Abstract
Mycoplasmas, the smallest free-living, self-replicating bacteria with diameters of 200 to 800 nm, have been reported to be associated with human diseases. It is well known that the mycoplasma lipoprotein/peptide is able to modulate the host immune system, whose N-terminal structure is an important factor in inducing immunity and distinguishing Toll-like receptors (TLRs). However, there is still no clear elucidation about the pathogenic mechanism of mycoplasma lipoprotein/peptide and the signaling pathway. Some researchers have focused on understanding the structures of these proteins and the relationships between their structure and biological function. This review provides an update on the research in this field.
Keywords: Mycoplasma lipoproteins/lipopeptides, Toll-like receptors, Signal pathway, Mycoplasmal pathogenic mechanism
INTRODUCTION
Mycoplasmas are physically the smallest bacteria and have the smallest genomes (580~2200 kb), with high A+T content (67%~76%) (You et al., 2006). With the basic genomes for survival, they have to derive most of their nutrients from host cells. Depending on the Mycoplasma species and respective hosts, these organisms can either occur as harmless commensals or cause inflammatory diseases, such as atypical pneumonia, nongonococcal urethritis, mastitis, salpingitis, and arthritis (Baseman and Tully, 1997). It is well known that mycoplasmas are wall-less bacteria, lacking the classical modulins such as lipopolysaccharide (LPS), lipoteichoic acid, and murein fragments (Henderson et al., 1996), but they are still potent activators of macrophages. One of the most important pathogenic elements is lipoproteins or lipopeptides (Feng and Lo, 1994; Mühlradt et al., 1998). To date, many mycoplasma lipoproteins or lipopeptides have been identified and studied, especially the Mycoplasma fermentans. Toll-like receptors (TLRs) are type I trans-membrane proteins that are evolutionarily conserved between insects and humans (Anderson, 2000). It is well known that TLRs are the important receptors for immune systems, especially for innate immunity (Lemaitre et al., 1996; Takeda et al., 2003). TLRs also play an important role in the mechanism of mycoplasma pathogenicity.
MYCOPLASMA MEMBRANE LIPOPROTEINS
Basic structure of the mycoplasma lipoprotein
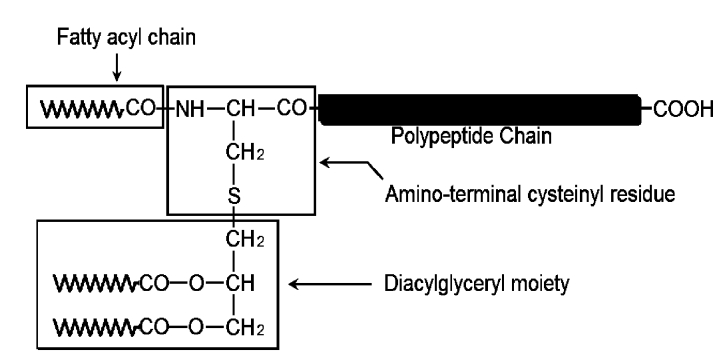
All membrane-anchored lipoproteins contain a lipoylated amino-terminal cysteinyl residue and proteins (Fig.1). In some cases, the residue is N-acylated. But it has been found that not all mycoplasma lipoproteins are N-acylated, such as lipoprotein p67 of the poultry pathogen Mycoplasma gallisepticum (Jan et al., 1996) and lipoproteins from Mycoplasma fermentans and Mycoplasma hyorhinis (Mühlradt et al., 1997). Also, an N-acyltransferase gene has not been found in Mycoplasma pneumoniae or Mycoplasma genitalium genome (Fraser et al., 1995; Himmelreich et al., 1996), and has not been identified in the Borrelia burgdorferi genome (Fraser et al., 1997). The loss of acylation might be the result of loss or mutation of N-acyltransferase gene. Therefore, some lipoproteins are triacylated, some are only diacylated, and some have the presence of both diacylated and triacylated forms (Jan et al., 1995). The property of being triacylated or diacylated will affect the recognition of the different TLRs, which will be covered in other paragraphs.
Fig. 1.
Chemical structure of the lipoylated amino-terminus of bacterial membrane-anchored lipoproteins. The lipoylation of the amino-terminal cysteinyl residue by a diacylglyceryl moiety via a thioether bond is a feature common to all known bacterial membrane lipoproteins. The amino group of the cysteinyl residue is blocked in certain lipoproteins by a third fatty acyl chain via an amide bond (Chambaud et al., 1999)
Categories and functions
In the early 1990s, Mühlradt and Schade (1991) extracted a high-molecular-weight material (MDHM) from M. fermentans and found that it could activate the macrophages’ release of interleukin-6 (IL-6) and increase the synthesis of cell-associated IL-1. Lipid-associated membrane proteins (LAMPs) have been found in different Mycoplasma species, such as P68 from M. bovis and P60 from M. capricolum. There are three forms of mycoplasma lipoproteins/peptides identified from M. fermentans, named macrophage-activating lipoprotein 2 (MALP-2), P48, and M161Ag (identical to MALP-404).
M161Ag is a 43-kDa M. fermentans lipoprotein of 428 amino acids with a signal sequence (Matsumoto et al., 1997), which is secreted as a 404-amino acid protein with a palmitoylated lipid anchor through posttranslational modification (Mühlradt et al., 1996; Matsumoto et al., 1997; Takeuchi et al., 2000). The P48 gene is 99% identical to the encoding of M161Ag. They have the similar N-terminal 114 amino acids including the 24-amino acid signal peptide, but diverge into distinct proteins by their respective C-terminal 315 and 71 amino acids. One common mycoplasma lipoprotein is a 2-kDa lipoprotein termed MALP-2. It has been found that the amino acid sequence of this lipopeptide (S-(2,3-bisacyloxypropyl)-cysteine-GNNDESNISFKEK) is entirely consistent with the N-terminal amino acid sequences of M161Ag and P48 (Mühlradt et al., 1997). It has also been found that the MALP-induced activation of intracellular signaling molecules is fully dependent on both TLR2 and myeloid differentiation primary response gene 88 (MyD88) (Takeuchi et al., 2000).
In addition to what has been introduced above, some other lipoproteins modulating host immune system have been also identified. For example, the N-terminal lipopeptide of a 44-kDa membrane-bound lipoprotein of M. salivarium is the active entity, whose structure was very similar to that of S-(2,3-bis-palmitoyloxypropyl)-cysteine-GDPKHPKSF (Shibata et al., 2000). M. arthritidis is a 41-kDa moiety and dependent on TLR2 and differentiation 14 (CD14) (Hasebe et al., 2007). Shimizu et al.(2005) found that the diacylated lipoprotein, F0F1-type ATPase (F0F1-ATPase), derived from M. pneumoniae, activated nuclear factors-kappaB (NF-κB) through TLR1, TLR2, and TLR6, but two more lipoproteins, N-ALP1 and N-ALP2, did that through TLR1 and TLR2, but not TLR6 (Shimizu et al., 2007). Among the three lipoproteins, F0F1-ATPase might be a key molecule in inducing acute inflammatory responses in the lungs of mice (Shimizu et al., 2008a). As another common mycoplasma that causes diseases in human, Ureaplasma parvum has been examined to have several lipoproteins, such as P75 and P55 containing UU012 and UU016, respectively. They were found to activate NF-κB through TLR1, TLR2, and TLR6, and to induce tumor necrosis factor-α (TNF-α) production in mouse peritoneal macrophages (Shimizu et al., 2008b).
