Abstract
Fukutin, a gene responsible for Fukuyama type congenital muscular dystrophy (FCMD), is presumably related to the glycosylation of α-dystroglycan (α-DG), involved in basement membrane formation. Hypoglycosylation of α-DG plays a key role for the pathogenesis of FCMD. On the other hand, fukutin and α-DG are also expressed in various non-neuromuscular tissues. Recently, a role of α-DG as a cancer suppressor has been proposed, because of a decrease of glycosylated α-DG in cancers. In this study, function of fukutin was investigated in two cancer cell lines, focusing on whether fukutin is involved in the glycosylation of α-DG in cancer cells and has any possible roles related to a cancer suppressor. Localization of fukutin and a result of laminin-binding assay after RNA interference suggest that fukutin may be involved in the glycosylation of α-DG in a small portion in these cancer cell lines. In Western blotting and immuno-electron microscopy, localization of fukutin in the nucleus was suggested in addition to the Golgi apparatus and/or endoplasmic reticulum. Immunohistochemically, there were more Ki-67-positive cells and more nuclear staining of phosphorylated c-jun after knockdown of fukutin in two cell lines. Fukutin appears to suppress cell proliferation through a system involving c-jun, although it is unclear this process is related to α-DG or not at present. The result may propose a possibility of another function of fukutin in addition to the glycosylation of α-DG in cancer cells.
Keywords: α-dystroglycan, cancer, cell proliferation, c-jun, fukutin, RNA interference
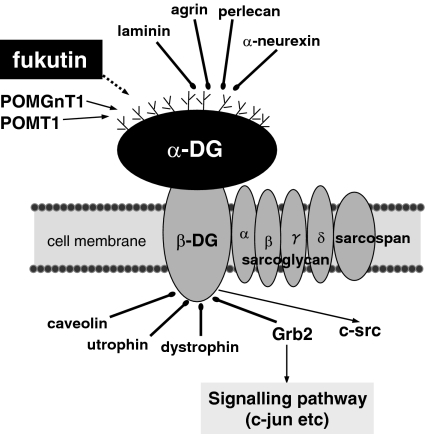
Fukutin is a gene responsible for Fukuyama type congenital muscular dystrophy (FCMD) (Kobayashi et al. 1998). FCMD is an autosomal recessive disease, showing muscular dystrophy, and central nervous system (CNS) and eye anomalies (Schessl et al. 2006), accompanying hypoglycosylation of α-dystroglycan (α-DG) (Hayashi et al. 2001; Yamamoto et al. 2004b; Martin 2005; Schessl et al. 2006). Muscular dystrophies exhibiting hypoglycosylation of α-DG are called as α-dystroglycanopathy, which include congenital muscular dystrophies such as FCMD, muscle-eye-brain disease (MEB) and Walker–Warburg syndrome (WWS) (Martin 2005). α-DG is one of the components of the dystrophin-glycoprotein complex (DGC), which is involved in basement membrane formation, linking intracellular and extracellular proteins (Figure 1) (Michele & Campbell 2003; Oak et al. 2003; Martin 2005; Schessl et al. 2006). It is heavily glycosylated and acts as a receptor for extracellular proteins such as laminin, agrin, perlecan and α-neurexin (Michele & Campbell 2003; Oak et al. 2003; Martin 2005; Schessl et al. 2006). MEB is caused by mutations in protein O-linked mannose β1,2-N-acetylglucosaminyltransferase (POMGnT1), which is an enzyme implicated in the glycosylation of α-DG (Takahashi et al. 2001; Yoshida et al. 2001; Manya et al. 2003). A part of WWS cases are caused by mutations in protein-O-mannosyltransferase 1 (POMT1), which is also an enzyme involved in the glycosylation of α-DG (Beltrán-Valero de Bernabéet al. 2002; Akasaka-Manya et al. 2004; Kim et al. 2004). Hypofunction of POMGnT1 or POMT1 may result in the hypoglycosylation of α-DG, and an abnormal basement membrane may be formed (Michele & Campbell 2003; Martin 2005; Schessl et al. 2006). Fukutin-null mice are lethal in utero (Kurahashi et al. 2005), and fukutin-deficient chimaeric mice exhibit brain lesions similar to those of a FCMD fetus (Chiyonobu et al. 2005). Both mice show an abnormal basement membrane with reduced glycosylation of α-DG (Chiyonobu et al. 2005; Kurahashi et al. 2005). Fukutin is considered to be related to the glycosylation of α-DG like POMGnT1 and POMT1 (Hayashi et al. 2001; Yamamoto et al. 2004b; Martin 2005; Schessl et al. 2006), but its function has not been directly proved.
Figure 1.
Dystrophin-glycoprotein complex in striated muscle.
On the other hand, both fukutin and α-DG are expressed in various tissues including epithelial cells, in addition to the striated muscle and nervous system (Saito et al. 2004; Yamamoto et al. 2004a). A model of a complex similar to DGC of the skeletal muscle has been considered in epithelial cells (Sgambato & Brancaccio 2005). Decrease of glycosylated of α-DG has been reported in various cancers, such as colon (Sgambato et al. 2003), breast (Muschler et al. 2002; Sgambato et al. 2003), oral (Jing et al. 2004), uterine cervical and vulvar cancers (Sgambato et al. 2006). As DG mRNA tends to be preserved, episodes during and/or postglycosylation may be a major cause of the decrease of glycosylated α-DG in cancer cells (Sgambato et al. 2003; Jing et al. 2004). As the decrease of glycosylated α-DG tends to be more conspicuous in higher grades and/or more advanced lesions (Sgambato et al. 2003, 2006), a role as a cancer suppressor has been proposed (Muschler et al. 2002; Sgambato & Brancaccio 2005). In this study, function of fukutin was investigated in cancer cell lines, focusing on whether fukutin is involved in the glycosylation of α-DG in cancer cells and has any possible roles related to a cancer suppressor. Expression and localization of fukutin were investigated in HeLa cells by Western blotting and immuno-electron microscopy. Moreover, RNA interference (RNAi) was performed in HeLa and breast cancer cells.
