Abstract
In submandibular gland atrophy, most acinar cells disappear by apoptosis, while many duct cells remain. The present study aimed to establish whether Bcl-2 and Bax, members of the Bcl-2 gene family, regulating the signalling pathway of apoptosis were involved in duct cell survival and acinar cell death in atrophic submandibular glands. The excretory duct of rat submandibular gland was doubly ligated with metal clips from 1 to 14 days to induce atrophy to the gland. The expressions of Bcl-2 and Bax in the atrophic submandibular gland were examined using immunohistochemistry and reverse transcriptase-polymerase chain reaction (RT-PCR). Immunohistochemically, Bcl-2 expression was identified in duct cells in the experimental glands at all time points. Some acinar cells showed Bax positivity 1 day after excretory duct ligation, and there were more Bax-positive acinar cells on days 3 and 5 when many apoptotic acinar cells were observed. Analysis by RT-PCR showed that the expression of mRNA for Bcl-2 became stronger as the glandular atrophy progressed and that Bax mRNA strongly expressed on days 1 and 3. These observations suggest that Bcl-2 inhibits duct cell apoptosis and Bax promotes apoptosis of acinar cells during atrophy of submandibular glands.
Keywords: apoptosis, atrophy, Bax, Bcl-2, duct-ligation, submandibular gland
Atrophy of salivary glands has repeatedly been examined using an experimental animal model with an excretory duct ligation of the salivary gland, as it causes serious clinical problems such as increases in dental caries, atrophy of the oral mucosa, difficulty in swallowing and speech and other problems (Hand 1986). The histological characteristics of the atrophic salivary glands are disappearance of acinar cells and prominence of duct cells (Tandler 1977; Matthews & Dardick 1988). It has been reported that apoptotic cell death and mitotic cell proliferation play important roles in the loss of acinar cells and increase in duct cell numbers respectively (Walker & Gobe 1987; Takahashi et al. 2000, 2002). Further, a recent study suggested that the Fas and Fas ligand (FasL) systems triggered apoptotic pathways during atrophy of the rat submandibular gland (Takahashi et al. 2007). However, the molecular mechanisms underlying the histopathological changes in the atrophy of salivary glands are not fully understood. Many aspects of the histopathological changes including how duct cells avoid apoptotic cell death and the mechanism of apoptosis of acinar cells still remains unexplained.
The Bcl-2 family is known to be a major protein family controlling cell survival or cell death in the molecular pathway of apoptosis. The Bcl-2 family mainly functions as a regulator of mitochondrial membrane permeability and controls the release of apoptogenic factors (Green 2000; Tsujimoto & Shimizu 2000). The Bcl-2 family is divided into three subfamilies: the Bcl-2 subfamily, the Bax subfamily and the Bcl-2 homology 3 (BH-3) only proteins, based on their structural and functional characteristics: the Bcl-2 subfamily is anti-apoptotic, the Bax subfamily and BH-3-only proteins are pro-apoptotic (Adams & Cory 1998; Tsujimoto & Shimizu 2000; Youle & Strasser 2008). The present study sheds light on the Bcl-2 protein, a member of the Bcl-2 subfamily, and on the Bax protein, a member of Bax subfamily, where it has been reported that Bcl-2 participates in cell survival and Bax in cell death during regressive changes of other exocrine glands such as the pancreas (Wada et al. 1997; Reid & Walker 1999; Scoggins et al. 2000) and mammary gland (Heermeier et al. 1996; Li et al. 1997; Venugopal et al. 1999).
To elucidate the molecular mechanism of the cell survival and cell death in atrophic salivary glands, the expression of Bcl-2 and Bax in atrophic rat submandibular glands was examined after excretory duct ligation, using immunohistochemistry with antibodies to these proteins and also mRNA analysis by reverse transcriptase-polymerase chain reaction (RT-PCR).
Materials and methods
Experimental procedures
Seven-week-old male Wistar rats, weighing 190–220 g, were provided by Hokudo Co. Ltd. (Sapporo, Japan). They were divided into experimental and control groups and housed with free access to pellet food and tap water at the Animal Facility of Hokkaido University Graduate School of Dental Medicine. All animal experimentation had the approval of the Animal Ethics Committee and complied with the Guide for the Care and Use of Laboratory Animals of Hokkaido University Graduate School of Dental Medicine.
