Abstract
Objective: To investigate cutaneous aging patterns of residents in Hangzhou, Zhejiang, China, and their contributing factors. Methods: Eight hundred and forty-eight Hangzhou residents received the survey between March 2004 and September 2004. Results: Facial wrinkling first occurred at 21 years of age and skin elasticity began to lose at 22 years of age. In middle-aged and old people, facial wrinkling and looseness escalated with the increase of ultraviolet (UV)-exposure time, indicating the accelerating effect of a higher accumulative dose of UV radiation on skin aging. Only Fitzpatrick types II, III and IV were found in the skin phototypes of residents in Hangzhou area, and Fitzpatrick type II seemed to be much more subject to severe wrinkling, elasticity destruction and skin tumors than types III and IV. The oily skin was more protected against wrinkling and facial looseness than dry skin. However, as to concomitant cutaneous diseases, no difference was found among different skin types. Conclusion: Age, solar-exposure time, Fitzpatrick type and skin type are the associated forces in promoting skin aging, and emotional factor seems to be another independent risk factor. The age of 49 years and 2 h/d of solar-exposure time seem to be the turning points responsible for dramatic changes of cutaneous appearance in the process of skin aging in Southeast China.
Keywords: Skin aging, Influential factor, Fitzpatrick type, Solar exposure, Skin type, Concomitant facial disease
INTRODUCTION
The patterns of skin aging are characterized by ethnic specificity among different peoples (Chung et al., 2001; Rijken et al., 2004); for example, pigmentation and wrinkling patterns in yellow skin share little common features with those in white skin (Roh et al., 2001). Nouveau-Richard et al.(2005) reported main skin aging features progress differently in Chinese and French women, and Zhao et al.(1998) investigated 470 healthy people in Northeast China to explore skin damage resulting from solar ultraviolet (UV) radiation. However, to our knowledge, the aging status and influential factors in the skins of Chinese people are still poorly understood. We, therefore, conducted a cross-sectional survey to investigate skin aging patterns and their attributing factors on residents in Hangzhou, a southeast city in China, using questionnaires and clinical assessments conducted by physician researchers.
SUBJECTS AND METHODS
Subjects
Advertisements were distributed in hospitals, supermarkets and other public places, and a preliminary interview was conducted to recruit the subjects. The interview included information such as age and vocation, medical history, mental condition, life style, and their usual skin reaction to sun exposure, both acute and prolonged. Eight hundred and forty-eight inhabitants (504 males and 344 females, mean age 35.8 years) in Hangzhou area without systemic disorders history were recruited in the survey after giving informed consent. They were divided into three age groups: young group (324, age range 18~29 years), middle-aged group (384, age range 30~49 years), and old group (140, age range 50~81 years). The effect of solar-exposure on facial aging status is one of our main concerns, so we further divided the subjects into 3 subgroups according to their solar-exposure time: 0~2, 2~4, and >4 h/d. Meanwhile, we also wanted to know the effect of different phototype or Fitzpatrick (1988) classification on facial aging process due to their different reactions to solar radiation, so the subjects in different age groups were further sub-grouped according to Fitzpatrick type. Finally, to