Abstract
Objective
To assess costs, effectiveness, and cost-effectiveness of inhaled corticosteroids (ICS) augmenting bronchodilator treatment for chronic obstructive pulmonary disease (COPD).
Data Sources
Claims between 1997 and 2005 from a large managed care database.
Study Design
Individual-level, fixed-effects regression models estimated the effects of initiating ICS on medical expenses and likelihood of severe exacerbation. Bootstrapping provided estimates of the incremental cost per severe exacerbation avoided.
Data Extraction Methods
COPD patients aged 40 or older with ≥15 months of continuous eligibility were identified. Monthly observations for 1 year before and up to 2 years following initiation of bronchodilators were constructed.
Principal Findings
ICS treatment reduced monthly risk of severe exacerbation by 25 percent. Total costs with ICS increased for 16 months, but declined thereafter. ICS use was cost saving 46 percent of the time, with an incremental cost-effectiveness ratio of $2,973 per exacerbation avoided; for patients ≥50 years old, ICS was cost saving 57 percent of time.
Conclusions
ICS treatment reduces exacerbations, with an increase in total costs initially for the full sample. Compared with younger patients with COPD, patients aged 50 or older have reduced costs and improved outcomes. The estimated cost per severe exacerbation avoided, however, may be high for either group because of uncertainty as reflected by the large standard errors of the parameter estimates.
Keywords: Chronic obstructive pulmonary disease, managed care, administrative data, cost-effectiveness analysis
Chronic obstructive pulmonary disease (COPD) is characterized by progressive airflow limitation and airway inflammation leading to a gradual loss in lung function (Global Initiative for Chronic Obstructive Lung Disease [GOLD] 2006). Acute exacerbations (i.e., sudden worsening of respiratory symptoms) are common for many COPD patients and are most often caused by respiratory tract infections (White, Gompertz, and Stockley 2003). These exacerbations are an important part of the morbidity, mortality, and disease progression. Patients with lower levels of lung function are more likely to experience more exacerbations, and frequent exacerbations may lead to reduced lung function (Seemungal et al. 2000; Calverley et al. 2005). Exacerbations are strongly related to increases in health care utilization, including inpatient admissions, emergency department visits, and use of rescue medications (Donaldson and Wedzicha 2006).
Because airway inflammation is one aspect of COPD, anti-inflammatory agents such as inhaled corticosteroids (ICS) may slow disease progression and prevent exacerbations. Current treatment guidelines recommend a stepwise increase in drug treatment depending on the disease severity (GOLD 2006). In particular, ICS treatment should be used to augment regular bronchodilator treatment for patients with advanced disease and repeated exacerbations. However, unlike the use of ICS in asthma treatment, the evidence for ICS efficacy is mixed for COPD treatment (Calverley 2004). In six randomized clinical studies in which COPD patients were followed for more than 12 months, ICS treatment was associated with a 4–26 percent reduction in exacerbation events defined by either respiratory-related hospitalizations, emergency department visits, worsening of respiratory symptoms, or their combination (Vestbo et al. 1999; Burge et al. 2000; Lung Health Study Research Group [LHSRG] 2000; Szafranski et al. 2003; Calverley, Pauwels et al. 2003; Calverley, Boonsawat et al. 2003). The most recent randomized clinical study (TORCH study) showed that the combination therapy of ICS and an inhaled bronchodilator reduced the risks of dying ( p=.052) and of moderate and severe exacerbations ( p<.001) during the 3-year follow-up (Calverley et al. 2007).
Because severe exacerbations are uncommon in COPD patients, large studies with adequate follow-up periods are required to detect statistically and clinically significant effects of drug treatments. Pooled analyses using more than 4,000 COPD patients from randomized clinical trials of ICS showed that ICS reduced exacerbation rates by 24–33 percent compared with a placebo (Sin et al. 2003; Gartlehner et al. 2006). On the other hand, observational studies using more than 4,000 COPD patients find no preventive effects associated with ICS (Bourbeau et al. 2003; Fan et al. 2003; de Melo, Ernst, and Suissa 2004; Suissa 2004) even though potential misclassification of ICS exposure status was carefully addressed by using either a time-dependent approach or a nested case–control study design.
One possible explanation for these mixed results may be the lack of a consistent definition of exacerbation. Because substantial individual variation occurs in exacerbation patterns, exacerbation episodes are operationally defined in various ways across studies, ranging from “mild exacerbation” defined by increased use of rescue therapy to “severe exacerbation” defined by respiratory conditions that require hospitalization (Calverley 2004; Pauwels et al. 2004). Other reasons for these mixed results may be related to individual factors that are not observed in claims data but that influence disease prognosis. Important unobserved characteristics that could bias the previous estimations include socioeconomic characteristics, smoking behavior, and chronic comorbid conditions (Burney et al. 2003; Spencer et al. 2004; Mannino et al. 2006).
In cross-sectional data analysis, omission of important factors from the regression model will lead to biased estimation. However, in longitudinal or panel data analysis, an individual has multiple observations of both exposure status and outcomes (Frees 2004). Each individual contributes time before drug exposure to the control group and time after drug exposure to the treatment group. Because each individual is his or her own control, potential bias due to person-specific omitted variables that do not change over time (e.g., education, historical health behaviors, and underlying health status) can be eliminated. With at least two observations per person, the treatment effect can be observed by measuring changes before and after the treatment (e.g., as a pre- and post-paired comparison), and thus time-invariant variables disappear by subtracting one from the other.
