Abstract
Objectives
Pelvic (ovarian) serous carcinomas frequently contain p53 mutations. Recently, a candidate serous cancer precursor (the p53 signature) with p53 mutations and other features in common with serous cancer has been discovered in distal fallopian tube mucosa. This study examined the relationship of putative ovarian cancer risk factors with the presence of p53 signatures in women with BRCA mutations (BRCA+).
Methods
Fallopian tubes from 75 BRCA+ women were immunostained for p53 signatures and correlated with age at first childbirth, parity, oral contraceptive use, body mass index BMI), and BRCA subtype (1 or 2). Statistical analysis was performed with the T-test or Chi-square analysis and logistic regression adjusting for age and parity.
Results
Thirty-eight percent of tubes contained p53 signatures, which were significantly associated with older age at first childbirth (mean 30.8 v. 28.4 yrs; p = 0.04) and lower parity (mean 1.4 v. 2.2; p=0.01) in univariate analyses. The unadjusted odds ratios were 3.8 (p-trend = 0.04) for first childbirth ≥ 30 years versus < 30 and 0.2 (p-trend = 0.01) for parity ≥ 3 versus nulliparous women. After adjusting for age and parity, the trend for age at first childbirth became non-significant (adjusted odds ratio 3.5; p-trend = 0.15), while that for parity remained significant (adjusted odds ratio 0.2; p-trend 0.02).
Conclusions
The p53 signature is significantly associated with lower parity and possibly higher age at first childbirth, further linking this entity to serous cancer via risk factors associated with ovulation. The p53 signature merits consideration as a surrogate marker for serous cancer risk.
Keywords: BRCA, p53, Fallopian tube neoplasms, Serous carcinoma, Ovarian neoplasms, Fimbria
Introduction
Ovarian cancer is diagnosed in approximately 22,200 women yearly in the United States and causes approximately 16,210 deaths, with a worldwide incidence and mortality of 190,000 and 114,000 respectively. 1 2 The pathogenesis of ovarian cancer is diverse. One pathway entails multiple genetic events paralleling a histologic progression from benign to malignant, including borderline and low-grade malignancies and endometrioid carcinomas. Defects in mismatch repair (microsatellite instability) and mutations in KRAS/BRAF, beta catenin, and pTEN characterize this pathway. The second involves mutations in the p53 tumor suppressor gene and rapid progression to malignancy, which characterizes serous carcinomas. 3 4 Because they involve serosal surfaces with rapid peritoneal spread, serous carcinomas are the most lethal form of epithelial ovarian cancer.5 6 7
Experimental data support an origin of ovarian carcinomas in the ovarian surface epithelium, and some prior reports identified focal accumulations of p53 protein in ovarian cortical inclusion cysts and tubal mucosa of women with, or at risk for, ovarian carcinoma. However, a precursor to high-grade serous ovarian cancer has not been demonstrated and universally accepted. 8 9 10 11 12 13 Three recent studies localized both BRCA+ and sporadic (non-familial) tubal carcinomas to the fimbria. 14 15 16 Protocols using systematic analysis of the fimbriated end have associated tubal intraepithelial carcinoma (TIC) with many ovarian serous carcinomas and shown that they often share the same p53 mutations. 17
DNA damage results in activation of specific response pathways, and the coordination of cell cycle arrest requires a functional p53 protein. The relationship between DNA damage and subsequent p53 mutations is unknown, but one study has proposed that the former exerts a selective pressure for the latter, ultimately leading to loss of cell cycle control.18 19 Mutations in p53 commonly occur in cancer but may be seen earlier in both preinvasive neoplasia and clonal epithelial expansion. 20 21 In a recent analysis of fallopian tubes from BRCA+ women, we discovered small segments of strongly p53 positive benign-appearing epithelium with a low proliferative index. On further study, these “p53 signatures” were found to share several attributes with tubal intraepithelial carcinoma, including: 1) strong nuclear localization of p53 protein, 2) location in the fimbria, 3) a secretory cell phenotype, 4) evidence of DNA damage, 5) p53 mutations, and 6) occasional direct continuity with tubal intraepithelial carcinoma.22 Except in rare cases, studies of cortical inclusion cysts in BRCA+ women have failed to demonstrate this entity.23 12 22 Based on these findings, the tubal p53 signature was proposed as an important precursor to pelvic serous carcinoma.
Factors associated with a lower risk of ovarian cancer include increased parity, oral contraceptive use and a prior tubal ligation.24 25 In some studies of BRCA+ women all three variables are associated with risk reduction. 26 Others have not seen significant correlations between with oral contraceptive use or tubal ligation. 27 28 Some have noted differences in the protective effect between BRCA1 and BRCA2 mutations carriers. 29 Overall, the most consistent risk factor for ovarian carcinoma in both BRCA+ and non-BRCA+ women is increased parity. 24 26–29 30
The purpose of this study was to determine whether variables associated with risk of epithelial ovarian cancer in BRCA+ women also were associated with the presence of p53 signatures. The importance of this study, which was conducted on 75 consecutive prophylactic salpingo-oophorectomies from BRCA+ women, lay in determining if the morphologic, immunohistochemical and genetic evidence supporting the precursor status of the p53 signature could be corroborated by epidemiologic data, thus identifying the p53 signature as a marker for serous cancer risk.
