Abstract
Background
Sebaceous tumors of the skin occurring in association with an internal malignancy characterize Muir-Torré syndrome (MTS), a variant of hereditary nonpolyposis colon cancer (Lynch syndrome). Limited information exists on incidence patterns of sebaceous carcinoma (SC) and no prior study has quantified risks of associated cancers.
Methods
We calculated cutaneous SC incidence rates (IRs) and IR ratios in nine U.S. Surveillance, Epidemiology and End Results Program registries (1973–2003). Indirectly standardized incidence ratios and 95% confidence intervals (CI) were calculated for subsequent cancers among two-month survivors of SC and for subsequent SC following other primary cancers.
Results
Among 664 cases of cutaneous SC, nearly 90% were diagnosed among Whites (IR=0.11/100,000 person-years), with significantly lower IR among Blacks (IR=0.04). Whereas eyelid SC IRs showed no gender differences and stabilized in recent years, IRs of non-eyelid SC predominated in males and rose steadily over time. Survivors of SC had a 43% (95%CI=15%–76%) increased risk of subsequent cancer, while risk of SC was elevated by 52% (95%CI=24%–84%) among survivors of other cancers. Whether prior to or following SC, the significant excesses of other primary cancers were limited to non-eyelid SC. Patterns suggestive of genetic predisposition included >20-fold risks for early-onset (<50 years) SC associated with colon, pancreatic, ovarian, or uterine corpus cancers, while late-onset SC (50+ years) predisposed to ureter cancer.
Conclusion
This population-based study of cutaneous SC revealed an association with a spectrum of early-onset cancers consistent with MTS. Etiologic heterogeneity was suggested by differences between eyelid and non-eyelid SC in incidence patterns and associated cancer risks.
Keywords: sebaceous carcinoma, Muir-Torré syndrome, multiple primary cancers, skin cancer
Sebaceous carcinoma (SC) is a rare adnexal tumor with sebocytic differentiation that usually involves the skin.1 Although SC primarily affects the elderly, a broad age range has been reported.2, 3 The clinical and pathologic recognition of SC is often delayed because it can mimic benign entities.4, 5 While SC is frequently reported to arise in the ocular region, particularly the eyelid, it also occurs at other cutaneous sites and occasionally elsewhere in the body.2–11
Muir-Torré syndrome (MTS) is an autosomal dominant condition characterized by the association of sebaceous tumors (adenoma, epithelioma, or carcinoma) and occasionally keratoacanthomas with a visceral neoplasm.12 Nearly half of individuals with MTS have been estimated to develop two or more malignancies prior to, concurrent with, or following a sebaceous tumor.13 The spectrum of cancers and the germline mutations characteristic of MTS have suggested it represents a phenotypic variant of hereditary nonpolyposis colorectal cancer (HNPCC) or Lynch syndrome.14, 15
While many hospital and clinic-based surveys of MTS have been reported (reviewed in13, 16–20) no study has quantified risks of cancers associated with SC. Furthermore, population-based studies of SC have been infrequent, usually involving the eyelid or ocular adnexa, with fewer than 100 cases in each study.21–24 Reports of extraocular SC have been mainly clinical surveys of fewer than 20 cases,8–10 with other descriptions based on case studies and literature reviews.6, 7, 11 To identify the epidemiologic patterns of SC, including the risks of visceral cancers, we examined data from the population-based cancer registries of the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program.
Methods
We included data from nine population-based cancer registries of the SEER Program (SEER-9) that represent approximately 10% of the U.S. population.25 The SEER Program classifies histology and topography according to the International Classification of Diseases for Oncology, 3rd edition (ICD-O-3).26 We considered all cases of invasive SC (M8410/3) of the skin (C440-449) diagnosed in SEER-9 (November 2005 submission) during 1973–2003. Information on cancer incidence in Asians and Pacific Islanders (APIs) in SEER registries became publically available in 1992. To enable evaluation of incidence of SC among APIs and to maximize the number of cases during this time period, analyses were limited to cases diagnosed between 1992 and 2003 and were undertaken using SEER-11, when the cancer registries in Los Angeles and San Jose-Monterey, CA were added to the SEER Program.25
Incidence Patterns
Age-specific and age-adjusted incidence rates (IRs), incidence rate ratios (IRRs), and 95% confidence intervals (CIs) were calculated using SEER*Stat (version 6.2.4). All IRs were age-adjusted to the 2000 U.S. population, and expressed per 100,000 person-years (PY). Age-adjusted rates are calculated to allow comparison of rates among populations that may differ in their age distribution and thus their crude rates (number of cases divided by the corresponding population during a defined time period); age-adjusted rates are a weighted average of the age-specific rates, where the weights are the proportional distribution of a standard population, which in this case is the age distribution of the U.S. population in 2000. Our analyses focused on SC of the eyelid (C441) and all other cutaneous sites (C440, C442-449) referred to as “non-eyelid.” Time trends and age-specific IRs were plotted using log-linear scales as previously described.27
Multiple Primary Cancers
We evaluated the risk of subsequent cancer among 500 two-month survivors of first primary cutaneous SC as well as the risk of subsequent SC among more than two million 2-month survivors of all cancers. Because heightened screening of cancer patients during the initial medical work-up tends to identify many simultaneous cancers (detection bias), we excluded from the calculations the first 2 months of follow-up and the new malignancies occurring within this initial 2-month period.28 The risk of nonmelanoma skin cancer (“other epithelial skin cancers”) occurring in association with SC was evaluated separately. Basal cell, papillary, and squamous cell cancers of the skin are not reportable to the SEER Program and thus are not available for analysis. We calculated the standardized incidence ratio (SIR) (observed (Obs.)-to-expected (E) number of subsequent cancers), exact 95%CI, and excess absolute risk (EAR) of second or higher order cancers among two-month survivors of a first primary neoplasm diagnosed between January 1, 1973 and December 31, 2003 using SEER*Stat. The SIR was calculated by compiling PY of observation according to age, gender, race, and calendar period beginning two months after the diagnosis of first primary cancer to the study end date, date of death, or date of last known follow-up, whichever occurred first. To estimate the expected number of subsequent cancers, IRs were calculated according to gender, race, 5-year age groups, and 5-year calendar intervals and multiplied by the PY at risk. The EAR was calculated by subtracting the expected from the observed number of subsequent cancers and then dividing the difference by the number of PY at risk. The EAR was expressed per 10,000 patients per year. Tests of homogeneity of differences (Pdiff) between SIRs or EARs were conducted using the methods of Breslow et al.29
Results
Incidence Patterns
A total of 664 cases (IR=0.11/100,000 PY) of SC of the skin were diagnosed in SEER-9 during 1973–2003 (Table 1) with a median age of 73 years (range 21–102 years). Incidence was significantly higher among males than females (IRR=1.55, 95%CI=1.32–1.81). Nearly 90% of cases occurred in Whites, with significantly lower rates among Blacks than Whites.