observe the relationship between aging status and skin type, subjects in different age groups were also divided into three subgroups according to skin types: oily, dry, and normal/combinative types. The skin type was determined by facial appearance and “skin test”. To perform the skin test, a few pieces of lens-cleaning tissue paper were pressed on different spots on the face. The skin was determined as “oily” if the paper picked up oily spots and became translucent, and the skin looked shiny with enlarged pores. If the paper did not pick up any oily spots, and the skin showed signs of dehydration and dryness, the skin would be determined as “dry”. If the paper was only stuck in the T-zone (forehead, nose and chin) and the skin had medium-sized pores and even texture on the cheeks, then the skin was defined as combinative or normal skin.
Investigation method
The evaluation of the facial aging status of subjects was based on questionnaire and clinical assessments. The questionnaire contained items such as age, gender, occupation, education background, lifestyle, diet habits, solar-exposure reaction and outdoor time. The clinical assessments covered facial wrinkling, Fitzpatrick type, looseness status, and concomitant facial disorders (including pigmented nevus, chloasma, freckles, solar lentigo, solar keratosis, seborrheic keratosis, and skin tumors associated with solar-exposure such as Bowen’s disease, basal cell carcinoma, squamous cell carcinoma and malignant melanoma). The interviewees were assessed by three physician researchers, and the predominant opinion was chosen as a final assessment.
The assessment on facial wrinkling was classified as below: no wrinkling—no visible line around the eyes and forehead (Fig.1a); fine wrinkling—thin lines around the eyes and forehead and getting obvious upon facial expressions (Fig.1b); moderate wrinkling—visible lines around the eyes and forehead and becoming deeper upon facial expressions (Fig.1c); severe wrinkling—deep and thick lines around the eyes and forehead even without facial expressions (Fig.1d).
Fig. 1.
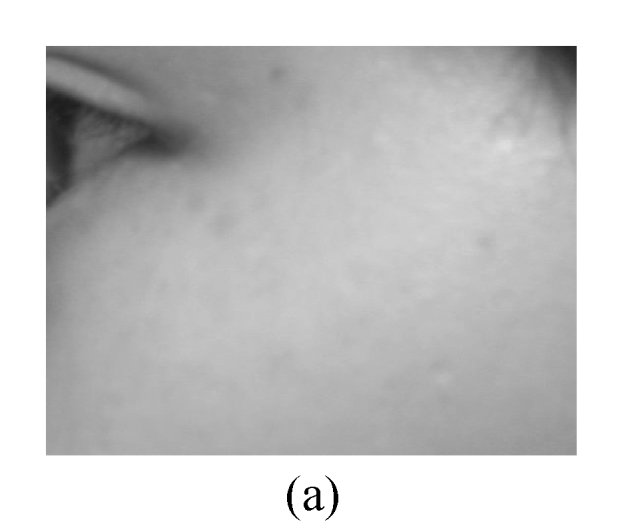
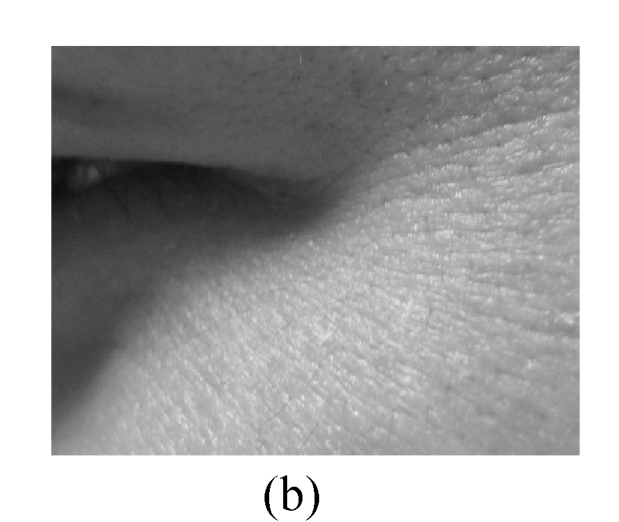
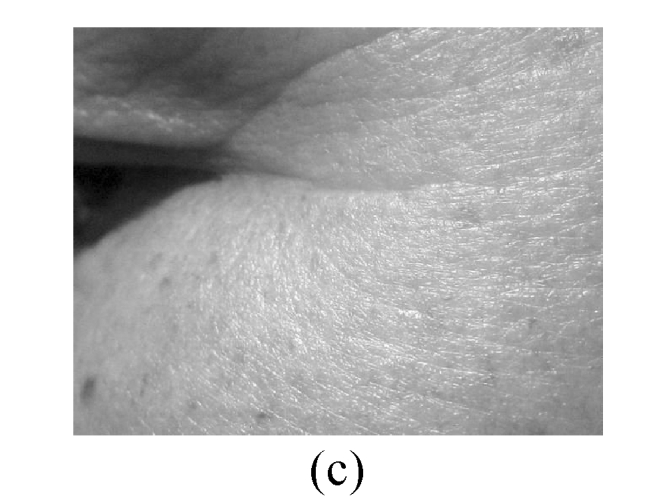
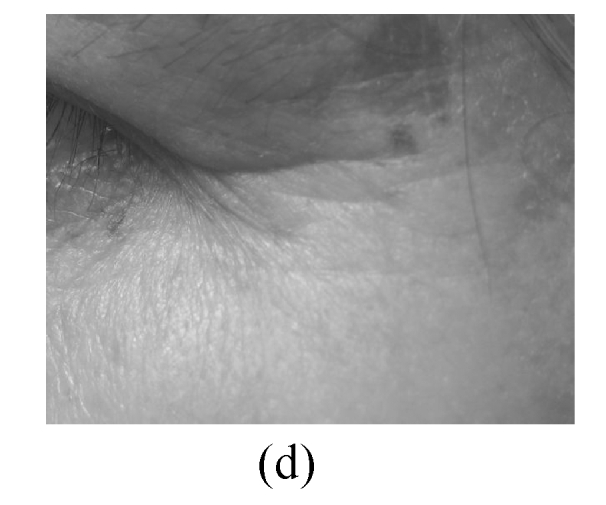
Facial wrinkling assessment
(a) No wrinkling; (b) Fine wrinkling; (c) Moderate wrinkling; (d) Severe wrinkling
The skin elasticity status was classified as: excellent—the skin is glazed and flat in appearance (Fig.2a); good—the skin looks a little slack (Fig.2b); not-good—the skin looks obviously slack (Fig.2c); bad—the skin looks extremely slack (Fig.2d).
Fig. 2.
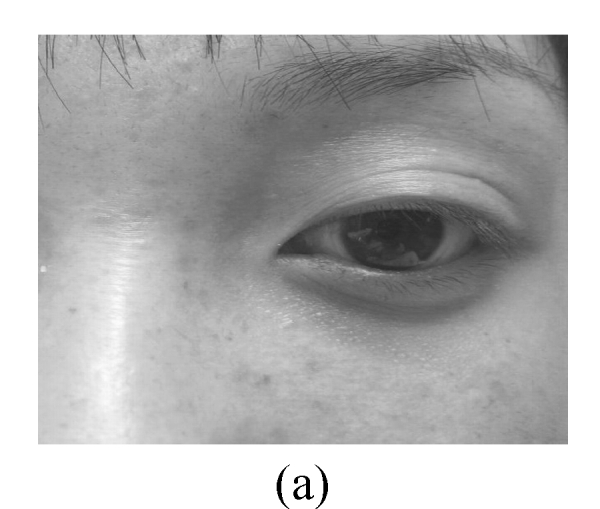

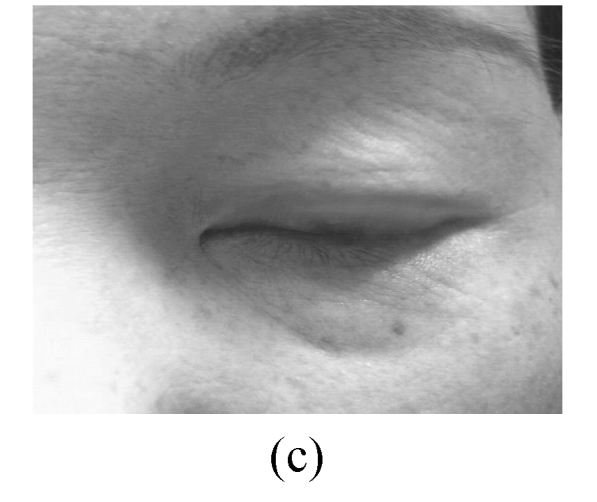
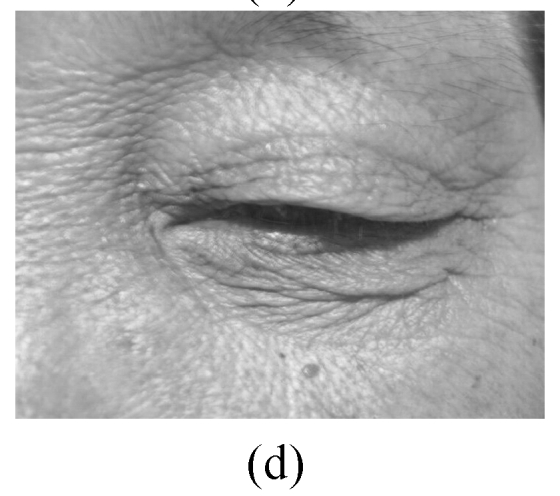
Facial elasticity assessment
(a) Excellent; (b) Good; (c) Not-good; (d) Bad
Statistical analysis
Differences in prevalence were tested using the χ 2 test, and P<0.05 was considered to be statistically significant.
RESULTS
Subjects information
In the 848 volunteers, oily skin type was found to be 38.6%, 32.0%, and 20.7% in the young, middle-aged, and old groups, respectively, while dry skin type was 30.8%, 34.9%, and 53.6%, respectively. Outdoor time in average was 2.49 h/d in all subjects, and the distribution of different solar-exposure time in different age groups is detailed in Table 1. As to Fitzpatrick type, types III and IV were dominant in all the age groups, and no type I, V, or VI was found. The constitutions of skin Fitzpatrick type were similar among different age groups (Table 1), suggesting that the influence of age on skin phototype was excluded (P>0.05).
Table 1.
