Abstract
Olfactory learning in humans leads to enhanced perceptual discrimination of odor cues. Examining mouse models of both aversive and appetitive conditioning, we demonstrate a mechanism which may underlie this adult learning phenomenon. Topographically unique spatial wiring of the olfactory system allowed us to demonstrate that emotional learning of odor cues alters the primary sensory representation within the nose and brain of adult mice. Transgenic mice labeled at the M71 odorant receptor (specifically activated by the odorant acetophenone) were behaviorally trained with olfactory-dependent fear conditioning or conditioned place preference using acetophenone. Odor-trained mice had larger M71-specific glomeruli and an increase in M71-specific sensory neurons within the nose compared with mice that were untrained, trained to a non-M71 activating odorant, or had nonassociative pairings of acetophenone. These data indicate that the primary sensory neuron population and its projections may remain plastic in adults, providing a structural mechanism for learning-enhanced olfactory sensitivity and discrimination.
Keywords: olfactory, glomerulus, fear, learning, plasticity, learning memory
Introduction
Recent data suggests that olfactory emotional learning alters the perceptual and cortical representations of previously indiscriminable odor cues in humans (Li et al., 2008). Although recent studies have examined olfactory learning and plasticity during development (Kerr and Belluscio, 2006; Marks et al., 2006), how such large sensory changes may occur in adults remains unknown. Furthermore, it raises the question of whether the olfactory piriform cortex is the first site of significant learning-induced synaptic plasticity, or can more early sensory areas also change with learning?
More generally, it has been assumed that long-term memories are mediated by structural changes in the brain, but it has been challenging to demonstrate this hypothesis in complex mammalian brains. There has been some evidence of higher-order synaptic rearrangement following developmental sensory deprivation, motor learning, and song learning (cf. Bailey and Kandel, 1993; Weiler et al., 1995; Knudsen et al., 2000), but not at the level of a primary sensory system and these changes are limited to development. However, the mammalian olfactory system, which is exquisitely organized with regards to its primary sensory receptive field map (Buck and Axel, 1991; Ressler et al., 1994; Mombaerts et al., 1996), may remain dynamic beyond the organizational “critical period” typical of other sensory systems. For example, granule cell count in the olfactory bulb can be altered with olfactory enrichment or deprivation (Corotto et al., 1994; Rochefort et al., 2002; Woo et al., 2006).
As a chief component of the olfactory epithelium, olfactory sensory neurons (OSNs) may exhibit a similar experience-dependent regulation. If so, the resultant changes might be seen in both the olfactory epithelium and bulb glomeruli. The M71–IRES–tauLacZ transgenic mouse (Vassalli et al., 2002) provides a powerful approach to visualize the changes within one specific population of OSNs during olfactory exposure and learning. These mice contain lacZ which encodes β-galactosidase in neurons which express the M71 odorant receptor. In vitro, the M71 receptor has been shown to respond to the odorant acetophenone (Bozza et al., 2002; Hague et al., 2004). Additionally, studies of rat olfactory bulb activity with 2-deoxyglucose (Johnson et al., 2005; Farahbod et al., 2006) have indicated that glomerular activation with acetophenone is localized to olfactory bulb regions containing M71-specific glomeruli.
Previously, our laboratory has effectively performed olfactory-dependent Pavlovian fear conditioning in mice, using acetophenone as an odorant conditioned stimulus paired with shock to create associative memories (Jones et al., 2005, 2007). As a mouse learns the associative relevance of an odor, it is likely that the olfactory system would become attuned to that stimulus. We propose that odor-specific fear learning leads to structure-function changes in the portions of the olfactory system encoding the odor, but not in portions of the olfactory system encoding unrelated odors. Evidence suggests that during early postnatal development, the olfactory system displays structural and functional plasticity (Mackay-Sim and Kittel, 1991a; Kerr and Belluscio, 2006). However, it is unknown whether the organization of the primary sensory pathway in the olfactory system, which is unique in its ongoing neurogenesis throughout an animal's life (Mackay-Sim and Kittel, 1991a,b), continues to be plastic in the adult. Thus, we used olfactory fear and appetitive conditioning in M71-LacZ transgenic mice to test the hypothesis that odor-specific alterations in the representation of the primary sensory pathway occur in a learning-dependent manner in adult animals.
Materials and Methods
Animals.
Adult male M71–IRES–tauLacZ transgenic mice (Vassalli et al., 2002) maintained in mixed 129/Sv × C57BL/6 background (Jackson Laboratories) were used in all studies, except adult male C57BL/6J mice were used to test acetophenone versus propanol discrimination. Mice were housed in a temperature controlled vivarium on a 12 h light/dark cycle in standard group cages (≤5/cage) and were given ad libitum access to food and water. All experiments were performed during the light cycle and approved by Emory University Institutional Review Board following National Institutes of Health Internal Animal Care and Use Committee standards.
Fear training and testing.