Table 1.
Age-adjusted incidence rates and incidence rate ratios of sebaceous carcinoma of the skin diagnosed in SEER-9 overall and according to gender by race, stage, and primary site, 1973–2003*
| Total |
Males |
Females |
Male:female | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | IR | IRR | (95%CI) | No. | IR | No. | IR | IRR | (95%CI) | |
| Total† | 664 | 0.11 | NA | NA | 348 | 0.13 | 316 | 0.09 | 1.55 | (1.32–1.81) |
| Race | ||||||||||
| White | 593 | 0.11 | 1.00 | (referent) | 310 | 0.14 | 283 | 0.09 | 1.57 | (1.33–1.85) |
| Black | 18 | 0.04 | 0.34 | (0.20–.55) | 11 | 0.05 | 7 | 0.03 | 1.84 | (0.64–5.75) |
| Other | 46 | 0.10 | 0.93 | (0.67–.26) | 22 | 0.11 | 24 | 0.10 | 1.07 | (0.57–2.01) |
| Unknown | 7 | ~ | ~ | NA | 5 | ~ | 2 | ~ | ~ | NA |
| Stage | ||||||||||
| Localized | 499 | 0.08 | 1.00 | (referent) | 260 | 0.10 | 239 | 0.07 | 1.49 | (1.24–1.79) |
| Regional | 81 | 0.01 | 0.16 | (0.13–0.21) | 39 | 0.02 | 42 | 0.01 | 1.37 | (0.85–2.17) |
| Distant | 13 | <0.01 | 0.03 | (0.01–0.05) | 7 | <0.01 | 6 | <0.01 | 1.82 | (0.51–6.40) |
| Not specified | 71 | 0.01 | 0.15 | (0.11–0.19) | 42 | 0.02 | 29 | 0.01 | 2.22 | (1.34–3.69) |
| Skin site | ||||||||||
| Eyelid | 305 | 0.05 | 1.00 | (referent) | 119 | 0.05 | 186 | 0.05 | 0.92 | (0.72–1.16) |
| Non-eyelid | 359 | 0.06 | 1.18 | (1.01–1.38) | 229 | 0.09 | 130 | 0.04 | 2.43 | (1.95–3.05) |
Abbreviations:No., number of incident cancers; IR, incidence rate; IRR, incidence rate ratio; CI, confidence interval; NA, not applicable; ~, IR and IRR not calculated for unknown race.
Incidence rates are age-adjusted to the 2000 U.S. standard population and expressed per 100,000 person-years. IRRs were calculated using unrounded rates.
Among the 664 cases of SC, 413 (62%) occurred as the only primary cancer, 97 (15%) were diagnosed in individuals who developed a subsequent primary cancer, and 153 (23%) occurred following another first primary cancer; the cancer sequence was unknown for one individual.
At initial diagnosis, 75% (n=499) of patients presented with localized disease, with similar findings for eyelid (73%) and non-eyelid (77%) SC. Eighty-one percent of SC (n=536) were diagnosed on the skin of the head/neck, of which more than half occurred on the eyelid (n=305). Compared to SC of the eyelid, the IR of SC occurring at non-eyelid skin sites was nearly 20% higher. Incidence of SC of the eyelid was similar among males and females, whereas incidence of non-eyelid SC was significantly higher among males than females. Compared to Whites, incidence of both eyelid (n=8; IRR=0.36, 95%CI=0.15–0.71) and non-eyelid cancers (n=10; IRR=0.33, 95%CI=0.15–0.62) was significantly lower among Blacks (data not shown).
In SEER-11 between 1992 and 2003, IRs of SC were 37% lower (95%CI=14%–54%) among APIs (n=46, IR=0.12) than among Whites (n=618, IR=0.19) (API data not shown in tables). Incidence of SC in APIs did not differ markedly between males and females (IRR=0.89, 95%CI=0.46–1.67). For males, IRs of SC were significantly lower (IRR 0.44, 95%CI=0.26–0.70) among APIs (n=19, IR=0.11) than Whites (n=344, IR=0.25), whereas for females, IRs were similar (IRR=0.88, 95%CI 0.56–1.31) among APIs (n=27, IR=0.13) and Whites (n=274, IR=0.14). Incidence of SC of the eyelid among APIs did not differ significantly from Whites (IRR=1.08, 95%CI=0.69–1.62), in contrast to non-eyelid SC which occurred less often among APIs than Whites (IRR 0.37, 95%CI=0.22–0.59).
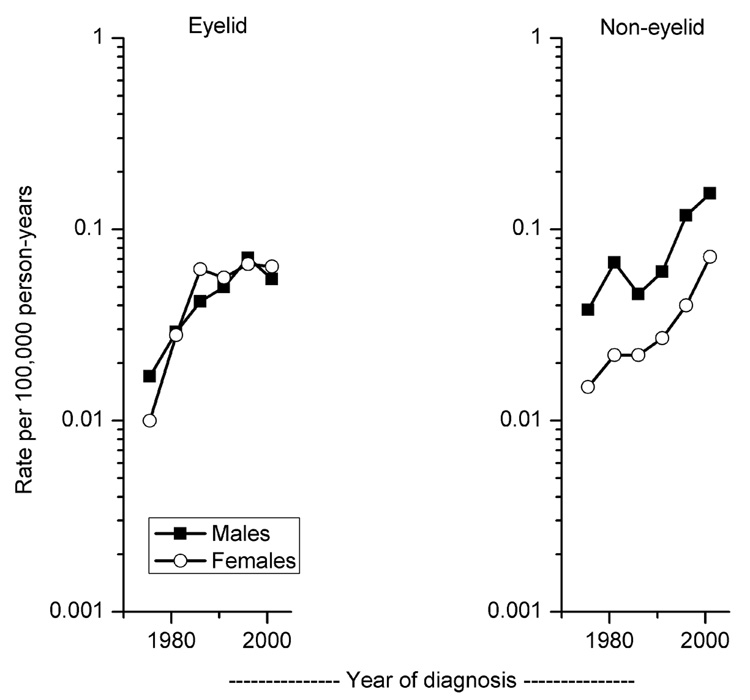
The incidence of all SC combined increased significantly at a rate of 5.2% per year (95%CI=4.1%–6.3%) from 0.04 during 1973 to 0.18 in 2003. However, IRs of eyelid tumors began to stabilize in recent years, earlier among females than males, while IRs of non-eyelid sites increased steadily with time (Figure 1). The male-to-female IRRs for eyelid SC remained close to 1.0 (range 0.7–1.7) over time. In contrast, the incidence of non-eyelid SC was consistently higher for males than females, with IRRs ranging from 2.1–3.0.