Distribution of skin type, solar-exposure time and Fitzpatrick type in different age groups of the 848 adults from Hangzhou City, China
| Age group | Distribution (%) |
||||||||
| Solar-exposure time |
Fitzpatrick type |
Skin type |
|||||||
| 0~2 h/d | 2~4 h/d | >4 h/d | II | III | IV | Oily | Dry | N/C | |
| Young (n=324) | 37.00 | 32.10 | 30.90 | 10.20 | 58.00 | 31.80 | 38.60 | 30.80 | 30.50 |
| Middle-aged (n=384) | 39.10 | 33.90 | 27.10 | 12.80 | 57.50 | 29.70 | 32.00 | 34.90 | 33.10 |
| Old (n=140) | 35.70 | 35.70 | 28.60 | 9.30 | 54.30 | 36.40 | 20.70 | 53.60 | 25.70 |
| Total (n=848) | 37.80 | 33.50 | 28.80 | 11.20 | 57.20 | 31.60 | 32.70 | 36.40 | 30.90 |
N/C: normal/combinative; χ 2 test was performed for comparison with the middle-aged group, P>0.05
Assessment on facial wrinkling
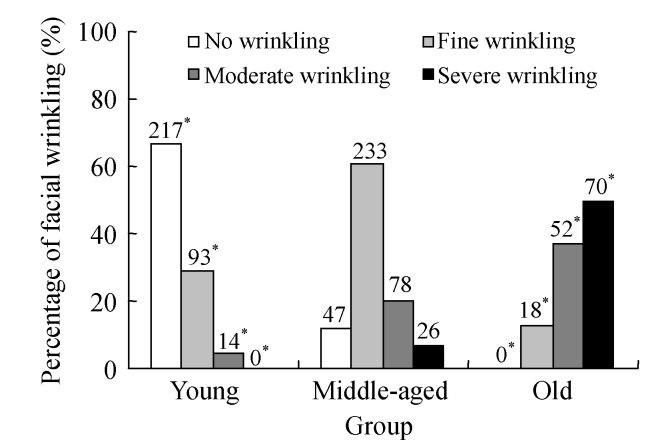
Skin wrinkling was found to be 33%, 87.8%, and 100% in the young, middle-aged, and old groups, respectively. The youngest age for fine wrinkling to occur was 21 years, and no severe wrinkling was found in the young group. Both the prevalence of skin wrinkling and wrinkling severity increased with the age (P<0.01), suggesting that age is one of the most important factors associated with both the occurrence and the extent of facial wrinkling. In the young group, 14 subjects complained of stress and pressure from routine life and 6 of them (42.9%) had moderate wrinkling, indicating that emotional factor needs to be taken into account for the occurrence of skin wrinkling. The assessment on facial wrinkling in different age groups is shown in Fig.3.
Fig. 3.
Percentages of no wrinkling, fine wrinkling, moderate wrinkling and severe wrinkling in different age groups of the 848 adults from Hangzhou City, China
χ 2 test was performed for comparison with the middle-aged group, * P<0.05
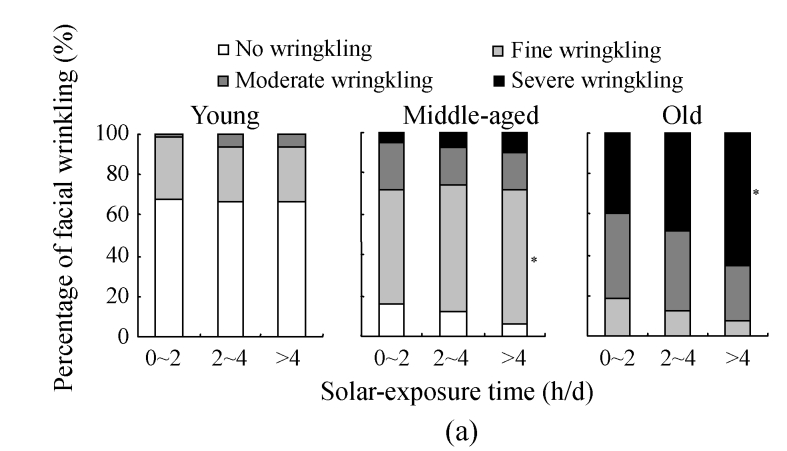
The assessment on skin wrinkling among solar-exposure subgroups of different age ranges is further detailed in Fig.4a. In the young group, although the constitution of wrinkling severity varied among solar-exposure groups, no statistical difference was found, indicating that the effect of solar exposure on the occurrence and development of skin wrinkling is marginal in the young group. In the middle-aged group, however, a marked reduction was found in the prevalence for no wrinkling in the >4 h/d solar-exposure subgroup when compared with that in the 0~2 h/d subgroup (P<0.05), suggesting that a higher accumulative dose of UV radiation resulting from chronic solar-exposure might accelerate the development of skin wrinkling. In the old group, the prevalence of skin wrinkling reached 100%, of which 50% was assessed as severe wrinkling. The percentage of severe wrinkling in the >4 h/d solar-exposure subgroup increased significantly when compared with that in the 0~2 h/d solar-exposure subgroup (P<0.05), indicating that UV-exposure of longer than 4 h/d is a driving force in both the appearance and the severity of skin wrinkling.
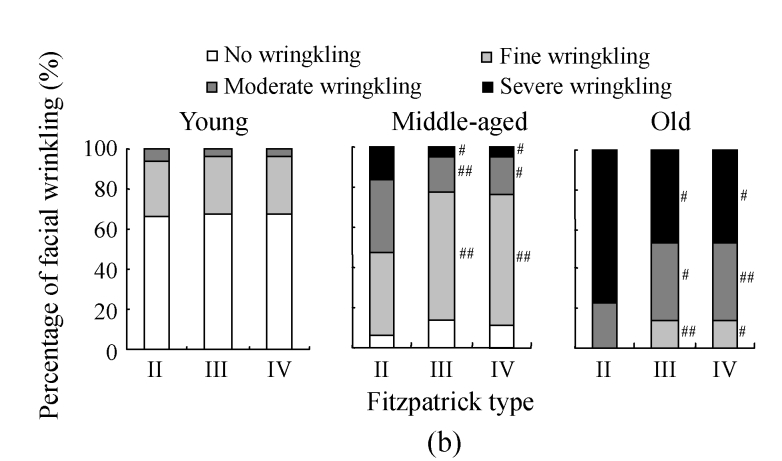
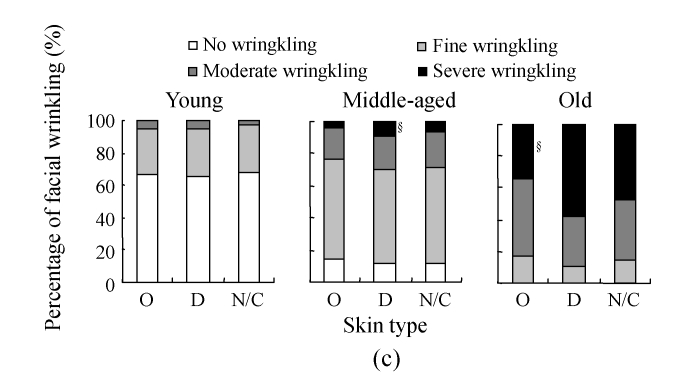
Fig. 4.