Methods
Case material
This study was approved by the institutional review board at Brigham and Women’s Hospital. Consecutive cases of prophylactic salpingo-oophorectomies for a hereditary mutation in the BRCA1 or BRCA2 gene accessioned between February 2005 and December 2006 were examined.
Tissue processing and pathologic review
All fallopian tubes and ovaries were entirely submitted for histologic exam. Fallopian tubes were analyzed using a procedure for sectioning and extensively examining the fimbriated end (SEE-FIM protocol). The fimbria was amputated, sectioned lengthwise and combined with cross sections of the proximal tube at 2–3 mm intervals.
Immunohistochemical detection of p53 signatures
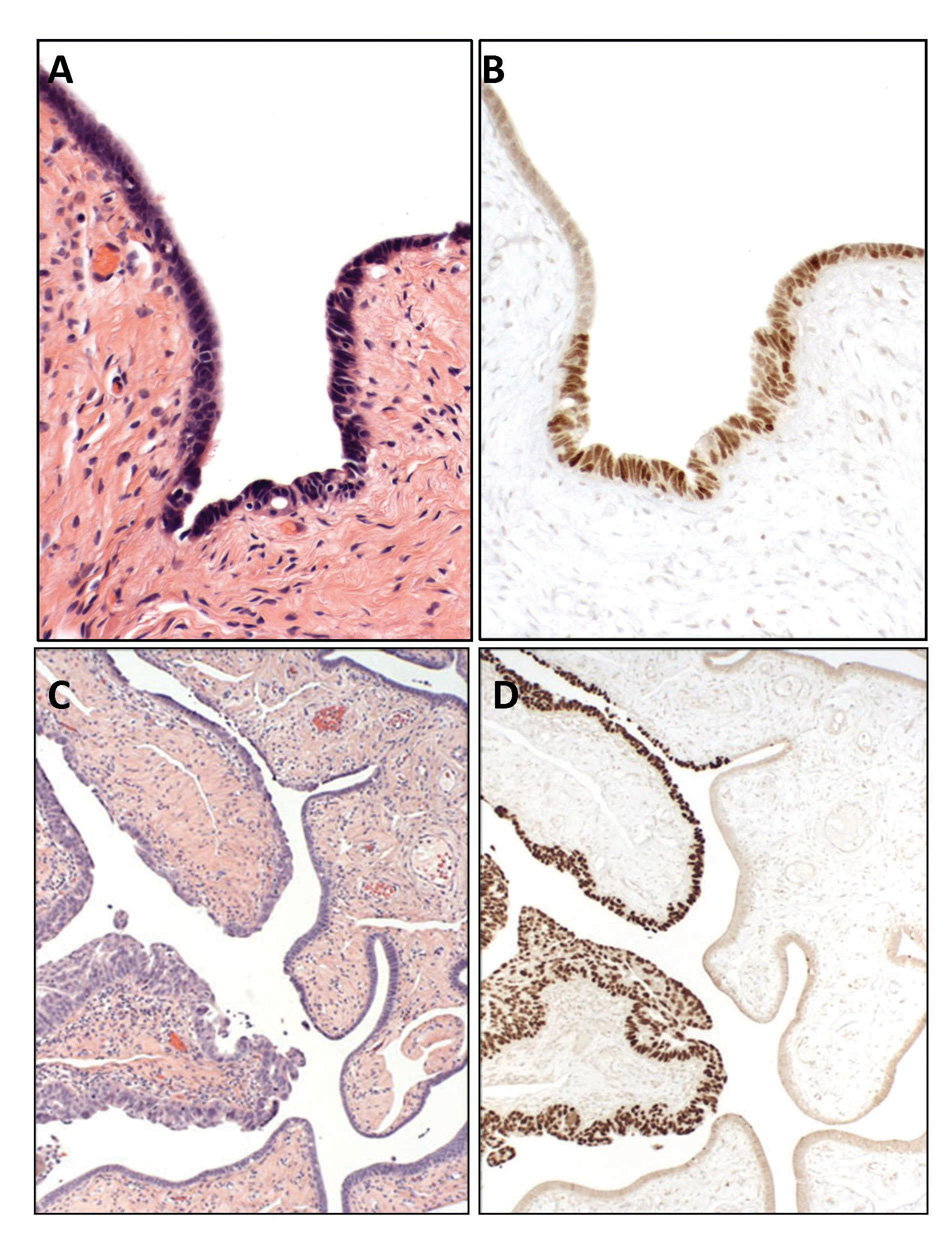
A monoclonal antibody to p53 was used to localize p53 protein (OP43, Oncogene Science, Cambridge, MA). A positive score required strong immunostaining obscuring nuclear detail. A requirement of at least 12 consecutive p53 positive secretory cell nuclei was arbitrarily imposed to exclude sporadic staining, which is common, presumably physiologic, and usually limited to no more than 2–3 consecutive nuclei in the tubal epithelium. This threshold was used in the prior studies in which the p53 signature was characterized.22 Although a threshold of 12 nuclei was established as a minimum, this number was typically exceeded (Figure 1).