Materials and methods
Cell lines
HeLa cells and human breast cancer cells (ZR-75-1) were grown in RPMI (Invitrogen, Carlsbad, CA, USA), supplemented with 10% fetal bovine serum (Thermo Fisher Scientific, Waltham, MA, USA) and 1% penicillin-streptomycin (Invitrogen). Cells were maintained at 37 °C in a humidified incubator with a CO2 atmosphere.
Western blotting
HeLa cells were homogenized with RIPA buffer (20 mM Tris-Cl, 150 mM NaCl, 2 mM EDTA, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS) containing protease inhibitor cocktail (Complete, Mini: Roche Diagnostics, Mannheim, Germany). After centrifugation at 12,500 g, 4 °C for 60 min, the supernatant was used as total extracts. Furthermore, nuclear and cytoplasmic proteins were separately extracted using NE-PER Nuclear and Cytoplasmic Extraction Reagents (Pierce, Rockford, IL, USA), according to the manufacturer's instructions.
The extracts (30 μg of total extracts, and 60 μg of nuclear or cytoplasmic extracts) were electrophoresed in a 9% SDS-polyacrylamide gel, and transferred onto a polyvinylidene fluoride membrane (Millipore, Billerica, MA, USA). After being treated overnight at 4 °C with TBS containing 0.2% Tween-20, 5% skim milk and 2% porcine serum, the membrane was incubated for 1 h at room temperature with the anti-fukutin antibody (polyclonal, 1:500; Yamamoto et al. 2002), anti-fukutin antisera (anti-C1; polyclonal, kindly provided by Dr Saito; Saito et al. 2000) and anti-β-actin antibody (monoclonal, 1:2000; Sigma-Aldrich, Tokyo, Japan). The anti-fukutin antibody was raised against approximately 70% of the C-terminal side of fukutin protein (Yamamoto et al. 2002). The anti-C1 antisera was made against a peptide corresponding to residues 448–461 (Saito; Saito et al. 2000). Immunoreactive signals were visualized by the chemiluminescense detection system using ECL kits (Amersham, Buckinghamshire, UK). Blots processed with the omission of the primary antibodies served as the negative reaction controls. Normal skin, pancreas, kidney and uterine cervix obtained by autopsy were used for positive controls of fukutin. Each specimen was treated in accordance with the Helsinki Declaration revised in 2000.
Immuno-electron microscopy
Immuno-electron microscopy by the postembedding method was performed in HeLa cells. Cells were fixed in 2.5% glutaraldehyde at 4 °C, overnight, and embedded in Epon 812 (TAAB, Berks, England). Ultrathin sections were treated with 3% H2O2 at room temperature (RT) for 10 min, and then, with normal serum at RT for 10 min. Sections were incubated with the anti-fukutin antibody (polyclonal, 1:20; Yamamoto et al. 2002) at 4 °C, overnight. After washing with PBS, sections were incubated with 15 nm gold-labelled goat anti-rabbit IgG (H + L) (Amersham Bioscience, Tokyo, Japan) at RT for 4 h. After washing with H2O, sections were stained with uranyl acetate and lead citrate, and examined using a TOPCON EM-002B electron microscope (TOPCON, Tokyo, Japan).
RNAi
Three types of Stealth siRNA duplex for the fukutin mRNA were designed and synthesized by Invitrogen. Target senses were 5′-UGCAAAUGCAGUAAAGUCUCUUGGA-3′ (F429), 5′-UUUGGAAGGGAACAAAUUUCCUGUC-3′ (F697) and 5′-UAAUGUUGCAUUGUCGAUACCAUCC-3′ (F1021). Scrambled negative control Stealth™ RNA (SNC, Invitrogen) was used as a negative control. Omission of siRNA was always performed as a negative control in each experiment. HeLa cells and ZR-75-1 cells were plated 1 day before transfection at a density of about 200,000 cells in each 35-mm dish. Antibiotics were omitted from the medium. siRNA (20 nM final concentration) was transfected into the cells using lipofectamine2000 (Invitrogen) and Opti-MEM (Invitrogen) according to the manufacturer's instructions. Four hours after transfection, 10% fetal bovine serum was added in the culture medium. The culture medium was changed to the regular formulation 1 day after transfection. Cells were harvested 1 and 5 days after transfection.
RT-PCR
RNA was extracted from HeLa and ZR-75-1 cells 1 day after RNAi, using the acid guanidium thiocyanate-phenol-chloroform method. Following the treatment of 2 μg RNA with M-MLV reverse transcriptase (Life Technologies, Rockville, MD, USA) at 37 °C for 2 h, cDNA of 2 ng/μl was amplified by PCR using the AccuPrime™ Taq DNA polymerase system (Invitrogen). The sequence of the primers for 112–731 bp of the fukutin cDNA (GenBank: AB008226) was 5′-ATGAGTAGAATCAATAAGAA-3′ (coding sense) and 5′-AACTGTAACTTTCGGAAGGG-3′ (anticoding sense), for 95–621 bp of POMGnT1 (GenBank: AB057356) was 5′-ATGGACGACTGGAAGCCCAG-3′ (coding sense) and 5′-GTGCAGATGAGCACTCGGCC-3′ (anticoding sense), for 180–590 bp of POMT1 (GenBank: AH007906) was 5′-ATGTGGGGATTTTTGAAGCG-3′ (coding sense) and 5′-AGCAGCTCCCATGGCGGCAC-3′ (anticoding sense), for 395–883 bp of α-DG (GenBank: XM_018223) was 5′-ATGAGGATGTCTGTGGGCCT-3′ (coding sense) and 5′-ATCACTGTGGTCTTCAGGGT-3′ (anticoding sense), and for GAPDH was 5′-TTTGGTCGTATTGGGCGCCT-3′ (coding sense) and 5′-GTGGTCATGAGTCCTTCCAC-3′ (anticoding sense). The reaction mixture was amplified for 35 cycles in a Zymoreactor thermo-cycler (ATTO Co., Tokyo, Japan). The amplification profiles consisted of denaturing at 94 °C for 1.5 min, annealing at 56 °C for 1 min and extension at 72 °C for 1 min. PCR products were electrophoresed using 1.2% agarose gels, stained with ethidium bromide, and photographed. The density of each band was measured using NIH image version 1.61, and ratio of each product against GAPDH was calculated.