The results of facial wrinkling assessment in different solar-exposure time subgroups (a), Fitzpatrick type subgroups (b), and skin type subgroups (O: oily; D: dry; N/C: normal/combinative) (c) of young, middle-aged and old participators
χ 2 test was performed for comparison with the 0~2 h/d subgroup, * P<0.05, ** P<0.01; comparison with the Fitzpatrick type II subgroup, # P<0.05, ## P<0.01; comparison with the dry subgroup, § P<0.05, §§ P<0.01
The assessment on skin wrinkling among Fitzpatrick groups is shown in Fig.4b. In the young group, no difference in skin wrinkling constitution was found, suggesting that the Fitzpatrick type had no effect on the occurrence and the severity of skin wrinkling in young ages. However, in the middle-aged group, fine wrinkling was 40.8% in the Fitzpatrick type II subgroup, significantly lower than that in the Fitzpatrick type III subgroup (62.9%, P<0.01), while the occurrences of both moderate wrinkling and severe wrinkling were much higher in the Fitzpatrick type II subgroup than those in the Fitzpatrick type III subgroup (P<0.01 and P<0.05, respectively). Significant differences were also found between the Fitzpatrick types II and IV subgroups for fine, moderate, and severe wrinkling in the middle-aged group, but no significant difference for the occurrence of skin wrinkling between the Fitzpatrick types III and IV subgroups was observed. In the old group, the prevalence for no wrinkling and fine wrinkling in the Fitzpatrick type II subgroup decreased to 0%. The percentage of moderate wrinkling also decreased to 23.1%, significantly lower than those in the Fitzpatrick type III (38.2%) and Fitzpatrick type IV (39.2%) subgroups (P<0.05 and P<0.01, respectively). Generally, severe wrinkling was found in all phototypes in the old group, but the percentage of severe wrinkling in the Fitzpatrick type II subgroup (76.9%) was significantly higher than those in the Fitzpatrick types III and IV subgroups (P<0.05). No significant difference was found for the percentage of skin wrinkling between the Fitzpatrick types III and IV subgroups in the old group.
Fig.4c shows the assessment results on skin wrinkling in different skin types. In the young group, the constitution of skin wrinkling in different skin types was similar, but in the middle-aged group, severe wrinkling accounted for only 3.3% in oily skin, significantly lower than that in dry skin (9.7%, P<0.05). And the percentage of severe wrinkling was also significantly lower in oily skin than that in dry skin (P<0.05) among the old people. In all the age groups, no significant difference was found for the percentage of skin wrinkling between oily skin and normal/combinative skin and between dry skin and normal/combinative skin, respectively.
Assessment on skin elasticity
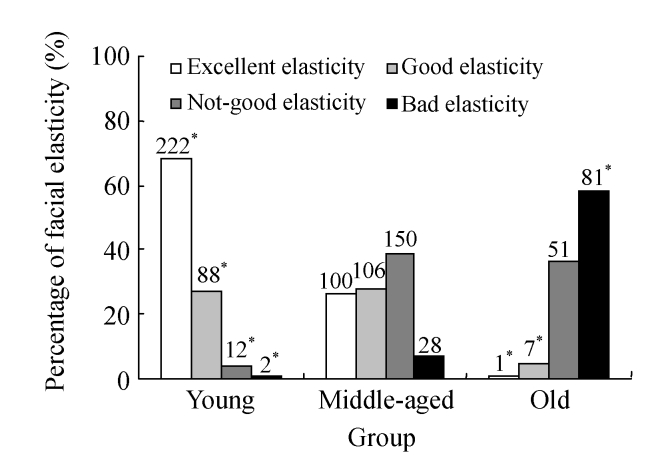
Facial skin elasticity was found excellent in the young group, only 12 subjects considered as not-good and 2 others bad, of which 1 seemed to be a victim from genetic cutis laxa, while the other led an extremely irregular life and was addicted to smoking and drinking. The youngest age for beginning to lose skin elasticity was 22 years old. The rate of excellent skin elasticity dramatically decreased to 26% (100/384) in the middle-aged group and only one subject was defined excellent in the old group. In the old group, 94.3% (132/140) of subjects were suffered from facial skin laxity, significantly higher than those in the young and middle-aged groups (P<0.01), suggesting that age factor affects facial elasticity. Assessment on skin elasticity among age groups is shown in Fig.5.
Fig. 5.
Percentage of excellent elasticity, good elasticity, not-good elasticity, and bad elasticity in different age groups of the 848 adults from Hangzhou City, China
χ 2 test was performed for comparison with the middle-aged group, * P<0.05
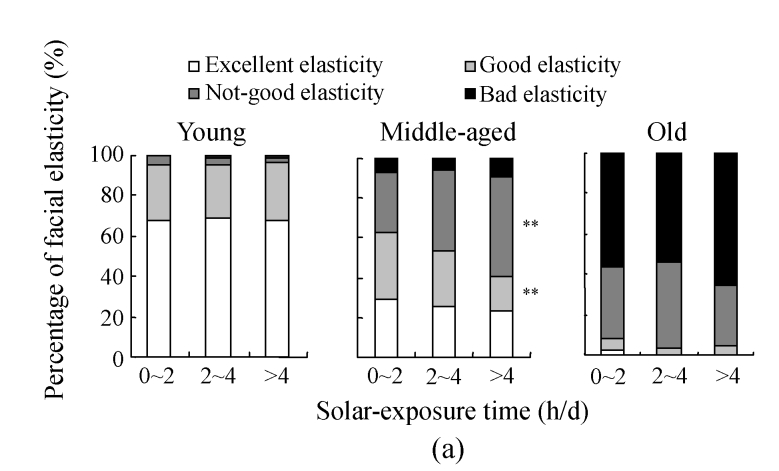
The assessment on skin elasticity in different solar-exposure subgroups of different age ranges is shown in Fig.6a. In the young group, bad elasticity was absent in the 0~2 h/d solar-exposure subgroup, one subject considered as bad elasticity in the 2~4 h/d solar-exposure subgroup and one in the >4 h/d solar-exposure subgroup. No significant differences in the percentages of excellent, good, and not-good elasticity, respectively, were found among the three solar-exposure subgroups. In the middle-aged group, the prevalence for good elasticity in the >4 h/d solar-exposure subgroup decreased significantly when compared with those in the 0~2 h/d and 2~4 h/d subgroups (P<0.05 and P<0.01, respectively), while the percentage of not-good elasticity in the >4 h/d subgroup increased to 50%, notably higher than that in the 0~2 h/d subgroup, suggesting a positive relation between solar-exposure time and skin laxity. In the old group, only one subject was considered as excellent elasticity in the 0~2 h/d subgroup, and no subject was found to be of excellent elasticity in both 2~4 h/d and >4 h/d subgroups. Bad elasticity accounted for the largest part in the old group, of which 56% (28/50) were defined as bad in the 0~2 h/d subgroup, 54% (27/50) in the 2~4 h/d subgroup, and 65% (26/40) in the >4 h/d subgroup. However, no significant differences in the percentages of good, not-good, and bad elasticity, respectively, were found among the three solar-exposure subgroups.