Figure 1.
Benign-appearing distal fallopian tube mucosa (A) from a BRCA+ subject contains discrete immunopositivity for p53 (B) characteristic of a p53 signature, a putative precursor to serous carcinoma. For comparison, an early (intra-epithelial) serous carcinoma of the fallopian tube (C) highlighted by p53 immunostaining (D).
Demographic data collection
Following institutional board approval, the hospital records were surveyed for some common variables that have been investigated as risk factors for ovarian carcinoma. The continuous variables included age at first childbirth (years), age at the time of prophylactic salpingo-oophorectomy (years), age at menopause (years), body mass index (kg/m2), gravida, age at menarche (years), oral contraceptive use (years), and parity. The categorical variables included type of BRCA mutation (1 or 2), history of chemotherapy, history of breast cancer, type of menopause, history of smoking, and use of tamoxifen. Information regarding history of tubal ligation was also collected but not included in the analysis.
Analysis of data
Each case was independently scored as positive or negative for a p53 signature. The data were entered on a spreadsheet and associations with epidemiologic variables were assessed. Student’s T-test and Chi-square tests were used on continuous and categorical variables, respectively, for the univariate analysis. Multivariate odds ratios (OR) and 95% confidence intervals (CI) were calculated using unconditional logistic regression with the p53 signature as the outcome and adjusting for age and parity continuously. Statistical significance was set at the p≤0.05 level, and all analyses were conducted using SAS 9.0 (Cary, NC).
Results
Histologic material
Ovaries and fallopian tubes from seventy-five women were included in the study. The mean age of the population was 48 years with a range from 35 to 76. All ovaries and fallopian tubes were examined completely. Six cases harbored a malignancy, including two invasive and four intraepithelial carcinomas. A total of 486 and 605 tissue blocks from fallopian tubes and ovaries, respectively, were evaluated by immunohistochemical staining for p53.
Detection of p53 signatures
Twenty-nine of the 75 BRCA cases (38%) harbored at least one p53 signature in the fallopian tube. The prevalence of p53 signatures in the BRCA+ women is similar to that reported in women whose BRCA status is negative or unknown. 22 A total of 40 signatures were identified in the fallopian tubes; nine cases contained more than one p53 signature. Of these 40 signatures, 30 occurred in the fimbriated end (75%) and 10 occurred in the proximal fallopian tube (25%).
Statistical analysis of demographic correlates of p53 signatures
Table 1–Table 3 illustrate the outcome of statistical analysis of continuous (Table 1), categorical (Table 2) and multivariate logistic regression (Table 3). In univariate analyses, we observed a significant association of the presence of p53 signatures with both increased age at first childbirth and decreased parity. BMI was suggestively associated with the presence of p53 signatures. Notably, there was no significant association with oral contraceptive use. No significant associations were observed in univariate analyses for age, age at menopause, gravidity, age at menarche, type of BRCA mutation, history of chemotherapy, personal history of breast cancer, type of menopause, smoking history, or tamoxifen use. Only 4 of the 75 women had a prior tubal ligation, so this variable could not be analyzed.
Table 1.
Univariate comparison of continuous ovarian cancer risk factors with p53 signature status among 75 BRCA+ women.
| Risk Factor | n | Mean (SD) | Mean, p53 negative | Mean, p53 positive | p-value, t-Test |
|---|---|---|---|---|---|
| Age at first birth, yr | 57 | 29.2 (4.1) | 28.4 | 30.8 | 0.04 |
| Age at surgery, yr | 75 | 48.6 (9.1) | 49.1 | 47.8 | 0.54 |
| Age at menopause, yr | 74 | 44.7 (5.7) | 44.7 | 44.7 | 0.98 |
| Body mass index, kg/m2 | 66 | 26.0 (5.5) | 26.9 | 24.7 | 0.13 |
| Gravidity | 75 | 2.3 (1.7) | 2.5 | 2.1 | 0.30 |
| Age at menarche, yr | 54 | 12.9 (1.5) | 12.9 | 12.8 | 0.72 |
| Oral contraceptive use, yr | 57 | 3.4 (5.1) | 2.9 | 4.2 | 0.38 |
| Parity | 75 | 1.9 (1.3) | 2.2 | 1.4 | 0.01 |
Table 3.