In the experimental animals, the right submandibular gland and its excretory duct were surgically dissected and the right excretory duct was doubly ligated with Ligaclips (Ethicon Endo-Surgery Inc., Cincinnati, OH, USA) near the hilum of the gland under general anaesthesia with ether. The experimental animals were killed at 1, 3, 5, 10 or 14 days after duct ligation (n = 4 at each time point) with deep inhalation of ether and the right submandibular glands were excised. The right submandibular glands of the untreated control rats (n = 4) were collected in the same way. The submandibular gland atrophy is caused by duct obstruction and damage to the corda tympani running on the excretory duct in the rodents (Harrison & Garrett 1972; Harrison et al. 2001).
Immunohistochemistry
The tissue for immunohistochemistry was fixed in 4% paraformaldehyde buffered at pH 7.4 with 0.1 M phosphate buffer for 24 h. After fixation, the specimens were embedded in paraffin and sectioned at 4 μm thickness. The sections were immersed in 0.3% hydrogen peroxide to inhibit the endogenous peroxidase and boiled in 10 mM citrate buffer at pH 9.8 for Bcl-2 or pH 6.0 for Bax (ChemMate Target Retrieval Solution; Dakocytomation, Kyoto, Japan) for 40 min. Then the sections were incubated successively with anti-Bcl-2 rabbit polyclonal antibody (N-19; Santa Cruz Biotechnology, Santa Cruz, CA, USA) or anti-Bax rabbit polyclonal antibody (N-20; Santa Cruz Biotechnology) overnight at 4 °C, biotinylated anti-rabbit swine polyclonal antibody (Dakocytomation) and streptavidin–biotin–horseradish peroxidase complex (Dakocytomation). The immunoreaction was developed with 3,3′-diaminobenzidine and the sections were lightly counterstained with haematoxylin.
Negative control sections were incubated with normal rabbit serum in the absence of primary antibodies.
RT-PCR
Fresh submandibular glands removed from experimental animals at 1, 3 or 14 days after duct ligation and from control animals were snap-frozen in liquid nitrogen and stored at −80 °C until required for RNA extraction. The samples were homogenized in ice-cold TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and total RNA were extracted according to the manufacturer's instructions. Then total RNA was quantified using a Nano-Drop spectrophotometer ND-1000 (NanoDrop Tech., Inc., Wilmington, DE, USA). First-strand cDNA was synthesized from total RNA with ReverTra Ace (Toyobo, Osaka, Japan) following the manufacturer's protocol and was subjected to PCR amplification using Amplitaq Gold DNA polymerase (Roche Molecular Systems, Braunchburg, NJ, USA) with a PCR System s9700 (Applied Biosystems, Foster City, CA, USA). The sequences of the primers used for PCR were as follows: Bax (F: 5′AAGAAGCTGAGCGAGTGTCT-3′; R: 5′CAAAGATGGTCACTGTCTGC-3′) (Rogerio et al. 2006), Bcl-2 (F: 5′GTATGATAACCGGGAGATCG-3′; R: 5′AGCCAGGAGAAATCAAACAG-3′) (Rogerio et al. 2006) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (F: 5′CGGAGTCAACGGATTTGGTCGTAT-3′; R: 5′AGCCTTCTCCATGGTGGTGAAGAC-3′) (Wong et al. 1994). The PCR condition was as follows: initial denaturation for 11 min at 95 °C; repeated cycles of denaturation for 1 min at 95 °C; annealing for 1 min at 58 °C (Bcl-2), 59 °C (Bax) or 63 °C (GAPDH); extension for 1 min at 72 °C; final extension for 10 min at 72 °C. The amplification was conducted for 34 cycles (Bcl-2), 29 cycles (Bax) or 25 cycles (GAPDH), and the expected sizes of the PCR products were 612 (Bcl-2), 361 (Bax) and 306 bp (GAPDH). The synthesized PCR products were separated by electrophoresis on a 1% agarose gel. The gels were stained with ethidium bromide and visualized under UV light.