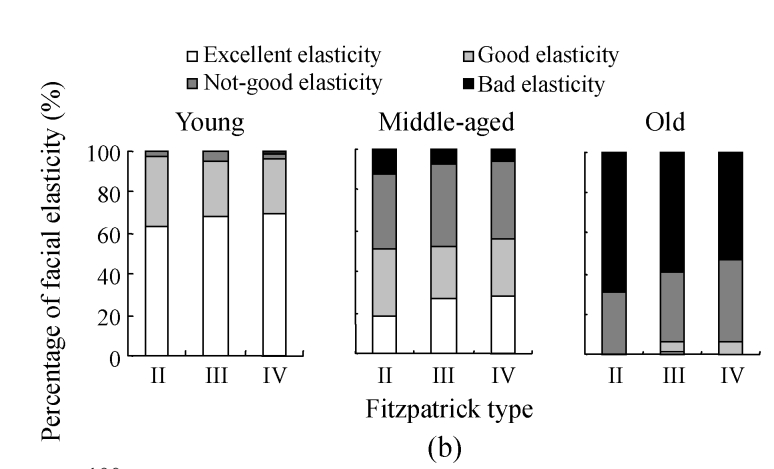
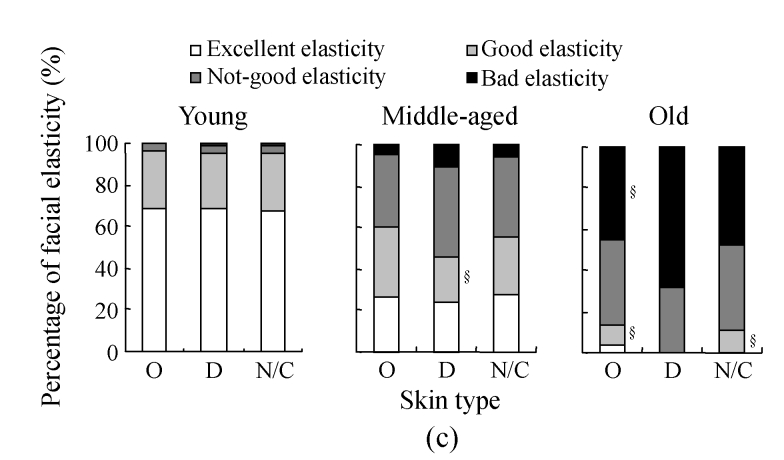
Fig. 6.
The results of facial elasticity assessment in different solar-exposure time subgroups (a), Fitzpatrick type subgroups (b), and skin type subgroups (O: oily; D: dry; N/C: normal/combinative) (c) of young, middle-aged and old participators
χ 2 test was performed for comparison with the 0~2 h/d subgroup, * P<0.05, ** P<0.01; comparison with the Fitzpatrick type II subgroup, # P<0.05, ## P<0.01; comparison with the dry subgroup, § P<0.05, §§ P<0.01
The assessment on skin elasticity among Fitzpatrick types of different age ranges is shown in Fig.6b. In the young group, bad elasticity was absent in the Fitzpatrick type II subgroup, and only one was found in the Fitzpatrick types III and IV subgroups, respectively. The prevalence of excellent elasticity in different Fitzpatrick types ranged from 63.6% (type II) to 70% (type IV), while 26.2% (type IV) to 33.3% (type II) for good elasticity and 2.9% (type IV) to 4.3% (type III) for not-good elasticity. No significant difference of skin elasticity was found among the three subgroups, suggesting that Fitzpatrick type has little effect on facial elasticity in the young group. In the middle-aged group, the prevalence and constitution for skin elasticity in different Fitzpatrick type subgroups were similar, suggesting that Fitzpatrick type has little effect on facial elasticity in the middle-aged group. In the old group, excellent elasticity was absent in all the subgroups except for one subject occurred in the Fitzpatrick type III subgroup, who was a vegetarian. In the Fitzpatrick type II subgroup, observed were no excellent elasticity (0%), not-good elasticity (30.8%, 4/13) and bad elasticity (69.2%, 9/13). Notably, no significant differences in the percentages of good, not-good, and bad elasticity, respectively, were found among solar-exposure subgroups in the old group, supporting that Fitzpatrick type might have limited the association of solar exposure with the alteration of facial elasticity.
The assessment on skin elasticity in different skin types of different age ranges is shown in Fig.6c. In the young group, no bad elasticity was found in the oily skin subgroup, while the dry and the normal/combinative skins subgroups had one subject with bad elasticity each. No significant differences in the percentages of skin elasticity were found among the skin type subgroups, suggesting that skin type is not a factor related to the decrease of facial elasticity in the young people. In the middle-aged group, the prevalence for good elasticity in the dry skin subgroup (21.6%, 29/134) decreased significantly when compared with that in the oily skin subgroup (33.3%, 41/123; P<0.05), while the percentage of bad elasticity in the dry skin subgroup increased to 10.5% (14/134), higher than that in the oily skin subgroup (4.9%, 6/123; P<0.05). These results indicate that facial skin gets loose in appearance more likely in dry skin than in oily skin in the middle ages. In the old group, no excellent elasticity was found in both the dry skin and the normal/combinative skin subgroups, and no good elasticity was found in the dry skin subgroup. In the oily skin subgroup, only one subject with excellent elasticity was observed. Bad elasticity was found 44.8% (13/29) in oily skin and 68.0% (51/75) in dry skin (P<0.05), and good elasticity in dry skin (0%) was much lower than that in oily skin (10.3%, 3/29) (P<0.05), further indicating that dry skin is much more likely to lose facial elasticity.
Assessment on concomitant facial diseases
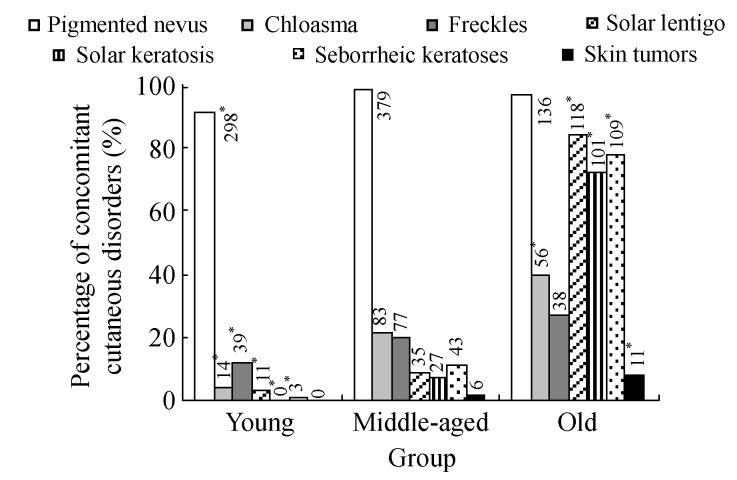
The results of investigation on concomitant facial diseases among the age groups are displayed in Fig.7. Pigmented nevus was the leading disorder in all the age ranges (>90%), and strikingly high prevalence of solar lentigo, solar keratosis and seborrheic keratoses was found in the old group, indicating that the prevalence of chloasma, freckles, solar lentigo, solar keratosis and seborrheic keratosis increased with age. Skin tumors were much more common in the old group, with the prevalence of 7.9%, significantly higher than that in the middle-aged group (1.56%, P<0.01). The youngest subject suffered from skin tumor was a 35-year-old female, and the tumor was pathologically confirmed as malignant melanoma.
Fig. 7.
