(98)00140-X. [DOI] [PubMed] [Google Scholar]
- 44.Spandorfer S, Chung P, Kligman I, Liu HC, Davis OK, Rosenwake Z. An analysis of the effect of age on implantation rates. J Assist Reprod Genet. 2000;17(6):303–306. doi: 10.1023/A:1009422725434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Toner JP, Brzyski RG, Oehninger S, Veeck LL, Simonetti S, Muasher SJ. Combined impact of the number of pre-ovulatory oocytes and cryopreservation on IVF outcome. Hum Reprod. 1991;14:284–289. doi: 10.1093/oxfordjournals.humrep.a137323. [DOI] [PubMed] [Google Scholar]
- 46.Toner JP, Philput CB, Jones GS, Muasher SJ. Basal follicle stimulating hormone level is a better predictor of in vitro fertilization performance than age. Fertil Steril. 1991;55(4):784–791. doi: 10.1016/s0015-0282(16)54249-6. [DOI] [PubMed] [Google Scholar]
- 47.Urbancsek J, Hauzman E, Klinga K, Rabe T, Papp Z, Strowitzki T. Use of serum inhibin B levels at the start of ovarian stimulation and at oocyte pickup in the prediction of assisted reproduction treatment outcome. Fertil Steril. 2005;83(2):341–348. doi: 10.1016/j.fertnstert.2004.06.065. [DOI] [PubMed] [Google Scholar]
- 48.Urbancsek J, Hauzman EE, Murber A, Lagarde AR, Rabe T, Papp Z, Strowitzki T. Serum CA-125 and inhibin B levels in the prediction of ovarian response to gonadotropin stimulation in in vitro fertilization cycles. Gynecol Endocrinol. 2005;21(1):38–44. doi: 10.1080/09513590500099438. [DOI] [PubMed] [Google Scholar]
- 49.Watt AH, Legedza AT, Ginsburg ES, Barbieri RL, Clarke RN, Hornstein MD. The prognostic value of age and follicle-stimulating hormone levels in women over forty years of age undergoing in vitro fertilization. J Assist Reprod Genet. 2000;17(5):264–268. doi: 10.1023/A:1009458332567. [DOI] [PMC free article] [PubMed] [Google Scholar]