Abstract
Background: Capsule endoscopy is a novel non-invasive method for visualization of the entire small bowel. The diagnostic yield of capsule endoscopy depends on the quality of visualization of the small bowel mucosa and its complete passage through the small bowel. To date, there is no standardized protocol for bowel preparation before capsule endoscopy. The addition of simethicone in the bowel preparation for the purpose of reducing air bubbles in the intestinal lumen had only been studied by a few investigators. Methods: Sixty-four participants were randomly divided into two groups to receive a bowel preparation of polyethylene glycol (PEG) solution (Group 1) and both PEG solution and simethicone (Group 2). The PEG solution and simethicone were taken the night before and 20 min prior to capsule endoscopy, respectively. Frames taken in the small intestine were examined and scored for luminal bubbles by two professional capsule endoscopists. Gastric emptying time and small bowel transit time were also recorded. Results: Simethicone significantly reduced luminal bubbles both in the proximal and distal small intestines. The mean time proportions with slight bubbles in the proximal and distal intestines in Group 2 were 97.1% and 99.0%, respectively, compared with 67.2% (P<0.001) and 68.8% (P<0.001) in Group 1. Simethicone had no effect on mean gastric emptying time, 32.08 min in Group 2 compared with 30.88 min in Group 1 (P=0.868), but it did increase mean small intestinal transit time from 227.28 to 281.84 min (P=0.003). Conclusion: Bowel preparation with both PEG and simethicone significantly reduced bubbles in the intestinal lumen and improved the visualization of the small bowel by capsule endoscopy without any side effects observed.
Keywords: Capsule endoscopy, Bowel preparation, Simethicone, Polyethylene glycol (PEG), Visualization, Air bubbles
INTRODUCTION
Capsule endoscopy has been used to examine small intestinal diseases, especially obscure gastrointestinal bleeding (OGIB) and inflammatory bowel disease (IBD). It is also useful in discovering rare intestinal diseases, such as intestinal lymphangiectasia (Fang et al., 2007). Capsule endoscopy has reduced the relative inaccessibility of the small bowel to endoscopy examination (Iddan et al., 2000; Keuchel and Hagenmuller, 2005). Video images are taken two frames per second over approximately eight hours’ working time. The recorded frames by a storing system can be studied after the procedure on a computer. However, certain limitations exist. Capsule endoscopy has a viewing field of only 140°, resulting in blind spots during visualization (Ginsberg et al., 2002). Furthermore, the actual extent of the lumen visualized by capsule endoscopy is limited by air bubbles, food, and other debris. Bile pigments in the upper segment of the small intestine and decreased light in the ileum will also impair the actual visualization. Such obscuring of the visual field may reduce the diagnostic yield. Therefore, it is essential to reduce these potential causes of poor visualization by optimizing the bowel preparation for capsule endoscopy. Unfortunately, no standardized protocol for preparation exists.
Normally, the small bowel moves more frequently than the colon when food and fluid are present. The rapid emptying of the small intestine soon after eating means that it is empty most of the time. When there is no fluid or air in the small intestine, the lumen is closed. Visualization of the small intestine by capsule endoscopy is improved by distending of the lumen and washing away of bubbles and debris with clear water. Most investigators used non-absorbable but osmotically active substrates such as polyethylene glycol (PEG) solution to distend the intestinal lumen and to wash away bile pigments and debris (Dai et al., 2005; Shiotani et al., 2007). PEG solution was also believed to assist in movement of the capsule through the small intestine, thereby increasing the likelihood that the entire small bowel was visualized while the capsule was still functional. Some investigators also administered oral simethicone in the preparation fluid to reduce bubbles in the lumen, thus improving the visualization of the small intestine (Albert et al., 2004; Ge et al., 2006). However, studies have differed in the volume, the dose of PEG solution used, the dose of simethicone used, the time at which the various solutions were taken, and the actual effects of the preparation (Albert et al., 2004; Viazis et al., 2004; Dai et al., 2005; Ge et al., 2006; Shiotani et al., 2007). In our experience, the ingestion of PEG solution the night prior to the capsule endoscopy and the addition of simethicone a short time before the capsule endoscopy greatly improved visualization.