Unadjusted and adjusted odds ratios (OR) and 95% confidence intervals (CI) for the presence of the p53 signature by various ovarian cancer risk factors, among BRCA+ women.
| Risk Factor | Unadjusted Odds Ratio (95% CI) | Unadjusted p-trend | Adjusted* Odds Ratio (95% CI) | Adjusted* p-trend (if applicable) |
|---|---|---|---|---|
| BRCA mutation: 1 | 1.0 | 1.0 | ||
| 2 | 1.9 (0.7–5.0) | - | 1.8 (0.6–5.3) | - |
| Chemotherapy: Never | 1.0 | 1.0 | ||
| Ever | 1.0 (0.4–2.7) | - | 0.7 (0.2–2.0) | - |
| Personal history of breast cancer: No | 1.0 | 1.0 | ||
| Yes | 0.7 (0.3–1.7) | - | 0.5 (0.2–1.4) | - |
| Menopause type: Surgery | 1.0 | 1.0 | ||
| Chemotherapy | 0.5 (0.1–2.1) | - | 0.7 (0.1–3.4) | - |
| Smoking history: Never | 1.0 | 1.0 | ||
| Ever | 0.8 (0.2–3.4) | - | 0.8 (0.2–3.8) | - |
| Tamoxifen use: Never | 1.0 | 1.0 | ||
| Ever | 0.8 (0.2–3.4) | - | 0.6 (0.1–2.8) | - |
| Age of first childbirth: < 30 yr | 1.0 | 1.0 | ||
| ≥ 30 yr | 3.8 (1.2–12) | 0.04 | 3.5 (0.9–12.9) | 0.15 |
| Age at oophorectomy: ≤ 45 yr | 1.0 | 1.0 | ||
| > 45–55 yr | 0.8 (0.3–2.1) | 0.5 (0.1–2.6) | ||
| > 55 yr | 0.6 (0.2–2.1) | 0.53 | 0.3 (0.01–3.2) | 0.69 |
| Age at menopause: < 50 yr | 1.0 | 1.0 | ||
| ≥ 50 yr | 1.3 (0.5–3.6) | 0.98 | 4.8 (0.9–24.0) | 0.49 |
| Body mass index: < 25 kg/m2 | 1.0 | 1.0 | ||
| 25 – <30 kg/m2 | 0.7 (0.2–2.2) | 0.8 (0.2–2.9) | ||
| ≥ 30 kg/m2 | 0.3 (0.1–1.4) | 0.13 | 0.3 (0.1–1.3) | 0.06 |
| Age at Menarche: ≤ 12 yr | 1.0 | 1.0 | ||
| > 12 yr | 1.0 (0.3–3.2) | 0.71 | 1.7 (0.4–6.3) | 0.65 |
| Oral Contraceptive Use: Never | 1.0 | 1.0 | ||
| Ever | 0.8 (0.3–2.4) | 0.38 | 0.9 (0.3–2.8) | 0.65 |
| Parity: Nulliparous | 1.0 | 1.0 | ||
| 1–2 children | 0.7 (0.2–2.2) | 0.7 (0.2–2.3) | ||
| 3+ children | 0.2 (0.04–0.9) | 0.01 | 0.2 (0.04–0.9) | 0.02 |
Adjusted for age and parity as continuous variables.
Table 2.
Univariate comparison of categorical ovarian cancer risk factors with p53 signature status among 75 BRCA+ women.
| Risk Factor | n | n (%), p53 negative | n (%), p53 positive | p-value, Chi-square |
|---|---|---|---|---|
| BRCA mutation: 1 | 39 | 24 (62) | 15 (38) | 0.35 |
| 2 | 33 | 23 (70) | 10 (30) | |
| History of chemotherapy: No | 47 | 30 (64) | 17 (36) | 0.93 |
| Yes | 28 | 17 (61) | 11 (39) | |
| Personal history of breast cancer: No | 42 | 27 (64) | 15 (36) | 0.40 |
| Yes | 33 | 20 (61) | 13 (39) | |
| Menopause Type: Surgery | 39 | 26 (67) | 13 (33) | 0.44 |
| Chemotherapy | 11 | 6 (55) | 5 (45) | |
| Smoking History: Never | 66 | 39 (59) | 27 (41) | 0.73 |
| Ever | 9 | 8 (89) | 1 (11) | |
| Tamoxifen Use: Never | 65 | 41 (63) | 24 (37) | 0.70 |
| Ever | 9 | 6 (67) | 3 (33) |
In general, results were similar for the unconditional logistic regression analyses, with an inverse association for parity and BMI and a positive association for age at first birth (Table 3). The unadjusted OR for the p53 signature was 3.8 (95% CI: 1.2, 12) for age of first childbirth ≥30 years versus <30 years (p-trend=0.04); this association was slightly attenuated and no longer statistically significant after adjustment for parity and age (p-trend=0.15). Women with 3 or more children had an OR of 0.2 (95% CI: 0.04, 0.9) versus nulliparous women (adjusted p-trend=0.02). Women with 1–2 children had a non-significantly lower risk of the p53 signature (OR: 0.7, 95% CI: 0.2, 2.3). After adjustment for age and parity, BMI was nearly significantly inversely associated with the p53 signature (p-trend=0.06) with an OR for women with a BMI ≥ 30 kg/m2 versus < 25 kg/m2 was 0.3 (95% CI: 0.1, 1.3).