Figure 1. Age-adjusted trends.
Age-adjusted trends across six calendar periods (1973–1978, 1979–1983, 1984–1988, 1989–1993, 1994–1998, 1999–2003) of sebaceous carcinoma of the skin diagnosed in SEER-9 according to primary site and gender, 1973–2003.
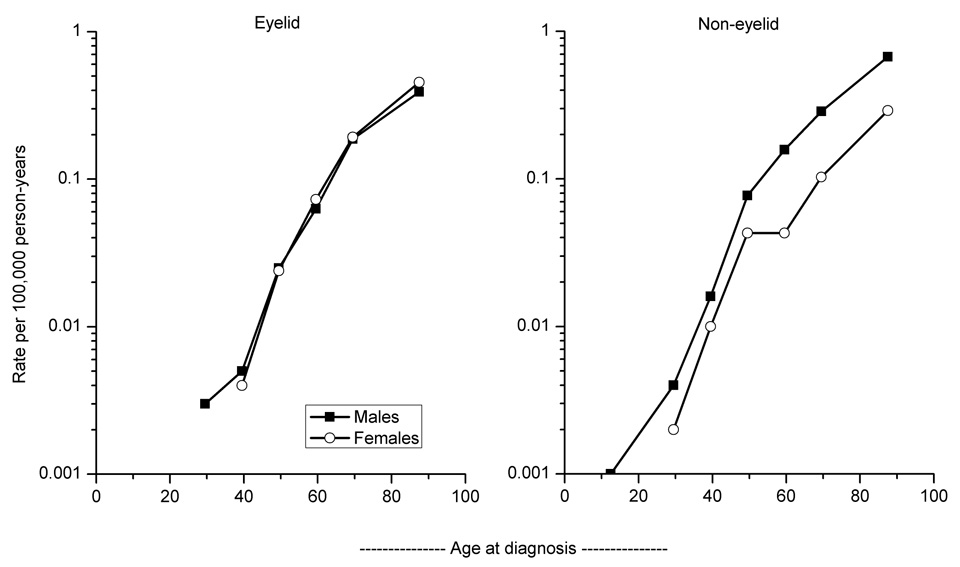
The incidence of eyelid and non-eyelid SC rose exponentially with advancing age among males and females (Figure 2). Across all age groups, IRs for eyelid SC did not differ significantly by gender, while the male predominance of non-eyelid SC persisted across all age groups, with significant differences at ages 55–64 years and older (data not shown).
Figure 2. Age-specific rates.
Age-specific incidence rates according to seven age groups (<25, 25–34, 35–44, 45–54, 55–64, 65–74, 75+ years) of sebaceous carcinoma of the skin diagnosed in SEER-9 according to primary site and gender, 1973–2003.
Multiple Primary Cancers
Among 500 two-month survivors of cutaneous SC, 91 subsequent primary cancers, including 12 third or higher order cancers, were diagnosed among 79 individuals during 1973–2003. Among those with two or more cancers, median age at SC was 68 years (range 35–87 years). The overall risk of developing a new malignancy following SC was 43% higher than expected based on SEER general population incidence (SIR=1.43, 95%CI=1.15–1.76) (Table 2). The subsequent cancer risk did not differ significantly between males (Obs.=54; SIR=1.52, 95%CI=1.14–1.99) and females (Obs.=37; SIR=1.32, 95%CI=0.93–1.81) (Pdiff=0.49), while the numbers of cases were too small to analyze by race. Over time, the SIR of developing a new cancer was relatively constant: 1.21 (Obs.=9; 95%CI=0.55–2.30), 1.51 (Obs.=39; 95%CI=1.07–2.06), 1.43 (Obs.=25; 95%CI=0.93–2.11), and 1.41 (Obs.=18; 95%CI=0.83–2.22) at <1, 1–4, 5–9, and 10+ years, respectively. Although based on small numbers, the subsequent risks for cancers of the salivary gland, nasal cavities/middle ear, ureter, and eye/orbit were more than 20-fold greater than expected.
Table 2.
Standardized incidence ratios of subsequent cancer among 2-month survivors of first primary sebaceous carcinoma of the skin diagnosed in SEER-9, overall and according to age, 1973–2003
| Age at diagnosis of first primary SC |
||||||||
|---|---|---|---|---|---|---|---|---|
| Total† |
<50 years |
50+ years |
||||||
| No. patients | 500 | 51 | 449 | |||||
| Person-years at risk |
3,287 |
493 |
2,794 |
|||||
| Subsequent cancer | Obs. | SIR | (95%CI) | Obs. | SIR | Obs. | SIR | Pdiff |
| All, excluding nonmelanoma skin | 91 | 1.43 | (1.15–1.76) | 13 | 4.58* | 78 | 1.28* | <0.001 |
| All solid | 84 | 1.49 | (1.19–1.85) | 13 | 5.08* | 71 | 1.32* | <0.001 |
| Tongue | 2 | 7.16 | (0.87–25.85) | 0 | ~ | 2 | 7.80 | ns |
| Salivary gland | 5 | 32.85 | (10.66–76.65) | 2 | 258.35* | 3 | 20.76* | <0.01 |
| Colon | 11 | 1.54 | (0.77–2.76) | 4 | 22.17* | 7 | 1.01 | <0.001 |
| Pancreas | 3 | 1.61 | (0.33–4.72) | 2 | 36.17* | 1 | 0.55 | <0.001 |
| Nose, nasal cavities, middle ear | 2 | 23.32 | (2.82–84.24) | 0 | ~ | 2 | 24.62* | ns |
| Lung | 13 | 1.40 | (0.74–2.39) | 1 | 2.59 | 12 | 1.35 | ns |
| Malignant melanoma, skin | 2 | 1.29 | (0.16–4.65) | 0 | ~ | 2 | 1.41 | ns |
| Female breast | 5 | 0.68 | (0.22–1.60) | 0 | ~ | 5 | 0.73 | ns |
| Uterine corpus | 4 | 2.71 | (0.74–6.95) | 2 | 20.95* | 2 | 1.45 | <0.001 |
| Prostate | 14 | 1.24 | (0.68–2.08) | 1 | 2.53 | 13 | 1.19 | ns |
| Urinary bladder | 7 | 1.90 | (0.76–3.91) | 0 | ~ | 7 | 1.96 | ns |
| Ureter | 3 | 28.09 | (5.79–82.09) | 0 | ~ | 3 | 28.73* | ns |
| Eye and orbit | 2 | 22.88 | (2.77–82.65) | 0 | ~ | 2 | 24.36* | ns |
| Brain | 2 | 3.63 | (0.44–13.12) | 0 | ~ | 2 | 3.92 | ns |
| Thyroid | 2 | 6.31 | (0.76–22.81) | 0 | ~ | 2 | 7.43 | ns |
| All lymphohematopoietic | 5 | 0.95 | (0.31–2.23) | 0 | ~ | 5 | 1.00 | ns |
| Leukemia, all | 3 | 1.62 | (0.34–4.75) | 0 | ~ | 3 | 1.68 | ns |
| Miscellaneous | 2 | 1.00 | (0.12–3.61) | 0 | ~ | 2 | 1.03 | ns |
Abbreviations:SC, sebaceous carcinoma; Obs., observed number of subsequent cancers; SIR, standardized incidence ratio; CI, confidence interval; Pdiff, test of homogeneity of difference; ns, not statistically significant (P>0.05); ~, SIR not calculated.