In order to compare the effects of different preparation methods for capsule endoscopy, we designed this prospective experiment to investigate the effects of a certain preparation on the quality of visualization of the small bowel and to determine the effect of the specific preparation on gastrointestinal transit time.
MATERIALS AND METHODS
Patients
The study was performed between May 2003 and June 2007. Sixty-four participants, including 45 males and 19 females with the age ranging from 21 to 75 years, were judged to be in acceptable health without severe system diseases that will affect the examination of capsule endoscopy, based on medical history and physical examination. Exclusion criteria included a history of gastric or intestinal surgery, clinical or suspected abnormalities in gastric emptying, diabetic gastroparesis, and medications that could affect gastrointestinal movement within one week. The study was approved by the local ethics committee.
Protocol
Study participants were randomly divided into Groups 1 and 2 (32 participants each). All participants had fasted for at least 12 h before swallowing the capsule. Group 1 received a bowel preparation of PEG solution and Group 2 a preparation of PEG solution and 600 mg of simethicone (Berlin-ChemieAG, Germany) in 15-ml solution. Simethicone was taken 20 min before ingesting the capsule. When swallowing the capsule, 200 ml of water was taken at the same time. Four hours after capsule ingestion, participants were allowed to ingest simple food, such as noodles and bread.
Bowel prep
The night before capsule endoscopy (M2A, Given Imaging Ltd., Yoqneam, Israel), 2 L of isotonic solution containing PEG 4000, sodium chloride, sodium bicarbonate, sodium carbonate, and potassium chloride (Na+ 125 mmol/L, K+ 10 mmol/L, HCO3 − 20 mmol/L, SO4 2− 40 mmol/L, Cl− 35 mmol/L, and PEG 4000 15 mmol/L) was ingested. The volunteers were told to take PEG solution within 2 h before the operation, and the first dose was 800 to 1000 ml and then no less than 250 ml every 10 to 15 min until finished. 2 L of PEG solution contains 120 g PEG 4000. All subjects tolerated the diarrhea caused by PEG. Group 2 took 600 mg of simethicone emulsion 20 min before capsule ingestion. The capsule was taken with 200 ml of water in both groups.
Scoring system
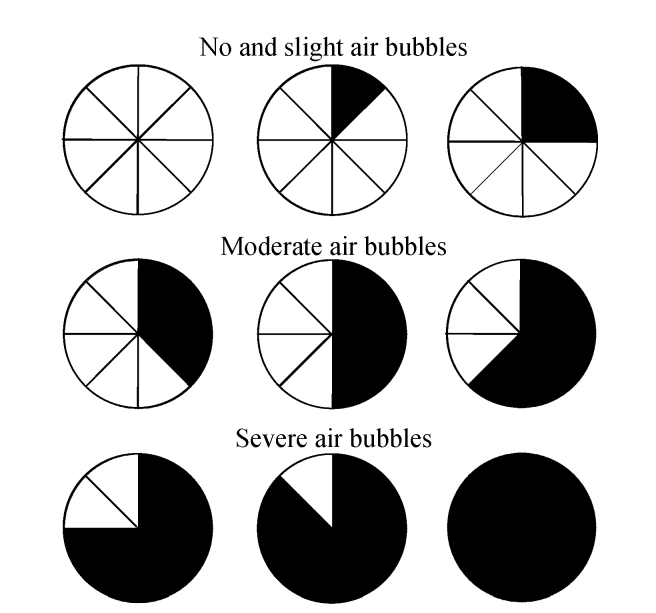
The visualization of the small intestinal lumen was equally divided into 8 parts; bubbles were measured using a 3-point scale—no and slight air bubbles: no bubbles and ≤25% bubbles; moderate air bubbles: 25%~50% bubbles; and severe air bubbles: ≥50% bubbles (Figs.1 and 2). We divided the entire small bowel examination into the following three segements based on transit time: Segment A began immediately after passage of the capsule through the pylorus; Segment B started 1 h before passage through the ileocecal valve, about 1 h in length for each segment; and Segment C was between Segments A and B. The frames from Segments A and B of each participant were scored individually by two observers. The frames for the first 2 min of every 5-min period were scored (e.g., from the 13 min after the capsule entered the small intestine, the frames of the following 2 min, i.e., the 13 and 14 min, will be observed and scored) and were observed at a speed of 40 frames/min. The observers were blinded to the group to which each participant was assigned. The degrees of visualization affected by air bubbles in the small intestinal lumen were scored. In addition, gastric and small bowel transit time was recorded.