Results
Histological changes during atrophy of submandibular glands have been reported (Takahashi et al. 2000), and briefly, ducts were dilated in the oedematous glandular tissue at 1 day after duct ligation. At 3 and 5 days, many apoptotic acinar cells were identified. At 10 and 14 days, most acinar cells disappeared and the parenchyma of atrophic glandular tissue comprised a few acini and many ducts.
Immunohistochemistry
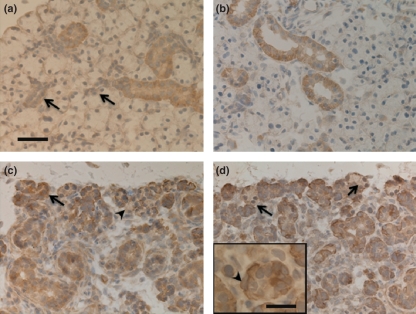
In both control and experimental submandibular glands at 1 day after duct ligation, most striated duct cells and a few intercalated duct cells showed positive reactions to Bcl-2, while all acinar cells were negative (Figure 1a,b). On days 3 and 5, most remaining duct cells exhibited immunoreactivity for Bcl-2; there were Bcl-2-positive cells in some acini; however, other acinar cells did not show a positive reaction to Bcl-2 antibodies (Figure 1c). On days 10 and 14, the remaining duct cells were positive and many acinar cells negative to Bcl-2. Spindle-shaped stellate cells at the periphery of the residual ducts and acini showed intense immunoreaction against Bcl-2 antibody (Figure 1d).
Figure 1.
Bcl-2 immunohistochemistry. Bar = 50 μm (a) Control. Striated duct cells are positive. Intercalated duct cells (arrows) and acinar cells are negative. (b) Day 1: Cells in dilated ducts show positive reaction. (c) Day 5: The remaining duct cells are immunoreactive to Bcl-2 antibody. There are some positive (arrow) and many negative (arrowhead) acinar cells in the atrophic gland. (d) Day 14: Most residual duct cells are positive and remaining acinar cells are negative (arrows). Inset: higher magnification of (d). Bar = 20 μm. The spindle-shaped (myoepithelial-like) cell is immunopositive (arrowhead).
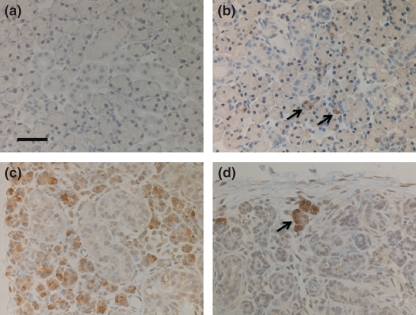
In Bax immunohistochemistry, there was no reaction in the control submandibular glands (Figure 2a). On day 1 following duct ligation, some Bax-positive cells appeared in acini (Figure 2b) and acinar cells showing a Bax-positive reaction were present in larger numbers at 3 and 5 days (Figure 2c). Thereafter, the number of Bax-positive cells decreased as acinar cells disappeared due to apoptosis; however, the residual acinar cells commonly showed intense positivity to Bax on days 10 and 14 (Figure 2d). No immunoreaction for Bax was identified in duct cells throughout the experimental period.
Figure 2.
Bax immunohistochemistry. Bar = 50 μm (a) Control. No immunoreaction is identified in normal submandibular glands. (b) Day 1: There are some positive acinar cells (arrows). (c) Day 3: Many acinar cells are Bax immunoreactive. (d) Day 14: The remaining acinar cells are reactive with the anti-Bax antibody (arrow).
Negative control sections for both immunohistochemical stainings showed no positive reactions.
RT-PCR
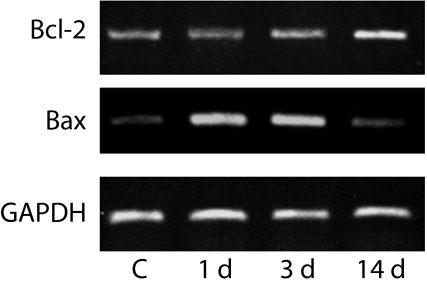
Figure 3 shows the data of the expression of Bcl-2 and Bax mRNA analysed with RT-PCR. Expressions of both mRNA were identified in the control and in all the experimental groups. The expression of Bcl-2 mRNA becomes stronger as the glandular atrophy progressed and the strongest band was identified at day 14 postobstruction. There was increased expression of Bax mRNA at 1 and 3 days.