Limited to cancer sites with >1 case.
P<0.05
Individuals diagnosed with SC before 50 years of age (early-onset) had significantly higher relative (Pdiff<0.001) and absolute risks (Pdiff<0.05) for all subsequent cancers combined (SIR=4.59, 95%CI=2.44–7.84, EAR=206) compared to individuals diagnosed with SC at age 50 years and older (late-onset) (SIR=1.28, 95%CI=1.02–1.60, EAR=62) (Table 2). Although based on small numbers, the SIRs and EARs were significantly greater in the younger than the older age group for cancers of the colon (<50 years: SIR=22.16, EAR=77.5; 50+ years: SIR=1.01, EAR=0.2), pancreas (<50 years: SIR=36.17, EAR=39.4; 50+ years: SIR=0.55, EAR=−2.9), and uterine corpus (<50 years: SIR=20.95, EAR=94.4; 50+ years: SIR=1.45, EAR=4.0). Following SC, individuals in both age groups had a significantly elevated risk of developing salivary gland cancer, while only those 50 years and older had excess risks of cancers of the ureter, nasal cavities/middle ear, and eye/orbit. The risk of lymphohematopoietic malignancies was not significantly increased following SC, and no cancer occurred significantly below expectation.
The risks of developing SC of the skin following another primary cancer are presented in Table 3. Among 2,322,728 two-month survivors of all primary cancers, the SIR of subsequent SC was 1.52 (Obs.=102). In 99 patients with subsequent SC, the median age at first cancer diagnosis was 65 years (range 30–99 years); 36% had two or more invasive cancers in addition to SC. Significantly increased risks of SC were observed following cancers of the colon, ovary, pharynx, nasal cavities/middle ear, and unspecified male genital sites, as well as NHL and CLL. The risk of SC was significantly higher following early-onset (SIR=5.64) compared with late-onset malignancy (SIR=1.32; Pdiff<0.001). Most notable were the 60-fold relative risks of developing a subsequent SC among individuals with early-onset cancers of the colon and ovary.
Table 3.
Standardized incidence ratios of subsequent sebaceous carcinoma of the skin among survivors of other first primary cancers diagnosed in SEER-9, overall and according to age, 1973–2003
| Age at diagnosis of first primary cancer |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| First primary cancer |
Total subsequent SC |
<50 years |
50+ years |
||||||
| Site | No. | Obs. | SIR | (95%CI) | Obs. | SIR | Obs. | SIR | Pdiff |
| All, excluding nonmelanoma skin | 2,322,728 | 102 | 1.52 | (1.24–1.84) | 17 | 5.64* | 85 | 1.32* | <0.001 |
| All solid | 2,088,301 | 90 | 1.42 | (1.14–1.75) | 17 | 6.17* | 73 | 1.21 | <0.001 |
| Pharynx | 11,324 | 2 | 12.65 | (1.53–45.70) | 1 | 75.59* | 1 | 6.90 | <0.05 |
| Colon | 200,781 | 25 | 3.27 | (2.12–4.83) | 9 | 59.97* | 16 | 2.14* | <0.001 |
| Rectum | 57,675 | 4 | 2.08 | (0.57–5.34) | 1 | 18.90 | 3 | 1.61 | <0.01 |
| Nose, nasal cavities, middle ear | 3,927 | 2 | 21.13 | (2.56–76.34) | 0 | ~ | 2 | 22.56* | ns |
| Lung | 286,059 | 2 | 0.75 | (0.09–2.70) | 0 | ~ | 2 | 0.77 | ns |
| Malignant melanoma, skin | 78,863 | 6 | 2.51 | (0.92–5.45) | 0 | ~ | 6 | 2.90* | ns |
| Female breast | 370,513 | 9 | 0.76 | (0.35–1.44) | 1 | 1.24 | 8 | 0.72 | ns |
| Uterine corpus | 83,021 | 3 | 0.77 | (0.16–2.26) | 0 | ~ | 3 | 0.80 | ns |
| Ovary | 42,330 | 6 | 9.04 | (3.32–19.68) | 4 | 60.07* | 2 | 3.35 | <0.001 |
| Prostate | 347,068 | 12 | 0.68 | (0.35–1.18) | 0 | ~ | 12 | 0.68 | ns |
| Other unspecified male genital† | 696 | 2 | 70.82 | (8.58–255.82) | 0 | ~ | 2 | 74.13* | ns |
| Urinary bladder | 106,581 | 6 | 1.20 | (0.44–2.60) | 0 | ~ | 6 | 1.24 | ns |
| Thyroid | 35,954 | 2 | 2.82 | (0.34–10.17) | 0 | ~ | 2 | 3.80 | ns |
| All lymphohematopoietic | 193,595 | 11 | 3.02 | (1.51–5.40) | 0 | ~ | 11 | 3.23* | ns |
| Non-Hodgkin lymphoma | 86,140 | 6 | 3.09 | (1.14–6.74) | 0 | ~ | 6 | 3.30* | ns |
| Chronic lymphocytic leukemia | 22,653 | 4 | 4.73 | (1.2912.11) | 0 | ~ | 4 | 4.82* | ns |
Abbreviations:SC, sebaceous carcinoma; No., number of first primary cancers; Obs., observed number of subsequent cancers; SIR, standardized incidence ratio; CI, confidence interval; Pdiff, test of homogeneity of difference; ns, not statistically significant (P>0.05); ~, SIR not calculated.
P<0.05
Includes International Classification for Oncology (third edition) topography codes C630–639.