Fig. 1.
The visual analogue scale for determining the amount of bubbles seen in the lumen
Luminal bubbles were scored using a 3-point scale: no and slight air bubbles (no bubbles and ≤25% bubbles), moderate air bubbles (25%~50% bubbles), severe air bubbles (≥50% bubbles)
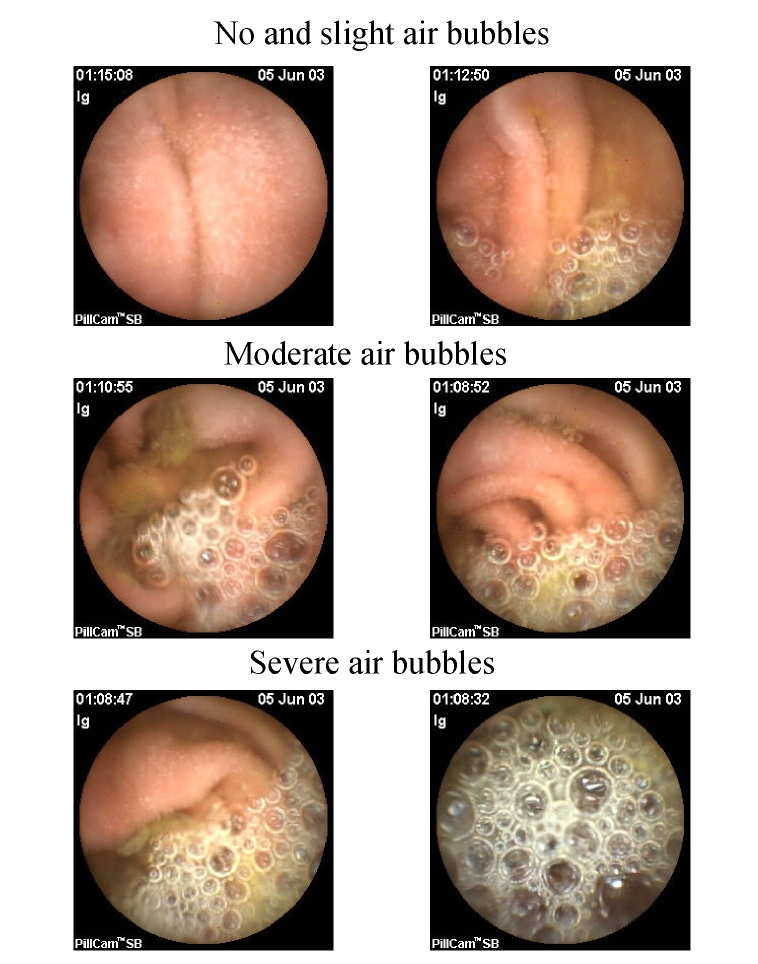
Fig. 2.
Grade of air bubbles in small bowel: no and slight air bubbles (no bubbles and ≤25% bubbles), moderate air bubbles (25%~50% bubbles), severe air bubbles (≥50% bubbles)
Data analysis
Values were expressed as mean±SD. Independent-samples t test was performed to compare the effect of simethicone on the capsule transit time and the time proportion of the luminal air bubbles. A two-sided P value of <0.05 was considered statistically significant. All statistical computations were performed using SPSS 13.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
All the subjects swallowed the capsules without any difficulty, and all the capsules reached the cecum within their functional time. The mean gastric emptying time was similar among participants in the two groups [gastric emptying time, (32.08±5.11) min in Group 2 vs (30.88±5.08) min in Group 1, P=0.868]. Group 2 showed an increased small bowel transit time [(281.84±12.91) min] compared with Group 1 [(227.28±12.27) min, P=0.003] (Table 1).