Discussion
Epidemiologic studies of ovarian cancer risk have traditionally focused on case control studies of women with ovarian cancer. Based on these studies, certain variables have emerged as either consistently associated with increased (family history, heritable BRCA mutation) or decreased (higher parity, tubal ligation, oral contraceptive use) ovarian cancer risk. 24 25 More recent studies have attempted to tease apart the genetic and environmental variables by focusing on women with BRCA mutations, with increased parity as the most consistent protective factor. 24 26–30
A consistent limitation to epidemiologic studies of ovarian cancer has been the absence of a defined precursor lesion. Because of this, it has not been possible to determine which demographic factor or factors exert their influence at the earliest stage of carcinogenesis. This is in stark contrast to cervical cancer, for which a precursor has been well defined and established not only as a surrogate for cervical cancer risk but also for cervical cancer prevention by vaccination. 31
Fundamental characteristics of serous ovarian cancers include a cell phenotype that recapitulates secretory cells of the fallopian tube and a mutation in the p53 tumor suppressor gene. In recent years, the link between these tumors and the fallopian tube has been strengthened by the discovery of early tubal carcinomas in women with BRCA mutations who are undergoing risk-reducing salpingo-oophorectomy. Several recent studies have verified that a high percentage of early cancers in these women actually arise in the fimbria, and have presented evidence that up to one-half of unselected ovarian serous carcinomas and so-called “primary peritoneal” serous carcinomas initiate in the distal tube as a non-invasive serous tubal intraepithelial carcinoma (STIC). Studies comparing p53 mutations between STICs and remote peritoneal or ovarian serous carcinomas have verified identical mutations in both. 17 32 More compelling has been the concurrent observation of p53 immuno-positive segments of benign appearing tubal epithelium in the fimbria which contain a secretory cell phenotype and share additional features with STIC, including evidence of DNA damage, p53 mutations, and occasionally, spatial continuity with STICs or serous cancers. The presence of the tubal p53 signature thus strengthens the concept that one form of serous carcinogenesis initiates in the distal fallopian tube. Interestingly, the p53 signature is equally common in both BRCA+ women and women without a family history of ovarian cancer or a current ovarian malignancy.22 23 The common occurrence of p53 signatures in women with and without BRCA mutations suggests that it is independent of BRCA status. However, women at high genetic risk may be more likely to progress from a p53 signature to an intraepithelial or invasive carcinoma. It is interesting to note that women with mutations in BRCA have up to a 46% lifetime risk of ovarian cancer and that the prevalence of p53 signatures is 38% in this population, suggesting that in fact the rate of progression is high.33 34 35 36
To our knowledge, this is the first study exploring the epidemiology of a non-malignant serous cancer precursor. The study was designed to both control for genetic risk (by targeting BRCA+ women only) and take advantage of the fact that the fallopian tubes from these women were examined in toto. Every tissue section was immunostained for p53 and two investigators reviewed all of the histologic material. Cases were grouped into those that were p53 signature positive and negative and compared to several known ovarian cancer risk factors. A positive association was observed between older age at first childbirth, which was slightly attenuated after adjustment for parity. This could have been due to the somewhat small sample size. In contrast, increased parity was strongly protective against the presence of the p53 signature. Interestingly, body mass index was inversely associated with the p53 signature, although the results were of borderline significance, and the biologic mechanism is unclear. No relationship between age, BRCA subtype (1 or 2), oral contraceptive use or other known ovarian cancer risk factor was observed. However, because of the limited sample size, larger studies should be conducted to rule out modest effects. One other limitation of this study is the collection of risk factor data via medical record abstraction, especially because there is frequently missing data for covariates of interest. Future questionnaire-based studies will be important to better understanding these relationships.
Recent studies have revealed that p53 signatures are most prevalent in the tubal mucosa in the vicinity of the ovarian surface (fimbria). Thus, they represent another possible result of ovulation-related oxidative stress, as has been proposed in some models.37 The data from this study are consistent with this model given the potential relationship between low parity and older age of first childbirth to ovulatory frequency. In the context of this and the experimental data, a prior report that linked p53-positive ovarian cancers to increased lifetime ovulations is an attractive endorsement for the concept that the p53 signatures is an early manifestation of this risk. 38 Nevertheless, this hypothesis has not been universally accepted and the role of ovulation or a related phenomenon in the genesis of the p53 signature – and serous cancer – remains to be clarified.39 More comprehensive studies will be required to confirm these findings and determine if other risk factors are related to the presence of the p53 signature. However, based on the findings in prior studies and in this report, the p53 signature merits consideration as both a precursor lesion and a surrogate endpoint for pelvic serous cancer.