One statistical approach to account for multiple observations per individual is called the “fixed-effects” model (Allison 2005). The model can be expressed as:
| (1) |
The notation i refers to different persons and t refers to different points in time; the term Yit is an outcome of interest, Xit is a main exposure of interest, αi is a dummy variable representing individual characteristics; Wit is a vector of time-variant variables of interest; and ɛit is a random error. In this model, because individual variations are considered fixed and are captured by the fixed-effect parameter, αi, omitted time-invariant variables are accounted for without measuring them. Wit, factors that change over time (e.g., disease symptoms) must be adjusted with common regression approaches, and unobserved time-varying factors may still cause bias.
In previous observational studies using claims data, limited information was available to measure and adjust for the effects of important predictors of prognosis of COPD, such as smoking history or chronic conditions (Sin and Tu 2001; Bourbeau et al. 2003; Suissa 2004). In this study, however, we address the potential bias that is likely to exist in the cross-sectional studies by using monthly data from a managed care claims database to obtain repeated measures over time for each individual. We use an individual-level, fixed-effects model to estimate the unbiased effects of adding ICS treatment on monthly medical expenses and likelihood of severe exacerbations among COPD patients who were initially under treatment with regular bronchodilators. We use parameter estimates from these two models to determine the incremental cost per severe exacerbation avoided.
METHODS
Data Source
Study data were obtained from the National Managed Care Benchmark Database (Integrated Healthcare Information Services [IHCIS], Waltham, MA) from January 1997 through December 2005. This database includes information on enrollment, facility, professional, and pharmacy services for more than 37 million patients covered by approximately 35 managed care health plans in nine census regions in the United States. For patients of Medicare age, the IHCIS dataset was able to capture pharmacy claims through health plan-sponsored Medicare programs that included pharmacy benefits. All data were HIPAA-compliant, with all health plan and personal information de-identified to assure confidentiality. IRB approval for the human subjects research was obtained from the Office of Human Research Ethics of the University of North Carolina at Chapel Hill.
IHCIS costs are provided in a standardized form in order to make comparisons easier across all services, data sources, and time periods. IHCIS uses different approaches to standardize pricing for each of the following major service categories: (1) facility inpatient costs are calculated based on primary diagnosis categories, length of stay, and presence of intensive care unit use/surgery; (2) facility outpatient costs are calculated based on requested (submitted) charges; (3) professional service costs are calculated using a standardized payment schedule based on a resource-based relative value scale; and (4) pharmacy service costs, including ICS costs, are calculated using First Data Bank pricing based on the National Drug Code and quantity of the prescription (IHCIS 2005). In order to reflect allowed payments among all service providers, deductibles, coinsurance, and other cost-sharing features are removed from the original data. All costs are adjusted to 2005 U.S. dollars using the Consumer Price Index for Medical Care Services.
Study Population
COPD patients were identified using both medical and pharmacy claims. Patients were included if they (1) had one or more medical claims of COPD (International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM): 491.xx, 492.xx or 496.xx in primary or secondary positions) between January 1998 and December 2004, (2) had regular treatment with inhaled bronchodilators (defined by at least three pharmacy claims of anticholinergics or long-acting β2-agonists), and (3) were aged 40 or older. All eligible patients were required to have continuous health plan coverage including pharmacy benefits from 3 months before the first pharmacy claim of inhaled bronchodilators through 12 months after the date of that claim. Patients were excluded from the analysis if they had a diagnosis of cystic fibrosis (ICD-9-CM: 277.0x) or respiratory tract cancer (ICD-9-CM: 160.xx-164.xx, or 231.xx), no ICS treatment during the follow-up period, or initiated ICS before the first bronchodilator treatment. To investigate whether effects of ICS varied by age, we also conducted all analyses for patients aged 50 and older.
Measures
Monthly observations were created for ICS treatment status, medical expenses, likelihood of a severe exacerbation event, and other explanatory variables from up to 12 months before the first bronchodilator claim through 24 months after that claim. The medical expenses were identified from inpatient, outpatient, and pharmacy claims (including ICS treatment expenses). Severe exacerbation events were identified by inpatient or emergency department claims, with primary diagnosis of COPD or with COPD-related procedures (e.g., oxygen therapy, a spirometry test, pulmonary rehabilitation, nebulized treatment, bronchoscopy, intubation, or tracheostomy).
The main exposure variable was the use of ICS. The period before the first ICS claim was defined as unexposed time, whereas the period after the first ICS claim was defined as ICS treatment time. To adjust for symptom changes over time, a continuous variable indicating “time” in months (coded from −12 to 23, with month 0 designating the initiation of bronchodilator treatment), dummy variables indicating “onset” (1 month before the first bronchodilator claim when many patients received medical services that led to the initiation of bronchodilator treatment, and therefore had extremely high medical costs), and “post onset” (months after patients receive the bronchodilator treatment) were included.
Covariates were age and selected comorbid conditions such as asthma and congestive heart failure. For patients with asthma or congestive heart failure, we defined time with the conditions in the following way: we identified the first month with an asthma claim (ICD-9-CM: 493.xx) or congestive heart failure claim (ICD-9-CM: 428.xx) and then defined the subsequent months as months with the conditions. In addition, to adjust for exacerbation episodes, the months with medications for acute exacerbations (i.e., antibiotics or oral corticosteroids) were identified. Proximity to death could not be controlled due to lack of mortality information in the database.
Analysis
An individual-level, fixed-effects linear regression model was used to estimate the impact of initiating ICS treatment on medical expenses and the likelihood of severe exacerbation among COPD patients. The models were:
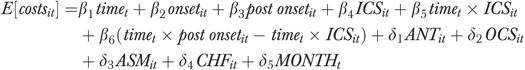 |
(2) |
| (3) |
where time is a continuous variable indicating the number of months from the first bronchodilator treatment, onset is a dummy variable indicating 1 month before the first bronchodilator treatment, post onset is a dummy variable indicating months after patients received the first bronchodilator treatment, ICS is a dummy variable indicating ICS use, ANT and OCS indicate use of rescue medications (i.e., antibiotics and oral corticosteroids, respectively), and ASM and CHF indicate having comorbid conditions (i.e., asthma and congestive heart failure, respectively). Costs were measured in dollars, and a dichotomous indicator was used to indicate the occurrence of a severe exacerbation in each month. The model for costs allowed the effect of ICS to vary with time; the effect of ICS on severe exacerbations did not vary with time so an interaction of time with ICS treatment was not included in the exacerbations model. A Hausman specification test was used to assess whether the fixed-effects model was preferred to a random-effects model (Kennedy 2003).