Table 1.
Effect of preparation on gastric emptying time and small bowel transit time
| Group | Gastric emptying time (min) | Small bowel transit time (min) |
| 1 (n=32) | 30.88±5.08 | 227.28±12.27 |
| 2 (n=32) | 32.08±5.11 | 281.84±12.91 |
| P | 0.868 (NS) | 0.003 |
Group 1 prepared with PEG without simethicone; Group 2 prepared with PEG and simethicone; NS: non-significant
For all participants, the administration of simethicone reduced luminal air bubbles in bowel Segments A and B. The proportions of time with no bubbles and slight bubbles in Segments A and B were 67.2% and 68.8%, respectively, in Group 1, and 97.1% and 99.0%, respectively, in Group 2. The proportions of time with moderate bubbles in Segments A and B were 20.1% and 14.6%, respectively, in Group 1, and 2.9% and 1.0%, respectively, in Group 2. The proportions of time with severe bubbles in Segments A and B were 12.8% and 16.7%, respectively, in Group 1, and no severe bubbles were seen in either Segment A or B of Group 2 (Table 2). No side effects related to the administration of simethicone were noted in any subjects.
Table 2.
Effect of preparation on intraluminal gas bubbles
| Group | Proportion of time (%) |
|||||
| Segment A |
Segment B |
|||||
| No and slight | Moderate | Severe | No and slight | Moderate | Severe | |
| 1 (n=32) | 67.2±25.7 | 20.1±19.4 | 12.8±11.6 | 68.8±28.8 | 14.6±17.1 | 16.7±20.4 |
| 2 (n=32) | 97.1±5.8 | 2.9±5.8 | 0.0±0.0 | 99.0±3.5 | 1.0±3.5 | 0.0±0.0 |
| P | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
DISCUSSION
Capsule endoscopy is a convenient method for examining the small intestinal mucosa, especially obscure intestinal bleeding. It is more effective than other imaging modalities, such as computed tomography (CT), plain film, or magnetic resonance imaging (MRI) for discovering intestinal bleeding lesions. Preparation of the small bowel can decrease the blind spots of capsule endoscopy caused by bubbles and debris. Bubbles appear especially in the distal and proximal segments of the small intestine. Studies have suggested that PEG distends the intestinal lumen and simethicone reduces air bubbles. Simethicone is said to decrease bubbles that form particularly from the detergent activity of bile salts and has been shown to be effective in controlled trials (Albert et al., 2004; Ge et al., 2006).
Simethicone is a detergent substance which can reduces the surface tension of air bubbles, thereby leading to their disruption. The substance is used to treat patients with symptoms caused by excess gas in the intestinal tract. Now, it is also used to improve the yield of capsule endoscopy. There are no known drug interactions, and side effects are rare except for allergic reactions in those who have atopy or asthma. In the present study, we did not observe any evidence of significant side effects for simethicone to be used in capsule endoscopy.
Bubbles and debris in the distal segment of small bowel had been considered of using PEG-contained solutions (Ben-Soussan et al., 2005; Dai et al., 2005). PEG-contained solutions are nonabsorbable but osmotically active substances, which are widely used in colonic cleansing for colonoscopy. Reasons for its use in capsule endoscopy include its ability to move through the bowel and potentially distend the lumen, wash out debris and bile, and possibly enhance small bowel transit time (Hammer et al., 1989).