Immunohistochemistry
HeLa and ZR-75-1 cells 5 days after RNAi were examined by immunohistochemistry. Cells cultured on culture slides (BD Falcon, Bedford, MA, USA) were fixed in 95% ethanol for 5 min at RT, and incubated with PBS containing 2% skim milk and 1% Triton X-100 for 20 min at RT. Cells were incubated with the anti-fukutin antibody (polyclonal, 1:100; Yamamoto et al. 2002) overnight at 4 °C, and then with Cy3-conjugated donkey anti-rabbit IgG (1:200; Jackson Immunoresearch Laboratory, West Grove, PA, USA) for 6 h. Immunostaining using a primary antibody pre-absorbed with full-length fukutin protein was performed to evaluate the specificity of the antibody. For the negative control experiment, the primary antibody was omitted from the solution. Specimens were examined using a fluorescence microscope (Nikon ECLIPSE TS100; Nikon, Tokyo, Japan).
For more immunohistochemistry using various primary antibodies, cell-blocks were made. Cells were detached by cell scraper (Iwaki, Tokyo, Japan), fixed in 4% paraformaldehyde/phosphate-buffered saline (pH 7.6) for 30 min at 4 °C, and then, were collected by centrifugation. Cells were gathered using 2% agarose, embedded in paraffin in a routine manner and sectioned at 3 μm. Sections were treated with 3% H2O2 in PBS for 10 min to block endogenous peroxidase and were transferred to normal serum for 30 min. After incubation with the primary antibody overnight at 4 °C, sections were treated with biotinylated anti-rabbit IgG or biotinylated anti-mouse IgG at RT for 30 min, and then with avidin–biotin complex at RT for 30 min. Colour was developed with chromogen diaminobenzidine tetrahydrochloride and the slides were counterstained by haematoxylin.
Primary antibodies used for cell-blocks were anti-α-DG (IIH6C4, monoclonal, 1:200; Upstate Biotechnology, Lake Placid, NY, USA), Ki-67 (MIB-1, monoclonal, 1:100; DaKo Cytomation, Glostrup, Denmark), p-c-jun (monoclonal, 1:100; Santa Cruz Biotechnology, Santa Cruz, CA, USA), c-fos (polyclonal, 1:50; Calbiochem, Cambridge, MA, USA), cyclin D1 (monoclonal, diluted, ZYMED Laboratories, San Francisco, CA, USA), p21 (monoclonal, 1:100; Santa Cruz), p27 (monoclonal, 1:500; Santa Cruz), c-src (monoclonal, 1:500; Santa Cruz), osteopontin (monoclonal, 1:2000; Santa Cruz), p53 (monoclonal, diluted, Immunotech, Cedex, France), and bcl-2 (monoclonal, DaKo, 1:500) antibodies. Sections were pretreated with trypsin for 30 min at 37 °C for fukutin and c-fos, and with microwave antigen retrieval for α-DG, Ki-67, cyclin D1, p21, p27, c-src, p53 and bcl-2. For Ki-67 immunostaining, positive cells per more than 500 cells were counted. For the negative control experiment, the primary antibody was omitted from the solution.
Laminin-binding assay
One day after the transfection of siRNA, HeLa cells and ZR-75-1 cells were trypsinized and inoculated on 35 mm laminin-coated dishes (BD Bioscience, Bedford, MA, USA) with RPMI (Invitrogen), supplemented with 10% fetal bovine serum (Thermo Fisher Scientific) and 1% penicillin-streptomycin (Invitrogen). Cells only treated with lipofectamine2000 were used for negative controls. Cells were cultured at 37 °C, and a condition of adhesion to the dish surface was examined from 2 to 8 h after the inoculation. A ratio between completely attached and non-attached cells was calculated on more than 300 cells.
Results
Western blotting of HeLa cells
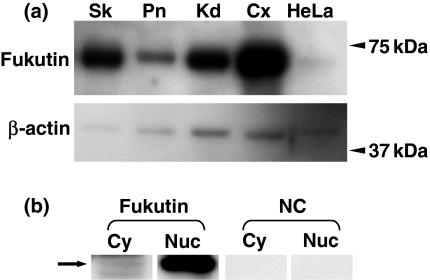
Total extracts of skin, pancreas, kidney and uterine cervix obtained by using RIPA buffer showed the band at about 60 kDa. A band at about 60 kDa was also observed in total extracts of HeLa cells (Figure 2). Cytoplasmic and nuclear extracts of HeLa cells also showed the band at the same molecular weight (Figure 2). The band was stronger in the nuclear extracts. Negative controls did not show positive reactions.
Figure 2.
Western blotting for fukutin in control tissues and HeLa cells (a, b). A band at about 60 kDa is seen in total extracts of skin, pancreas, kidney, uterine cervix and HeLa cells (a). Both nuclear and cytoplasmic extracts of HeLa cells exhibit a band at about 60 kDa (arrow), and nuclear extracts show stronger reaction compared with cytoplastic extracts (b). There are no bands in negative controls. Sk, skin; Pn, pancreas; Kd, kidney; Cx, uterine cervix; NC, negative control; Cy, cytoplasmic extracts; Nuc, nuclear extracts.