Figure 3.
RT-PCR analysis of mRNA expression of Bcl-2, Bax and GAPDH. C, control (normal submandibular gland); 1d, 1 day after duct ligation; 3d, day 3 and 14d, day 14. The bands correspond to 612 (Bcl-2), 361 (Bax) and 306 bp (GAPDH).
Discussion
The Bcl-2 gene was discovered at the t (14; 18) chromosome translocation breakpoint in human B-cell lymphoma (Tsujimoto et al. 1985) and it inhibits apoptotic cell death by preventing release of apoptogenic factors such as cytochrome c and apoptosis-inducing factor from the intermembrane space of mitochondria (Tsujimoto & Shimizu 2000). Bax promotes apoptosis by inducing release of the apoptogenic factors (Youle & Strasser 2008) and forms heterodimers with Bcl-2 (Oltvai et al. 1993). Considering this, it has been considered that the ratio of Bcl-2/Bax determines the fate of a cell, survival or death (Korsmeyer et al. 1993; Oltvai et al. 1993). In the atrophic submandibular gland, acinar cell apoptosis is most frequently observed on day 3 after duct ligation (Takahashi et al. 2000). In the present study, the positive acinar cells against Bax antibody appeared on day 1 and many acinar cells expressed Bax especially on day 3. A few acinar cells were Bcl-2 positive throughout the experimental period. In addition, the sample obtained from days 1 and 3 clearly expressed mRNA of Bax in RT-PCR. These results suggest that Bax accelerates acinar cell apoptosis in the atrophy of submandibular glands. In the control gland, no Bax-positive reaction was immunohistochemically identified, although a weak Bax reaction was observed in RT-PCR. The reason for this difference is not exactly clear. Bax is inactive by binding to 14-3-3 protein under normal condition and becomes active by dissociation from this protein on apoptotic stimulation (Nomura et al. 2003). It may be possible to speculate that such a protein prevents anti-Bax antibody from recognizing the epitope of Bax protein.
Analysis by RT-PCR in the present study showed the expression of mRNA of Bcl-2 during the course of the glandular atrophy and the expression became stronger as the glandular atrophy progressed. Immunohistochemically, most duct cells expressed Bcl-2, but there were few duct cells expressing Bax in the atrophic gland. This showed that Bcl-2 was predominant over Bax in the remaining duct cells. These findings suggest that Bcl-2 contributes to the survival of duct cells in the atrophic submandibular gland. Several acinar cells were also Bcl-2 positive during atrophy. Such acinar cells would resist apoptotic stimuli and survive in the atrophied glandular tissue.
It has been reported that apoptosis in the atrophy of rat submandibular glands following duct ligation is triggered by the Fas/FasL system (Takahashi et al. 2007). Two types of cells (so-called types I and II cells) involved in Fas/FasL system-mediated apoptosis are known. Although the pathway to apoptosis does not proceed through mitochondria in type I cells, the apoptotic signals were transmitted to mitochondria in type II cells (Scaffidi et al. 1998; Barnhart et al. 2003). Participation of Bcl-2 and Bax regulating the mitochondrial membrane permeability (Green 2000; Tsujimoto & Shimizu 2000) was shown in the present study, suggesting that parenchymal cells of the submandibular gland may behave as type II cells in the glandular atrophy.
Expressions of Bax and Bcl-2 have also been reported in the regressive process of other exocrine glands. After weaning of pups, the mammary glands involute with disappearance of secretory alveolar cells by apoptosis (Walker et al. 1989), which is promoted by Bax (Heermeier et al. 1996; Venugopal et al. 1999). In pancreatitis induced by duct obstruction (Wada et al. 1997; Scoggins et al. 2000) or caerulein administration (Reid & Walker 1999), Bcl-2-expressing duct cells are not subject to apoptosis and remain, although acinar cells undergo apoptosis. These findings coincide with the results of the present study, suggesting that Bcl-2 and Bax may both be important regulators of the apoptotic pathway and common to the regressive alterations of exocrine glands.