Prevalence of concomitant cutaneous disorders in different age groups of the 848 adults from Hangzhou City, China
χ 2 test was performed for comparison with the middle-aged group, * P<0.05. The distribution of other concomitant cutaneous disorders in the young, middle-aged, and old groups: Bowen’s disease: 0, 2, 2; basal cell carcinoma: 0, 1, 4; squamous cell carcinoma: 0, 1, 2; malignant melanoma: 0, 2, 3
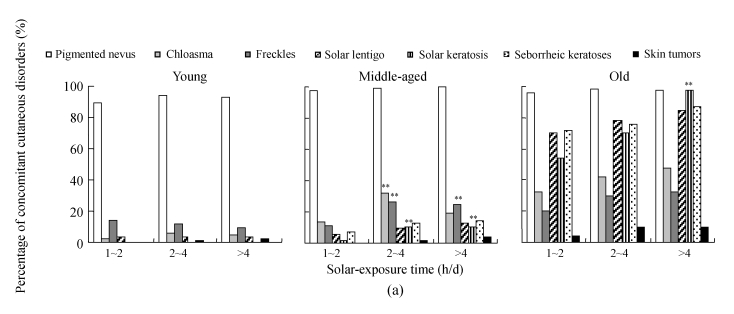
The assessment on concomitant cutaneous disorders among the solar-exposure time subgroups of different age ranges is shown in Fig.8a. In the young group, no solar keratosis or skin tumor was found, no seborrheic keratosis was found in the 0~2 h/d solar-exposure subgroup, and only 1 seborrheic keratosis in the 2~4 h/d subgroup and 2 seborrheic keratoses in the >4 h/d subgroup were observed. The prevalence of pigmented nevus reached 89.2% (107/120) in the 0~2 h/d subgroup, 94.2% (98/104) in the 2~4 h/d subgroup, and 93% (93/100) in the >4 h/d subgroup. The occurrence of freckles or solar lentigo was found without significant difference among the different solar exposure subgroups, suggesting that solar-exposure time might not be the only factor that affects the prevalence of so-called UV-related cutaneous disorders in young people. In the middle-aged group, pigmented nevus was the most prevalent disease, 97.3% (146/150) in the 0~2 h/d subgroup, 99.2% (129/130) in the 2~4 h/d subgroup and 100% (104/104) in the >4 h/d subgroup. Skin tumor was not found in the 0~2 h/d subgroup, while 2 and 4 cases were found in the 2~4 h/d and >4 h/d subgroups, respectively, indicating that the occurrence of skin tumors was positively associated with UV exposure time. Chloasma increased significantly from 14% in the 0~2 h/d subgroup to 32.3% in the 2~4 h/d subgroup (P<0.01), and fell to 19.2% in the >4 h/d subgroup (P<0.05). Freckles and solar keratosis were much more common in the 2~4 h/d and >4 h/d subgroups when compared with those in the 0~2 h/d subgroup (P<0.01), while the occurrence of solar lentigo remained unchanged in the three subgroups. In the old group, the pigmented nevus was found 96% (48/50) in the 0~2 h/d subgroup, 98% (49/50) in the 2~4 h/d subgroup, and 97.5% (39/40) in the >4 h/d subgroup. Solar keratosis was found more common in the >4 h/d subgroup (97.5%, 39/40) than in the 2~4 h/d subgroup (70%, 35/50) and 0~2 h/d subgroup (54%, 27/50).
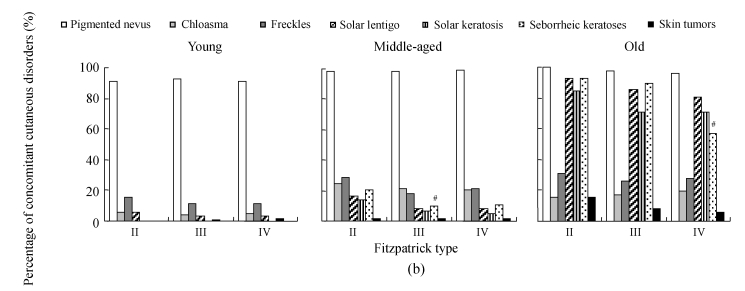
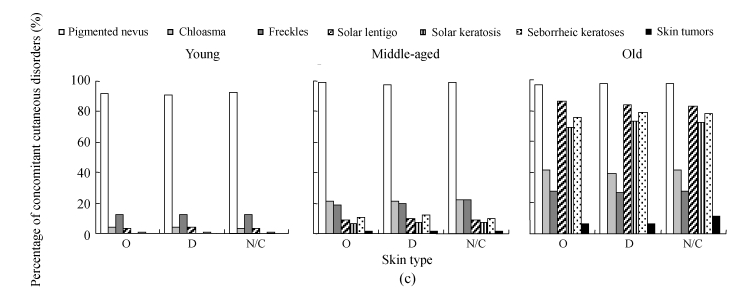
Fig. 8.
The results of investigation on concomitant cutaneous disorders in different solar-exposure time subgroups (a), Fitzpatrick type subgroups (b), and skin type subgroups (O: oily; D: dry; N/C: normal/combinative) (c) of young, middle-aged and old participators
χ 2 test was performed for comparison with the 0~2 h/d subgroup, * P<0.05, ** P<0.01; comparison with the Fitzpatrick type II subgroup, # P<0.05, ## P<0.01; comparison with the dry subgroup, § P<0.05, §§ P<0.01
The assessment on concomitant skin diseases among Fitzpatrick types of different age ranges is shown in Fig.8b. In the young group, seborrheic keratoses were rare and no case of solar keratosis or skin tumor was found. No differences of the occurrences of pigmented nevus, chloasma, freckles, and solar lentigo, respectively, were found in the three different skin types. In the middle-aged group, 6 cases were diagnosed with skin tumors, 1 in the Fitzpatrick type II subgroup, 3 in the Fitzpatrick type III subgroup and 2 in the Fitzpatrick type IV subgroup. Seborrheic keratosis was found 20.4% (10/49) in the Fitzpatrick type II subgroup, higher than 9.5% (21/221) in the Fitzpatrick type III subgroup (P<0.05) and 10.5% (12/114) in the Fitzpatrick type IV subgroup (P<0.05). In the old group, seborrheic keratosis was found 92.3% (45/49) in the Fitzpatrick type II subgroup and 89.5% (198/221) in the Fitzpatrick type III subgroup, higher than 56.9% (65/114) in the Fitzpatrick type IV subgroup (P<0.05 and P<0.01, respectively), indicating that Fitzpatrick skin type affected the occurrence of seborrheic keratoses.
The assessment on concomitant skin diseases among skin types of different age ranges is shown in Fig.8c. In the young group, no solar keratosis or skin tumor was found, and only one case of seborrheic keratosis was found in each skin type, indicating that skin type might not affect the prevalence of skin diseases in young people. All concomitant facial diseases were found without deference among skin types in both the middle-aged and the old groups, suggesting that skin type is not a factor related to skin diseases mentioned above.
DISCUSSION
The symptoms of skin aging process include wrinkling, hyperpigmentation, telangiectasia, enlarged pores and actinic keratoses, and wrinkling and skin elasticity are thought to be the most evident features (El-Domyati et al., 2002; McCullough and Kelly, 2006). Meanwhile, a series of photo-damages caused by chronic UV radiation including texture abnormalities, irregular pigmentation (such as solar keratosis, solar lentigo, and seborrheic keratosis) and even skin tumors, intertwine with the inherent influences and contribute to skin aging in the long run (Rabe et al., 2006; Fisher, 2005; Stern, 2004). However, factors that play important roles in skin aging and the aging patterns in different races or areas are largely unknown. A better understanding of both the intrinsic and extrinsic influences on the skin aging, as well as epidemic information of skin aging, is crucial to taking protective measures. In the present study based on the investigation of 848 subjects from Hangzhou area, we wish to objectively assess the skin aging status of Hangzhou residents, and examine a few influential factors (including solar-exposure time, Fitzpatrick type and skin type) in certain ages.