Immuno-electron microscopy of HeLa cells
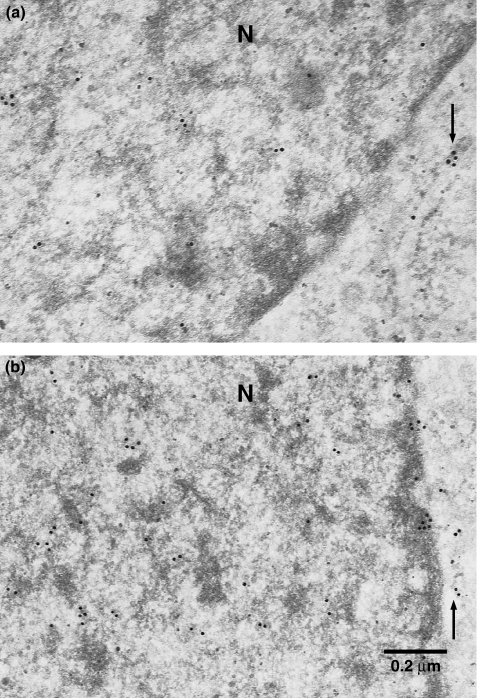
Gold-particles were observed in cytoplasmic organelles, presumably in the endoplasmic reticulum and/or Golgi apparatus. Moreover, particles were seen in the nucleus predominantly in the heterochromatin. Particles in the nucleus appeared to be more compared with those in the endoplasmic reticulum and/or Golgi apparatus, although a quantitative analysis was not performed (Figure 3).
Figure 3.
Immuno-electron microscopy for fukutin in HeLa cells (a, b). Gold particles are seen in the nucleus, predominantly in the heterochromatin, and presumably in the Golgi apparatus and/or endoplasmic reticulum (arrows). N, nucleus.
RNAi for HeLa and ZR-75-1 cells: RT-PCR
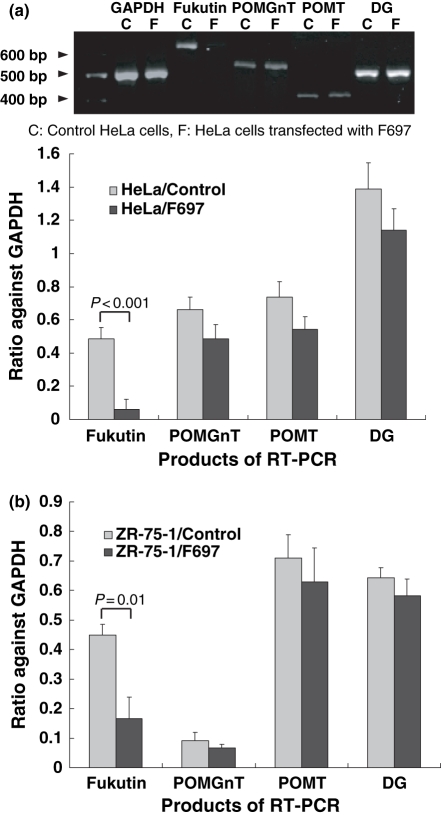
mRNA of fukutin, POMGnT1, POMT1 and DG was amplified by RT-PCR. Amplification products of fukutin were reduced in HeLa and ZR-75-1 cells transfected with F429, F697 and F1021. No reduction was induced by SNC. In spite of the transfection of siRNA, there was no significant decrease in RT-PCR products of POMGnT1, POMT1 or DG in both cell lines (Figure 4). The result indicates that fukutin was knocked-down, specifically. As F697 induced the highest reduction of fukutin, cells transfected F697 was used for further examinations.
Figure 4.
Results of RT-PCR in HeLa (a) and ZR-75-1 cells (b) after RNAi. In RT-PCR using primers for fukutin, POMGnT1, POMT1 and DG cDNA, amplification products for fukutin are reduced significantly in both cell lines after RNAi, but those for POMGnT1, POMT1 or DG are not. Control, cells only treated with lipofectamine; F697, cells transfected with F697.
RNAi for HeLa and ZR-75-1 cells: morphology and immunohistochemistry
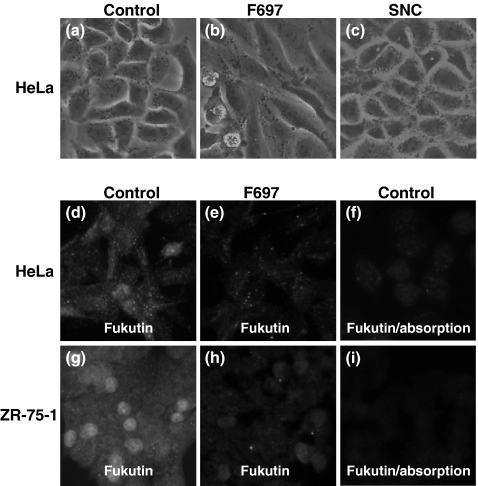
HeLa cells transfected with F429, F697 and F1021 tended to show spindled appearances, but there were no morphological alterations in cells only treated with lipofectamine or cells transfected with SNC (Figure 5). ZR-75-1 cell tended to grow making clusters, and it was difficult to observe an alteration of shape in each cell.
Figure 5.
Cell morphology (a–c) and results of immunohistochemistry for fukutin (d–i) in HeLa and ZR-75-1 cells after RNAi. Compared with controls (a), HeLa cells exhibit an elongated appearance after transfection of F697 (b). Cells transfected with SNC, siRNA for negative control, do not show any morphological changes (c). Immunohistochemically, fukutin is positive in the nucleus and cytoplasm in control HeLa and ZR-75-1 cells (d, g), and the immunoreaction in the nucleus and cytoplasm is reduced in cells transfected with F697 (e, h). Immunoreaction is also reduced when the primary antibody is pre-absorbed with fukutin protein (f, i). Control, cells only treated with lipofectamine; F697, cells transfected with F697; SNC, cells transfected with SNC.
In immunohistochemistry for fukutin, positive immunoreaction was seen in the cytoplasm and nucleus of control HeLa and ZR-75-1 cells, and immunoreaction in both the cytoplasm and nucleus was reduced in cells transfected with F697 (Figure 5). Immunoreaction was diminished when the fukutin antibody was pre-absorbed with fukutin protein, and when the fukutin antibody was omitted from the solution.