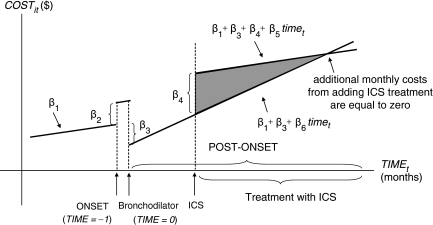
The interpretation of estimated coefficients from the cost model (Equation 2) is summarized in Figure 1. The coefficient β1 describes the impact of natural disease progression on medical expenses. The coefficient β2 accounts for a change in expenses in the month before the first bronchodilator treatment. Changes in monthly expenses after the first bronchodilator treatment are expressed by β1+β3+β6×time; changes after the first ICS treatment are expressed by β1+β3+β4+β5×time. Thus, the ICS treatment effect on monthly expenses is expressed by differences in the equations before and after the first ICS treatment, β4+(β5–β6) ×time. If adding ICS treatment increases average monthly costs (at least initially), then β4 would be positive (as shown in Figure 1). If monthly costs increase more slowly with ICS than without ICS, then the line representing the increase in expected costs with time will be flatter (i.e., the sign of β5–β6 would be negative), also shown in Figure 1. Thus, the area expressed by the difference in the two equations (shaded in gray) represents additional medical expenses associated with adding ICS treatment. The lines cross at the point where the estimated additional monthly expenses are equal to zero and total expenses due to ICS initiation decrease in subsequent months. If β4 is negative, then adding ICS treatment results in reduced costs in each month (i.e., the line β1+β3+β4+β5×time will be below the line β1+β3+β6×time everywhere).
Figure 1.
Graphical Representation of Estimated Coefficients from Fixed-Effects Models
The parameter estimates from the regressions were used to estimate the effect of initiating ICS on monthly medical expenses and the likelihood of avoiding a severe exacerbation event during the months following ICS initiation. Nonparametric bootstrapping, with repeated samples of 1,000 (Efron and Tibshirani 1993), was conducted to estimate the likelihood that ICS treatment is cost saving and to characterize the likelihood that ICS treatment is cost effective relative to different thresholds of what a decision maker might be willing to pay for a gain in health outcomes. Results of the bootstrapping process are displayed in a cost-effectiveness acceptability curve (Van Hout et al. 1994; Briggs 2000; Briggs, Sculpher, and Claxton 2006). This curve displays the likelihood or probability that adopting a new technology (in this case, simultaneous prescribing of ICS with bronchodilators) is the correct decision (Drummond et al. 2005).
Specifically, we bootstrapped the incremental cost-effectiveness ratio (ICER), which provides an estimate of the additional cost per severe exacerbation avoided. The ICER can be interpreted as the monetary value of avoiding a severe exacerbation beyond the medical costs directly involved in treatment (i.e., what one might be willing to pay to avoid the psychological trauma and indirect costs to the individual and family members of a severe exacerbation). Bootstrapping the model accounts for the correlation between the numerator (i.e., expected costs from Equation 2) and the denominator (i.e., risk of exacerbation from Equation 3) (Chaudhary and Stearns 1996). All analyses were conducted using STATA version 9.2 (StataCorp LP, College Station, TX).
RESULTS
We identified 785,759 COPD patients without diagnoses of cystic fibrosis or respiratory tract cancer in the IHCIS database between 1998 and 2004. Reductions in the sample occurred because of the lack of at least 15 months of data (n=473,691), lack of pharmacy benefit coverage (n=234,411), lack of required medication regimens (n=54,340), or patient age younger than 40 years (n=4,748). A total of 10,271 eligible patients met all inclusion and exclusion criteria.
Patient demographics, comorbid conditions, prior utilization (during 3 months before initiating bronchodilator treatment), and post-term treatments (during 24 months after initiating bronchodilator treatment) are summarized in Table 1. Approximately half of the study sample was male; 85 percent of patients were aged 50 and older, whereas 53 percent were aged 60 and older. Approximately 20 percent of patients received services from a pulmonary medicine specialist and a spirometry test during the 3 months before initiating bronchodilator treatment. Patients had a variety of comorbid conditions, including hypertension (37 percent), asthma (37 percent), diabetes (14 percent), congestive heart failure (12 percent), and depression (12 percent). In terms of resource utilization, fewer than 20 percent of patients used either respiratory-related inpatient services or emergency department visits. Patients aged 50 and older were similar in their use of resources and the presence of comorbid conditions.
Table 1.