Fear training and testing were conducted using startle response systems (SR-LAB, San Diego Instruments), modified to deliver discrete odor stimuli as previously described (Jones et al., 2005) (Fig. 1A). Odorants consisted of 10% acetophenone or 10% propanol (both from Sigma-Aldrich) in propylene glycol. Briefly, mice were habituated to the startle chambers three times (10 min per day) before training. Mice then received 2 training sessions per week for 3 weeks to ensure strong and stable odor-shock association. Each odor + shock training session consisted of 5 trials of 10 s odor conditioned stimulus coterminating with a 0.25 s, 0.4 mA footshock, presented with an average 120 s intertrial interval (ITI) (range 90–150 s).
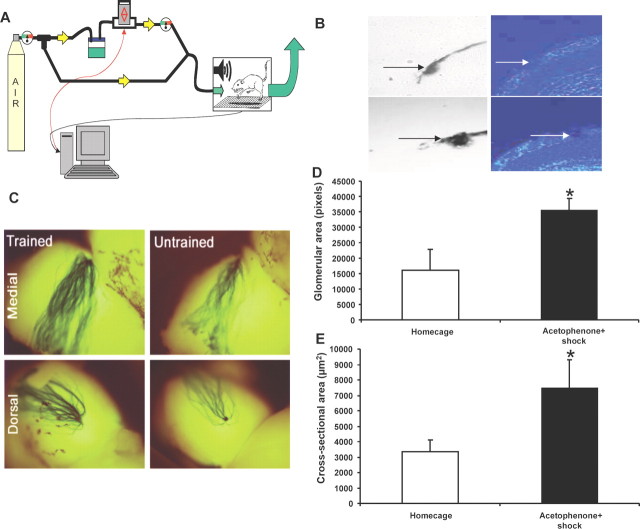
Figure 1.
Increased M71 axon density and glomerular size with fear conditioning. A, SR-Lab Response software controls a solenoid switch (red arrows) which allows compressed air to flow through the odorant jar and into the startle chamber. When closed, clean air flows with no difference in airflow. Backflow is prevented by a series of one-way valves (yellow arrows). The odor is removed via an exhaust hose (green arrow) by outflow fan. Shock is generated by a programmable animal shocker and is delivered through the bars in the cage floor. During behavioral testing, startle is elicited by a 105 dB white noise burst. Activity and startle amplitude are measured by a piezoelectronic device beneath the floor of the cage. B, Cross-section of an olfactory bulb from a homecage mouse (top left, LacZ; top right, DAPI stain) or from an acetophenone + shock paired mouse (bottom left, LacZ; bottom right, DAPI stain). C, Medial and dorsal pairs of M71 glomeruli in X-gal stained olfactory bulbs from M71–IRES–tauLacZ mice. D, Glomerular surface area was larger in the acetophenone + shock trained group than in the homecage group (p < 0.05). E, Glomerular cross-sectional area was larger in the acetophenone-trained versus the homecage mice, *p < 0.05.
In Experiment 1, one group (n = 6 mice, 10 glomeruli) received odor (acetophenone) + shock pairing while a second group (n = 6 mice, 10 glomeruli) remained in the home cage and received handling on training days. In Experiment 2, an acetophenone-alone no-shock group (n = 4 mice, 14 glomeruli) was added in addition to the acetophenone + shock (n = 4 mice, 12 glomeruli) and homecage (n = 5 mice, 18 glomeruli). To evaluate odor discrimination between propanol and acetophenone, separate C57BL/6J wild-type mice (n = 12) were trained using the same procedure for only one session. The following day, mice were presented with either 10 acetophenone-startle trials or 10 propanol-startle trials randomly intermingled with 10 startle-alone trials and separated by 90 s ITIs. Each odor-startle trial consisted of a 10 s odor presentation coterminated with 50 ms, 105 dB noise burst. For each animal, a fear-potentiated startle score was computed by subtracting the mean of the startle-alone trials from the mean of the odor-startle trials. In Experiment 3, we compared M71-lacZ mice that received acetophenone + shock training (n = 4 mice, 13 glomeruli), propanol + shock training (n = 3 mice, 8 glomeruli), acetophenone exposure only in their home cage (n = 4 mice, 12 glomeruli), and home cage exposure alone (n = 3 mice, 7 glomeruli). Mice received two training sessions per week for 3 weeks. In Experiment 4, we compared M71-lacZ mice fear trained to the same acetophenone odor-shock pairings over 3 weeks as described above (n = 5 mice, 19 glomeruli), with mice that were trained to the same number of acetophenone odor-shock pairings over 3 consecutive days (10 odor-shock pairings per training session per day) (n = 6 mice, 16 glomeruli), and with home cage control mice (n = 14 mice, 51 glomeruli).
Olfactory conditioned place preference training.
Conditioned place preference (CPP) apparatuses (Med Associates) each consisted of 2 conditioning chambers (black with rod floor and white with mesh floor) connected by a central chamber with vertical sliding manual doors. Using pilot mice the chamber adjustable light levels were optimized for balanced preference between black and white chambers. Fifteen photo-beams recorded time spent in each compartment.
To investigate whether the M71-IRES-LacZ mice may demonstrate glomerular plasticity to appetitive learning, in Experiment 5, adult male mice were trained for cocaine CPP with acetophenone (n = 7 mice, 18 glomeruli) or without the presence of acetophenone pairing with cocaine (n = 8 mice, 26 glomeruli).