All of these lipoproteins serve as potent cytokine inducers for monocytes/macrophages and have cytolytic activity. It was proven that the M161Ag could induce maturation of dendritic cells (DCs) and activate host complement on affected cells. The TLR2 mediated stimulation by MALP-2-induced cyclo-oxygenase 2 (COX-2) and prostaglandin E2 (PGE2) in human placental trophoblast cells via NF-κB and mitogen activated protein (MAP) kinase pathways (Mitsunari et al., 2006).
TOLL-LIKE RECEPTORS AND IMMUNITY
TLRs and their sub-cellular location
In 1997, the first human homologue of the Drosophila protein Toll was identified. Thirteen members of the TLR family have been identified in mammals and are responsible for recognizing pathogens as diverse as gram-positive and gram-negative bacteria, viruses, and fungi, as well as protozoa (Takeda et al., 2003). Among these TLRs, TLR1~TLR10 are functional in humans; however, TLR7 and TLR10 have no known natural ligands (Hasan et al., 2005), and TLR11 is a pseudogene (Zhang et al., 2004). TLRs recognize various conserved pathogen-associated molecules, including triacylated lipoproteins (TLR1, TLR2), diacylated lipoproteins (TLR2, TLR6), double-stranded (DS) RNA (TLR3), LPS (TLR4), flagellin (TLR5), single-stranded (SS) RNA (TLR7, TLR8) (Kawai and Akira, 2007), and unmethylated DNA (TLR9) (Liu et al., 2007).
Binding with their own ligands, TLRs recruit signaling molecules to their intracellular signaling domains, leading to the activation of the NF-κB and the secretion of proinflammatory cytokines (Triantafilou et al., 2006). Individual TLRs have different locations in cells. For example, TLR1, TLR2, and TLR4 are expressed on the cell surface, which has been confirmed by positive staining of the cell surface by specific antibodies. In contrast, TLR3, TLR7, TLR8, and TLR9 have been shown to be expressed in intracellular fractions, such as endosomes (Heil et al., 2003; Matsumoto et al., 2003; Latz et al., 2004). It has been reported that TLR3-, TLR7-, or TLR9-mediated recognition of their ligands requires endosomal maturation (Heil et al., 2004; Funami et al., 2004). Whether the TLR is on or in the cell, studies have shown that either phagosomal/lysosomal or endosomal/lysomal compartments may be the main sites for TLR recognition of microbial components (Latz et al., 2004; Leifer et al., 2004; Underhill et al., 1999).
Structure and function of the TLRs
The TLRs are type I integral membrane glycoproteins with molecular weights ranging from 90 to 115 kDa. TLRs are related to IL-1 receptors (IL-1Rs), based on the similarity in the cytoplasmic portions (designated the Toll-IL-1R, or TIR, domain), but the extra-cellular portions of TLRs and IL-1Rs are quite different. The extra-cellular portion of TLRs contains leucine-rich repeats (LRRs), whereas IL-1Rs contain three immunoglobulin-like domains. With regard to the structure, TLRs have three domains: extra-cellular, trans-membrane, and cytoplasmic regions (Gay and Gangloff, 2008). The TLR extra-cellular domain (ectodomain) consists of three discrete secondary structural elements: the LRRs, N-capping and C-capping structures (West et al., 2006) (Fig.2). Bell et al.(2005) found that the TLRs contain a unique consensus LRR, xLxxLxLxx[N/L]x+xx+xxxxFxxLx, where x represents any amino acid. They characterized each LRR in TLR1~TLR10 and concluded that diverse LRRs containing insertions after residues 10 and 15 confer ligand specificity. It has been illustrated that the deletion of the first 7 N-terminal LRRs did not drastically affect their ability to transduce signals for NF-κB in the human embryonic kidney 293 (HEK293) cells by mutational analysis of the TLR2 extra-cellular domain. Therefore, their ability to interact with other components, such as microbial polypeptides, was not affected. N-terminal mutant TLR2, however, showed downstream signaling deficiencies in response to many other TLR2 ligands, which provided the specific LRR domains on TLR2 interacting with different LAMPs to increase recognition capacity. Therefore, LRRs on each TLR function to make unique ligand specificities for the respective receptors.
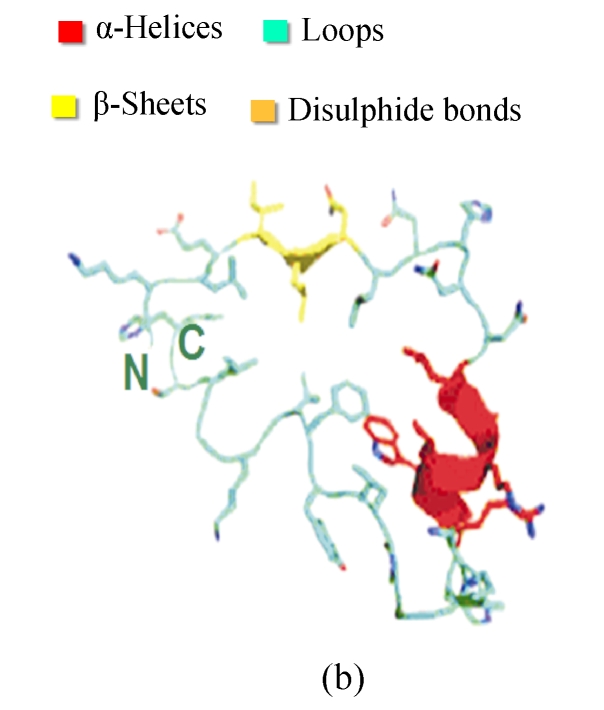
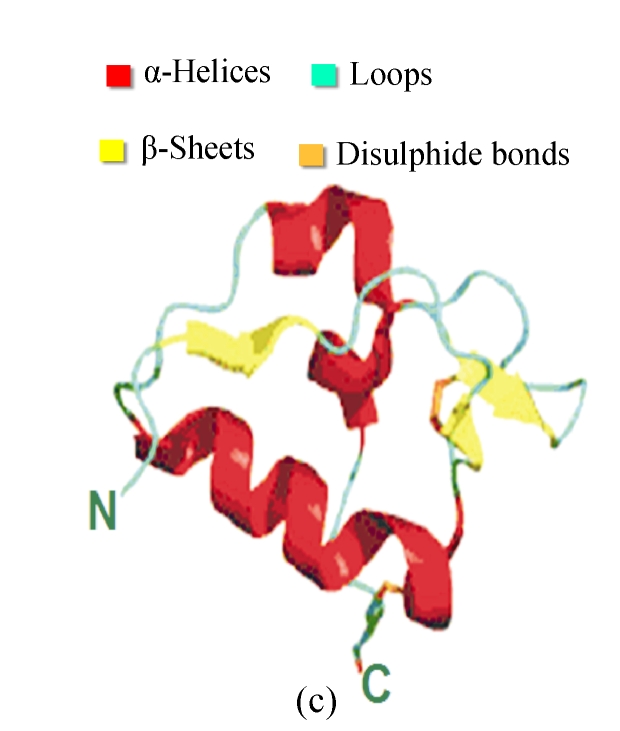
Fig. 2.