Summary Statistics for Full Sample (n = 10,271) and Persons Aged 50 and Older (n = 8,738)
| Mean (SD) | ||
|---|---|---|
| Full Sample | Age 50 and Older | |
| Outcomes | ||
| Medical expenses per month (excluding pharmacy expenses) | $1,131.50 ($2,037.31) | $1,179.86 ($2,098.18) |
| Pharmacy expenses per month | $323.16 ($309.07) | $323.71 ($291.23) |
| Likelihood of having severe exacerbation event per month | 0.0140 (0.0386) | 0.0150 (0.0401) |
| Demographics | ||
| Gender: male | 0.4596 | 0.4754 |
| Age 40–49 years | 0.1493 | — |
| Age 50–59 years | 0.3246 | 0.3816 |
| Age 60–69 years | 0.2871 | 0.3375 |
| Age 70–79 years | 0.2390 | 0.2810 |
| Insurance: Medicaid/Medicare (ref: any other) | 0.1532 | 0.1737 |
| Census region: Northeast | 0.5174 | 0.5113 |
| Census region: South | 0.1216 | 0.1217 |
| Census region: Midwest | 0.1119 | 0.1181 |
| Census region: West/others | 0.2491 | 0.2489 |
| Comorbid conditions (during 3 months before and after initiating bronchodilator treatment) | ||
| Myocardial infarction | 0.0363 | 0.0399 |
| Congestive heart failure | 0.1243 | 0.1402 |
| Peripheral vascular disease | 0.0430 | 0.0480 |
| Cerebrovascular disease | 0.0570 | 0.0643 |
| Rheumatologic disease | 0.0230 | 0.0236 |
| Diabetes | 0.1435 | 0.1551 |
| Renal disease | 0.0200 | 0.0227 |
| Malignancy/AIDS | 0.0720 | 0.0799 |
| Asthma | 0.3702 | 0.3393 |
| Depression | 0.1195 | 0.1055 |
| Nonvertebral fracture | 0.0326 | 0.0346 |
| Hypertension | 0.3746 | 0.3992 |
| Prior utilization (during 3 months before initiating bronchodilator treatment) | ||
| Pulmonologist care | 0.1952 | 0.1999 |
| Inpatient admission for any reason | 0.1920 | 0.2000 |
| Inpatient admissions for respiratory-relatedconditions | 0.1173 | 0.1198 |
| Emergency department visit for any reason | 0.2136 | 0.2119 |
| Emergency department visits for respiratory-related conditions | 0.1332 | 0.1310 |
| Oxygen therapy | 0.0815 | 0.0871 |
| Spirometry test | 0.1939 | 0.1928 |
| Pulmonary rehabilitation | 0.0216 | 0.0198 |
| Nebulized therapy | 0.0270 | 0.0271 |
| Bronchoscopy, intubation, or tracheostomy | 0.0124 | 0.0129 |
| Antibiotics | 0.3356 | 0.3222 |
| Oral corticosteroids | 0.1708 | 0.1638 |
| Short-acting β2 agonists | 0.2183 | 0.2082 |
| Theophylline | 0.0471 | 0.0500 |
| Post-term treatments (during 24 months after initiating bronchodilator treatment) | ||
| Follow-up time after initiating bronchodilator treatment (in months) | 21.2 (3.9) | 21.1 (3.9) |
| Follow-up time with ICS treatment (in months) | 16.6 (7.0) | 16.4 (7.0) |
| ICS costs per month | $53.62 (50.42) | $54.76 ($51.17) |
| Number of ICS prescriptions during follow-up | 5.8 (5.2) | 5.8 (5.2) |
| ICS costs per prescription | $150.35 ($96.06) | $153.05 ($99.47) |
| Days of supply per ICS prescription | 34.8* (21.1) | 35.6† (21.8) |
287 have missing data.
239 have missing data.
AIDS, acquired immunodeficiency syndrome; ICS, inhaled corticosteroids; SD, standard deviation.
In this analysis, we used follow-up data for up to 24 months after initiating bronchodilator treatment. Sixty-one percent of patients had complete 24-month follow-up data, and the mean duration of the follow-up was 21.2 months (standard deviation [SD], 3.9 months). Patients used ICS for 16.6 months (SD, 7.0 months) on average, with an average of 5.8 ICS prescriptions costing $150 per prescription. Unadjusted mean ICS expenses per person per month were $53.62 (SD, $50.42). Unadjusted outcomes also are summarized in Table 1. COPD patients (in the full sample) incurred medical expenses of $1,131 (SD, $2,037) per month (excluding pharmacy expenses) and pharmacy expenses of $323 (SD, $309) per month, respectively. The likelihood of having a severe exacerbation per month was 0.0140 (SD, 0.0386). Although total medical expenses (exclusive of pharmacy expenses) were slightly higher for older COPD patients, pharmacy expenses and the likelihood of exacerbation were similar.
The effects of ICS treatment on the monthly medical expenses and likelihood of severe exacerbation for the full sample (40–79 years old) and the older patient group (ages 50–79 years old) are summarized in Table 2. For the full sample, ICS treatment was associated with increased medical expenses during the first month even though the amount ($23.08, the coefficient β4) was not statistically significant at the 5 percent level. The expected additional monthly expenses, expressed by β4+(β5 – β6) ×time=23.08–1.37 ×time, decreased over time, with total costs expected to decrease beyond month 16. ICS treatment reduced severe exacerbation risks significantly ( p<.001). The estimated likelihood of having a severe exacerbation event with ICS treatment was 1.12 percent per month whereas the likelihood without ICS treatment was 1.49 percent per month, which translated into a 0.37-percentage-point absolute risk reduction (1.12–1.49=−0.37) or a 25-percent relative risk reduction (1.12/1.49=0.75).
Table 2.