To our knowledge, bowel preparation for capsule endoscopy in most published studies was not mentioned or only consisted of a 12-h fast before ingestion of the capsule (Costamagna et al., 2002; Ell et al., 2002; Hahne et al., 2002; Fireman et al., 2003; Liangpunsakul et al., 2003). The Given Imaging Ltd. recommendation was a 12-h fast prior to capsule endoscopy with no other preparation. The air bubbles and debris in the lumen significantly limit the field of visualization. Bowel preparation with PEG prior to capsule endoscopy reduced bowel debris. However, PEG could not effectively reduce air bubbles. Simethicone has been used as preparation for capsule endoscopy in a few studies and demonstrated a significant reduction of gas bubbles in the intestinal lumen compared with controls.
Dai et al.(2005) found that simethicone not only enhanced the visibility of capsule endoscopy but also shortened the procedure time and led to a higher rate of complete capsule endoscopy. In contrast, Wei et al.(2008) showed that simethicone shortened neither gastric emptying time nor small bowel transit time. In our study, the administration of simethicone did increase transit time through the small bowel, but did not demonstrate changes in gastric emptying time. The intestinal transit time in the simethicone group increased greatly compared with the non-simethicone group [281.84 min vs 227.28 min (P=0.003)].
Gastric emptying and small bowel transit time depend upon a number of factors, such as the composition of food and fluid in the gastrointestinal tract, gastrointestinal diseases, etc. In addition, transit time differs depending on whether the individual was in a postprandial or fasting state. Initial capsule endoscopy experiences showed that the cecum could not be viewed in approximately 20% of a study population, due mainly to the stasis of the capsule in the stomach for more than 60 min (Fireman et al., 2003; Mylonaki et al., 2003).
In initial studies, bowel preparation with PEG-contained solutions and other colonic cleaning substances indicated decreased bowel transit time. Other studies suggested that 2 L of PEG did not have any effect on either the quality of capsule images or its diagnostic performance. Moreover, studies showed that PEG tended to increase gastric emptying time and, therefore, may constitute a limitation for small bowel complete examination (Ben-Soussan et al., 2005). In a study by Fireman et al.(2003), 3 L of PEG (1.5 L 12 h before capsule swallowing, and another 1.5 L 1 h after capsule swallowing) significantly shortened the gastric emptying time and small bowel transit time. Dai et al.(2005) reported that the administration of 3 L of PEG solution the day before and 1 L 3 h before swallowing the capsule shortened the small intestinal transit time but not the gastric emptying time. van Tuyl et al.(2007) showed that 1 L or 2 L of PEG the night before capsule endoscopy reduced both gastric emptying time and small bowel transit time, which, however, was statistically insignificant.
According to these studies, the effects of PEG on gastric emptying time and small bowel transit time were dependent on the volume of PEG and the time the PEG was administered. PEG administered before capsule endoscopy was intended to clean the small bowel, while administration after the swallowing of the capsule was intended to distend the small bowel and wash away debris. In our study, we administered 2 L of PEG solution the night before capsule endoscopy. The small intestinal transit time and gastric emptying time were 227.28 and 30.88 min, respectively, for the volunteers who ingested only PEG solution, compared with 281.84 and 32.08 min in those who took both PEG and simethicone. The small bowel transit time in both groups was much longer than that in other studies conducted in Western countries, which may be attributable to physiological differences between Western and Asian individuals. However, since the volume of PEG solution and the time when the solution was taken were arbitrarily discrepant in published studies, further work should be done to reach the goal of improved visualization with minimal adverse effects.
Our results indicated that gas bubbles in the small bowel were significantly decreased both in the proximal and distal small intestine with the addition of 600 mg simethicone compared with controls. Several randomized studies of bowel preparation for colonoscopy had found that administration of simethicone with colonic cleaning substances can improve image quality. Kark et al.(1995) found that simethicone enhanced visibility, shortened procedure time, and produced no adverse effects. In a few studies, simethicone was administered as preparation for capsule endoscopy, and the results indicate that simethicone could significantly reduce luminal bubbles in the small bowel and shorten the small bowel transit time without producing any adverse effects (Dai et al., 2005; Ge et al., 2006). Albert et al.(2004) used 80 mg of simethicone without any other preparation and Ge et al.(2006) used 200 mg simethicone; they both found the improvement of the visualization of capsule endoscopy compared with the control groups. In the present study, we used 600 mg simethicone and demonstrated that the time proportion with no bubbles was significantly reduced both in the proximal and distal small bowel in the simethicone group compared with the non-simethicone group, which was similar to Wei et al.(2008)’s results.