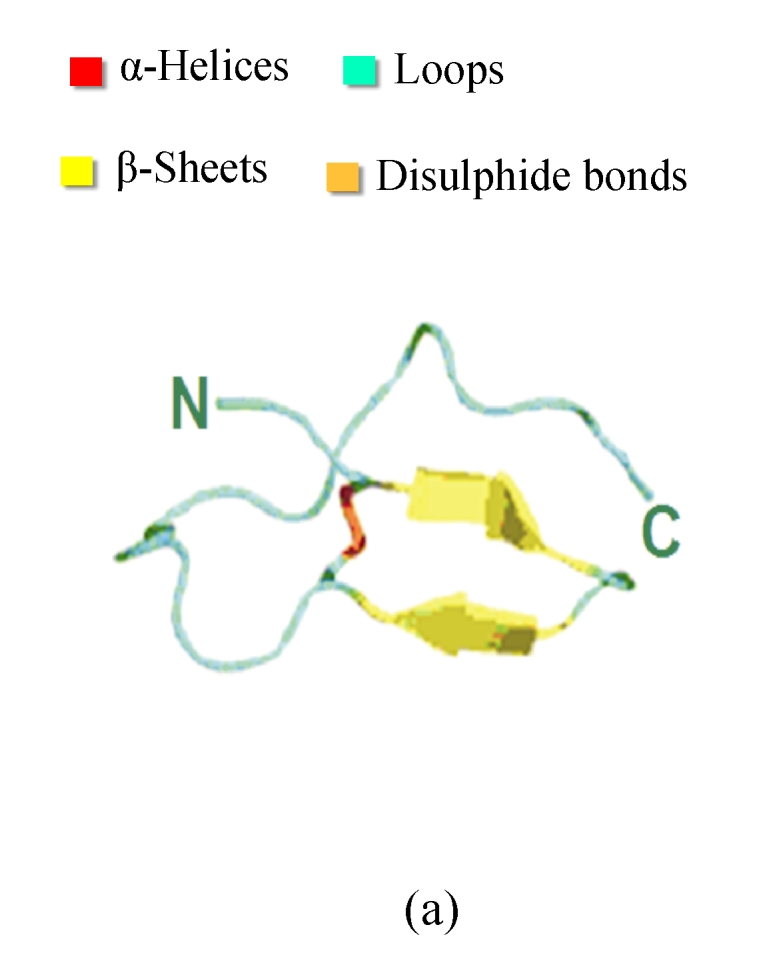
Structures and motifs involved in Toll signaling. (a) N-terminal capping structure taken from the Toll-like receptor 3 (TLR3) ectodomain structure (2A0Z); (b) A single leucine-rich repeat (LRR) unit taken from the TLR3. The short β-sheet (yellow) forms the inside surface of the solenoid. The conserved leucine residues point into the structure to form a hydrophobic core; (c) C-terminal capping structure from TLR3 (Gay and Gangloff, 2007)
Each LRR has a short β-sheet region, and a more variable secondary structure (Fig.2). The conserved leucine residues pack together to form a hydrophobic core, and asparagine is required for the turn that connects the two secondary structure elements. Each of LRR units folds together to form a solenoidal structure with a characteristic curvature. The fold is stabilized by the formation of a parallel β-sheet, with one strand being contributed by each LRR. This forms the internal or convex surface of the solenoid (Gay and Gangloff, 2008). For the TLRs, the repeat blocks are often flanked by cysteine-rich capping structures. The purpose of these researches may be to shield the hydrophobic residues of the terminal repeats, but there is also evidence that they participate in protein-protein interactions. As for the N-cap and C-cap, they also have both structural and functional roles for signaling the extra-cellular information (Huizinga et al., 2002; Nishitani et al., 2006).
The TLR ectodomains are connected to the cytoplasm by a hydrophobic trans-membrane sequence of about 22 amino acids, which probably form an α-helix. A short linker connects the C-terminal capping structure of the ectodomain and the membrane. However, on the cytoplasmic side of the membrane, there is a much longer linker connecting the trans-membrane sequence to the first secondary structure element of the TIR domain, varying from 20 amino acids in TLR4 to 30 amino acids in TLR5. The TIR domain consists of about 200 amino acids and folds into an α-β structure similar to that of the bacterial chemotaxis protein Che Y. It can be divided into two groups. The first group consists of type I trans-membrane receptors, the 10 TLRs and IL-1 family. They function in the second phase of the innate immune response. The second group constitutes the five signaling adapters for the TLRs, i.e., MyD88 adaptor-like/TIR-associated protein (Mal/TIRAP), MyD88, sterile-alpha and armadillo motif containing protein (SARM), TIR domain-containing adaptor inducing IFN-β (TRIF), and TRIF-related adaptor molecule (TRAM) (O′Neill et al., 2003). MyD88 functions as a critical adaptor for signaling pathway of TLR1/2/6, TLR5, and TLR7~TLR9. TRIF is an adaptor for TLR3 and TLR4 (Yamamoto et al., 2003). In all, the TIR domain has evolved as a general protein-protein interaction domain that later acquired some specialized functions in development and in immune processes.