The mice received pretraining test for individual preference by placing the mouse in the neutral compartment and allowing 20 min of free access to all 3 chambers. Three days of CPP training were performed, where each day animals received one cocaine-paired session [10 mg/kg of cocaine-hydrochloride IP (Sigma) dissolved in sterile 0.9% saline] in the white chamber, and one saline vehicle-pairing session in the black chamber (distributed and alternating between 9:00 and 11:00 A.M. and 2:00 and 4:00 P.M.) for 30 min.
The day after the completion of CPP training, all mice received post-training test (identical to pretraining test), in which animals were allowed to freely explore all three chambers for 20 min. CPP data were calculated as percent time spent on cocaine-paired chamber over the total time.
Beta-galactosidase staining.
Three weeks following the onset of odor-shock fear training or odor-cocaine CPP training, mice were perfused with 4% paraformaldehyde in PBS. For olfactory epithelium cell counts, the nasal cavity was placed in 0.5 m EDTA at 4°C for 3 weeks for decalcification. Olfactory epithelium were cut at 30 μm on a Leica cryostat and mounted on 4 parallel series of slides. M71-LacZ was stained for b-gal, using 45 mg of X-gal (1 mg/ml) dissolved in 600 μl of DMSO and 45 ml of a solution of 5 mm potassium ferricyanide, 5 mm potassium ferrocyanide, and 2 mm MgCl in 1 M PBS, applied to slides and incubated overnight at 37°C.
M71 olfactory glomerular quantification.
Using a microscope-mounted digital camera, high-resolution images were captured at 40× magnification of the M71-positive glomeruli. Images were converted to grayscale and equalized for background brightness. The distribution of pixel brightness was exported in Scion Image as gray levels from 0 = black to 255 = white. X-gal-labeled glomerular area was quantified as pixels, less than a set threshold gray level of 150 (optimized for axon vs background).
X-gal-labeled glomerular cross-sectional area was obtained as follows: frozen olfactory bulbs were sectioned at 30 μm on a cryostat and mounted on 4 parallel series of slides. Microscope-mounted digital camera, high resolution images were captured of every section that contained X-gal labeled glomerulus. The largest cross-section for each glomerulus was selected for measurement. Each glomerulus was traced using the lasso tool in Photoshop and the area was recorded from the histogram tool. Glomerular cross-sectional areas were quantified as μm2 and mirrored similar quantification of glomerular area from the surface area of the bulbs. A total of 104 glomeruli were analyzed in the morphometric studies shown in Figures 1 and 2.
Figure 2.
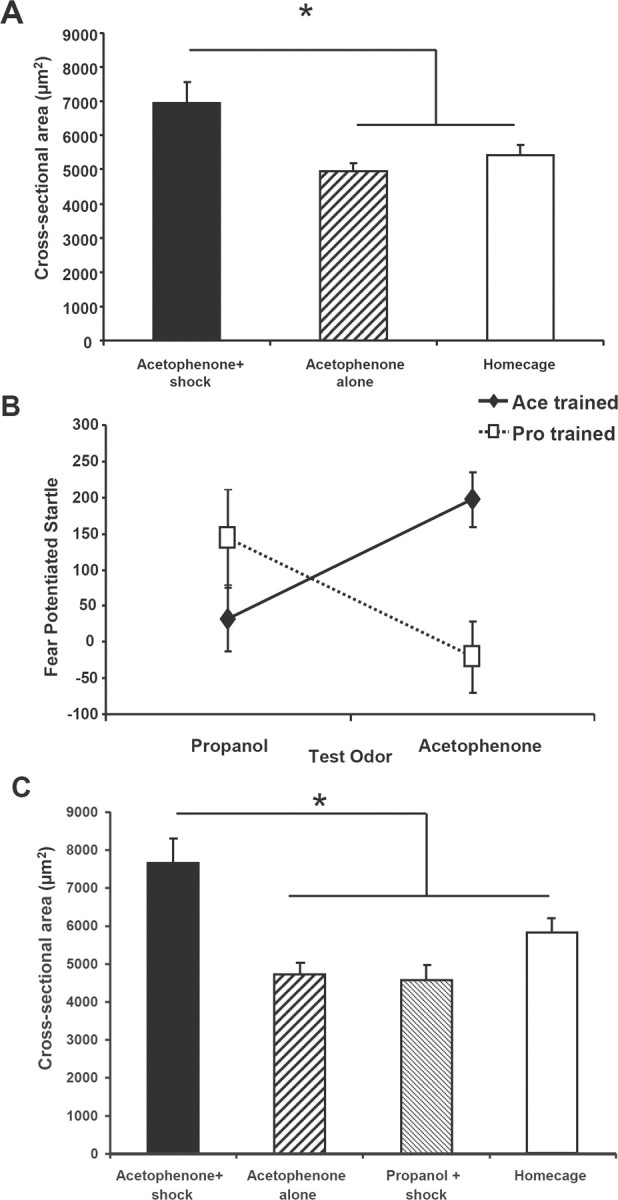
M71 glomerular size increases require associative learning. A, Glomerular cross-sectional area was larger in the acetophenone + shock group than either the acetophenone-alone or the homecage groups; *p < 0.01 for acetophenone + shock versus homecage; *p < 0.05 for acetophenone + shock versus acetophenone-alone. B, Mice learn an odor-shock association to both propanol and acetophenone and can discriminate between the two odors, p < 0.001. C, Glomerular cross-sectional areas were greater in the acetophenone + shock versus homecage, acetophenone-alone, and propanol-shock groups; *p < 0.05 for each group versus acetophenone + shock.