Risk of all subsequent cancers combined was significantly higher following SC of non-eyelid sites (SIR=1.84) than for eyelid SC (SIR=1.08; Pdiff<0.05) (Table 4). Among individuals with an initial SC of the eyelid, only the risk of subsequent salivary gland cancer (Obs.=4, SIR=49.07) was significantly increased. Based on small numbers of cases, increased risks after non-eyelid SC were seen for cancers of the uterine corpus, ureter, eye/orbit, and thyroid. In the reverse direction, following all other primary cancers combined, the risk of SC was significantly greater for non-eyelid sites (SIR=1.89) than the eyelid (SIR=1.07; Pdiff<0.01). The risk of developing non-eyelid SC was significantly elevated following cancers of the colon, rectum, ovary, and nasal cavities/middle ear, as well as NHL and CLL.
Table 4.
Standardized incidence ratios of subsequent cancer among 2-month survivors of first primary sebaceous carcinoma and standardized incidence ratios of subsequent sebaceous carcinoma following other primary cancers diagnosed in SEER-9 according to skin site, 1973–2003
| SC as first primary cancer |
SC as second or higher order cancer |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Eyelid |
Non-eyelid |
Eyelid |
Non-eyelid |
|||||||
| No. patients | 33 | 46 | 33 | 66 | ||||||
| Median age at first cancer (range), years | 69 (35–84) | 67.5 (42–87) | 67 (37–99) | 64.5 (30–87) | ||||||
| Male:female ratio |
0.6:1 |
2.8:1 |
0.9:1 |
2.5:1 |
||||||
| Cancer site | Obs. | SIR | Obs. | SIR | Pdiff | Obs. | SIR | Obs. | SIR | Pdiff |
| All, excluding nonmelanoma skin | 37 | 1.08 | 54 | 1.84* | <0.05 | 33 | 1.07 | 69 | 1.89* | <0.01 |
| All solid | 36 | 1.19 | 48 | 1.84* | <0.05 | 30 | 1.03 | 60 | 1.75* | <0.05 |
| Salivary gland | 4 | 49.07* | 1 | 14.13 | ns | 1 | 11.94 | 0 | ~ | ns |
| Colon | 7 | 1.70 | 4 | 1.33 | ns | 4 | 1.11 | 21 | 5.19* | <0.01 |
| Rectum | 0 | ~ | 0 | ~ | ns | 0 | ~ | 4 | 3.85* | ns |
| Pancreas | 1 | 0.94 | 2 | 2.52 | ns | 0 | ~ | 0 | ~ | ns |
| Nose, nasal cavities, middle ear | 1 | 21.50 | 1 | 25.48 | ns | 0 | ~ | 2 | 38.16* | ns |
| Uterine corpus | 1 | 1.02 | 3 | 6.12* | ns | 1 | 0.43 | 2 | 1.29 | ns |
| Ovary | 0 | ~ | 0 | ~ | ns | 2 | 5.05 | 4 | 14.97* | ns |
| Urinary bladder | 3 | 1.61 | 4 | 2.19 | ns | 3 | 1.48 | 3 | 1.00 | ns |
| Ureter | 0 | ~ | 3 | 61.25* | ns | 0 | ~ | 1 | 18.54 | ns |
| Eye and orbit† | 0 | ~ | 2 | 48.01* | ns | 0 | ~ | 0 | ~ | ns |
| Thyroid | 0 | ~ | 2 | 13.73* | ns | 2 | 5.75 | 0 | ~ | ns |
| All lymphohematopoietic | 1 | 0.34 | 4 | 1.71 | ns | 2 | 1.22 | 9 | 4.50* | ns |
| Non-Hodgkin lymphoma | 1 | 0.74 | 0 | ~ | ns | 1 | 1.13 | 5 | 4.74* | ns |
| Chronic lymphocytic leukemia | 0 | ~ | 1 | 2.93 | ns | 0 | ~ | 4 | 8.57* | ns |
Abbreviations: SC, sebaceous carcinoma; No., number; Obs., observed number of subsequent cancers; SIR, standardized incidence ratio; Pdiff, test of homogeneity of difference; ns, not statistically significant (P>0.05); ~, SIR not calculated.
P<0.05.
Includes squamous cell carcinoma (n=1) and malignant melanoma (n=1).
Discussion
To our knowledge, this is the largest population-based study of cutaneous SC reported to date, enabling an evaluation of age-specific incidence patterns according to eyelid and non-eyelid sites. We found a predominance of cutaneous SC among males, Whites, and at non-eyelid sites. Many,3, 5, 21–24, 30–32 but not all,33, 34 studies of sebaceous tumors of the eyelid have reported a female predominance, often based on relative frequencies (which may reflect the generally longer lifespan in women). Surveys of extraocular SC have been limited by small numbers of cases.8–10 Although we did not find significant gender differences for eyelid SC in any age group, the incidence of non-eyelid SC was consistently higher among males, particularly at older ages. Eyelid and non-eyelid SC are considered biologically similar,1 however, the variation in incidence patterns by gender suggests etiologic heterogeneity.
We also observed a significantly lower incidence of both eyelid and non-eyelid SC among Blacks compared to Whites, which resembles the racial differences reported for keratinocyte-derived skin cancers as well as cutaneous melanoma.35, 36 Our findings suggest that the protective effects of cutaneous pigmentation on various forms of skin cancer may extend to sebaceous tumors of the eyelid and other sites.
Sebaceous carcinoma of the eyelid has been reported to occur more frequently30 and to comprise a greater proportion of eyelid tumors among Asian than Western populations.21 However, there are limited data on actual incidence rates for eyelid SC in Asia,22 and no information on incidence of non-eyelid SC. Our SEER population-based data revealed that, similar to Blacks, the risks of SC were significantly lower among APIs than Whites, but the deficit was limited to non-eyelid tumors. Taken together, the patterns in the U.S. point to racial differences in susceptibility to SC according to anatomic site.
Although the incidence trend for eyelid SC rose after the SEER Program began in 1973, the IRs seemed to plateau beginning in the mid-1980s, resembling the stable trend reported in Taiwan between 1979 and 1999.22 In contrast, the incidence of non-eyelid SC in SEER has continued to climb in both sexes. These findings may reflect site-specific differences in risk factors for SC and/or differential detection by medical specialists.