In this research, facial wrinkling and elasticity damage were rarely found in the young group, and no difference was found among people subjected to different outdoor time, Fitzpatrick type or skin type. Facial wrinkling was first observed at age of 21 years and slight sagging at age of 22 years, indicating that aging changes could occur at an early age. However, in the young group, the effects of outdoor time, Fitzpatrick type and skin type on the aging signs seemed limited. Notably, 6 out of 14 subjects with moderate wrinkling in the young group complained of spiritual stress, and 1 with bad elasticity had strong addictions to smoking and drinking. In accordance with previous reports (Gupta and Gilchrest, 2005; Akiba et al., 1999; Helfrich et al., 2007), the facial aging seems to be accelerated by emotional deterioration and awful life styles in young people. However, although data of the young group suggested that mental conditions or life styles should be taken into account during aging process, we failed to find out the relationship between these factors and facial aging changes in the elder groups (data not shown). This might be due to our tendency in selecting volunteers without systemic disorders (including psychological diseases), or to our insufficient classification and grading in psychological problems and life styles. Thus, to further investigate the effect of mental health or life styles on skin aging, a more professional and detailed epidemiological survey involving mental stress, life styles and related factors needs to be conducted.
Facial wrinkling and sagging became prominent in the middle-aged group, and statistical analysis strongly illustrated that solar-exposure time had a positive relation with the skin aging status. It was observed that solar exposure time was highly significant for increasing signs of skin aging in the middle-aged subjects, but not in the young ones. This could most likely be explained by the fact that the middle-aged group had been exposed to a higher cumulative dose of UV radiation, whereas only a very small number of youth were exposed to commensurable sun radiation. The prevalence of concomitant skin disorders also increased dramatically in the middle-aged group, and most skin disorders such as chloasma, freckles and solar keratosis were much more common in the 2~4 h/d solar-exposure group than in the 0~2 h/d group (P<0.01), suggesting that 2 h/d of solar-exposure time might be a critical threshold for pathogenic UV stimulation in Hangzhou area. Thus, sun avoidance and effective photoprotections should be the fundamental measures for prevention against skin aging and UV-associated skin disorders in Southeast China.
Fitzpatrick types III and IV constituted major parts of Hangzhou people, and only 49 subjects (12.8%) were classified as Fitzpatrick type II in the middle-aged group. Elasticity destruction and concomitant skin disorders were more common in Fitzpatrick type II of the middle-aged subjects, although no significant difference was found among the three subgroups. As to facial wrinkling, however, a significantly higher percent was observed in the Fitzpatrick type II subgroup, while no difference is found between the types III and IV subgroups. We concluded that Fitzpatrick type is another influential factor on skin aging status, though not significant, and that it might be attributed to varied reactions of different skin phototypes to solar exposure (Phan et al., 2006).
In the middle-aged group, although no difference was found for concomitant cutaneous diseases among different skin types, lower percentages of facial wrinkling and laxity were observed in the oily skin subgroup than in the dry skin subgroup (P<0.05). Our data suggest that skin type may be associated with the skin aging to some extent, and the results reveal a relatively delayed tendency of facial aging in the oily skin subgroup than in the dry skin subgroup. This corresponds well to the viewpoint that dry environment often induces epidermal hyperplasia or inflammation and accelerates skin aging process (Tagami, 2008; Makrantonaki and Zouboulis, 2007). The possible mechanism lies in that stratum corneum hydration plays an important role in performing skin functions and keeping the skin surface soft and smooth, and slower formation of neutral lipids and decreased water content in the dry skin are responsible for impaired skin functions and wrinkle, laxity and roughness of skin appearance (Hashizume, 2004). Our data indicated a relationship between dry skin and cutaneous aging, and this may provide implications on skin care application in the prevention of skin aging.
The percentages of severe facial wrinkling and sagging increased remarkably in the old group, and similar influences of solar-exposure time, Fitzpatrick type and skin type on skin aging changes were also observed. It is worth noting that skin tumors accounted for a striking percentage of 7.9% in the old group, strongly indicating a close link between skin aging and skin tumors (especially the malignant tumors). Aging changes contributing to the development of cutaneous tumors have been previously mentioned (Goukassian and Gilchrest, 2004; Pons and Quintanilla, 2006), and Omari et al.(2006) reported that median age at onset of malignant melanoma for female patients in Jordan was 49 years. In this survey, however, the youngest female suffered from malignant melanoma in Hangzhou City was 35 years old, and the median age at diagnosis of malignant melanoma was 46.4 years. It is of special interest for us to learn whether the youngest patient with malignant melanoma had a family cancer history, but she denied family histories of any kind of cancers. Also our data showed the overall prevalence of skin tumors in Hangzhou City was about 2%, which is rather higher when compared with data of Stang et al.(2007). They reported an investigation covering a population of about 75 000 individuals in Germany from July 1998 to June 2003, and showed the case incidence rates of invasive skin cancers, including basal cell carcinoma, squamous cell carcinoma and skin melanoma, were about 0.2%. Considering the different area location and data construction (our skin tumor data contained not only malignant skin tumors but also other skin tumors such as Bowen’s disease), and the fact that over the 12-year period there was an extremely fast increase in the overall number of skin cancer samples (Hoey et al., 2007), the discrepancy in prevalence of skin tumors might be interpreted to a large extent. However, a larger number of samples seem necessary in order to find out the relationship between the unusually early onset of skin tumors and family cancer history, and to reflect the latest incidence rates of skin tumors in China.
A marked reduction of chloasma was observed in the old group than in the middle-aged group, suggesting that the occurrence of chloasma is not merely relevant to age. It is previously implicated that estrogen plays an important role in the pathogenesis of pigmentation and skin aging (Krause, 2006; Hall and Phillips, 2005; Verdier-Sevrain et al., 2006), and our data seems to support this viewpoint.
Overall, our data silhouette the facial aging statues in a moderate number of samples of Hangzhou people and highlight the independent influential factors (solar-exposure time, Fitzpatrick type and skin type) linked to skin aging, which may be helpful in fully understanding the skin aging progress and other aging-related aspects, including skin tumors.
Acknowledgments
We are grateful to Dr. Ya-xian Zhen, L′Oreal Research Center, Shanghai, China, for providing her kind advice and interest in the research.