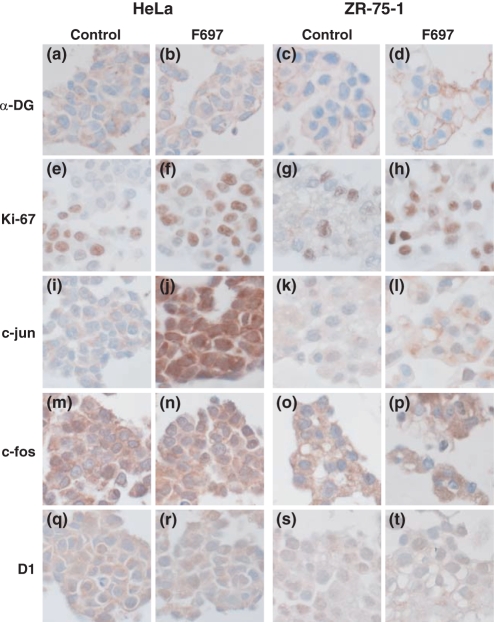
In immunohistochemistry using cell-blocks after RNAi, similar results were obtained in both HeLa and ZR-75-1 cells (Figure 6). No significant differences could be observed in the immunohistochemistry of α-DG between cells transfected with F697 and control cells only treated with lipofectamine. α-DG was not positive in the nucleus. Ki-67-positive cells were increased in cells transfected with F697 (Figure 6). Positive cells were 56.9 ± 1.67% (mean ± SD) in fukutin-suppressed HeLa cells, but only 35.2 ± 3.05% in control cells (P = 0.015). Ki-67-positive cells comprised about 45.5 ± 2.37% of fukutin-suppressed ZR-75-1 cells, but only 27.8 ± 2.08% of the control cells (P = 0.011). Fukutin-suppressed HeLa cells showed stronger nuclear and cytoplasmic staining of p-c-jun compared with control cells (Figure 6). Slight increase of p-c-jun could be seen in ZR-75-1 cells (Figure 6). No clear difference could be found in the immunohistochemistry of c-fos, cyclin D1, p21, p27, c-src, osteopontine, p53 or bcl-2 in either cell line (Figure 6).
Figure 6.
Results of immunohistochemistry using various antibodies in HeLa and ZR-75-1 cells after RNAi. No clear differences are found in α-DG immunohistochemistry (a–d). Ki-67-positive cells increases after knockdown of fukutin in both HeLa and ZR-75-1 cells (e–h). After knockdown of fukutin, immunoreaction for c-jun becomes stronger in the nucleus and cytoplasm of HeLa cells, (i, j) and increases slightly in ZR-75-1 cells (k, l). There are no apparent differences in c-fos or cyclin D1 (m–t). Control, cells only treated with lipofectamine; F697, cells transfected with F697; D1, cyclin D1.
RNAi for HeLa and ZR-75-1 cells: laminin-binding assay
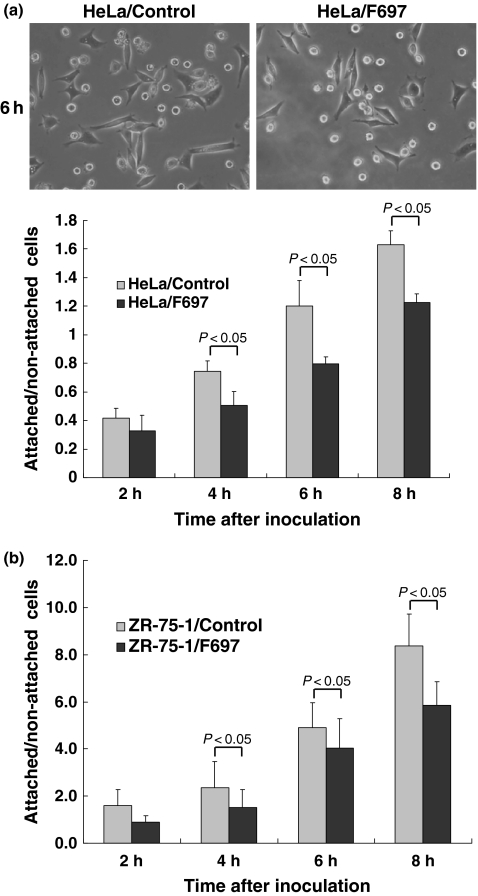
Laminin-binding assay was performed as an indirect method to evaluate the glycosylation of α-DG. After the inoculation, HeLa cells gradually attached to the dishes and showed elongated appearances with time, so that elongated cells were regarded as attached cells, and rounded cells were as non-attached cells (Figure 7). ZR-75-1 cell also gradually attached to the dishes, but did not show elongated appearance clearly. In ZR-75-1 cells, cells or clusters that did not move with vibration were regarded as attached cells, because it was difficult to distinguish the difference by morphology. A ratio between attached and non-attached cells looked slightly reduced when fukutin was knocked down in both HeLa and ZR-75-1 cells (Figure 7).
Figure 7.
Results of laminin-binding assay after RNAi. Elongated cells and rounded cells were observed in HeLa cells after inoculation in laminin-coated dishes. Photos show appearance of cells 6 h after inoculation. A ratio of attached cells to non-attached ones was tended to be low in fukutin-suppressed HeLa (a) and ZR-75-1 cells (b). Control, cells only treated with lipofectamine; F697, cells transfected with F697.
Discussion
Fukutin is expressed in various somatic organs such as the lung, the liver, the kidney, the pancreas, the skin and the intestine as well as in neuromuscular tissues (Kobayashi et al. 1998; Saito et al. 2004; Yamamoto et al. 2004a). Expression of fukutin in HeLa cells was confirmed by Western blotting, exhibiting a band about 60 kDa (Yamamoto et al. 2002), which was also observed in the skin, pancreas, kidney and uterine cervix. In cultured myocytes (Matsumoto et al. 2004) and COS-7 cells (Kobayashi et al. 1998) transfected with fukutin, fukutin protein originated from the transfected gene is localized in the cis-Golgi compartment, an organelle generally required for the synthesis of glycoproteins. In the present immuno-electron microscopy, existence of gold particles in the Golgi apparatus and/or endoplasmic reticulum of HeLa cells may be compatible with the presumable function of fukutin in relation to the glycosylation of α-DG.