Our study is the first to quantitatively assess the risk of other cancers occurring in patients with SC. Several literature reviews of MTS have reported cancers occurring prior to, concurrently with, or subsequent to sebaceous tumors.13, 16, 19, 20 Most frequently reported have been colorectal and genitourinary cancers, accounting for 56% and 22% of cases, respectively.16 In our population-based study, patients with SC had a 43% significantly increased risk of developing a new malignancy compared to the general population. In the opposite direction, a significant 52% elevated risk of subsequent SC was seen among those diagnosed with other forms of cancer. Over all ages combined, we observed excess risks for cancers of the ureter, salivary gland, nasal cavities/middle ear, and eye/orbit following SC, while increased risks of SC were found after cancers of the colon, ovary, pharynx, and nasal cavities/middle ear, as well as NHL and CLL. In contrast to prior clinical surveys, colorectal cancer accounted for only 12% of cancers following SC and 28% of cancers associated with subsequent SC. This may reflect differences in the types of sebaceous tumors included in earlier studies (e.g., we did not assess sebaceous adenomas or epitheliomas) and/or reporting bias in the clinical literature. In addition, among patients previously reported with multiple cancers, 40–50% of SC have involved the eyelid,16, 19, 20 a slightly higher estimate than the 36% we observed in the SEER data. In the SEER population, however, we found that the risk of other cancers was significantly higher in association with non-eyelid than eyelid SC. Notably, individuals with SC in our study did not differ from the general population in their risk for several common cancers, such as breast, lung, and prostate.
It is noteworthy that, following cutaneous SC, risk of all subsequent cancers combined was markedly higher among those diagnosed with SC before rather than after age 50 years. In particular, individuals with early-onset SC had significantly increased risks of colon, uterine corpus, and pancreatic cancers, whereas those with early-onset cancers of the colon and ovary had significantly elevated risks of subsequent SC. In addition, individuals with late-onset SC had significantly increased risk of ureter cancer, while those with late-onset colon cancer had an excess of subsequent SC. Cancers of the colon, ureter, uterine corpus, ovary and, less commonly, pancreas have been reported in previous series of SC,13, 16, 19, 20 and all are considered HNPCC-related tumors.37 As with HNPCC, individuals with MTS often have germline mutations in mismatch repair genes (e.g., MLH1, MSH2) with resulting microsatellite instability (MSI) in all tumors, including SC.15, 38–42 In contrast, most sporadic sebaceous tumors have not been associated with MSI.38, 41, 43 In our study, the spectrum of early-onset cancers associated with SC, the greatly elevated risk estimates, and the reciprocal increases in the risks of colon cancer and SC, are consistent with the constellation of tumors reported in MTS.
In addition to MTS, genetic predisposition may contribute to the excess risk of SC of the eyelid reported in patients with retinoblastoma treated with radiotherapy.44 Furthermore, SC has been reported in immunosuppressed populations, including organ transplant recipients and patients infected with human immunodeficiency virus (HIV),45–47 which suggests that immunologic impairment may have contributed to the 3-fold or greater risks of SC that we observed following NHL, CLL, and melanoma at age 50 or older. Since immune alterations predispose to infectious agents, further consideration in SC should be given to the possible role of oncogenic viruses.48, 49 Ultraviolet radiation may also play a role in the development of SC, as suggested by the racial disparities in our study, and the predominance of tumors occurring on the head and neck, although sebaceous glands are also more common in this area.2, 45
Cancers of the salivary gland, nasal cavities/middle ear, and eye/orbit have been reported rarely in surveys of SC.7, 16, 50 It is uncertain whether the excess risks we observed for these cancer sites reflect a true association or are due to misclassified metastases,2, 4 multicentric tumors,5, 7 histologic transformation,50 or radiotherapy administered for the initial cancer. Although none of the cancers associated with SC in our series were reported to have sebaceous histology, the ability of SC to masquerade as other neoplasms must be considered.2, 4, 5, 33
The population-based design and magnitude of our study allowed us to estimate with some precision the incidence patterns and cancer risks associated with SC, while avoiding the biases that may result from clinical series. Limitations included the lack of centralized pathology review, particularly given diagnostic challenges associated with SC; however, the SEER database has high reliability and 93% of patients with multiple primary cancers have microscopic confirmation.28 We included ICD-O-3 topography codes for other and unspecified parts of the face (C443) and unspecified skin site (C449) within the non-eyelid category, which may have resulted in eyelid lesions being included within the non-eyelid category. Nevertheless, misclassification of tumor site would have yielded attenuated risk differences between eyelid and non-eyelid SC. Our analysis also included multiple comparisons, and small numbers were involved in several risk estimates, so that some of the observed associations may represent chance findings.
In summary, this population-based study of SC revealed a higher incidence in Whites than Blacks or APIs, consistent with the racial differences seen with other forms of skin cancer. In contrast to the patterns for eyelid SC, the non-eyelid tumors predominated among males, rose steadily over time, and were associated with significantly increased risk of other primary cancers, either preceding or following a diagnosis of SC. The associations with SC were most pronounced for early-onset cancers of the colon, uterine corpus, ovary, and pancreas, as well as late-onset ureter cancer, all of which are components of MTS, a variant of HNPCC (Lynch syndrome). In addition, a role for immunologic factors was suggested by the elevated risks of SC following NHL, CLL, and cutaneous melanoma, consistent with previous reports of SC following organ transplantation and HIV infection. Further studies of SC by site of origin should clarify the genetic, immunologic, and environmental determinants of this tumor and help design screening programs aimed at the early detection of SC and visceral cancers associated with MTS.
Acknowledgments
The authors thank Nathan Appel, Information Management Services, Inc., Silver Spring, Maryland, for careful review of the data.
Acknowledgement of research support:This research was supported by the Intramural Research Program of the National Cancer Institute/National Institutes of Health and the Department of Veterans Affairs.
Footnotes
The authors have no financial conflicts of interest.
Bibliography
- 1.Rutten A, Wick MR, Sangueza OP, Wallace C. LeBoit PE, Burg G, Weedon D, Sarasin A, editors. Tumours with sebaceous differentiation. Lyon: IARC Press; World Health Organization Classification of Tumours: Pathology and Genetics of Skin Tumours. 2006:160–163.