Signaling pathways of TLRs
The signaling pathways associated with each TLR are not identical and may result in different biological responses, such as antigen-presenting cell (APC) activation, DCs maturation, and B cell activation (Anders et al., 2004). It is well known that there exist MyD88-dependent and -independent signaling pathways of TLRs. Here, MyD88-dependent signaling pathway related to mycoplasma lipopeptide-induced signaling pathway shall be introduced. After ligand binding, the intracellular adaptor molecule MyD88 is recruited to the receptor complex (Medzhitov et al., 1998; Muzio et al., 1998). MyD88 has a C-terminal TIR domain that mediates its homophilic interaction with the receptor and an N-terminal death domain that engages the death domain of its downstream target IL-1 receptor-associated kinase (IRAK) (Wesche et al., 1997). IRAK1 and IRAK4 join the receptor complex, which involves in the phosphorylation and activation of TNF receptor-associated factor 6 (TRAF6). In contrast, IRAK-M lacks kinase activity and plays different roles. IRAK-M negatively regulates TLR signaling by preventing dissociation of phosphorylated IRAK1 and IRAK4 from MyD88. Upon dimerization, TRAF6 forms a complex with Ubc13 and Uev1A, both of which facilitate lysine 63 ubiquitination of TRAF6 (Chen, 2005). Ub-TRAF6 then binds and phosphorylates another complex composed of transforming growth factor-activated kinase 1 (TAK1) and TAK1-binding proteins 1 and 2. Once it is activated, TAK1 in turn activates IκB kinase (IKK), p38, and c-Jun N-terminal kinase (JNK). JNK induces activator protein-1 (AP-1) activation (Wang et al., 2001). IKK phosphorylates IκB, an NF-κB inhibitor, and leads to its ubiquitination and degradation, unmasking the nuclear localization domain of NF-κB. Freed NF-κB translocates into the nucleus and turns on the transcription of multiple proinflammatory genes, including TNF-α, IL-1, and IL-6 (Cook et al., 2004; Kawai and Akira, 2005). Similar to the NF-κB pathway, TAK1 activates mitogen-activated protein kinase (MAPK) pathways, and leads to the expression of multiple proinflammatory genes.
INTERACTION BETWEEN MYCOPLASMA LIPOPROTEIN AND TLRS
Signaling pathway triggered by mycoplasma lipoproteins through TLRs
It has been shown that the treatment with proteinase K fails to decrease the activity of M. fermentans and M. penetrans, while the treatment of lipoprotein lipase decreases the ability to induce NF-κB. This suggests that the active components are attributed to lipid moieties, although the moieties of proteins might have other functions (Shimizu et al., 2004). The acylated proteins of mycoplasmas are abundant cell-surface antigens, which are recognized by extra-cellular domains of TLRs. As described above, LRRs on each TLR can recognize different ligands. The extra-cellular regions of Ser40-Ile64 and leucine residues at positions 107, 112, and 115 in a LRR motif of TLR2 are involved in the recognition of mycoplasmal diacylated lipoproteins and lipopeptides (Fujita et al., 2003). So, we could conclude that LRRs on TLR1 or TLR6 discriminate the diacylated and triacylated lipoproteins. When the signal arrives at the TIR region of the cytoplasmic domain through a much longer linker connecting the trans-membrane sequence to the TIR domain, it forms a receptor complex activating the signaling pathways of TLRs. It was found that the MALP-induced activation of intracellular signaling molecules was fully dependent on both TLR2 and MyD88 (Takeuchi et al., 2000), and that TLR2-mediated stimulation by MALP-2 induced COX-2 and PGE2 in human placental trophoblast cells via NF-κB and MAPK pathways. Another finding is that domain-negative forms of MyD88 and Fas-associated protein with death domain (FADD), but not IRAK-4, reduced the cytocidal activity of the mycoplasma lipoproteins in transfected-HEK293 and the forms also down-regulated the activation of both NF-κB and caspase 8. Also, a selective inhibitor of p38 MAPK attenuated the cytocidal activity sufficiently in mycoplasmal membrane diacylated lipoproteins inducer tests. It could be concluded that mycoplasmal membrane diacylated lipoproteins not only initiate proinflammatory responses through TLR2 and TLR6 via the activation of the transcriptional factor NF-κB as an early event, but also initiate apoptotic responses as a latter event (Into et al., 2004).
Cooperative interactions among TLRs
Studies have focused more on cooperation of TLR2 and TLR6 or TLR1 to recognize the varied Mycoplasma species. Takeuchi et al.(2001) found that TLR6-deficient cells did not produce any inflammatory cytokines in response to mycoplasma-derived diacylated lipoproteins, but retained their normal response to triacylated lipopeptides of other bacterial origins. Experiments demonstrated that TLR6 cooperates with TLR2 to recognize diacylated lipoprotein, and to discriminate between the N-terminal lipoylated structures of MALP-2 and lipopeptides derived from other bacteria. Lipopeptides from M. salivarium got a different activation toward the human acute monocytic leukemia cell line (THP-1). It is suggested that both the fatty acids of diacylglycerol and the amino acid sequence of the peptide portion are indispensable for TLR2/6-mediated signaling, and the size of the peptide portion of lipopeptide affects the recognition of the lipopeptide by TLR2/6 (Okusawa et al., 2004). In contrast, TLR1-deficient cells show a normal response to mycoplasma-derived diacylated lipopeptides, but an impaired response to triacylated lipopeptides. Shimizu et al.(2004) found that the purified lipoprotein of M. penetrans LAMPs was able to activate NF-κB through TLR1 and TLR2. On the other hand, the activation of NF-κB by the purified lipoproteins of M. fermentans LAMPs was mediated through TLR2 and TLR6, but not TLR1. These results indicate that M. fermentans LAMPs may contain several active components, one of which is recognized by TLR2 and TLR6, while other components might be recognized by TLR1 and TLR2.
Another finding is the existence of adjacent receptors for mycoplasma lipoproteins in Mycoplasma species. For example, Hoebe et al.(2005) put forth the opinion that TLR2/6 heterodimers require cluster of differentiation 36 (CD36) to sense diacylated lipoproteins, whereas TLR1/2 heterodimers do not. Almost at the same time, the findings by Hasebe et al.(2007) showed that four macrophage-activating components from M. arthritidis were dependent on TLR2 and cluster of CD14. M. tuberculosis, M. leprae, and M. bovis Bacille Calmette-Guerin (BCG) all activated adaptive immune by triggering dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN)- and TLR-signal pathways on DCs (Gringhuis et al., 2007). Besides, the Dectin-1 was reported to be a co-receptor of TLR2, which recognized the biosynthetic lipoprotein, Pam3CSK4, on DCs (Gantner et al., 2003). In these adjacent receptors, CD14 is necessary for the recognition of triacylated lipoproteins by TLR1 and TLR2, but not for that of diacylated lipoproteins. For other Mycoplasmas species, a triacylated pattern is necessary but not sufficient to render a lipopeptide TLR1-dependent, and a diacylation pattern is necessary but not sufficient to render a lipopeptide TLR6-dependent (Buwitt-Beckmann et al., 2006). These results show the importance of the amide-bound acyl residue, the two ester-bound acyl residues, and the amino acid sequence of the peptide chain within lipoproteins for the induction of signaling through TLR2 hetero- or homo-dimers. Another explanation for the discrimination of diacylated or triacylated lipoproteins from TLR structures is the LRR domains. Omueti et al.(2005) have shown that LRR9~LRR12 of human TLR1 and TLR6 mediate the ability to discriminate between acylated lipoproteins, as the domain exchange between the two receptors alters lipopeptide responsiveness in transfected cells. Additionally, Grabiec et al.(2004) showed that LRR7~LRR10 of TLR2 were critical for responses to tri-lauroylated lipopeptides.