Footnotes
Project supported by the Skin Grant Program (2004) of L′ Oreal-Chinese Medical Association (CMA), China
References
- 1.Akiba S, Shinkura R, Miyamoto K, Hillebrand G, Yamaguchi N, Ichihashi M. Influence of chronic UV exposure and lifestyle on facial skin photo-aging: results from a pilot study. J Epidermiol. 1999;9(6 Suppl.):S136–S142. doi: 10.2188/jea.9.6sup_136. [DOI] [PubMed] [Google Scholar]
- 2.Chung JH, Lee SH, Youn CS, Park BJ, Kim KH, Park KC, Cho KH, Eun HC. Cutaneous photodamage in Koreans: influence of sex, sun exposure, smoking, and skin color. Arch Dermatol. 2001;137(8):1043–1051. [PubMed] [Google Scholar]
- 3.El-Domyati M, Attia S, Saleh F, Brown D, Birk DE, Gasparro F, Ahmad H, Uitto J. Intrinsic aging vs. photoaging: a comparative histopathological, immunohistochemical, and ultrastructural study of skin. Exp Dermatol. 2002;11(5):398–405. doi: 10.1034/j.1600-0625.2002.110502.x. [DOI] [PubMed] [Google Scholar]
- 4.Fisher GJ. The pathophysiology of photoaging of the skin. Cutis. 2005;75(2 Suppl):5–8. [PubMed] [Google Scholar]
- 5.Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124(6):869–871. doi: 10.1001/archderm.124.6.869. [DOI] [PubMed] [Google Scholar]
- 6.Goukassian DA, Gilchrest BA. The interdependence of skin aging, skin cancer, and DNA repair capacity: a novel perspective with therapeutic implications. Rejuvenation Res. 2004;7(3):175–185. doi: 10.1089/rej.2004.7.175. [DOI] [PubMed] [Google Scholar]
- 7.Gupta MA, Gilchrest BA. Psychosocial aspects of aging skin. Dermatol Clin. 2005;23(4):643–648. doi: 10.1016/j.det.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 8.Hall G, Phillips TJ. Estrogen and skin: the effects of estrogen, menopause, and hormone replacement therapy on the skin. J Am Acad Dermatol. 2005;53(4):555–568. doi: 10.1016/j.jaad.2004.08.039. [DOI] [PubMed] [Google Scholar]
- 9.Hashizume H. Skin aging and dry skin. J Dermatol. 2004;31(8):603–609. doi: 10.1111/j.1346-8138.2004.tb00565.x. [DOI] [PubMed] [Google Scholar]
- 10.Helfrich YR, Yu L, Ofori A, Hamilton TA, Lambert J, King A, Voorhees JJ, Kang S. Effect of smoking on aging of photoprotected skin: evidence gathered using a new photonumeric scale. Arch Dermatol. 2007;143(3):397–402. doi: 10.1001/archderm.143.3.397. [DOI] [PubMed] [Google Scholar]
- 11.Hoey SE, Devereux CE, Murray L, Catney D, Gavin A, Kumar S, Donnelly D, Dolan OM. Skin cancer trends in Northern Ireland and consequences for provision of dermatology services. Br J Dermatol. 2007;156(6):1301–1307. doi: 10.1111/j.1365-2133.2007.07936.x. [DOI] [PubMed] [Google Scholar]
- 12.Krause W. Skin diseases in consequence of endocrine alterations. Aging Male. 2006;9(2):81–95. doi: 10.1080/13685530600708573. [DOI] [PubMed] [Google Scholar]
- 13.Makrantonaki E, Zouboulis CC. Molecular mechanisms of skin aging: state of the art. Ann N Y Acad Sci. 2007;1119(1):40–50. doi: 10.1196/annals.1404.027. [DOI] [PubMed] [Google Scholar]
- 14.McCullough JL, Kelly KM. Prevention and treatment of skin aging. Ann N Y Acad Sci. 2006;1067(1):323–331. doi: 10.1196/annals.1354.044. [DOI] [PubMed] [Google Scholar]
- 15.Nouveau-Richard S, Yang Z, Mac-Mary S, Li L, Bastien P, Tardy I, Bouillon C, Humbert P, de Lacharriere O. Skin ageing: a comparison between Chinese and European populations. A pilot study. J Dermatol Sci. 2005;40(3):187–193. doi: 10.1016/j.jdermsci.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Omari AK, Khammash MR, Matalka I. Skin cancer trends in northern Jordan. Int J Dermatol. 2006;45(4):384–388. doi: 10.1111/j.1365-4632.2004.02444.x. [DOI] [PubMed] [Google Scholar]
- 17.Phan TA, Halliday GM, Barnetson RS, Damian DL. Melanin differentially protects from the initiation and progression of threshold UV-induced erythema depending on UV waveband. Photodermatol Photoimmunol Photomed. 2006;22(4):174–180. doi: 10.1111/j.1600-0781.2006.00226.x. [DOI] [PubMed] [Google Scholar]
- 18.Pons M, Quintanilla M. Molecular biology of malignant melanoma and other cutaneous tumors. Clin Transl Oncol. 2006;8(7):466–474. doi: 10.1007/s12094-006-0046-4. [DOI] [PubMed] [Google Scholar]
- 19.Rabe JH, Mamelak AJ, McElgunn PJ, Morison WL, Sauder DN. Photoaging: mechanisms and repair. J Am Acad Dermatol. 2006;55(1):1–19. doi: 10.1016/j.jaad.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 20.Rijken F, Bruijnzeel PL, van Weelden H, Kiekens RC. Responses of black and white skin to solar-simulating radiation: differences in DNA photodamage, infiltrating neutrophils, proteolytic enzymes induced, keratinocyte activation, and IL-10 expression. J Invest Dermatol. 2004;122(6):1448–1455. doi: 10.1111/j.0022-202X.2004.22609.x. [DOI] [PubMed] [Google Scholar]
- 21.Roh K, Kim D, Ha S, Ro Y, Kim J, Lee H. Pigmentation in Koreans: study of the differences from caucasians in age, gender and seasonal variations. Br J Dermatol. 2001;144(1):94–99. doi: 10.1046/j.1365-2133.2001.03958.x. [DOI] [PubMed] [Google Scholar]
- 22.Stang A, Ziegler S, Büchner U, Ziegler B, Jöckel KH, Ziegler V. Malignant melanoma and nonmelanoma skin cancers in Northrhine-Westphalia, Germany: a patient- vs. diagnosis-based incidence approach. Int J Dermatol. 2007;46(6):564–570. doi: 10.1111/j.1365-4632.2006.03056.x. [DOI] [PubMed] [Google Scholar]
- 23.Stern RS. Treatment of photoaging. N Engl J Med. 2004;350(15):1526–1534. doi: 10.1056/NEJMcp023168. [DOI] [PubMed] [Google Scholar]
- 24.Tagami H. Functional characteristics of the stratum corneum in photoaged skin in comparison with those found in intrinsic aging. Arch Dermatol Res. 2008;300(S1):1–6. doi: 10.1007/s00403-007-0799-9. [DOI] [PubMed] [Google Scholar]
- 25.Verdier-Sevrain S, Bonte F, Gilchrest B. Biology of estrogens in skin: implications for skin aging. Exp Dermatol. 2006;15(2):83–94. doi: 10.1111/j.1600-0625.2005.00377.x. [DOI] [PubMed] [Google Scholar]
- 26.Zhao P, Zhu X, Liu Y, Wang B, Wang C, Burns FJ. Solar ultraviolet radiation and skin damage: an epidemiological study among a Chinese population. Arch Environ Health. 1998;53(6):405–409. doi: 10.1080/00039899809605728. [DOI] [PubMed] [Google Scholar]