- 2.Nelson BR, Hamlet KR, Gillard M, Railan D, Johnson TM. Sebaceous carcinoma. J Am Acad Dermatol. 1995 Jul;33(1):1–15. doi: 10.1016/0190-9622(95)90001-2. quiz 16–18. [DOI] [PubMed] [Google Scholar]
- 3.Shields JA, Demirci H, Marr BP, Eagle RC, Jr, Shields CL. Sebaceous carcinoma of the eyelids: personal experience with 60 cases. Ophthalmology. 2004 Dec;111(12):2151–2157. doi: 10.1016/j.ophtha.2004.07.031. [DOI] [PubMed] [Google Scholar]
- 4.McLean IW, Burnier MN, Zimmerman LE, Jakobiec FA. Tumors of the eye and ocular adnexa. Washington, D.C: Armed Forces Institute of Pathology; 1994. [Google Scholar]
- 5.Wolfe JT, 3rd, Yeatts RP, Wick MR, Campbell RJ, Waller RR. Sebaceous carcinoma of the eyelid. Errors in clinical and pathologic diagnosis. Am J Surg Pathol. 1984 Aug;8(8):597–606. doi: 10.1097/00000478-198408000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Khan Z, Misra G, Fiander AN, Dallimore NS. Sebaceous carcinoma of the vulva. Bjog. 2003 Feb;110(2):227–228. [PubMed] [Google Scholar]
- 7.Shulman J, Waisman J, Morledge D. Sebaceous carcinoma of the parotid gland. Arch Otolaryngol. 1973 Dec;98(6):417–421. doi: 10.1001/archotol.1973.00780020431015. [DOI] [PubMed] [Google Scholar]
- 8.Rulon DB, Helwig EB. Cutaneous sebaceous neoplasms. Cancer. 1974 Jan;33(1):82–102. doi: 10.1002/1097-0142(197401)33:1<82::aid-cncr2820330115>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 9.Bassetto F, Baraziol R, Sottosanti MV, Scarpa C, Montesco M. Biological behavior of the sebaceous carcinoma of the head. Dermatol Surg. 2004 Mar;30(3):472–476. doi: 10.1111/j.1524-4725.2004.30025.x. [DOI] [PubMed] [Google Scholar]
- 10.Wick MR, Goellner JR, Wolfe JT, 3rd, Su WP. Adnexal carcinomas of the skin. II. Extraocular sebaceous carcinomas. Cancer. 1985 Sep 1;56(5):1163–1172. doi: 10.1002/1097-0142(19850901)56:5<1163::aid-cncr2820560533>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 11.Bailet JW, Zimmerman MC, Arnstein DP, Wollman JS, Mickel RA. Sebaceous carcinoma of the head and neck. Case report and literature review. Arch Otolaryngol Head Neck Surg. 1992 Nov;118(11):1245–1249. doi: 10.1001/archotol.1992.01880110113020. [DOI] [PubMed] [Google Scholar]
- 12.Ponti G, Ponz de Leon M. Muir-Torre syndrome. Lancet Oncol. 2005 Dec;6(12):980–987. doi: 10.1016/S1470-2045(05)70465-4. [DOI] [PubMed] [Google Scholar]
- 13.Cohen PR, Kohn SR, Kurzrock R. Association of sebaceous gland tumors and internal malignancy: the Muir-Torre syndrome. Am J Med. 1991 May;90(5):606–613. [PubMed] [Google Scholar]
- 14.Lynch HT, Lynch PM, Pester J, Fusaro RM. The cancer family syndrome. Rare cutaneous phenotypic linkage of Torre's syndrome. Arch Intern Med. 1981 Apr;141(5):607–611. [PubMed] [Google Scholar]
- 15.South CD, Hampel H, Comeras I, Westman JA, Frankel WL, de la Chapelle A. The frequency of Muir-Torre syndrome among Lynch syndrome families. J Natl Cancer Inst. 2008 Feb 20;100(4):277–281. doi: 10.1093/jnci/djm291. [DOI] [PubMed] [Google Scholar]
- 16.Akhtar S, Oza KK, Khan SA, Wright J. Muir-Torre syndrome: case report of a patient with concurrent jejunal and ureteral cancer and a review of the literature. J Am Acad Dermatol. 1999 Nov;41(5 Pt 1):681–686. doi: 10.1016/s0190-9622(99)70001-0. [DOI] [PubMed] [Google Scholar]
- 17.Rishi K, Font RL. Sebaceous gland tumors of the eyelids and conjunctiva in the Muir-Torre syndrome: a clinicopathologic study of five cases and literature review. Ophthal Plast Reconstr Surg. 2004 Jan;20(1):31–36. doi: 10.1097/01.IOP.0000103009.79852.BD. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz RA, Torre DP. The Muir-Torre syndrome: a 25-year retrospect. J Am Acad Dermatol. 1995 Jul;33(1):90–104. doi: 10.1016/0190-9622(95)90017-9. [DOI] [PubMed] [Google Scholar]
- 19.Cohen PR, Kohn SR, Davis DA, Kurzrock R. Muir-Torre syndrome. Dermatol Clin. 1995 Jan;13(1):79–89. [PubMed] [Google Scholar]
- 20.Serleth HJ, Kisken WA. A Muir-Torre syndrome family. Am Surg. 1998 Apr;64(4):365–369. [PubMed] [Google Scholar]
- 21.Lee SB, Saw SM, Au Eong KG, Chan TK, Lee HP. Incidence of eyelid cancers in Singapore from 1968 to 1995. Br J Ophthalmol. 1999 May;83(5):595–597. doi: 10.1136/bjo.83.5.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin HY, Cheng CY, Hsu WM, Kao WH, Chou P. Incidence of eyelid cancers in Taiwan: a 21-year review. Ophthalmology. 2006 Nov;113(11):2101–2107. doi: 10.1016/j.ophtha.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 23.Margo CE, Mulla ZD. Malignant tumors of the eyelid: a population-based study of non-basal cell and non-squamous cell malignant neoplasms. Arch Ophthalmol. 1998 Feb;116(2):195–198. doi: 10.1001/archopht.116.2.195. [DOI] [PubMed] [Google Scholar]
- 24.Muqit MM, Roberts F, Lee WR, Kemp E. Improved survival rates in sebaceous carcinoma of the eyelid. Eye. 2004 Jan;18(1):49–53. doi: 10.1038/sj.eye.6700523. [DOI] [PubMed] [Google Scholar]
- 25.Surveillance, Epidemiology and End Results (SEER) Program. SEER*Stat Database: Incidence - SEER Regs Public-Use, Nov 2005 Sub (1973–2003) National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2006, based on the November 2005 submission. ( www.seer.cancer.gov)
- 26.Fritz A, Percy C, Jack A, et al., editors. International Classification of Diseases for Oncology. 3rd ed. Geneva (Switzerland): World Health Organization; 2000. [Google Scholar]
- 27.Devesa SS, Donaldson J, Fears T. Graphical presentation of trends in rates. Am J Epidemiol. 1995 Feb 15;141(4):300–304. doi: 10.1093/aje/141.4.300. [DOI] [PubMed] [Google Scholar]