Effects of Inhaled Corticosteroids (ICS) Treatment on Monthly Medical Costs and Monthly Likelihood of Severe Exacerbation
| Medical Costs | Severe Exacerbation | |||
|---|---|---|---|---|
| Coefficient | SE | Coefficient | SE | |
| Ages 40–79 (n = 10,271) | ||||
| β1 (follow-up months, time) | 44.17 | 5.90** | 0.00007 | 0.00004 |
| β2 (1 month before the first bronchodilator claim, onset) | 1251.17 | 61.45** | 0.05112 | 0.00115** |
| β3 (months after the first bronchodilator claim, post onset) | −210.08 | 56.97** | 0.00292 | 0.00082** |
| β4 (months after the first inhaled corticosteroid claim, ICS) | 23.08 | 50.41 | −0.00370 | 0.00078** |
| β5 (time×ICS) | −53.55 | 6.20** | ||
| β6 (time×post onset–time×ICS) | −52.18 | 7.92** | ||
| β5−β6 | −1.37 | 5.59 | ||
| δ1 (month with an antibiotic prescription) | 877.67 | 29.64** | 0.02518 | 0.00062** |
| δ2 (month with an oral corticosteroid prescription) | 824.86 | 39.21** | 0.06330 | 0.00082** |
| δ3 (months after the first asthma diagnosis) | 452.95 | 36.42** | 0.00327 | 0.00076** |
| δ4 (months after the first congestive hart failure diagnosis) | 1943.67 | 51.81** | 0.01731 | 0.00109** |
| Ages 50–79 (n = 8,738) | ||||
| β1 (follow-up months, time) | 49.15 | 6.62** | 0.00008 | 0.00004* |
| β2 (1 month before the first bronchodilator claim, onset) | 1329.56 | 68.95** | 0.05465 | 0.00129** |
| β3 (months after the first bronchodilator claim, post onset) | −238.60 | 63.74** | 0.00270 | 0.00091** |
| β4 (months after the first inhaled corticosteroid claim, ICS) | −4.78 | 56.44 | −0.00377 | 0.00088** |
| β5 (time×ICS) | −58.22 | 6.97** | ||
| β6 (time×post onset–time×ICS) | −58.06 | 8.89** | ||
| β5−β6 | −0.15 | 6.26 | ||
| δ1 (month with an antibiotic prescription) | 914.94 | 33.73** | 0.02778 | 0.00071** |
| δ2 (month with an oral corticosteroid prescription) | 798.38 | 44.47** | 0.06803 | 0.00093** |
| δ3 (months after the first asthma diagnosis) | 483.88 | 41.66** | 0.00355 | 0.00087** |
| δ4 (months after the first congestive heart failure diagnosis) | 1963.58 | 55.61** | 0.01782 | 0.00117** |
Statistical significance at the 5% level;
Statistical significance at the at the 1% level.
A Hausman specification test confirmed that the fixed-effects model was appropriate.
estimated coefficients; SE, robust standard errors.
For patients aged 50 or older, ICS treatment was associated with a statistically nonsignificant reduction in total medical expenses during the first month of ICS treatment (−$4.78, the coefficient β4). Expected monthly expenses decreased further with time, expressed by β4+(β5–β6) ×time=−4.78–0.15 ×time. In Figure 1, the line representing costs with ICS treatment would therefore lie below the line representing costs without ICS treatments. The statistically significant decrease in the estimated monthly likelihood of a severe exacerbation was similar to the effect for the full sample.
Although the reductions in monthly costs are modest and take some time to occur for the full sample, the larger standard errors associated with parameter estimates (i.e., costs and the likelihood of a severe exacerbation) and the implications for the cost effectiveness of ICS treatment are best understood by conducting an economic evaluation using bootstrapping with the parameter estimates from the regression results. This analysis calculates the empirical distribution of the ICER, which estimates the additional cost per severe exacerbation avoided. Because total costs increased through 16 months following the initiation of ICS treatment for the full sample (i.e., because the estimate of β4 was positive), we calculated the cost-effectiveness implications for the full sample for 17 months following initiation of ICS treatment. More specifically, we calculated the increase in costs as the shaded area in Figure 1 for the numerator of the ICER and used the cumulative reduction in the likelihood of avoiding an exacerbation event (assuming independence between months) as the denominator. In addition, we conducted the same analysis for the subgroup ages 50 and older to compare the results. The estimates, therefore, represent the maximum cost to avoid a severe exacerbation for 17 months after ICS initiation (because total costs are expected to decrease 17 months after ICS initiation for the full sample).
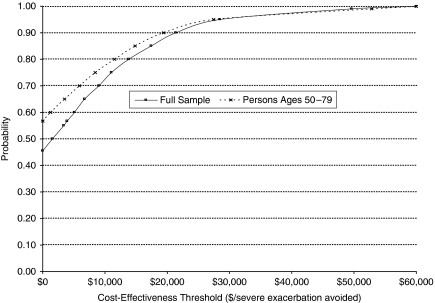
Figure 2 summarizes the results from the analysis. Incremental medical expenses were calculated as the sum of additional monthly expenses from the ICS treatment for 17 months following initiation (mean, $190.34 and SD, $1,266.96 for the full sample; mean, −$203.55 and SD, $1,406.52 for persons aged 50 and older). Incremental effectiveness was calculated as the sum of monthly risk reductions of having a severe exacerbation event (mean, 0.0982 and SD, 0.0252 for the full sample; mean, 0.0965 and SD, 0.0277 for the group aged 50 and older). For the full sample, the likelihood that ICS treatment is cost saving is 45.5 percent. The ICER was $2,973 per severe exacerbation avoided. The cost to avoid a severe exacerbation with a high degree of certainty is substantial. For example, at a cost of $30,000 to avoid an acute exacerbation, the likelihood that ICS treatment is cost effective is about 96 percent. The likelihood that ICS treatment is cost saving is higher for the older sample, at approximately 56.7 percent, indicating that on average ICS treatment in persons aged 50 and older reduces total costs and improves outcomes; however, the threshold for cost effectiveness becomes similar to that for the full sample (about $30,000 with 95 percent confidence).
Figure 2.
Cost-Effectiveness Acceptability Curve for Inhaled Corticosteroids (ICS) Treatment to Prevent Severe Exacerbation Events, Based on 17 Months of Follow-Up
DISCUSSION
Our findings using longitudinal data demonstrate that adding ICS treatment to regular inhaled bronchodilators for patients with COPD most likely increases total medical expenses initially, although severe exacerbation risks decline and total costs eventually decline. The likelihood of reductions in both costs and exacerbations is greater for older patients, although the cost per avoided severe exacerbation may still be high. Yet when one considers unmeasured consequences of repeated exacerbations (i.e., decreased quality of life, productivity loss, or caregiver time), the additional expenses may be compensated by additional benefits (D'Souza et al. 2006; Simoens and Decramer 2007).