Olfactory epithelium cell counts.
One series of LacZ-positive OSNs were restained with X-gal as above and counted on a microscope at 100× magnification only in cells in the mature layer of the entire olfactory epithelium. Approximately 1400 olfactory epithelium sections were individually counted in total. The total number of lacZ+ cells counted was divided by the number of sections analyzed to derive the average number of lacZ+ cells per section as in Figure 3B. A second experimenter who was fully blind to all experimental conditions confirmed the counts by sampling a lesser number of slides and applying the same criteria to count cells. Inter-rater reliability of this correlation (supplemental Fig. 2, available at www.jneurosci.org as supplemental material) was confirmed by a Pearson's correlation coefficient of 0.95 (p < 0.05).
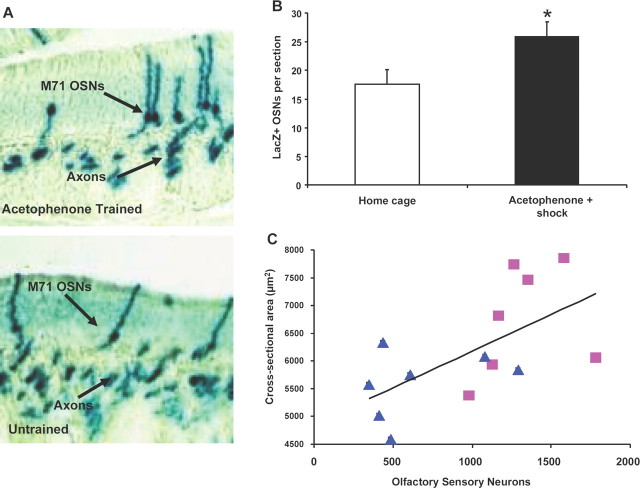
Figure 3.
Associative learning leads to increases in olfactory sensory neuron number. A, Olfactory sensory neurons in the olfactory epithelium of an acetophenone + shock trained (top) and homecage mouse (bottom). B, More M71-LacZ+ olfactory sensory neurons were found in acetophenone-trained than homecage mice (p < 0.05). C, Glomerular size is positively correlated with increasing olfactory sensory neuron number (triangles, home cage; squares, acetophenone + shock; Pearson's correlation coefficient = 0.62, p < 0.05).
Statistical analyses.
Glomerular area and glomerular cross-sectional area were analyzed by Student's t test (Experiments 1 and 5) or one-way ANOVA (Experiments 2, 3, and 4). Fear potentiated startle was analyzed by one-way ANOVA with repeated measures. Olfactory sensory neuron number was analyzed by Student's t test, and glomerular size to olfactory sensory neuron number correlation was analyzed by linear regression. CPP was analyzed by two-way ANOVA with repeated measures (training). All ANOVA main effects or interactions were followed by Tukey post hoc tests.
Results
Increased M71 axon density and glomerular size with fear conditioning using acetophenone
Although olfactory fear conditioning can be accomplished in as little as one training session (Cousens and Otto, 1998; Paschall and Davis, 2002), the experimental design was to optimize the robustness and stability of any possible learning adaptations by conditioning the mice to thirty total trials of acetophenone paired with shock spread over 3 weeks (Fig. 1A). After the 3 week training period, brains were collected and stained with X-gal, to label and analyze M71 (LacZ-positive) glomeruli from the bulb surface (Fig. 1C). M71 glomerular area, quantified from the surface of the bulbs, was significantly larger in trained than in homecage mice, p < 0.05 (Fig. 1C,D). Alone, this could be the result of differences in the nature of axon paths ranging over the surface of the bulb, rather than an actual increase in axonal innervation. To address this, we quantified the cross-sectional area of each glomerulus, where glomeruli from odor-trained mice were also larger than those from homecage mice, *p < 0.05 (Fig. 1B,E).
Increased glomerular size requires associative olfactory learning
It was unclear whether the increase in glomerular size was due to odor exposure alone (a nonspecific result of olfactory enrichment), or was specific to the odor-shock association. To address this, in Experiment 2, we measured M71 glomerular cross-sectional area in mice receiving acetophenone + shock pairings versus those that received acetophenone exposures alone without shocks, and homecage handled mice. Glomerular cross-sectional area was larger in the acetophenone + shock group than either the acetophenone-alone or the homecage groups (F(2,43) = 6.99, p < 0.01, post hoc Tukey tests, p < 0.01 for acetophenone + shock vs homecage, and p < 0.05 for acetophenone + shock vs acetophenone-alone) (Fig. 2A).