Acknowledgments
This work was supported by grants from the NCI (P50 CA105009 [SPORE]: D. Cramer, PI), NCI KO8 CA108748 (R Drapkin, PI), NCI 1R21CA124688-01A1 (CP Crum, PI), The Charlotte Geyer Foundation (CP Crum, PI), The Columbia Hospital For Women Research Foundation (CP Crum, PI), and the Francis Ward Paine and TSA Pemberton Funds from the Division of Women’s and Perinatal Pathology, Brigham and Women’s Hospital.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Quirk JT, Natarajan N, Mettlin CJ. Age-specific ovarian cancer incidence rate patterns in the United States. Gynecol Oncol. 2005;99:248–250. doi: 10.1016/j.ygyno.2005.06.052. [DOI] [PubMed] [Google Scholar]
- 2.Stewart BW, Kleihues P, editors. Lyon (France): IARC Press; WHO world cancer report. 2003
- 3.Dalrymple JC, Bannatyne P, Russell P, Solomon HJ, Tattersall MH, Atkinson K, Carter J, Duval P, Elliott P, Friedlander M, et al. Extraovarian peritoneal serous papillary carcinoma. A clinicopathologic study of 31 cases. Cancer. 1989;64:110–115. doi: 10.1002/1097-0142(19890701)64:1<110::aid-cncr2820640120>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 4.Shih IeM, Kurman RJ. Ovarian tumorigenesis: a proposed model based on morphological and molecular genetic analysis. Am J Pathol. 2004;164:1511–1518. doi: 10.1016/s0002-9440(10)63708-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cannistra SA. Cancer of the ovary. N Engl J Med. 2004;351:2519–2529. doi: 10.1056/NEJMra041842. [DOI] [PubMed] [Google Scholar]
- 6.Rabban JT, Bell DA. Current issues in the pathology of ovarian cancer. J Reprod Med. 2005;50:467–474. [PubMed] [Google Scholar]
- 7.Singer G, Stohr R, Cope L, Dehari R, Hartmann A, Cao DF, Wang TL, Kurman RJ, Shih IeM. Patterns of p53 mutations separate ovarian serous borderline tumors and low- and high-grade carcinomas and provide support for a new model of ovarian carcinogenesis: a mutational analysis with immunohistochemical correlation. Am J Surg Pathol. 2005;29:218–224. doi: 10.1097/01.pas.0000146025.91953.8d. [DOI] [PubMed] [Google Scholar]
- 8.Brewer MA, Johnson K, Follen M, Gershenson D, Bast R. Prevention of ovarian cancer: intraepithelial neoplasia. Clin Cancer Res. 2003;9:20–30. [PubMed] [Google Scholar]
- 9.Hutson R, Ramsdale J, Wells M. p53 protein expression in putative precursor lesions of epithelial ovarian cancer. Histopathology. 1995;27:367–371. doi: 10.1111/j.1365-2559.1995.tb01528.x. [DOI] [PubMed] [Google Scholar]
- 10.Piek JM, van Diest PJ, Zweemer RP, Jansen JW, Poort-Keesom RJ, Menko FH, Gille JJ, Jongsma AP, Pals G, Kenemans P, Verheijen RH. Dysplastic changes in prophylactically removed fallopian tubes of women predisposed to developing ovarian cancer. J Pathol. 2001;195:451–456. doi: 10.1002/path.1000. [DOI] [PubMed] [Google Scholar]
- 11.Bell DA, Scully RE. Early de novo ovarian carcinoma. A study of fourteen cases. Cancer. 1994;73:1859–1864. doi: 10.1002/1097-0142(19940401)73:7<1859::aid-cncr2820730714>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 12.Barakat RR, Federici MG, Saigo PE, Robson ME, Offit K, Boyd J. Absence of premalignant histologic, molecular, or cell biologic alterations in prophylactic oophorectomy specimens from BRCA1 heterozygotes. Cancer. 2000;89:383–390. doi: 10.1002/1097-0142(20000715)89:2<383::aid-cncr25>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 13.Drapkin RL, Hecht JL. Pathogenesis of ovarian cancer. In: Crum CP, Lee KR, editors. Diagnostic Gynecologic and Obstetric Pathology. Philadelphia: Elsevier-Saunders; 2006. pp. 793–809. [Google Scholar]
- 14.Colgan TJ, Murphy J, Cole DE, Narod S, Rosen B. Occult carcinoma in prophylactic oophorectomy specimens: prevalence and association with BRCA germline mutation status. Am J Surg Pathol. 2001;25:1283–1289. doi: 10.1097/00000478-200110000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Medeiros F, Muto MG, Lee Y, Elvin JA, Callahan MJ, Feltmate C, Garber JE, Cramer DW, Crum CP. The Tubal Fimbria is a Preferred Site for Early Adenocarcinoma in Women with Familial Ovarian Cancer Syndrome. Am J Surg Pathol. 2006;30:230–236. doi: 10.1097/01.pas.0000180854.28831.77. [DOI] [PubMed] [Google Scholar]