It is difficult to compare our estimates of the additional expenses to avoid a severe exacerbation with those from prior studies. Ayres, Price, and Efthimiou (2003) used data from a randomized, controlled trial to assess the cost effectiveness of adding ICS in the form of fluticasone propionate to regular bronchodilator therapy in symptomatic patients with moderate-to-severe COPD. Their estimate from the United Kingdom's National Health Service perspective was £7.5 per month (approximately $11) to have a moderate/severe exacerbation-free day. Sin, Golmohammadi, and Jacobs (2004) reported an estimate of $10,000 per moderate and severe exacerbation avoided in a 3-year Markov model. Yet the groups analyzed by these studies were substantially sicker (having a monthly rate of exacerbations of 15 percent, compared to 1.4 percent in our study) and the focus of those calculations was not on the incremental cost of early initiation of ICS.
Several important limitations associated with claims data deserve discussion (Schneeweiss and Avorn 2005). Because no clinical information about COPD or other comorbid conditions were available, we estimated severity of COPD from resource utilization patterns in the claims data. Severe exacerbation events were identified using COPD-related hospital admissions or emergency department visits, which often are used to relieve severe exacerbations (Ayres, Price, and Efthimiou 2003; Sin, Golmohammadi, and Jacobs 2004). Comorbid conditions were identified with the first medical claim associated with the comorbidity diagnosis, and we assumed that the conditions would continue until the end of the study period. These proxy measures could cause bias due to the misclassification of explanatory variables. However, this type of measurement error should be random (i.e., not associated with drug selection or outcomes) and will result in bias toward the null (i.e., no difference between the groups).
In addition, we did not have access to data that might explain the reason for initiating ICS treatment. We assume some physicians may prescribe ICS to prevent exacerbations, whereas others may prescribe it only to patients having frequent exacerbations (Collet and Boivin 2000). Without clinical information, it was difficult to distinguish whether estimated expenses were the result of the treatment or whether ICS treatment was initiated due to the expected outcomes. To minimize the bias from this temporal causal relationship, we used a narrow time frame to measure both drug exposure status and outcomes and used monthly values in the analysis. Also, we included month indicator variables to adjust for seasonality. If patients used ICS because they experienced frequent exacerbations, ICS should be associated with increased medical expenses. We believe, therefore, that the direction of the bias would be one way only: ICS treatment effects on medical expenses and exacerbation risks would be underestimated and our estimated benefits would be conservative.
Finally, to identify an appropriate study sample, we used various inclusion and exclusion criteria such as diagnosis codes, COPD-related medications (at least three bronchodilator prescriptions with an interquartile range of 2.3–9.6 months), and patient age (40 years or older). To assess impact of changes in ICS treatment status over time, all patients were required to have at least 15 months of continuous eligibility; failure to meet this criteria was the main cause for exclusion from the original study population. As a consequence of this restriction, patients who did not survive and continue enrollment for at least 15 months were automatically excluded. Therefore, the findings from this study are only generalizable to COPD patients from a managed care population who survive and continue to be covered by that managed care organization, including pharmacy benefits for at least 15 months after initiation of bronchodilator treatment.
CONCLUSIONS
Current clinical guidelines recommend that ICS treatment should be initiated in patients who do not have adequate symptom control using a regular bronchodilator regimen. Our findings provide additional information that may support early initiation of ICS, especially in older patients. Increased medical expenses due to regular ICS treatment are anticipated initially for patients on average. However, these expenses may be compensated by additional health benefits from avoiding repeated exacerbations and by preventing disease progression in long-term disease management. Although patients were only followed for up to 2 years following initiation of bronchodilator treatment, the analyses suggest that initiation of ICS treatment eventually leads to reductions in total costs for longer-surviving patients.
Acknowledgments
Joint Acknowledgment/Disclosure Statement: The authors thank Drs. John E. Paul, Kourtney J. Davis, and Richard H. Stanford for their comments and suggestions on this manuscript. We also appreciate the efforts of two anonymous reviewers who provided valuable suggestions to enhance the manuscript.
Disclosure: This study was conducted as the first author's dissertation research at the University of North Carolina at Chapel Hill (UNC). Although his doctoral training was funded in part through a GlaxoSmithKline (GSK)/UNC Health Outcomes Fellowship, the choice of dissertation topic and the right to publish results are not contingent on approval of GSK. Other authors have no disclosures.
Supporting Information
The following supporting information for this article is available online:
Author Matrix.
This material is available as part of the online article from http://www.blackwell-synergy.com/doi/abs/10.1111/j.1475-6773.2008.00879.x (this link will take you to the article abstract).