In 2007, Jin et al.(2007), by using the hybrid LRR technique as previously described (Kim et al., 2007), revealed the mode of lipopeptide binding to TLR1 and TLR2 heterodimer, which provided a structural explanation for why both TLR1 and TLR2 are required for triacylated lipopeptide binding and signaling. It is well known that the diacylated glyceryl is attached to the N-terminal cysteine via a thioether bond, and that the third lipid chain is connected to the cysteine via an amide bond in triacylated lipoproteins. When triacylated lipoproteins bound to the complex of TLR1 and TLR2, TLR1 channel and TLR2 pocket were connected at the dimer interface and the two C-terminal domains covered in the middle, shaping a form of the letter “m”. And then the two ester-bound lipid chains were inserted into the TLR2 pocket in extended conformation, and the amide-bound lipid chain was exposed to the outside of the pocket and inserted into a narrow channel in TLR1. As for TLR6, it does not contain the lipid-binding channel analyzed by modeling the structure of TLR6 with an automatic homology modeling server using the TLR1 structure as template (Tosatto, 2005). Therefore, the amide-bound lipid chain of the triacylated lipopeptide cannot interact with TLR6 so as to initiate signaling through the TLR2 and TLR6 complex.
Apoptosis and mycoplasmas lipoproteins
Aliprantis et al.(1999) found several bacterial lipoproteins could induce apoptosis in both transfected HEK293 and THP-1 monocytic cells through TLR2. It also appears that apoptosis induced by TLR2 signaling involves an interaction between the death domain of MyD88 and the TNF-pathway-component FADD, leading in turn to the activation of caspase 8. Brightbill et al.(1999) could not observe cell death in HEK293, suggesting that the activation of apoptosis is sensitive to culture conditions and the nature of the treatment. As described above, mycoplasma lipoproteins triggered TLR2- and TLR6-mediated events leading to activation of NF-κB and apoptosis. The apoptosis was regulated by p38 MAPK, MyD88, and FADD. Into and Shibata (2005) examined roles of apoptosis signal-regulating kinase (ASK1), an upstream activator of p38 MAPK, in TLR2 signaling. They found that a kinase-inactive mutant of ASK1 impaired the sustained phosphorylation of p38 MAPK induced by mycoplasma lipoproteins and also attenuated mycoplasma lipoprotein-induced transcriptional activities of NF-κB and activatorprotein-1 (AP-1) by inhibition of p38 MAPK activation. Mycoplasma lipoprotein-induced cell death, including DNA fragmentation and caspase 3/7 activation, was also down-regulated by the ASK1 mutant by p38 MAPK inhibition. Different experiments have shown some controversial evidence that mycoplasmal infections inhibit apoptosis. For example, Gerlic et al. (2007) found M. fermentans inhibits TNF-α-induced apoptosis in the human myelomonocytic U937 cell line; its effect is upstream of mitochondria and upstream of caspase 8. And their further study showed that the M. fermentans lipoproteins could inhibit the apoptosis induced by TNF-α in U937 cells. One possible explanation for these seemingly contradictory conclusions is that HEK293 cells over-express the function of TLRs and may exaggerate the activation of apoptosis using mycoplasma lipoproteins as inducers. Another explanation is that other components of mycoplasmas may exist to inhibit the apoptosis through other signal pathways.
CONCLUSION AND PERSPECTIVES
The mycoplasma lipoproteins play a key role in infection, although some of them do not cause clinical symptoms in humans. They are important factors in the inflammation process and are probably also involved in leukocyte recruitment to the infected tissue. Their ability to undergo size or phase variation at a high frequency appears to be a means of adapting to different conditions, including the host’s immune response. The conjugation, diacylated or triacylated lipopeptides, and the size of the C-terminal sequence have the effect on the immunity through TLRs. Some studies have revealed the basic structure, signaling pathway, and cooperation functions of TLRs in innate immune reactions. Over-expression studies are often used to know about the function of signal transduction molecules, but sometimes their results can be mislieading. Canparent to it, using knockout mice or RNA interference might get better results. Also, the sample components should be highly purified and synthetic compounds be used whenever possible, so that the interaction between mycoplasma lipoproteins and TLRs in vitro will be better examined (Akira et al., 2001). As for structural biology research, more X-ray crystallography and NMR spectroscopy studies are necessary to evaluate the interaction.
The current understanding of the cellular mechanisms involved in the TLR-mediated anti-mycoplasmas activity is minimal, and the signaling pathway of TLRs is still elusive. However, as long as researchers continue to explore and understand the biological structure and components of mycoplasma lipoproteins and TLR proteins, it will be possible to develop strategies to obtain antibodies or vaccines for defense.