Increased glomerular size requires pairing with an M71-specific odor
It was unknown whether stress from shocks may generally increase olfactory representations, independent of the specific nature of the acetophenone-receptive pathway. Furthermore, olfactory learning may lead to overall increases in glomerular size or innervation regardless of the odorant specificity. To test these hypotheses in Experiment 3, a propanol + shock training group was added, since propanol activates areas of the bulb distinct from acetophenone (supplemental Fig. 1, available at www.jneurosci.org as supplemental material) (Johnson and Leon, 2000), and would control both for the specificity of the odorant, as well as the effect of receiving shocks not associated with acetophenone. The mice differentially learned either acetophenone + shock or propanol + shock associations (interaction: F(1,22) = 20, p < 0.001) (Fig. 2B), showing that acetophenone and propanol are perceptually different to a mouse and that both can support fear conditioning. These robust discrimination training results were similar to our previous behavioral findings using acetophenone and amyl acetate (Jones et al., 2005). Following training, the acetophenone + shock trained group had larger glomerular cross-sectional areas compared with the propanol + shock, acetophenone alone, and homecage control groups F(3,39) = 9.91, p < 0.001, post hoc Tukey tests p < 0.05 for each group versus acetophenone + shock (Fig. 2C).
Increased glomerular size is associated with increased number of olfactory sensory neurons
We hypothesized that increased glomerular size was due to an increase in the number of M71 receptor-expressing OSNs. To test this, M71 OSNs (LacZ-positive) were quantified within the olfactory epithelium of acetophenone + shock trained and homecage no-odor control mice (Fig. 3A). The acetophenone-trained mice had a higher number of LacZ-positive OSNs than the homecage controls, p < 0.05 (Fig. 3B). There was also a correlation between the number of M71-specific olfactory neurons and the cross-sectional areas of M71 glomeruli in the olfactory bulb, Pearson's correlation coefficient = 0.62, F(1,13) = 7.62, p < 0.05 (Fig. 3C).
Massed olfactory training results in long-lasting glomerular size increases
In Experiment 4, we investigated whether it was the number of trainings or length of the overall training period that determined the increase in glomerular area. Thus we compared 3 weeks of spaced training to the same number of acetophenone + shock pairings provided over 3 consecutive training days, followed by 2.5 weeks without intervention. Three weeks after the onset of training, glomerular area was greater in the 3 d and 3 weeks acetophenone + shock trained groups versus homecage (main effect of training: F(2,83) = 10.74, p < 0.001, post hoc Tukey tests, p < 0.05 vs homecage) (Fig. 4A,B).
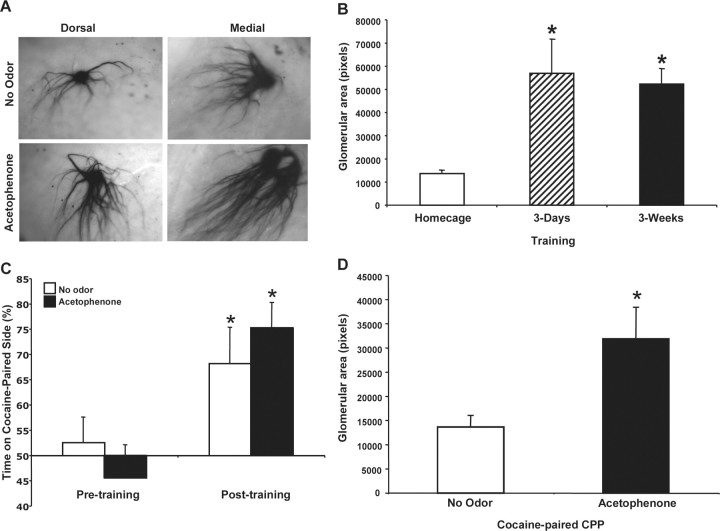
Figure 4.
Long-lasting glomerular changes result from aversive and appetitive learning. A, Dorsal and medial M71 glomeruli in X-gal stained olfactory bulbs from acetophenone + shock paired mice trained in only 3 consecutive days compared with untrained (no odor) control mice examined 2.5 weeks after training. B, Glomerular area was greater in the 3 d and 3 weeks acetophenone + shock trained versus the homecage mice, *p < 0.05 versus homecage. C, M71 mice learned cocaine CPP equally well with either no odor, or acetophenone paired with the cocaine, *p < 0.05 versus pretrained. D, Glomerular area was greater in mice that were CPP-trained with acetophenone paired with cocaine, versus no odor paired with cocaine, *p < 0.05.