Please note: Wiley-Blackwell is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
REFERENCES
- Allison P. Fixed Effects Regression Methods for Longitudinal Data Using SAS. Cary, NC: SAS Institute; 2005. [Google Scholar]
- Ayres J G, Price M J, Efthimiou J. Cost-Effectiveness of Fluticasone Propionate in the Treatment of Chronic Obstructive Pulmonary Disease: A Double-Blind Randomized, Placebo-Controlled Trial. Respiratory Medicine. 2003;97(3):212–20. doi: 10.1053/rmed.2003.1441. [DOI] [PubMed] [Google Scholar]
- Bourbeau J, Ernst P, Cockcoft D, Suissa S. Inhaled Corticosteroids and Hospitalization Due to Exacerbation of COPD. Pharmacoeconomics. 2003;17:479–500. doi: 10.1183/09031936.03.00113802. [DOI] [PubMed] [Google Scholar]
- Briggs A. Handling Uncertainty in Cost-Effectiveness Models. PharmacoEconomics. 2000;17:479–500. doi: 10.2165/00019053-200017050-00006. [DOI] [PubMed] [Google Scholar]
- Briggs A, Sculpher M, Claxton K. Decision Modelling for Health Economic Evaluation. New York: Oxford University Press; 2006. [Google Scholar]
- Burge P S, Calverley P M, Jones P W, Spencer S, Anderson J A, Maslen T K. Randomized, Double Blind, Placebo Controlled Study of Fluticasone Propionate in Patients with Moderate to Severe Chronic Obstructive Pulmonary Disease: The ISOLDE Trial. British Medical Journal. 2000;320(7245):1297–303. doi: 10.1136/bmj.320.7245.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burney P, Suissa S, Soriano J B, Vollmer W M, Viegi G, Sullivan S D, Fabbri L M, Sin D D, Ernst P, Coultas D, Bourbeau J, Mapel D W, Weiss K, McLaughlin T, Price D, Sturkenboom M C, Taylor R, Hagan G W. The Pharmacoepidemiology of COPD: Recent Advances and Methodological Discussion. European Respiratory Journal. 2003;43(suppl):1s–44s. [PubMed] [Google Scholar]
- Calverley P, Anderson J A, Celli B, Ferguson G T, Jenkins C, Jones P W, Yates J C, Vestbo J (TORCH Investigators. Salmeterol and Fluticasone Propionate and Survival in Chronic Obstructive Pulmonary Disease. New England Journal of Medicine. 2007;356(8):775–89. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- Calverley P, Pauwels D R, Lofdahl C G, Svensson K, Higenbottam T, Carlsson L G, Stahl E. Relationship between Respiratory Symptoms and Medical Treatment in Exacerbations of COPD. European Respiratory Journal. 2005;26(3):406–13. doi: 10.1183/09031936.05.00143404. [DOI] [PubMed] [Google Scholar]
- Calverley P, Pauwels R, Vestbo J, Jones P, Pride N, Gulsvik A, Anderson J, Maden C, and the Trial of Inhaled Steroids and Long-Acting β2 Agonists Study Group Combined Salmeterol and Fluticasone in the Treatment of Chronic Obstructive Pulmonary Disease: A Randomised Controlled Trial. Lancet. 2003;361(9356):449–56. doi: 10.1016/S0140-6736(03)12459-2. [DOI] [PubMed] [Google Scholar]
- Calverley P M. Effect of Corticosteroids on Exacerbations of Asthma and Chronic Obstructive Pulmonary Disease. Proceedings of the American Thoracic Society. 2004;1(3):161–6. doi: 10.1513/pats.200402-008MS. [DOI] [PubMed] [Google Scholar]
- Calverley P M, Boonsawat W, Cseke Z, Zhong N, Peterson S, Olsson H. Maintenance Therapy with Budesonide and Fometerol in Chronic Obstructive Pulmonary Disease. European Respiratory Journal. 2003;22(6):912–9. doi: 10.1183/09031936.03.00027003. [DOI] [PubMed] [Google Scholar]
- Chapman K R, Mannino D M, Soriano J B, Vermeire P A, Buist A S, Thun M J, Connell C, Jemal A, Lee T A, Miravitlles M, Aldington S, Beasley R. Epidemiology and Costs of Chronic Obstructive Pulmonary Disease. European Respiratory Journal. 2006;27(1):188–207. doi: 10.1183/09031936.06.00024505. [DOI] [PubMed] [Google Scholar]
- Chaudhary M A, Stearns SC. Estimating Confidence Intervals for Cost-Effectiveness Ratios: An example from a Randomized Trial. Statistics in Medicine. 1996;15:1447–58. doi: 10.1002/(SICI)1097-0258(19960715)15:13<1447::AID-SIM267>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Collet J P, Boivin J F. Bias and Confounding in Pharmacoepidemiology. In: Strom B L, editor. Pharmacoepidemiology. 3d Edition. Chichester, UK: Wiley & Sons Ltd; 2000. pp. 765–84. [Google Scholar]
- de Melo M N, Ernst P, Suissa S. Inhaled Corticosteroids and the Risk of a First Exacerbation in COPD Patients. European Respiratory Journal. 2004;23(5):692–7. doi: 10.1183/09031936.04.00049604. [DOI] [PubMed] [Google Scholar]
- Donaldson G C, Wedzicha J A. COPD Exacerbations .1: Epidemiology. Thorax. 2006;61(2):164–8. doi: 10.1136/thx.2005.041806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond M F, Sculpher M J, Torrance G W, O'Brien B J, Stoddart G L. Methods for Economic Evaluation of Health Care Programmes. 3d Edition. New York: Oxford University Press; 2005. [Google Scholar]
- D'Souza A O, Smith M J, Miller L A, Kavookjian J. An Appraisal of Pharmacoeconomic Evidence of Maintenance Therapy for COPD. Chest. 2006;129(6):1693–708. doi: 10.1378/chest.129.6.1693. [DOI] [PubMed] [Google Scholar]
- Efron B, Tibshirani T R. An Introduction to the Bootstrap. New York: Chapman and Hall; 1993. [Google Scholar]