Footnotes
Project (No. 30770115) supported by the National Natural Science Foundation of China
References
- 1.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2(8):675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 2.Aliprantis AO, Yang RB, Mark MR, Suggett S, Devaux B, Radolf JD, Klimpel GR, Godowski P, Zychlinsky A. Cell activation and apoptosis by bacterial lipoproteins through Toll-like receptor-2. Science. 1999;285(5428):736–739. doi: 10.1126/science.285.5428.736. [DOI] [PubMed] [Google Scholar]
- 3.Anders HJ, Banas B, Schlondorff D. Signaling danger: Toll-like receptors and their potential roles in kidney disease. J Am Soc Nephrol. 2004;15(4):854–867. doi: 10.1097/01.ASN.0000121781.89599.16. [DOI] [PubMed] [Google Scholar]
- 4.Anderson KV. Toll signaling pathways in the innate immune response. Curr Opin Immunol. 2000;12(1):13–19. doi: 10.1016/S0952-7915(99)00045-X. [DOI] [PubMed] [Google Scholar]
- 5.Baseman JB, Tully JG. Mycoplasmas: sophisticated, reemerging, and burdened by their notoriety. Emerg Infect Dis. 1997;3(1):21–32. doi: 10.3201/eid0301.970103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bell JK, Botos I, Hall PR, Askins J, Shiloach J, Segal DM, Davies DR. The molecular structure of the Toll-like receptor 3 ligand-binding domain. Proc Natl Acad Sci USA. 2005;102(31):10976–10980. doi: 10.1073/pnas.0505077102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brightbill HD, Libraty DH, Krutzik SR, Yang RB, Belisle JT, Bleharski JR, Maitland M, Norgard MV, Plevy SE, Smale ST, et al. Host defense mechanisms triggered by microbial lipoproteins through Toll-like receptors. Science. 1999;285(5428):732–736. doi: 10.1126/science.285.5428.732. [DOI] [PubMed] [Google Scholar]
- 8.Buwitt-Beckmann U, Heine H, Wiesmüller KH, Jung G, Brock R, Akira S, Ulmer AJ. TLR1- and TLR6-independent recognition of bacterial lipopeptides. J Biol Chem. 2006;281(14):9049–9057. doi: 10.1074/jbc.M512525200. [DOI] [PubMed] [Google Scholar]
- 9.Chambaud I, Wróblewski H, Blanchard A. Interactions between mycoplasma lipoproteins and the host immune system. Trends Microbiol. 1999;7(12):493–499. doi: 10.1016/S0966-842X(99)01641-8. [DOI] [PubMed] [Google Scholar]
- 10.Chen ZJ. Ubiquitin signalling in the NF-kappaB pathway. Nat Cell Biol. 2005;7(8):758–765. doi: 10.1038/ncb0805-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cook DN, Pisetsky DS, Schwartz DA. Toll-like receptors in the pathogenesis of human disease. Nat Immunol. 2004;5(10):975–979. doi: 10.1038/ni1116. [DOI] [PubMed] [Google Scholar]
- 12.Feng SH, Lo SC. Induced mouse spleen B-cell proliferation and secretion of immunoglobulin by lipid-associated membrane proteins of Mycoplasma fermentans incognitus and Mycoplasma penetrans . Infect Immun. 1994;62(9):3916–3921. doi: 10.1128/iai.62.9.3916-3921.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fraser CM, Gocayne JD, White O, Adams MD, Clayton RA, Fleischmann RD, Bult CJ, Kerlavage AR, Sutton G, Kelley JM, et al. The minimal gene complement of Mycoplasma genitalium . Science. 1995;270(5235):397–403. doi: 10.1126/science.270.5235.397. [DOI] [PubMed] [Google Scholar]
- 14.Fraser CM, Casjens S, Huang WM, Sutton GG, Clayton R, Lathigra R, White O, Ketchum KA, Dodson R, Hickey EK, et al. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi . Nature. 1997;390(6660):580–586. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- 15.Fujita M, Into T, Yasuda M, Okusawa T, Hamahira S, Kuroki Y, Eto A, Nisizawa T, Morita M, Shibata K. Involvement of leucine residues at positions 107, 112, and 115 in a leucine-rich repeat motif of human Toll-like receptor 2 in the recognition of diacylated lipoproteins and lipopeptides and Staphylococcus aureus peptidoglycans. J Immunol. 2003;171(7):3675–3683. doi: 10.4049/jimmunol.171.7.3675. [DOI] [PubMed] [Google Scholar]
- 16.Funami K, Matsumoto M, Oshiumi H, Akazawa T, Yamamoto A, Seya T. The cytoplasmic ‘linker region’ in Toll-like receptor 3 controls receptor localization and signaling. Int Immunol. 2004;16(8):1143–1154. doi: 10.1093/intimm/dxh115. [DOI] [PubMed] [Google Scholar]
- 17.Gantner BN, Simmons RM, Canavera SJ, Akira S, Underhill DM. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J Exp Med. 2003;197(9):1107–1117. doi: 10.1084/jem.20021787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gay NJ, Gangloff M. Structure and function of Toll receptors and their ligands. Annu Rev Biochem. 2007;76(1):141–165. doi: 10.1146/annurev.biochem.76.060305.151318. [DOI] [PubMed] [Google Scholar]
- 19.Gay NJ, Gangloff M. Handbook of Experimental Pharmacology. Vol. 183. Springer Berlin Heidelberg; 2008. Structure of Toll-like Receptors; pp. 181–200. [DOI] [PubMed] [Google Scholar]
- 20.Gerlic M, Horowitz J, Farkash S, Horowitz S. The inhibitory effect of Mycoplasma fermentans on tumor necrosis factor (TNF)-alpha-induced apoptosis resides in the membrane lipoproteins. Cell Microbiol. 2007;9(1):142–153. doi: 10.1111/j.1462-5822.2006.00774.x. [DOI] [PubMed] [Google Scholar]
- 21.Grabiec A, Meng G, Fichte S, Bessler W, Wagner H, Kirschning CJ. Human but not murine Toll-like receptor 2 discriminates between tri-palmitoylated and tri-lauroylated peptides. J Biol Chem. 2004;279(46):48004–48012. doi: 10.1074/jbc.M405311200. [DOI] [PubMed] [Google Scholar]
- 22.Gringhuis SI, den Dunnen J, Litjens M, van Het Hof B, van Kooyk Y, Geijtenbeek TB. C-type lectin DC-SIGN modulates Toll-like receptor signaling via Raf-1 kinase-dependent acetylation of transcription factor NF-kappaB. Immunity. 2007;26(5):605–616. doi: 10.1016/j.immuni.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 23.Hasan U, Chaffois C, Gaillard C, Saulnier V, Merck E, Tancredi S, Guiet C, Brière F, Vlach J, Lebecque S, et al. Human TLR10 is a functional receptor, expressed by B cells and plasmacytoid dendritic cells, which activates gene transcription through MyD88. J Immunol. 2005;174(5):2942–2950. doi: 10.4049/jimmunol.174.5.2942. [DOI] [PubMed] [Google Scholar]
- 24.Hasebe A, Mu HH, Washburn LR, Chan FV, Pennock ND, Taylor ML, Cole BC. Inflammatory lipoproteins purified from a toxigenic and arthritogenic strain of Mycoplasma arthritidis are dependent on Toll-like receptor 2 and CD14. Infect Immun. 2007;75(4):1820–1826. doi: 10.1128/IAI.00516-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heil F, Ahmad-Nejad P, Hemmi H, Hochrein H, Ampenberger F, Gellert T, Dietrich H, Lipford G, Takeda K, Akira S, et al. The Toll-like receptor 7 (TLR7)-specific stimulus loxoribine uncovers a strong relationship within the TLR7, 8 and 9 subfamily. Eur J Immunol. 2003;33(11):2987–2997. doi: 10.1002/eji.200324238. [DOI] [PubMed] [Google Scholar]