For further examination, RNAi was performed to elucidate whether a knockdown of fukutin reduces the glycosylation of α-DG or not. If fukutin was greatly involved in the glycosylation of α-DG, immunohistochemistry and Western blotting would reveal a decrease of the glycosylation after RNAi, because much DG mRNA was expressed in HeLa and ZR-75-1 cells. However, the present immunohistochemistry using IIH6C4, the antibody for the glycosylated epitope of α-DG (Ervasti & Campbell 1993; Martin 2005), could not show apparent reduction of glycosylation of α-DG in fukutin-suppressed HeLa or ZR-75-1 cells, so as Western blotting (data not shown). Therefore, laminin-binding assay was performed to evaluate the glycosylation of α-DG indirectly, as the glycosylated site of α-DG is a receptor for laminin (Michele & Campbell 2003). Although several proteins bind to the glycosylated site (Michele & Campbell 2003; Oak et al. 2003; Martin 2005; Schessl et al. 2006), the reduced glycosylation can result in a loss of laminin-binding ability (Michele et al. 2002; Kim et al. 2004). The laminin-binding ability looked slightly decreased in fukutin-suppressed HeLa and ZR-75-1 cells. Together with the findings of Western blotting and immuno-electron microscopy, showing a band about 60 kDa on the cytoplasmic extracts and gold particles in the Golgi apparatus and/or endoplasmic reticulum, fukutin may be able to work for the glycosylation of α-DG in these cancer cell lines, but may be limited in a small portion.
An interesting finding is that the band about 60 kDa was seen in nuclear and cytoplasmic extracts, and the former looked stronger than the latter. The result of Western blotting seems to be consistent with that of immuno-electron microscopy, in which gold particles appeared to be more in the nucleus compared with those in the Golgi apparatus and/or endoplasmic reticulum of HeLa cells. Although fukutin protein originated from the transfected gene is localized to the cis-Golgi compartment (Kobayashi et al. 1998; Matsumoto et al. 2004), an in situ subcellular localization of fukutin has not been clearly determined. Positive nuclear staining of fukutin was often observed in immunohistochemistry using various normal human tissues and cancer tissues (data not shown). Possibilities remain that the nuclear staining of fukutin is an artifact or cross-reaction or detecting isoforms, but specificity of the anti-fukutin antibody has been examined by ELISA and by absorption tests on Western blotting and immunohistochemistry (Yamamoto et al. 2002). In this study, immunoreaction was diminished by absorption test as well. Moreover, RNAi is considered to be one of the good tools to prove a specificity of an antibody. Reduction of immunoreaction for fukutin in the nucleus after RNAi seems to support that at least a part of the nuclear staining reveals fukutin protein itself. Nuclear localization may propose a new function of fukutin in addition to the glycosylation of α-DG.
As fukutin seems to be expressed in the nucleus, effects of fukutin suppression for cell proliferation were examined. There were more Ki-67-positive cells after suppression of fukutin in HeLa cells and ZR-75-1 cells. The anti-Ki-67 antibody detects cells in G1, S, G2 and M phase of the cell cycle (Gerdes et al. 1984; Cattoretti et al. 1992), and it has been used widely to evaluate cell proliferation in the field of routine surgical pathology. The increase of Ki-67-positive cells after fukutin suppression suggests that fukutin suppresses cell proliferation. An elongated appearance observed in fukutin-suppressed HeLa cells might reflect a sarcomatoid feature of cancer, which is one of the subtypes of poorly differentiated carcinoma.
Immunohistochemistry for several proteins relating to cell proliferation was performed to investigate a mechanism for the increase of cell proliferation after the fukutin suppression. As a lot of proteins are complicatedly involved in the cell proliferation, several proteins were selected in this experiment, especially ones relating to the cell cycle or presumably relating to DGC. Cyclin D1, p21, p27 and p53 regulate the cell cycle in the nucleus (Morgan et al. 2002). In DGC of the striated muscle, the C-terminus of α-DG binds to the N-terminus of β-DG in the extracellular area (Michele & Campbell 2003; Oak et al. 2003). β-DG, a transmembrane protein, binds to dystrophin and other proteins such as growth factor receptor bound protein 2 (Grb2) at its C-terminus in the cytoplasm (Figure 1) (Russo et al. 2000; Michele & Campbell 2003; Oak et al. 2003). Moreover, c-jun is probably in the downstream section of the signalling pathway involving DGC, together with c-jun NH2-terminal kinase (JNK) (Oak et al. 2003). Tyrosine phosphorylation of the C-terminus of β-DG is dependent on c-src (Sotgia et al. 2001; Oak et al. 2003). Thus, c-jun and c-src are involved in signal transduction relating to DGC (Oak et al. 2003). Osteopontin is regulated by c-src and is a direct target of p53 (El-Tanani et al. 2006). In this examination, only c-jun showed a difference after the suppression of fukutin. No apparent differences were found in the other proteins examined.
c-Jun is a component of the transcription factor activator protein 1 (AP-1), forming homodimers or heterodimers with c-fos or other bZip proteins (Vleugel et al. 2006), and is phosphorylated by c-JNK (Oak et al. 2003; Nateri et al. 2005). After activation by phosphorylation, c-jun works as a transcription factor in the nucleus and is involved in cell proliferation (Vleugel et al. 2006). Inhibition of c-jun expression or inactivation of c-jun suppresses tumour growth (Nateri et al. 2005; Zhang et al. 2006). Because the anti-c-jun antibody used in this study detects phosphorylated c-jun, an activated form, c-jun is considered to be activated after knockdown of fukutin in HeLa and ZR-75-1 cells. Fukutin may suppress epithelial cell proliferation through a system involving c-jun.