- Fan V S, Bryson C L, Curtis J R, Fihn S D, Bridevaux P O, McDonell M B, Au D H. Inhaled Corticosteroids in Chronic Obstructive Pulmonary Disease and Risk of Death and Hospitalization: Time-Dependent Analysis. American Journal of Respiratory and Critical Care Medicine. 2003;168(12):1488–94. doi: 10.1164/rccm.200301-019OC. [DOI] [PubMed] [Google Scholar]
- Frees E W. Longitudinal and Panel Data: Analysis and Applications in the Social Sciences. Cambridge, UK: Cambridge University Press; 2004. [Google Scholar]
- Gartlehner G, Hansen R A, Carson S S, Lohr K N. The Efficacy and Safety of Inhaled Corticosteroids in Patients with COPD: A Systematic Review and Meta-Analysis. Annals of Family Medicine. 2006;4(3):253–62. doi: 10.1370/afm.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global Initiative for Chronic Obstructive Lung Disease (GOLD) Global Strategy for the Diagnosis, Management, and Prevention of COPD: Published November 2006. 2006 [accessed on March 12, 2007]. Available at http://www.goldcopd.org/
- IHCIS (Integrated Healthcare Information System) National Managed Care Benchmark Database, User Manual. Eden Prairie, MN: Ingenix; 2005. [Google Scholar]
- Kennedy P. A Guide to Econometrics. 5th Edition. Cambridge, MA: The MIT Press; 2003. [Google Scholar]
- Lung Health Study Research Group (LHSRG) Effect of Inhaled Triamcinolone on the Decline in Pulmonary Function in Chronic Obstructive Pulmonary Disease. New England Journal of Medicine. 2000;343(26):1902–9. doi: 10.1056/NEJM200012283432601. [DOI] [PubMed] [Google Scholar]
- Mannino D M, Watt G, Hole D, Gillis C, Hart C, McConnachie A, Davey S G, Upton M, Hawthorne V, Sin D D, Man S F, Van Eeden S, Mapel D W, Vestbo J. The Natural History of Chronic Obstructive Pulmonary Disease. European Respiratory Journal. 2006;27(3):627–43. doi: 10.1183/09031936.06.00024605. [DOI] [PubMed] [Google Scholar]
- Pauwels R, Calverley P, Buist A S, Rennard S, Fukuchi Y, Stahl E, Lofdahl C G. COPD Exacerbations: The Importance of a Standard Definition. Respiratory Medicine. 2004;98(2):99–107. doi: 10.1016/j.rmed.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Schneeweiss S, Avorn J. A Review of Uses of Health Care Utilization Databases for Epidemiologic Research on Therapeutics. Journal of Clinical Epidemiology. 2005;58(4):323–37. doi: 10.1016/j.jclinepi.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Seemungal T A, Donaldson G C, Bhowmik A, Jeffries D J, Wedzicha J A. Time Course and Recovery of Exacerbations in Patients with Chronic Obstructive Pulmonary Disease. American Journal of Respiratory and Critical Care Medicine. 2000;161(5):1608–13. doi: 10.1164/ajrccm.161.5.9908022. [DOI] [PubMed] [Google Scholar]
- Simoens S, Decramer M. Pharmacoeconomics of the Management of Acute Exacerbations of Chronic Obstructive Pulmonary Disease. Expert Opinions in Pharmacotherapy. 2007;8(5):633–48. doi: 10.1517/14656566.8.5.633. [DOI] [PubMed] [Google Scholar]
- Sin D D, Golmohammadi K, Jacobs P. Cost-Effectiveness of Inhaled Corticosteroids for Chronic Obstructive Pulmonary Disease According to Disease Severity. American Journal of Medicine. 2004;116(5):325–31. doi: 10.1016/j.amjmed.2003.09.027. [DOI] [PubMed] [Google Scholar]
- Sin D D, McAlister F A, Man S F, Anthonisen N R. Contemporary Management of Chronic Obstructive Pulmonary Disease: Scientific Review. Journal of the American Medical Association. 2003;290(17):2301–12. doi: 10.1001/jama.290.17.2301. [DOI] [PubMed] [Google Scholar]
- Sin D D, Tu J V. Inhaled Corticosteroids and the Risk of Mortality and Readmission in Elderly Patients with Chronic Obstructive Pulmonary Disease. American Journal of Respiratory and Critical Care Medicine. 2001;164(4):580–4. doi: 10.1164/ajrccm.164.4.2009033. [DOI] [PubMed] [Google Scholar]
- Spencer S, Calverley P M, Burge P S, Jones P W. Impact of Preventing Exacerbations on Deterioration of Health Status in COPD. European Respiratory Journal. 2004;23(5):698–702. doi: 10.1183/09031936.04.00121404. [DOI] [PubMed] [Google Scholar]
- Suissa S. Inhaled Steroids and Mortality in COPD: Bias from Unaccounted Immortal Time. European Respiratory Journal. 2004;23(3):391–5. doi: 10.1183/09031936.04.00062504. [DOI] [PubMed] [Google Scholar]
- Szafranski W, Cukier A, Ramirez A, Menga G, Sansores R, Nahabedian S, Peterson S, Olsson H. Efficacy and Safety of Budesonide/Fometerol in the Management of Chronic Obstructive Pulmonary Disease. European Respiratory Journal. 2003;21(1):74–81. doi: 10.1183/09031936.03.00031402. [DOI] [PubMed] [Google Scholar]
- Van Hout B, Al M J, Gordon G S, Rutten F F. Costs, Effects, and C/E Ratios Alongside a Clinical Trial. Health Economics. 1994;3:309–19. doi: 10.1002/hec.4730030505. [DOI] [PubMed] [Google Scholar]
- Vestbo J, Sorensen T, Lange P, Brix A, Torre P, Viskum K. Long-Term Effect of Inhaled Budesonide in Mild and Moderate Chronic Obstructive Pulmonary Disease: A Randomised Controlled Trial. Lancet. 1999;353(9167):1819–23. doi: 10.1016/s0140-6736(98)10019-3. [DOI] [PubMed] [Google Scholar]
- White A J, Gompertz S, Stockley R A. Chronic Obstructive Pulmonary Disease. Thorax. 2003;58(1):73–80. doi: 10.1136/thorax.58.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Author Matrix.