- 26.Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, Bauer S. Species-specific recognition of single-stranded RNA via Toll-like receptor 7 and 8. Science. 2004;303(5663):1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 27.Henderson B, Poole S, Wilson M. Bacterial modulins: a novel class of virulence factors which cause host tissue pathology by inducing cytokine synthesis. Microbiol Rev. 1996;60(2):316–341. doi: 10.1128/mr.60.2.316-341.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Himmelreich R, Hilbert H, Plagens H, Pirkl E, Li BC, Herrmann R. Complete sequence analysis of the genome of the bacterium Mycoplasma pneumoniae . Nucleic Acids Res. 1996;24(22):4420–4449. doi: 10.1093/nar/24.22.4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoebe K, Georgel P, Rutschmann S, Du X, Mudd S, Crozat K, Sovath S, Shamel L, Hartung T, Zähringer U, et al. CD36 is a sensor of diacylglycerides. Nature. 2005;433(7025):523–527. doi: 10.1038/nature03253. [DOI] [PubMed] [Google Scholar]
- 30.Huizinga EG, Tsuji S, Romijn RAP, Schiphorst ME, de Groot PG, Sixma JJ, Gros P. Structures of glycoprotein Ib alpha and its complex with von Willebrand factor A1 domain. Science. 2002;297(5584):1176–1179. doi: 10.1126/science.107355. [DOI] [PubMed] [Google Scholar]
- 31.Into T, Shibata K. Apoptosis signal-regulating kinase 1-mediated sustained p38 mitogen-activated protein kinase activation regulates mycoplasmal lipoprotein- and staphylococcal peptidoglycan-triggered Toll-like receptor 2 signalling pathways. Cell Microbiol. 2005;7(9):1305–1317. doi: 10.1111/j.1462-5822.2005.00558.x. [DOI] [PubMed] [Google Scholar]
- 32.Into T, Kiura K, Yasuda M, Kataoka H, Inoue N, Hasebe A, Takeda K, Akira S, Shibata K. Stimulation of human Toll-like receptor (TLR) 2 and TLR6 with membrane lipoproteins of Mycoplasma fermentans induces apoptotic cell death after NF-kappaB activation. Cell Microbiol. 2004;6(2):187–199. doi: 10.1046/j.1462-5822.2003.00356.x. [DOI] [PubMed] [Google Scholar]
- 33.Jan G, Fontenelle C, Le Hénaff M, Wróblewski H. Acylation and immunological properties of Mycoplasma gallisepticum membrane proteins. Res Microbiol. 1995;146(9):739–750. doi: 10.1016/0923-2508(96)81070-9. [DOI] [PubMed] [Google Scholar]
- 34.Jan G, Brenner C, Wróblewski H. Purification of Mycoplasma gallisepticum membrane proteins p52, p67 (pMGA), and p77 by high-performance liquid chromatography. Protein Expr Purif. 1996;7(2):160–166. doi: 10.1006/prep.1996.0023. [DOI] [PubMed] [Google Scholar]
- 35.Jin MS, Kim SE, Heo JY, Lee ME, Kim HM, Paik SG, Lee H, Lee JO. Crystal structure of the TLR1-TLR2 heterodimer induced by binding of a tri-acylated lipopeptide. Cell. 2007;130(6):1071–1082. doi: 10.1016/j.cell.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 36.Kawai T, Akira S. Toll-like receptor downstream signaling. Arthritis Res Ther. 2005;7(1):12–19. doi: 10.1186/ar1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kawai T, Akira S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol Med. 2007;13(11):460–469. doi: 10.1016/j.molmed.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 38.Kim HM, Park BS, Kim JI, Kim SE, Lee J, Oh SC, Enkhbayar P, Matsushima N, Lee H, Yoo OJ, et al. Crystal structure of the TLR4-MD-2 complex with bound endotoxin antagonist Eritoran. Cell. 2007;130(5):906–917. doi: 10.1016/j.cell.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 39.Latz E, Schoenemeyer A, Visintin A, Fitzgerald KA, Monks BG, Knetter CF, Lien E, Nilsen NJ, Espevik T, Golenbock DT. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat Immunol. 2004;5(2):190–198. doi: 10.1038/ni1028. [DOI] [PubMed] [Google Scholar]
- 40.Leifer CA, Kennedy MN, Mazzoni A, Lee C, Kruhlak MJ, Segal DM. TLR9 is localized in the endoplasmic reticulum prior to stimulation. J Immunol. 2004;173(2):1179–1183. doi: 10.4049/jimmunol.173.2.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spätzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86(6):973–983. doi: 10.1016/S0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- 42.Liu PT, Krutzik SR, Modlin RL. Therapeutic implications of the TLR and VDR partnership. Trends Mol Med. 2007;13(3):117–124. doi: 10.1016/j.molmed.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 43.Matsumoto M, Takeda J, Inoue N, Hara T, Hatanaka M, Takahashi K, Nagasawa S, Akedo H, Seya T. A novel protein that participates in nonself discrimination of malignant cells by homologous complement. Nat Med. 1997;3:1266–1270. doi: 10.1038/nm1197-1266. [DOI] [PubMed] [Google Scholar]
- 44.Matsumoto M, Funami K, Tanabe M, Oshiumi H, Shingai M, Seto Y, Yamamoto A, Seya T. Subcellular localization of Toll-like receptor 3 in human dendritic cells. J Immunol. 2003;171(6):3154–3162. doi: 10.4049/jimmunol.171.6.3154. [DOI] [PubMed] [Google Scholar]
- 45.Medzhitov R, Preston-Hurlburt P, Kopp E, Stadlen A, Chen C, Ghosh S, Janeway CAJ. MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol Cell. 1998;2(2):253–258. doi: 10.1016/S1097-2765(00)80136-7. [DOI] [PubMed] [Google Scholar]
- 46.Mitsunari M, Yoshida S, Shoji T, Tsukihara S, Iwabe T, Harada T, Terakawa N. Macrophage-activating lipopeptide-2 induces cyclooxygenase-2 and prostaglandin E(2) via Toll-like receptor 2 in human placental trophoblast cells. J Reprod Immunol. 2006;72(1-2):46–59. doi: 10.1016/j.jri.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 47.Mühlradt PF, Schade U. MDHM, a macrophage-stimulatory product of Mycoplasma fermentans, leads to in vitro interleukin-1 (IL-1), IL-6, tumor necrosis factor, and prostaglandin production and is pyrogenic in rabbits. Infect Immun. 1991;59(11):3969–3974. doi: 10.1128/iai.59.11.3969-3974.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mühlradt PF, Meyer H, Jansen R. Identification of S-(2,3-dihydroxypropyl) cystein in a macrophage-activating lipopeptide from Mycoplasma fermentans . Biochemistry. 1996;35(24):7781–7786. doi: 10.1021/bi9602831. [DOI] [PubMed] [Google Scholar]