Is the activation of c-jun related to the glycosylation of α-DG in HeLa and ZR-75-1 cells? Hypoglycosylation of α-DG might induce the activation, because this experiment suggested a slight involvement of fukutin for the glycosylation of α-DG in these cells, and c-jun is located in the downstream section of the signalling pathway related to the DGC (Oak et al. 2003). However, if a loss of fukutin caused hypoglycosylation of α-DG, our result showing increased proliferation after knockdown of fukutin would be contradictory to the data showing binding of laminin to the DGC induces activation of the signalling involving c-jun and increases proliferation in myoblasts (Oak et al. 2003; Zhou et al. 2007). There might be a difference between muscle and epithelial cells. On the other hand, based on the nuclear expression, more functions of fukutin might be proposed in relation to the activation of c-jun, because activated c-jun works in the nucleus and glycosylated α-DG has not been detected in the nucleus. Although significance of the nuclear localization of fukutin is uncertain at present, more roles of fukutin in addition to the glycosylation of α-DG can be suspected especially in epithelial cells.
Acknowledgments
The authors wish to thank Dr Yoshiaki Saito, Division of Child Neurology, Institute of Neurological Sciences, Faculty of Medicine, Tottori University, for kindly providing the anti-fukutin antisera. The authors are also grateful to Mr Mizuho Karita, Mr Hideyuki Takeiri, Mr Fumiaki Muramatsu, Mrs Noriko Sakayori and Mr Shuichi Iwasaki for their excellent technical assistance and help in the preparation of the paper.
References
- Akasaka-Manya K, Manya H, Endo T. Mutations of the POMT1 gene found in patients with Walker-Warburg syndrome lead to a defect of protein O-mannosylation. Biochem. Biophys. Res. Commun. 2004;325:75–79. doi: 10.1016/j.bbrc.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Beltrán-Valero de Bernabé D, Currier S, Steinbrecher A, et al. Mutations in the O-mannosyltransferase gene POMT1 give rise to the severe neuronal migration disorder Walker-Warburg syndrome. Am. J. Hum. Genet. 2002;71:1033–1043. doi: 10.1086/342975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattoretti G, Becker MHG, Key G, et al. Monoclonal antibodies against recombinant parts of the Ki-67 antigen (MIB 1 and MIB 3) detect proliferating cells in microwave-processed formalin-fixed paraffin sections. J. Pathol. 1992;168:357–363. doi: 10.1002/path.1711680404. [DOI] [PubMed] [Google Scholar]
- Chiyonobu T, Sasaki J, Nagai Y, et al. Effects of fukutin deficiency in the developing brain. Neuromuscul. Disord. 2005;15:416–426. doi: 10.1016/j.nmd.2005.03.009. [DOI] [PubMed] [Google Scholar]
- El-Tanani MK, Campbell FC, Kurisetty V, Jin D, McCann M, Rudland PS. The regulation and role of osteopontin in malignant transformation and cancer. Cytokine Growth Factor Rev. 2006;17:463–474. doi: 10.1016/j.cytogfr.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Ervasti JM, Campbell KP. A role for the dystrophin-glycoprotein complex as a transmembrane linker between laminin and actin. J. Cell Biol. 1993;122:809–823. doi: 10.1083/jcb.122.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes J, Lemke H, Baisch H, Wacker H-H, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J. Immunol. 1984;133:1710–1715. [PubMed] [Google Scholar]
- Hayashi YK, Ogawa M, Tagawa K, et al. Selective deficiency of α-dystroglycan in Fukuyama-type congenital muscular dystrophy. Neurology. 2001;57:115–121. doi: 10.1212/wnl.57.1.115. [DOI] [PubMed] [Google Scholar]
- Jing J, Lien CF, Sharma S, Rice J, Brennan PA, Górecki DC. Aberrant expression, processing and degradation of dystroglycan in squamous cell carcinomas. Eur. J. Cancer. 2004;40:2143–2151. doi: 10.1016/j.ejca.2004.05.018. [DOI] [PubMed] [Google Scholar]
- Kim D-S, Hayashi YK, Matsumoto H, et al. POMT1 mutation results in defective glycosylation and loss of laminin-binding activity in α-DG. Neurology. 2004;62:1009–1011. doi: 10.1212/01.wnl.0000115386.28769.65. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Nakahori Y, Miyake M, et al. An ancient retrotransposal insertion causes Fukuyama-type congenital muscular dystrophy. Nature. 1998;394:388–392. doi: 10.1038/28653. [DOI] [PubMed] [Google Scholar]
- Kurahashi H, Taniguchi M, Meno C, et al. Basement membrane fragility underlies embryonic lethality in fukutin-null mice. Neurobiol. Dis. 2005;19:208–217. doi: 10.1016/j.nbd.2004.12.018. [DOI] [PubMed] [Google Scholar]
- Manya H, Sakai K, Kobayashi K, et al. Loss-of-function of an N-acetylglucosaminyltransferase, POMGnT1, in muscle-eye-brain disease. Biochem. Biophys. Res. Commun. 2003;306:93–97. doi: 10.1016/s0006-291x(03)00924-0. [DOI] [PubMed] [Google Scholar]
- Martin PT. The dystroglycanopathies: the new disorders of O-linked glycosylation. Semin. Pediatr. Neurol. 2005;152:152–158. doi: 10.1016/j.spen.2005.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto H, Noguchi S, Sugie K, et al. Subcellular localization of fukutin and fukutin-related protein in muscle cells. J. Biochem. 2004;135:709–712. doi: 10.1093/jb/mvh086. [DOI] [PubMed] [Google Scholar]
- Michele DE, Campbell KP. Dystrophin-glycoprotein complex: post-transcriptional processing and dystroglycan function. J. Biol. Chem. 2003;278:15457–15460. doi: 10.1074/jbc.R200031200. [DOI] [PubMed] [Google Scholar]