In previous studies, the grading systems used were admittedly subjective. They were dependent on the examiner and were semiquantitative. Due to the large volume of images generated from each patient, evaluation of all the frames proves difficult and time consuming. Thus far, there is no precise quantitative evaluation of visibility. In our study, we divided the field of visualization into 8 equal parts and recorded the proportions of time with no bubbles, bubbles less than 25%, between 25% and 50%, and more than 50%. Moreover, in our study, relatively healthy participants without gastric or intestinal surgery, clinical or suspected abnormalities in gastric emptying, or diabetic gastroparesis were included in order to reduce the effects on gastric emptying and small bowel transit time caused by gastrointestinal diseases. Thus, our results may better reflect the real effect of simethicone on gastric emptying time and small bowel transit time.
In conclusion, we found that bowel preparation with both PEG and simethicone significantly reduced bubbles in the intestinal lumen and improved the visualization of the small bowel by capsule endoscopy without any side effects observed. Additional study is needed to further identify the effects of simethicone, such as the visibility effect of different doses, in capsule endoscope preparation.
Footnotes
Project (No. 20070230) supported by the Department of Education of Zhejiang Province, China
References
- 1.Albert J, Göbel CM, Lesske J, Lotterer E, Nietsch H, Fleig WE. Simethicone for small bowel preparation for capsule endoscopy: a systematic, single-blinded, controlled study. Gastrointestinal Endoscopy. 2004;59(4):487–491. doi: 10.1016/S0016-5107(04)00003-3. [DOI] [PubMed] [Google Scholar]
- 2.Ben-Soussan E, Savoye G, Antonietti M, Ramirez S, Ducrotté P, Lerebours E. Is a 2-liter PEG preparation useful before capsule endoscopy? Journal of Clinical Gastroenterology. 2005;39(5):381–384. doi: 10.1097/01.mcg.0000159271.43233.45. [DOI] [PubMed] [Google Scholar]
- 3.Costamagna G, Shah SK, Riccioni ME, Foschia F, Mutignani M, Perri V, Vecchioli A, Brizi MG, Picciocchi A, Marano P. A prospective trial comparing small bowel radiographs and video capsule endoscopy for suspected small bowel disease. Gastroenterology. 2002;123(4):999–1005. doi: 10.1053/gast.2002.35988. [DOI] [PubMed] [Google Scholar]
- 4.Dai N, Gubler C, Hengstler P, Meyenberger C, Bauerfeind P. Improved capsule endoscopy after bowel preparation. Gastrointestinal Endoscopy. 2005;61(1):28–31. doi: 10.1016/S0016-5107(04)02444-7. [DOI] [PubMed] [Google Scholar]
- 5.Ell C, Remke S, May A, Helou L, Henrich R, Mayer G. The first prospective controlled trial comparing wireless capsule endoscopy with push enteroscopy in chronic gastrointestinal bleeding. Endoscopy. 2002;34(9):685–689. doi: 10.1055/s-2002-33446. [DOI] [PubMed] [Google Scholar]
- 6.Fang YH, Zhang BL, Wu JG, Chen CX. A primary intestinal lymphangiectasia patient diagnosed by capsule endoscopy and confirmed at surgery: a case report. World Journal of Gastroenterology. 2007;13(15):2263. doi: 10.3748/wjg.v13.i15.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fireman Z, Mahajna E, Broide E, Shapiro M, Fich L, Sternberg A, Kopelman Y, Scapa E. Diagnosing small bowel Crohn’s disease with wireless capsule endoscopy. British Society of Gastroenterology. 2003;52:390–392. doi: 10.1136/gut.52.3.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ge ZZ, Chen HY, Gao YJ, Hu YB, Xiao SD. The role of simeticone in small-bowel preparation for capsule endoscopy. Endoscopy. 2006;38(8):836–840. doi: 10.1055/s-2006-944634. [DOI] [PubMed] [Google Scholar]