- 49.Mühlradt PF, Kiess M, Meyer H, Süssmuth R, Jung G. Isolation, structure elucidation, and synthesis of a macrophage stimulatory lipopeptide from Mycoplasma fermentans acting at picomolar concentration. J Exp Med. 1997;185(11):1951–1958. doi: 10.1084/jem.185.11.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mühlradt PF, Kiess M, Meyer H, Süssmuth R, Jung G. Structure and specific activity of macrophage-stimulating lipopeptides from Mycoplasma hyorhinis . Infect Immun. 1998;66(10):4804–4810. doi: 10.1128/iai.66.10.4804-4810.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Muzio M, Natoli G, Saccani S, Levrero M, Mantovani A. The human Toll signaling pathway: divergence of nuclear factor kappaB and JNK/SAPK activation upstream of tumor necrosis factor receptor-associated factor 6 (TRAF6) J Exp Med. 1998;187(12):2097–2101. doi: 10.1084/jem.187.12.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nishitani C, Mitsuzawa H, Sano H, Shimizu T, Matsushima N, Kuroki Y. Toll-like receptor 4 region Glu24-Lys47 is a site for MD-2 binding: importance of CYS29 and CYS40. J Biol Chem. 2006;281(50):38322–38329. doi: 10.1074/jbc.M606904200. [DOI] [PubMed] [Google Scholar]
- 53.Okusawa T, Fujita M, Nakamura J, Into T, Yasuda M, Yoshimura A, Hara Y, Hasebe A, Golenbock DT, Morita M, et al. Relationship between structures and biological activities of mycoplasmal diacylated lipopeptides and their recognition by Toll-like receptors 2 and 6. Infect Immun. 2004;72(3):1657–1665. doi: 10.1128/IAI.72.3.1657-1665.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Omueti KO, Beyer JM, Johnson CM, Lyle EA, Tapping RI. Domain exchange between human Toll-like receptors 1 and 6 reveals a region required for lipopeptide discrimination. J Biol Chem. 2005;280(44):36616–36625. doi: 10.1074/jbc.M504320200. [DOI] [PubMed] [Google Scholar]
- 55.O′Neill LAJ, Fitzgerald KA, Bowie AG. The Toll-IL-1 receptor adaptor family grows to five members. Trends in Immunol. 2003;24:287–290. doi: 10.1016/S1471-4906(03)00115-7. [DOI] [PubMed] [Google Scholar]
- 56.Shibata K, Hasebe A, Into T, Yamada M, Watanabe T. The N-terminal lipopeptide of a 44-kDa membrane-bound lipoprotein of Mycoplasma salivarium is responsible for the expression of intercellular adhesion molecule-1 on the cell surface of normal human gingival fibroblasts. J Immunol. 2000;165:6538–6544. doi: 10.4049/jimmunol.165.11.6538. [DOI] [PubMed] [Google Scholar]
- 57.Shimizu T, Kida Y, Kuwano K. Lipid-associated membrane proteins of Mycoplasma fermentans and M. penetrans activate human immunodeficiency virus long-terminal repeats through Toll-like receptors. Immunology. 2004;113(1):121–129. doi: 10.1111/j.1365-2567.2004.01937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shimizu T, Kida Y, Kuwano K. A dipalmitoylated lipoprotein from Mycoplasma pneumoniae activates NF-kappaB through TLR1, TLR2, and TLR6. J Immunol. 2005;175(7):4641–4646. doi: 10.4049/jimmunol.175.7.4641. [DOI] [PubMed] [Google Scholar]
- 59.Shimizu T, Kida Y, Kuwano K. Triacylated lipoproteins derived from Mycoplasma pneumoniae activate nuclear factor-kappaB through Toll-like receptors 1 and 2. Immunology. 2007;121(4):473–483. doi: 10.1111/j.1365-2567.2007.02594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shimizu T, Kida Y, Kuwano K. Mycoplasma pneumoniae-derived lipopeptides induce acute inflammatory responses in the lungs of mice. Infect Immun. 2008;76(1):270–277. doi: 10.1128/IAI.00955-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shimizu T, Kida Y, Kuwano K. Ureaplasma parvum lipoproteins, including MB antigen, activate NF-κB through TLR1, TLR2 and TLR6. Microbiology. 2008;154(5):1318–1325. doi: 10.1099/mic.0.2007/016212-0. [DOI] [PubMed] [Google Scholar]
- 62.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21(1):335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 63.Takeuchi O, Kaufmann A, Grote K, Kawai T, Hoshino K, Morr M, Mühlradt PF, Akira S. Cutting edge: preferentially the R-stereoisomer of the mycoplasmal lipopeptide macrophage-activating lipopeptide-2 activates immune cells through a Toll-like receptor 2- and MyD88-dependent signaling pathway. J Immunol. 2000;164(2):554–557. doi: 10.4049/jimmunol.164.2.554. [DOI] [PubMed] [Google Scholar]
- 64.Takeuchi O, Kawai T, Mühlradt PF, Morr M, Radolf JD, Zychlinsky A, Takeda K, Akira S. Discrimination of bacterial lipoproteins by Toll-like receptor 6. Int Immunol. 2001;13(7):933–940. doi: 10.1093/intimm/13.7.933. [DOI] [PubMed] [Google Scholar]
- 65.Tosatto SC. The victor/FRST function for model quality estimation. J Comput Biol. 2005;12(10):1316–1327. doi: 10.1089/cmb.2005.12.1316. [DOI] [PubMed] [Google Scholar]
- 66.Triantafilou M, Gamper FG, Haston RM, Mouratis MA, Morath S, Hartung T, Triantafilou K. Membrane sorting of Toll-like receptor (TLR)-2/6 and TLR2/1 heterodimers at the cell surface determines heterotypic associations with CD36 and intracellular targeting. J Biol Chem. 2006;281(41):31002–31011. doi: 10.1074/jbc.M602794200. [DOI] [PubMed] [Google Scholar]
- 67.Underhill DM, Ozinsky A, Hajjar AM, Stevens A, Wilson CB, Bassetti M, Aderem A. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature. 1999;401(6755):811–815. doi: 10.1038/44605. [DOI] [PubMed] [Google Scholar]
- 68.Wang C, Deng L, Hong M, Akkaraju GR, Inoue J, Chen ZJ. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature. 2001;412(6844):346–351. doi: 10.1038/35085597. [DOI] [PubMed] [Google Scholar]
- 69.Wesche H, Henzel WJ, Shillinglaw W, Li S, Cao Z. MyD88: an adapter that recruits IRAK to the IL-1 receptor complex. Immunity. 1997;7(6):837–847. doi: 10.1016/S1074-7613(00)80402-1. [DOI] [PubMed] [Google Scholar]
- 70.West AP, Koblansky AA, Ghosh S. Recognition and signaling by Toll-like receptors. Annu Rev Cell Dev Biol. 2006;22(1):409–437. doi: 10.1146/annurev.cellbio.21.122303.115827. [DOI] [PubMed] [Google Scholar]
- 71.Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, et al. Role of adaptor TRIF in the MyD88-independent Toll-like receptor signaling pathway. Science. 2003;301(5633):640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 72.You XX, Zeng YH, Wu YM. Interactions between mycoplasma lipid-associated membrane proteins and the host cells. J Zhejiang Univ Sci B. 2006;7(5):342–350. doi: 10.1631/jzus.2006.B0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang D, Zhang G, Hayden MS, Greenblatt MB, Bussey C, Flavell RA, Ghosh S. A Toll-like receptor that prevents infection by uropathogenic bacteria. Science. 2004;303(5663):1522–1526. doi: 10.1126/science.1094351. [DOI] [PubMed] [Google Scholar]