At 10 and 14 days after duct ligation, the residual duct and acini were surrounded by spindle-shaped cells showing intensely positive against Bcl-2. The distribution and morphological characteristic of these cells are coincident with those of the remaining myoepithelial cells (Takahashi et al. 2001). Therefore, the remaining myoepithelial cells are considered to express Bcl-2 in the late stage of submandibular gland atrophy. It has been reported that TUNEL-positive myoepithelial cells were most frequently observed at 5 days after duct ligation and that thereafter apoptosis of myoepithelial cells was rare (Takahashi et al. 2001). Taken together with the results of this study, myoepithelial cells passing through the early phase of atrophy without apoptosis are thought to survive by expressing Bcl-2 in the late phase of atrophy.
In conclusion, strong expressions of Bcl-2 and Bax were identified in duct cells and acinar cells, respectively, during atrophy of the submandibular gland in the present study, showing that duct cells are not subject to apoptosis due to Bcl-2 and that acinar cell apoptosis is accelerated by Bax. The molecular mechanism of atrophy of salivary glands has been better understood by our series of studies (Takahashi et al. 2007); however, further study will be needed to elucidate all the details of the event and to establish the therapeutic strategies for salivary gland atrophy.
References
- Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- Barnhart BC, Alappat EC, Peter ME. The CD95 type I/type II model. Semin. Immunol. 2003;15:185–193. doi: 10.1016/s1044-5323(03)00031-9. [DOI] [PubMed] [Google Scholar]
- Green DR. Apoptotic pathways: paper wraps stone blunts scissors. Cell. 2000;102:1–4. doi: 10.1016/s0092-8674(00)00003-9. [DOI] [PubMed] [Google Scholar]
- Hand AR. Salivary glands. In: Provenza DV, Seibel W, editors. Oral Histology. 2. PA: Lea & Febiger; 1986. pp. 388–417. [Google Scholar]
- Harrison JD, Garrett JR. Mucocele formation in cats by glandular duct ligation. Arch. Oral Biol. 1972;17:1403–1414. doi: 10.1016/0003-9969(72)90099-4. [DOI] [PubMed] [Google Scholar]
- Harrison JD, Fouad HMA, Garrett JR. Variation in the response to ductal obstruction of feline submandibular and sublingual salivary glands and the importance of the innervation. J. Oral Pathol. Med. 2001;30:29–34. doi: 10.1034/j.1600-0714.2001.300105.x. [DOI] [PubMed] [Google Scholar]
- Heermeier K, Benedict M, Li M, Furth P, Nunez G, Hennighausen L. Bax and Bcl-Xs are induced at the onset of apoptosis in involuting mammary epithelial cells. Mech. Dev. 1996;56:197–207. doi: 10.1016/0925-4773(96)88032-4. [DOI] [PubMed] [Google Scholar]
- Korsmeyer SJ, Shutter JR, Veis DJ, Merry DE, Oltvai ZN. Bcl-2/Bax: a rheostat that regulates an anti-oxidant pathway and cell death. Semin. Cancer Biol. 1993;4:327–332. [PubMed] [Google Scholar]
- Li M, Liu X, Robinson G, et al. Mammary-derived signals activate programmed cell death during the first stage of mammary gland involution. Proc. Natl. Acad. Sci. U. S. A. 1997;94:3425–3430. doi: 10.1073/pnas.94.7.3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews TW, Dardick I. Morphological alterations of salivary gland parenchyma in chronic sialadenitis. J. Otolaryngol. 1988;17:385–398. [PubMed] [Google Scholar]
- Nomura M, Shimizu S, Sugiyama T, et al. 14-3-3 interacts directly with and negatively regulates pro-apoptotic Bax. J. Biol. Chem. 2003;278:2058–2065. doi: 10.1074/jbc.M207880200. [DOI] [PubMed] [Google Scholar]
- Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- Reid LE, Walker NI. Acinar cell apoptosis and the origin of tubular complexes in caerulein-induced pancreatitis. Int. J. Exp. Pathol. 1999;80:205–215. doi: 10.1046/j.1365-2613.1999.00116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogerio F, Jordao H, Jr, Vieira AS, et al. Bax and Bcl-2 expression and TUNEL labeling in lumbar enlargement of neonatal rats after sciatic axotomy and melatonin treatment. Brain Res. 2006;1112:80–90. doi: 10.1016/j.brainres.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Scaffidi C, Fulda S, Srinivasan A, et al. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoggins CR, Meszoely IM, Wada M, Means AL, Yang L, Leach SD. p53-Dependent acinar cell apoptosis triggers epithelial proliferation in duct-ligated murine pancreas. Am. J. Physiol. Gastrointest. Liver Physiol. 2000;279:827–836. doi: 10.1152/ajpgi.2000.279.4.G827. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Nakamura S, Suzuki R, et al. Apoptosis and mitosis of parenchymal cells in the duct-ligated rat submandibular gland. Tissue Cell. 2000;32:457–463. doi: 10.1016/s0040-8166(00)80002-6. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Nakamura S, Shinzato K, Domon T, Yamamoto T, Wakita M. Apoptosis and proliferation of myoepithelial cells in atrophic rat submandibular glands. J. Histochem. Cytochem. 2001;49:1557–1563. doi: 10.1177/002215540104901209. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Shinzato K, Nakamura S, Domon T, Yamamoto T, Wakita M. The roles of apoptosis and mitosis in atrophy of the rat sublingual gland. Tissue Cell. 2002;34:297–304. doi: 10.1016/s0040816602000034. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Gobe GC, Yoshimura Y, Kohgo T, Yamamoto T, Wakita M. Participation of the Fas and Fas ligand systems in apoptosis during atrophy of the rat submandibular glands. Int. J. Exp. Pathol. 2007;88:9–17. doi: 10.1111/j.1365-2613.2006.00511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandler B. Ultrastructure of chronically inflamed human submandibular glands. Arch. Pathol. Lab. Med. 1977;101:425–431. [PubMed] [Google Scholar]
- Tsujimoto Y, Shimizu S. Bcl-2 family: life-or-death switch. FEBS Lett. 2000;466:6–10. doi: 10.1016/s0014-5793(99)01761-5. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y, Jaffe E, Cossman J, Gorham J, Nowell PC, Croce CM. Clustering of breakpoints on chromosome 11 in human B-cell neoplasms with t (11;14) chromosome translocation. Nature. 1985;315:340–343. doi: 10.1038/315340a0. [DOI] [PubMed] [Google Scholar]
- Venugopal M, Callaway A, Snyderwine EG. 2-Amino-1-methyl-6-phenylimidazo (4,5-6)pyridine (PhIP) retards mammary gland involution in lactating Sprague–Dawley rats. Carcinogenesis. 1999;20:1309–1314. doi: 10.1093/carcin/20.7.1309. [DOI] [PubMed] [Google Scholar]
- Wada M, Doi R, Hosotani R, et al. Expression of bcl-2 and PCNA in duct cells after pancreatic duct ligation in rats. Pancreas. 1997;15:176–182. doi: 10.1097/00006676-199708000-00010. [DOI] [PubMed] [Google Scholar]
- Walker NI, Gobe GC. Cell death and cell proliferation during atrophy of the rat parotid gland induced by duct obstruction. J. Pathol. 1987;153:333–344. doi: 10.1002/path.1711530407. [DOI] [PubMed] [Google Scholar]
- Walker NI, Bennett RE, Kerr JFR. Cell death by apoptosis during involution of the lactating breast in mice and rats. Am. J. Anat. 1989;185:19–32. doi: 10.1002/aja.1001850104. [DOI] [PubMed] [Google Scholar]
- Wong H, Anderson WD, Cheng T, Riabowol KT. Monitoring mRNA expression by polymerase chain reaction: the ‘primer-dropping’ method. Anal. Biochem. 1994;223:251–258. doi: 10.1006/abio.1994.1581. [DOI] [PubMed] [Google Scholar]
- Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]