Abstract
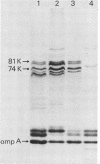
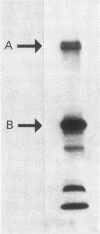
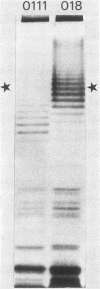
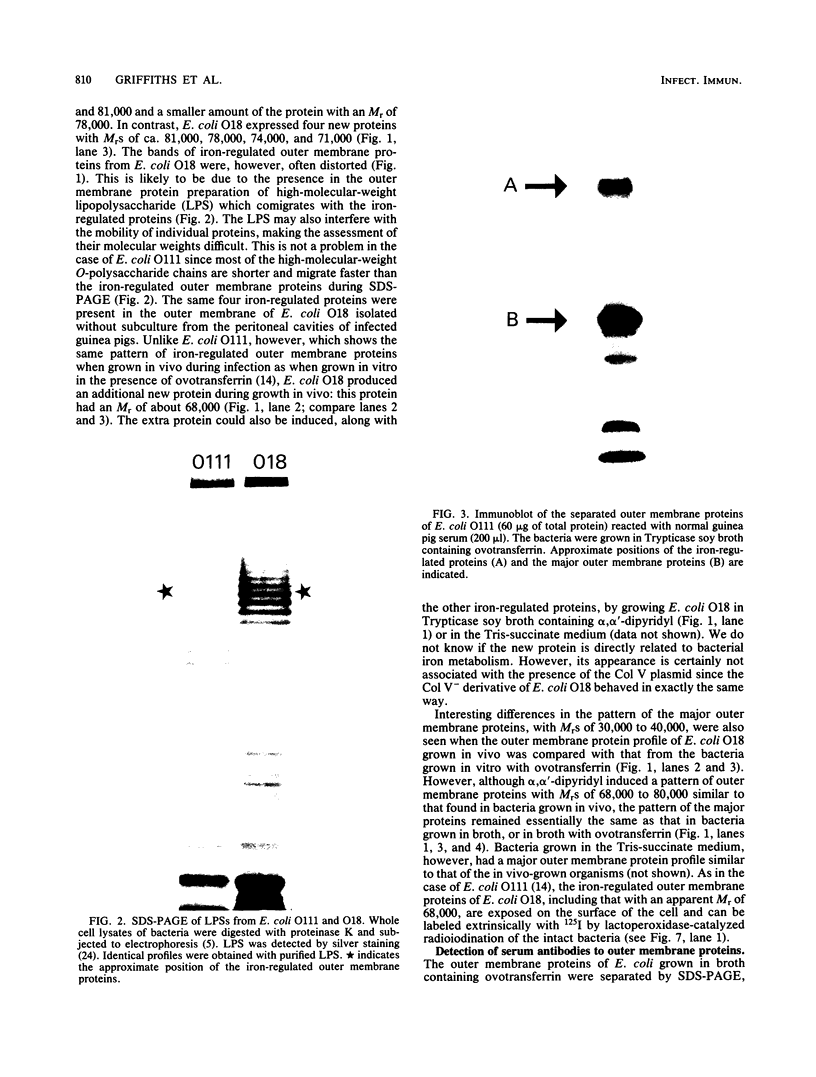
Sera from normal healthy human adults and infants, as well as sera from mice, rabbits, and guinea pigs, were examined by immunoblotting for naturally occurring antibodies reacting with outer membrane proteins of two Escherichia coli strains, O111 and O18. Some individuals had antibodies reacting very strongly with the iron-regulated outer membrane proteins, including the ferric-enterochelin receptor protein (Mr, 81,000), as well as with ompA. However, sera from infants contained predominantly antibodies to ompA; antibodies recognizing the iron-regulated outer membrane proteins were either absent or barely detectable. In human serum the antibodies were mainly of the immunoglobulin G class. No serotype-specific antibodies to the lipopolysaccharide of E. coli O111 or O18 were found in the sera tested.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bullen J. J. The significance of iron in infection. Rev Infect Dis. 1981 Nov-Dec;3(6):1127–1138. doi: 10.1093/clinids/3.6.1127. [DOI] [PubMed] [Google Scholar]
- Carlsson B., Gothefors L., Ahlstedt S., Hanson L. A., Winberg J. Studies of Escherichia coli O antigen specific antibodies in human milk, maternal serum and cord blood. Acta Paediatr Scand. 1976 Mar;65(2):216–224. doi: 10.1111/j.1651-2227.1976.tb16541.x. [DOI] [PubMed] [Google Scholar]
- Chart H., Shaw D. H., Ishiguro E. E., Trust T. J. Structural and immunochemical homogeneity of Aeromonas salmonicida lipopolysaccharide. J Bacteriol. 1984 Apr;158(1):16–22. doi: 10.1128/jb.158.1.16-22.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulton J. W. The ferrichrome-iron receptor of Escherichia coli K-12. Antigenicity of the fhuA protein. Biochim Biophys Acta. 1982 Jul 16;717(1):154–162. doi: 10.1016/0304-4165(82)90393-2. [DOI] [PubMed] [Google Scholar]
- Emödy L., Batai I., Jr, Kerényi M., Székely J., Jr, Polyak L. Anti-Escherichia coli alpha-hemolysin in control and patient sera. Lancet. 1982 Oct 30;2(8305):986–986. doi: 10.1016/s0140-6736(82)90183-0. [DOI] [PubMed] [Google Scholar]