- 16.Cass I, Holschneider C, Datta N, Barbuto D, Walts AE, Karlan BY. BRCA-Mutation-Associated Fallopian Tube Carcinoma: A Distinct Clinical Phenotype? Obstet Gynecol. 2005;106:1327–1334. doi: 10.1097/01.AOG.0000187892.78392.3f. [DOI] [PubMed] [Google Scholar]
- 17.Kindelberger D, Lee Y, Miron A, et al. Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: evidence for a causal relationship. Am J Surg Pathol. 2007;31(2):161–169. doi: 10.1097/01.pas.0000213335.40358.47. [DOI] [PubMed] [Google Scholar]
- 18.Gorgoulis VG, Vassiliou LV, Karakaidos P, Zacharatos P, Kotsinas A, Liloglou T, Venere M, Ditullio RA, Jr, Kastrinakis NG, Levy B, Kletsas D, Yoneta A, Herlyn M, Kittas C, Halazonetis TD. Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature. 2005;434:907–913. doi: 10.1038/nature03485. [DOI] [PubMed] [Google Scholar]
- 19.Latonen L, Laiho M. Cellular UV damage responses--functions of tumor suppressor p53. Biochim Biophys Acta. 2005;1755:71–89. doi: 10.1016/j.bbcan.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 20.Alrawi SJ, Schiff M, Carroll RE, Dayton M, Gibbs JF, Kulavlat M, Tan D, Berman K, Stoler DL, Anderson GR. Aberrant crypt foci. Anticancer Res. 2006;26:107–119. [PubMed] [Google Scholar]
- 21.Ren ZP, Ahmadian A, Ponten F, Nister M, Berg C, Lundeberg J, Uhlen M, Ponten J. Benign clonal keratinocyte patches with p53 mutations show no genetic link to synchronous squamous cell precancer or cancer in human skin. Am J Pathol. 1997;150:1791–1803. [PMC free article] [PubMed] [Google Scholar]
- 22.Lee Y, Miron A, Drapkin R, Nucci MR, Medeiros F, Saleemuddin A, Garber J, Birch C, Mou H, Gordon RW, Cramer DW, McKeon FD, Crum CP. A candidate precursor to serous carcinoma that originates in the distal fallopian tube. J Pathol. 2007;211:26–35. doi: 10.1002/path.2091. [DOI] [PubMed] [Google Scholar]
- 23.Folkins AK, Jarboe EA, Saleemuddin A, Lee Y, Callahan MJ, Drapkin R, Garber JE, Muto MG, Tworoger S, Crum CP. A candidate precursor to pelvic serous cancer (p53 signature) and its prevalence in ovaries and fallopian tubes from women with BRCA mutations. Gynecol Oncol. doi: 10.1016/j.ygyno.2008.01.012. (March 2008 in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGuire V, Felberg A, Mills M, Ostrow KL, DiCioccio R, John EM, West DW, Whittemore AS. Relation of contraceptive and reproductive history to ovarian cancer risk in carriers and noncarriers of BRCA1 gene mutations. Am J Epidemiol. 2004;160:613–618. doi: 10.1093/aje/kwh284. [DOI] [PubMed] [Google Scholar]
- 25.Tworoger SS, Fairfield KM, Colditz GA, Rosner BA, Hankinson SE. Association of oral contraceptive use, other contraceptive methods, and infertility with ovarian cancer risk. Am J Epidemiol. 2007;166(8):894–901. doi: 10.1093/aje/kwm157. [DOI] [PubMed] [Google Scholar]
- 26.Whittemore AS, Balise RR, Pharoah PD, Dicioccio RA, Oakley-Girvan I, Ramus SJ, Daly M, Usinowicz MB, Garlinghouse-Jones K, Ponder BA, Buys S, Senie R, Andrulis I, John E, Hopper JL, Piver MS. Oral contraceptive use and ovarian cancer risk among carriers of BRCA1 or BRCA2 mutations. Br J Cancer. 2004;91:1911–1915. doi: 10.1038/sj.bjc.6602239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Modan B, Hartge P, Hirsh-Yechezkel G, Chetrit A, Lubin F, Beller U, Ben-Baruch G, Fishman A, Menczer J, Ebbers SM, Tucker MA, Wacholder S, Struewing JP, Friedman E, Piura B National Israel Ovarian Cancer Study Group. Parity, oral contraceptives, and the risk of ovarian cancer among carriers and noncarriers of a BRCA1 or BRCA2 mutation. N Engl J Med. 2001;345:235–240. doi: 10.1056/NEJM200107263450401. [DOI] [PubMed] [Google Scholar]
- 28.McLaughlin JR, Risch HA, Lubinski J, Moller P, Ghadirian P, Lynch H, Karlan B, Fishman D, Rosen B, Neuhausen SL, Offit K, Kauff N, Domchek S, Tung N, Friedman E, Foulkes W, Sun P, Narod SA Hereditary Ovarian Cancer Clinical Study Group. Reproductive risk factors for ovarian cancer in carriers of BRCA1 or BRCA2 mutations: a case-control study. Lancet Oncol. 2007;8:26–34. doi: 10.1016/S1470-2045(06)70983-4. [DOI] [PubMed] [Google Scholar]