Appetitive olfactory conditioned place preference results in similar glomerular size increases
Finally, we hypothesized that an alternate type of olfactory-dependent emotional learning may also lead to glomerular adaptation. Experiment 5 aimed to investigate whether appetitive learning from acetophenone-cocaine paired CPP would lead to similar adaptations as the aversive fear learning described above. M71-IRES-LacZ mice were trained in the cocaine conditioned place preference task, with M71-activating acetophenone, or no odor, in the reinforced chamber. These mice learned CPP by spending more time in the cocaine paired side post-training with either no odor, or acetophenone paired with the cocaine (main effect of test, F(1,13) = 10.64, p < 0.05, post hoc Tukey tests, p < 0.05 vs pretrained), but there was no effect of odor on cocaine CPP (Fig. 4C). Thus, the odor is not directly required for the CPP behavior. Notably, 3 weeks following this appetitive learning task, the cocaine + acetophenone paired mice had increased glomerular area compared with the no odor CPP-trained mice, t test, p < 0.05 (Fig. 4D). We think that it is likely that the olfactory learning occurred in parallel with the place preference learning, but that we are at a “ceiling effect” of CPP. We expect that other measures of olfactory sensitivity would show differences between the group. Overall, these data demonstrate that appetitive olfactory learning supports enhanced M71 representations within the olfactory bulb as well as aversive olfactory learning.
Discussion
In summary, we demonstrated that an M71-specific odor (acetophenone) paired with fear conditioning increased the size of M71 glomeruli. This result suggests that the primary olfactory sensory pathway in adult mice is capable of learning-dependent structural plasticity. Additionally, we confirmed that odor-exposure alone is insufficient for the increases in glomerular size, but that olfactory-dependent associative learning was necessary. We then found that the increased representation of M71 odorant receptor-specific glomeruli was due to the specific associative learning process produced by pairing acetophenone with shock, and not due to nonspecific effects of shock. Importantly, this experiment also demonstrated that odor-shock associative learning with a non-M71 activating odorant (propanol) does not lead to increased M71 sensory representation within the olfactory bulb. Interestingly, analyses at the level of the olfactory epithelium indicated that the increase in M71 glomerular size was a result of increased number of M71-specific sensory neurons within the olfactory epithelium. We next determined that 3 consecutive days of olfactory dependent fear training replicated the glomerular plasticity observed over 3 weeks of training, which also suggests that the increase in glomerular area is long-lasting, remaining for at least 3 weeks following the 3 d training regimen. Therefore, we hypothesized that 3 consecutive days of CPP training would also lead to changes in glomerular size 3 weeks later. In the final experiment, we demonstrated that long-lasting glomerular plasticity in adult mice can occur with both appetitive and aversive associative olfactory learning.
These data suggest that the olfactory system is dynamically regulated, even at the level of the primary sensory pathway, in an activity-dependent manner in adult mice. These odor-specific adaptations occur with associative learning in a way that does not depend on odor exposure alone, nonspecific odor learning, shock-stress, or handling procedures. The final experiment with cocaine-mediated CPP demonstrates that the valence of the emotional learning is unlikely the crucial factor in glomerular plasticity, but instead it is potentially the emotional salience. Finally, the data suggest that the learning-dependent changes we find in the olfactory bulb are accompanied by an increased number of odorant-specific neurons in the olfactory epithelium. Although glomerular enlargement with olfactory stimulation has been observed in developing young rats (Coopersmith and Leon, 1984; Woo et al., 1987) or in insect models (Faber et al., 1999; Sachse et al., 2007), this study is novel in the demonstration of glomerular plasticity not just following olfactory stimulation, but after olfactory-dependent associative learning, in the adult mammalian olfactory system.
Since OSNs periodically die off and are replaced, the cell number increase in the olfactory epithelium could indicate a learning-dependent increase in neurogenesis of the M71 neurons. However, given that odorant receptor specificity is thought to be determined postmitotically by an irreversible stochastic process (Li et al., 2004; Shykind et al., 2004), such a selective neurogenesis would require the CNS to influence OR gene expression in immature neurons by a yet undiscovered mechanism. Alternatively, longer survival time may exist for the M71 neurons, perhaps through learning-dependent release of neurotrophins from the olfactory bulb. This hypothesis seems more likely since mature OSNs have neurotrophin receptor-expressing axon projections to the OB (Roskams et al., 1996) and the connection to OB is known to be required for OSN survival (Schwob et al., 1992).
Our results may explain enhanced odorant sensitivity and increased intranasal olfactory-evoked responses to repetitive odorant exposure as has been demonstrated in mice (Wang et al., 1993), rats (Youngentob and Kent, 1995) and humans (Wang et al., 2004). These increased odor responses were seen after 23–90 d, approximately equal or longer than our own protocol. Of course, more rapid changes in odor perception might be associated with higher olfactory areas and may not require this mechanism (Li et al., 2008).
The data may also explain multiple chemical sensitivity syndrome, in which a person becomes extremely hypersensitive to certain odorants (Bell et al., 1992). This idiopathic disorder is thought to occur with aversive olfactory experience and subsequent odor sensitization. Our data suggest a hypothesis that in such patients, aversive olfactory experiences may lead to enhanced structural representation and sensitivity of specific olfactory receptor pathways at the primary sensory level. Finally this phenomenon, occurring at the primary olfactory synapse, will provide a very powerful model of adult learning-dependent plasticity, in which differential cues within the same modality can differentially modify structural representation. This approach also provides a powerful tool for examination of top–down regulation of sensory systems. For example, our group has shown that acquisition, but not expression, of olfactory-mediated fear-conditioning is blocked by local infusion of NMDA antagonists into the amygdala (Walker et al., 2005). Hence, we can now test whether the primary sensory changes we have seen in the current experiments are also amygdala-dependent. If so, this would provide a striking example of how the amygdala can modulate learning-dependent plasticity in different brain areas (McGaugh, 2004), in this case, perhaps via direct centrifugal projections to the olfactory bulb and/or via amygdala-dependent modulation of monoamine cell groups that project to the bulb.