- Filip C., Fletcher G., Wulff J. L., Earhart C. F. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J Bacteriol. 1973 Sep;115(3):717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths E. K., Yoonessi S., Neter E. Antibody response to enterobacterial lipoprotein of patients with varied infections due to Enterobacteriaceae. Proc Soc Exp Biol Med. 1977 Feb;154(2):246–249. doi: 10.3181/00379727-154-39647. [DOI] [PubMed] [Google Scholar]
- Griffiths E., Humphreys J. Alterations in tRNAs containing 2-methylthio-N6-(delta2-isopentenyl)-adenosine during growth of enteropathogenic Escherichia coli in the presence of iron-binding proteins. Eur J Biochem. 1978 Jan 16;82(2):503–513. doi: 10.1111/j.1432-1033.1978.tb12044.x. [DOI] [PubMed] [Google Scholar]
- Griffiths E., Humphreys J., Leach A., Scanlon L. Alterations in the tRNA's of Escherichia coli recovered from lethally infected animals. Infect Immun. 1978 Nov;22(2):312–317. doi: 10.1128/iai.22.2.312-317.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Enhanced autoradiographic detection of 32P and 125I using intensifying screens and hypersensitized film. FEBS Lett. 1977 Oct 15;82(2):314–316. doi: 10.1016/0014-5793(77)80609-1. [DOI] [PubMed] [Google Scholar]
- Neilands J. B. Microbial envelope proteins related to iron. Annu Rev Microbiol. 1982;36:285–309. doi: 10.1146/annurev.mi.36.100182.001441. [DOI] [PubMed] [Google Scholar]
- O'Hanley P. D., Cantey J. R. Immune response of the ileum to invasive Escherichia coli diarrheal disease in rabbits. Infect Immun. 1981 Jan;31(1):316–322. doi: 10.1128/iai.31.1.316-322.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBINET H. G. Relationship of host antibody to fluctuations of Escherichia coli serotypes in the human intestine. J Bacteriol. 1962 Nov;84:896–901. doi: 10.1128/jb.84.5.896-901.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIMON E. H., TESSMAN I. THYMIDINE-REQUIRING MUTANTS OF PHAGE T4. Proc Natl Acad Sci U S A. 1963 Sep;50:526–532. doi: 10.1073/pnas.50.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciortino C. V., Finkelstein R. A. Vibrio cholerae expresses iron-regulated outer membrane proteins in vivo. Infect Immun. 1983 Dec;42(3):990–996. doi: 10.1128/iai.42.3.990-996.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Weinberg E. D. Iron withholding: a defense against infection and neoplasia. Physiol Rev. 1984 Jan;64(1):65–102. doi: 10.1152/physrev.1984.64.1.65. [DOI] [PubMed] [Google Scholar]