- 29.Narod SA, Sun P, Ghadirian P, Lynch H, Isaacs C, Garber J, Weber B, Karlan B, Fishman D, Rosen B, Tung N, Neuhausen SL. Tubal ligation and risk of ovarian cancer in carriers of BRCA1 or BRCA2 mutations: a case-control study. Lancet. 2001;357:1467–1470. doi: 10.1016/s0140-6736(00)04642-0. [DOI] [PubMed] [Google Scholar]
- 30.Riska A, Leminen A. Updating on primary fallopian tube carcinoma. Acta Obstet Gynecol Scand. 2007;86:1419–1426. doi: 10.1080/00016340701771034. [DOI] [PubMed] [Google Scholar]
- 31.Garland SM, Hernandez-Avila M, Wheeler CM, Perez G, Harper DM, Leodolter S, Tang GW, Ferris DG, Steben M, Bryan J, Taddeo FJ, Railkar R, Esser MT, Sings HL, Nelson M, Boslego J, Sattler C, Barr E, Koutsky LA Females United to Unilaterally Reduce Endo/Ectocervical Disease (FUTURE) I Investigators. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356:1928–1943. doi: 10.1056/NEJMoa061760. [DOI] [PubMed] [Google Scholar]
- 32.Carlson JW, Miron A, Jarboe EA, Parast M, Hirsch MS, Lee Y, Muto MG, Kindelberger D, Crum CP. Serous Tubal Intraepithelial Carcinoma (STIC): Its Potential Role in Primary Peritoneal Serous Carcinoma (PPSC) and Serous Cancer Prevention. J Clin Oncol. doi: 10.1200/JCO.2008.16.4814. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ford D, Easton DF, Stratton M, Narod S, Goldgar D, Devilee P, Bishop DT, Weber B, Lenoir G, Chang-Claude J, Sobol H, Teare MD, Struewing J, Arason A, Scherneck S, Peto J, Rebbeck TR, Tonin P, Neuhausen S, Barkardottir R, Eyfjord J, Lynch H, Ponder BAJ, Gayther SA, Birch JM, Lindblom A, Stoppa-Lyonnet D, Bignon Y, Borg A, Hamann U, Haites N, Scott RJ, Maugard CM, Vasen H, Seitz S, Cannon-Albright LA, Schofield A, Zelada-Hedman M Breast Cancer Linkage Consortium. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. Am J Hum Genet. 1998;62:676–689. doi: 10.1086/301749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Antoniou A, Pharoah PD, Narod SA, Risch HA, Eyfjord JE, Hopper JL, Loman N, Olsson H, Johannsson O, Borg A, Pasini B, Radice P, Manoukian S, Eccles DM, Tang N, Olah E, Anton-Culver H, Warner E, Lubinski J, Gronwald J, Gorski B, Tulinius H, Thorlacius S, Eerola H, Nevanlinna H, Syrjakoski K, Kallioniemi OP, Thompson D, Evans C, Peto J, Lallloo F, Evans DG, Easton DF. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72:1117–1130. doi: 10.1086/375033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lancaster JM, Powell CB, Kauff ND, Cass I, Chen LM, Lu KH, Mutch DG, Berchuck A, Karlan BY, Herzog TJ the Society of Gynecologic Oncologists Hereditary Cancer Education Resource Panel. Society of Gynecologc Oncologists Education Committee statement of risk assessment for inherited gynecologic cancer predispositions. Gynecol Oncol. 2007;107:159–162. doi: 10.1016/j.ygyno.2007.09.031. [DOI] [PubMed] [Google Scholar]
- 36.Whittemore AS, Gong G, Itnyre J. Prevalence and contribution of BRCA1 mutations in breast cancer and ovarian cancer: results from three US population-based case-control studies of ovarian cancer. Am J Hum Gent. 1997;60:496–504. [PMC free article] [PubMed] [Google Scholar]
- 37.Murdoch WJ, Martinchick JF. Oxidative damage to DNA of ovarian surface epithelial cells affected by ovulation: carcinogenic implication and chemoprevention. Exp Biol Med. 2004;229:546–552. doi: 10.1177/153537020422900613. [DOI] [PubMed] [Google Scholar]
- 38.Schildkraut JM, Bastos E, Berchuck A. Relationship between lifetime ovulatory cycles and overexpression of mutant p53 in epithelial ovarian cancer. J Natl Cancer Inst. 1997;89:932–938. doi: 10.1093/jnci/89.13.932. [DOI] [PubMed] [Google Scholar]
- 39.Webb PM, Green A, Cummings MC, Purdie DM, Walsh MD. Chenevix-Trench G.Relationship between number of ovulatory cycles and accumulation of mutant p53 in epithelial ovarian cancer. J Natl Cancer Inst. 1998;90:1729–1734. doi: 10.1093/jnci/90.22.1729. [DOI] [PubMed] [Google Scholar]