- 9.Ginsberg GG, Barkun AN, Bosco JJ, Isenberg GA, Nguyen CC, Petersen BT, Silverman WB, Slivka A, Taitelbaum G. Wireless capsule endoscopy. Gastrointestinal Endoscopy. 2002;56(5):621–624. doi: 10.1016/S0016-5107(02)70106-5. [DOI] [PubMed] [Google Scholar]
- 10.Hahne M, Adamek HE, Schilling D, Riemann JF. Wireless capsule endoscopy in a patient with obscure occult bleeding. Endoscopy. 2002;34(7):588–590. doi: 10.1055/s-2002-33209. [DOI] [PubMed] [Google Scholar]
- 11.Hammer HF, Santa Ana CA, Schiller LR, Fordtran JS. Studies of osmotic diarrhea induced in normal subjects by ingestion of polyethylene glycol and lactulose. Journal of Clinical Investigation. 1989;84(4):1056–1062. doi: 10.1172/JCI114267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iddan G, Meron G, Glukhovsky A, Swain P. Wireless capsule endoscopy. Nature. 2000;405(6785):417. doi: 10.1038/35013140. [DOI] [PubMed] [Google Scholar]
- 13.Kark W, Krebs-Richter H, Hotz J. Improving the effect of orthograde colonic lavage with golytely solution by adding dimethicone. Zeitschrift Gastroenterologie. 1995;33(1):20–23. [PubMed] [Google Scholar]
- 14.Keuchel M, Hagenmuller F. Small bowel endoscopy. Endoscopy. 2005;37(2):122–132. doi: 10.1055/s-2004-826155. [DOI] [PubMed] [Google Scholar]
- 15.Liangpunsakul S, Chadalawada V, Rex DK, Maglinte D, Lappas J. Wireless capsule endoscopy detects small bowel ulcers in patients with normal results from state of the art enteroclysis. American Journal of Gastroenterology. 2003;98(6):1295–1298. doi: 10.1111/j.1572-0241.2003.07471.x. [DOI] [PubMed] [Google Scholar]
- 16.Mylonaki M, Fritscher-Ravens A, Swain P. Wireless capsule endoscopy: a comparison with push enteroscopy in patients with gastroscopy and colonoscopy negative gastrointestinal bleeding. British Society of Gastroenterology. 2003;52:1122–1126. doi: 10.1136/gut.52.8.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shiotani A, Opekun AR, Graham DY. Visualization of the small intestine using capsule endoscopy in healthy subjects. Digestive Diseases and Sciences. 2007;52(4):1019–1025. doi: 10.1007/s10620-006-9558-6. [DOI] [PubMed] [Google Scholar]
- 18.van Tuyl SA, den Ouden H, Stolk MF, Kuipers EJ. Optimal preparation for video capsule endoscopy: a prospective, randomized, single-blind study. Endoscopy. 2007;39(12):1037–1040. doi: 10.1055/s-2007-966988. [DOI] [PubMed] [Google Scholar]
- 19.Viazis N, Sgouros S, Papaxoinis K, Vlachogiannakos J, Bergele C, Sklavos P, Panani A, Avgerinos A. Bowel preparation increases the diagnostic yield of capsule endoscopy: a prospective, randomized, controlled study. Gastrointestinal Endoscopy. 2004;60(4):534–538. doi: 10.1016/S0016-5107(04)01879-6. [DOI] [PubMed] [Google Scholar]
- 20.Wei W, Ge ZZ, Lu H, Gao YJ, Hu YB, Xiao SD. Purgative bowel cleansing combined with simethicone improves capsule endoscopy imaging. The American Journal of Gastroenterology. 2008;103(1):77–82. doi: 10.1111/j.1572-0241.2007.01633.x. [DOI] [PubMed] [Google Scholar]