- Michele DE, Barresi R, Kanagawa M, et al. Post-transcriptional disruption of dystroglycan-ligand interactions in congenital muscular dystrophies. Nature. 2002;418:417–421. doi: 10.1038/nature00837. [DOI] [PubMed] [Google Scholar]
- Morgan D, Murray A, Hunt T, Nurse P. The cell cycle and programmed cell death. In: Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P, editors. Molecular Biology of the Cell. 4. New York: Garland Science; 2002. pp. 983–1026. [Google Scholar]
- Muschler J, Levy D, Boudreau R, Henry M, Campbell K, Bissell MJ. A role for dystroglycan in epithelial polarization: loss of function in breast tumor cells. Cancer Res. 2002;62:7102–7109. [PubMed] [Google Scholar]
- Nateri AS, Spencer-Dene B, Behrens A. Interaction of phosphorylated c-Jun with TCF4 regulates intestinal cancer development. Nature. 2005;437:281–285. doi: 10.1038/nature03914. [DOI] [PubMed] [Google Scholar]
- Oak SA, Zhou YW, Jarrett HW. Skeletal muscle signaling pathway through the dystrophin glycoprotein complex and Rac1. J. Biol. Chem. 2003;278:39287–39295. doi: 10.1074/jbc.M305551200. [DOI] [PubMed] [Google Scholar]
- Russo K, Di Stasio E, Macchia G, Rosa G, Brancaccio A, Petrucci TC. Characterization of the β-dystroglycan-growth factor receptor 2 (Grb2) interaction. Biochem. Biophys. Res. Commun. 2000;274:93–98. doi: 10.1006/bbrc.2000.3103. [DOI] [PubMed] [Google Scholar]
- Saito Y, Mizuguchi M, Oka A, Takashima S. Fukutin protein is expressed in neurons of the normal developing human brain but is reduced in fukuyama-type congenital muscular dystrophy brain. Ann. Neurol. 2000;47:756–764. [PubMed] [Google Scholar]
- Saito Y, Yamamoto T, Ohtsuka-Tsurumi E, et al. Fukutin expression in mouse non-muscle somatic organs: its relationship to the hypoglycosylation of α-dystroglycan in Fukuyama-type congenital muscular dystrophy. Brain Dev. 2004;26:469–479. doi: 10.1016/j.braindev.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Schessl J, Zou Y, Bönnemann CG. Congenital muscular dystrophies and the extracellular matrix. Semin. Pediatr. Neurol. 2006;13:80–89. doi: 10.1016/j.spen.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Sgambato A, Brancaccio A. The dystroglycan complex: from biology to cancer. J. Cell. Physiol. 2005;205:163–169. doi: 10.1002/jcp.20411. [DOI] [PubMed] [Google Scholar]
- Sgambato A, Migaldi M, Montanari M, et al. Dystroglycan expression is frequently reduced in human breast and colon cancers and is associated with tumor progression. Am. J. Pathol. 2003;162:849–860. doi: 10.1016/S0002-9440(10)63881-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgambato A, Tarquini E, Resci F, et al. Aberrant expression of α-dystroglycan in cervical and vulvar cancer. Gynecol. Oncol. 2006;103:397–404. doi: 10.1016/j.ygyno.2006.03.059. [DOI] [PubMed] [Google Scholar]
- Sotgia F, Lee H, Bedford MT, Petrucci T, Sudol M, Lisanti MP. Tyrosine phosphorylation of β-dystroglycan at its WW domain binding motif, PPxY, recruits SH2 domain containing proteins. Biochemistry. 2001;40:14585–14592. doi: 10.1021/bi011247r. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Sasaki T, Manya H, et al. A new β1,2-N-acetylglucosaminyltransferase that may play a role in the biosynthesis of mammalian O-mannosyl glycans. Glycobiology. 2001;11:37–45. doi: 10.1093/glycob/11.1.37. [DOI] [PubMed] [Google Scholar]
- Vleugel MM, Greijer AE, Bos R, van der Wall E, van Diest PJ. c-Jun activation is associated with proliferation and angiogenesis in invasive breast cancer. Hum. Pathol. 2006;37:668–674. doi: 10.1016/j.humpath.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Kato Y, Karita M, et al. Fukutin expression in glial cells and neurons: implication in the brain lesions of Fukuyama congenital muscular dystrophy. Acta Neuropathol. 2002;104:217–224. doi: 10.1007/s00401-002-0542-8. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Kato Y, Karita M, Kawaguchi M, Shibata N, Kobayashi M. Expression of genes related to muscular dystrophy with lissencephaly. Pediatr. Neurol. 2004a;31:183–190. doi: 10.1016/j.pediatrneurol.2004.03.020. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Kato Y, Kawaguchi M, Shibata N, Kobayashi M. Expression and localization of fukutin, POMGnT1 and POMT1 in the central nervous system: consideration for functions of fukutin. Med. Electron Microsc. 2004b;37:200–207. doi: 10.1007/s00795-004-0260-5. [DOI] [PubMed] [Google Scholar]
- Yoshida A, Kobayashi K, Manya H, et al. Muscular dystrophy and neuronal migration disorder caused by mutations in a glycosyltransferase, POMGnT1. Dev. Cell. 2001;1:717–724. doi: 10.1016/s1534-5807(01)00070-3. [DOI] [PubMed] [Google Scholar]
- Zhang G, Luo X, Sumithran E, et al. Squamous cell carcinoma growth in mice and in culture is regulated by c-Jun and its control of matrix metalloproteinase-2 and –9 expression. Oncogene. 2006;25:7260–7266. doi: 10.1038/sj.onc.1209726. [DOI] [PubMed] [Google Scholar]
- Zhou YW, Jiang D, Thomason DB, Jarrett HW. Laminin-induced activation of Rac1 and JNKp46 is initiated by src family kinases and mimics the effects of skeletal muscle contraction. Biochemistry. 2007;46:14907–14916. doi: 10.1021/bi701384k. [DOI] [PMC free article] [PubMed] [Google Scholar]