- 28.Fraumeni JF, Jr, Curtis RE, Edwards BK, Tucker MA. Introduction. In: Curtis RE, Freedman DM, Ron E, et al., editors. New malignancies among cancer survivors: SEER cancer registries, 1973–2000. Bethesda, MD: National Cancer Institute; 2006. pp. 1–7. NIH Publ. No. 05-5302. [Google Scholar]
- 29.Breslow NE, Lubin JH, Marek P, Langholz B. Multiplicative models and cohort analysis. J Am Stat Assoc. 1983;78:1–12. [Google Scholar]
- 30.Ni C, Searl SS, Kuo PK, Chu FR, Chong CS, Albert DM. Sebaceous cell carcinomas of the ocular adnexa. Int Ophthalmol Clin. 1982 Spring;22(1):23–61. doi: 10.1097/00004397-198202210-00006. [DOI] [PubMed] [Google Scholar]
- 31.Rao NA, Hidayat AA, McLean IW, Zimmerman LE. Sebaceous carcinomas of the ocular adnexa: A clinicopathologic study of 104 cases, with five-year follow-up data. Hum Pathol. 1982 Feb;13(2):113–122. doi: 10.1016/s0046-8177(82)80115-9. [DOI] [PubMed] [Google Scholar]
- 32.Wang JK, Liao SL, Jou JR, et al. Malignant eyelid tumours in Taiwan. Eye. 2003 Mar;17(2):216–220. doi: 10.1038/sj.eye.6700231. [DOI] [PubMed] [Google Scholar]
- 33.Doxanas MT, Green WR. Sebaceous gland carcinoma. Review of 40 cases. Arch Ophthalmol. 1984 Feb;102(2):245–249. doi: 10.1001/archopht.1984.01040030195025. [DOI] [PubMed] [Google Scholar]
- 34.Sihota R, Tandon K, Betharia SM, Arora R. Malignant eyelid tumors in an Indian population. Arch Ophthalmol. 1996 Jan;114(1):108–109. doi: 10.1001/archopht.1996.01100130104031. [DOI] [PubMed] [Google Scholar]
- 35.Gruber SB, Armstrong BK. Cutaneous and ocular melanoma. In: Schottenfeld D, Fraumeni JF Jr, editors. Cancer Epidemiology and Prevention. New York: Oxford University Press; 2006. pp. 1196–1229. [Google Scholar]
- 36.Karagas MR, Weinstock MA, Nelson HH. Keratinocyte carcinomas (basal and squamous cell carcinomas of the skin) In: Schottenfeld D, Fraumeni JF Jr, editors. Cancer Epidemiology and Prevention. New York: Oxford University Press; 2006. pp. 1230–1250. [Google Scholar]
- 37.Umar A, Boland CR, Terdiman JP, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004 Feb 18;96(4):261–268. doi: 10.1093/jnci/djh034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Entius MM, Keller JJ, Drillenburg P, Kuypers KC, Giardiello FM, Offerhaus GJ. Microsatellite instability and expression of hMLH-1 and hMSH-2 in sebaceous gland carcinomas as markers for Muir-Torre syndrome. Clin Cancer Res. 2000 May;6(5):1784–1789. [PubMed] [Google Scholar]
- 39.Honchel R, Halling KC, Schaid DJ, Pittelkow M, Thibodeau SN. Microsatellite instability in Muir-Torre syndrome. Cancer Res. 1994 Mar 1;54(5):1159–1163. [PubMed] [Google Scholar]
- 40.Machin P, Catasus L, Pons C, et al. Microsatellite instability and immunostaining for MSH-2 and MLH-1 in cutaneous and internal tumors from patients with the Muir-Torre syndrome. J Cutan Pathol. 2002 Aug;29(7):415–420. doi: 10.1034/j.1600-0560.2002.290705.x. [DOI] [PubMed] [Google Scholar]
- 41.Ponti G, Losi L, Di Gregorioc C, et al. Identification of Muir-Torre syndrome among patients with sebaceous tumors and keratoacanthomas: role of clinical features, microsatellite instability, and immunohistochemistry. Cancer. 2005 Mar 1;103(5):1018–1025. doi: 10.1002/cncr.20873. [DOI] [PubMed] [Google Scholar]
- 42.Ponti G, Losi L, Pedroni M, et al. Value of MLH1 and MSH2 mutations in the appearance of Muir-Torre syndrome phenotype in HNPCC patients presenting sebaceous gland tumors or keratoacanthomas. J Invest Dermatol. 2006 Oct;126(10):2302–2307. doi: 10.1038/sj.jid.5700475. [DOI] [PubMed] [Google Scholar]
- 43.Kruse R, Rutten A, Schweiger N, et al. Frequency of microsatellite instability in unselected sebaceous gland neoplasias and hyperplasias. J Invest Dermatol. 2003 May;120(5):858–864. doi: 10.1046/j.1523-1747.2003.12125.x. [DOI] [PubMed] [Google Scholar]
- 44.Howrey RP, Lipham WJ, Schultz WH, et al. Sebaceous gland carcinoma: a subtle second malignancy following radiation therapy in patients with bilateral retinoblastoma. Cancer. 1998 Aug 15;83(4):767–771. [PubMed] [Google Scholar]
- 45.Harwood CA, Swale VJ, Bataille VA, et al. An association between sebaceous carcinoma and microsatellite instability in immunosuppressed organ transplant recipients. J Invest Dermatol. 2001 Feb;116(2):246–253. doi: 10.1046/j.1523-1747.2001.01233.x. [DOI] [PubMed] [Google Scholar]
- 46.Bordea C, Wojnarowska F, Millard PR, Doll H, Welsh K, Morris PJ. Skin cancers in renal-transplant recipients occur more frequently than previously recognized in a temperate climate. Transplantation. 2004 Feb 27;77(4):574–579. doi: 10.1097/01.tp.0000108491.62935.df. [DOI] [PubMed] [Google Scholar]
- 47.Frantz S, Greiner A, Schoen C, Langmann P, Klinker H. A sebaceous tumor in a patient with acquired immunodeficiency syndrome. Eur J Med Res. 2002 Mar 28;7(3):135–137. [PubMed] [Google Scholar]
- 48.Feng H, Shuda M, Chang Y, Moore PS. Clonal Integration of a Polyomavirus in Human Merkel Cell Carcinoma. Science. 2008 Jan 17; doi: 10.1126/science.1152586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hayashi N, Furihata M, Ohtsuki Y, Ueno H. Search for accumulation of p53 protein and detection of human papillomavirus genomes in sebaceous gland carcinoma of the eyelid. Virchows Arch. 1994;424(5):503–509. doi: 10.1007/BF00191436. [DOI] [PubMed] [Google Scholar]
- 50.Jakobiec FA, Zimmerman LE, La Piana F, Hornblass A, Breffeilh RA, Lackey JK. Unusual eyelid tumors with sebaceous differentiation in the Muir-Torre syndrome. Rapid clinical regrowth and frank squamous transformation after biopsy. Ophthalmology. 1988 Nov;95(11):1543–1548. doi: 10.1016/s0161-6420(88)32975-1. [DOI] [PubMed] [Google Scholar]