Footnotes
This work was supported by National Institutes of Health (NIH) Grants MH069884, DA019624 (K.J.R.), and R37 MH47840 (M.D.), National Alliance for Research on Schizophrenia and Depression, the Center for Behavioral Neuroscience (National Science Foundation agreement IBN-987675), Burroughs Wellcome Fund, and by NIH Grant P51RR000165 to Yerkes National Primate Research Center.
References
- Bailey CH, Kandel ER. Structural changes accompanying memory storage. Annu Rev Physiol. 1993;55:397–426. doi: 10.1146/annurev.ph.55.030193.002145. [DOI] [PubMed] [Google Scholar]
- Bell IR, Miller CS, Schwartz GE. An olfactory-limbic model of multiple chemical sensitivity syndrome: possible relationships to kindling and affective spectrum disorders. Biol Psychiatry. 1992;32:218–242. doi: 10.1016/0006-3223(92)90105-9. [DOI] [PubMed] [Google Scholar]
- Bozza T, Feinstein P, Zheng C, Mombaerts P. Odorant receptor expression defines functional units in the mouse olfactory system. J Neurosci. 2002;22:3033–3043. doi: 10.1523/JNEUROSCI.22-08-03033.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck L, Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. 1991;65:175–187. doi: 10.1016/0092-8674(91)90418-x. [DOI] [PubMed] [Google Scholar]
- Coopersmith R, Leon M. Enhanced neural response to familiar olfactory cues. Science. 1984;225:849–851. doi: 10.1126/science.6474157. [DOI] [PubMed] [Google Scholar]
- Corotto FS, Henegar JR, Maruniak JA. Odor deprivation leads to reduced neurogenesis and reduced neuronal survival in the olfactory bulb of the adult mouse. Neuroscience. 1994;61:739–744. doi: 10.1016/0306-4522(94)90397-2. [DOI] [PubMed] [Google Scholar]
- Cousens G, Otto T. Both pre- and posttraining excitotoxic lesions of the basolateral amygdala abolish the expression of olfactory and contextual fear conditioning. Behav Neurosci. 1998;112:1092–1103. doi: 10.1037//0735-7044.112.5.1092. [DOI] [PubMed] [Google Scholar]
- Faber T, Joerges J, Menzel R. Associative learning modifies neural representations of odors in the insect brain. Nat Neurosci. 1999;2:74–78. doi: 10.1038/4576. [DOI] [PubMed] [Google Scholar]
- Farahbod H, Johnson BA, Minami SS, Leon M. Chemotopic representations of aromatic odorants in the rat olfactory bulb. J Comp Neurol. 2006;497:350–366. doi: 10.1002/cne.20982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hague C, Uberti MA, Chen Z, Bush CF, Jones SV, Ressler KJ, Hall RA, Minneman KP. Olfactory receptor surface expression is driven by association with the beta2-adrenergic receptor. Proc Natl Acad Sci U S A. 2004;101:13672–13676. doi: 10.1073/pnas.0403854101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Leon M. Modular representations of odorants in the glomerular layer of the rat olfactory bulb and the effects of stimulus concentration. J Comp Neurol. 2000;422:496–509. doi: 10.1002/1096-9861(20000710)422:4<496::aid-cne2>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Farahbod H, Leon M. Interactions between odorant functional group and hydrocarbon structure influence activity in glomerular response modules in the rat olfactory bulb. J Comp Neurol. 2005;483:205–216. doi: 10.1002/cne.20409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SV, Heldt SA, Davis M, Ressler KJ. Olfactory-mediated fear conditioning in mice: simultaneous measurements of fear-potentiated startle and freezing. Behav Neurosci. 2005;119:329–335. doi: 10.1037/0735-7044.119.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SV, Stanek-Rattiner L, Davis M, Ressler KJ. Differential regional expression of brain-derived neurotrophic factor following olfactory fear learning. Learn Mem. 2007;14:816–820. doi: 10.1101/lm.781507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr MA, Belluscio L. Olfactory experience accelerates glomerular refinement in the mammalian olfactory bulb. Nat Neurosci. 2006;9:484–486. doi: 10.1038/nn1673. [DOI] [PubMed] [Google Scholar]
- Knudsen EI, Zheng W, DeBello WM. Traces of learning in the auditory localization pathway. Proc Natl Acad Sci U S A. 2000;97:11815–11820. doi: 10.1073/pnas.97.22.11815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Ishii T, Feinstein P, Mombaerts P. Odorant receptor gene choice is reset by nuclear transfer from mouse olfactory sensory neurons. Nature. 2004;428:393–399. doi: 10.1038/nature02433. [DOI] [PubMed] [Google Scholar]
- Li W, Howard JD, Parrish TB, Gottfried JA. Aversive learning enhances perceptual and cortical discrimination of indiscriminable odor cues. Science. 2008;319:1842–1845. doi: 10.1126/science.1152837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay-Sim A, Kittel P. Cell dynamics in the adult mouse olfactory epithelium: a quantitative autoradiographic study. J Neurosci. 1991a;11:979–984. doi: 10.1523/JNEUROSCI.11-04-00979.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay-Sim A, Kittel PW. On the life span of olfactory receptor neurons. Eur J Neurosci. 1991b;3:209–215. doi: 10.1111/j.1460-9568.1991.tb00081.x. [DOI] [PubMed] [Google Scholar]
- Marks CA, Cheng K, Cummings DM, Belluscio L. Activity-dependent plasticity in the olfactory intrabulbar map. J Neurosci. 2006;26:11257–11266. doi: 10.1523/JNEUROSCI.2805-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- Mombaerts P, Wang F, Dulac C, Chao SK, Nemes A, Mendelsohn M, Edmondson J, Axel R. Visualizing an olfactory sensory map. Cell. 1996;87:675–686. doi: 10.1016/s0092-8674(00)81387-2. [DOI] [PubMed] [Google Scholar]
- Paschall GY, Davis M. Olfactory-mediated fear-potentiated startle. Behav Neurosci. 2002;116:4–12. doi: 10.1037//0735-7044.116.1.4. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Sullivan SL, Buck LB. Information coding in the olfactory system: evidence for a stereotyped and highly organized epitope map in the olfactory bulb. Cell. 1994;79:1245–1255. doi: 10.1016/0092-8674(94)90015-9. [DOI] [PubMed] [Google Scholar]
- Rochefort C, Gheusi G, Vincent JD, Lledo PM. Enriched odor exposure increases the number of newborn neurons in the adult olfactory bulb and improves odor memory. J Neurosci. 2002;22:2679–2689. doi: 10.1523/JNEUROSCI.22-07-02679.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roskams AJ, Bethel MA, Hurt KJ, Ronnett GV. Sequential expression of Trks A, B, and C in the regenerating olfactory neuroepithelium. J Neurosci. 1996;16:1294–1307. doi: 10.1523/JNEUROSCI.16-04-01294.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachse S, Rueckert E, Keller A, Okada R, Tanaka NK, Ito K, Vosshall LB. Activity-dependent plasticity in an olfactory circuit. Neuron. 2007;56:838–850. doi: 10.1016/j.neuron.2007.10.035. [DOI] [PubMed] [Google Scholar]
- Schwob JE, Szumowski KE, Stasky AA. Olfactory sensory neurons are trophically dependent on the olfactory bulb for their prolonged survival. J Neurosci. 1992;12:3896–3919. doi: 10.1523/JNEUROSCI.12-10-03896.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shykind BM, Rohani SC, O'Donnell S, Nemes A, Mendelsohn M, Sun Y, Axel R, Barnea G. Gene switching and the stability of odorant receptor gene choice. Cell. 2004;117:801–815. doi: 10.1016/j.cell.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Vassalli A, Rothman A, Feinstein P, Zapotocky M, Mombaerts P. Minigenes impart odorant receptor-specific axon guidance in the olfactory bulb. Neuron. 2002;35:681–696. doi: 10.1016/s0896-6273(02)00793-6. [DOI] [PubMed] [Google Scholar]
- Walker DL, Paschall GY, Davis M. Glutamate receptor antagonist infusions into the basolateral and medial amygdala reveal differential contributions to olfactory vs. context fear conditioning and expression. Learn Mem. 2005;12:120–129. doi: 10.1101/lm.87105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HW, Wysocki CJ, Gold GH. Induction of olfactory receptor sensitivity in mice. Science. 1993;260:998–1000. doi: 10.1126/science.8493539. [DOI] [PubMed] [Google Scholar]
- Wang L, Chen L, Jacob T. Evidence for peripheral plasticity in human odour response. J Physiol. 2004;554:236–244. doi: 10.1113/jphysiol.2003.054726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler IJ, Hawrylak N, Greenough WT. Morphogenesis in memory formation: synaptic and cellular mechanisms. Behav Brain Res. 1995;66:1–6. doi: 10.1016/0166-4328(94)00116-w. [DOI] [PubMed] [Google Scholar]
- Woo CC, Coopersmith R, Leon M. Localized changes in olfactory bulb morphology associated with early olfactory learning. J Comp Neurol. 1987;263:113–125. doi: 10.1002/cne.902630110. [DOI] [PubMed] [Google Scholar]
- Woo CC, Hingco EE, Taylor GE, Leon M. Exposure to a broad range of odorants decreases cell mortality in the olfactory bulb. Neuroreport. 2006;17:817–821. doi: 10.1097/01.wnr.0000215780.84226.2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngentob SL, Kent PF. Enhancement of odorant-induced mucosal activity patterns in rats trained on an odorant identification task. Brain Res. 1995;670:82–88. doi: 10.1016/0006-8993(94)01275-m. [DOI] [PubMed] [Google Scholar]