Abstract
The N-methyl-d-aspartate (NMDA) subtype of ionotropic glutamate receptors comprises both NR1 and NR2 subunits, and plays numerous roles in both physiological and pathophysiological processes in the central nervous system (CNS). NR2C-containing NMDA receptors are most abundant in cerebellum, thalamus and olfactory bulb, and are also expressed in oligodendrocytes and hippocampal interneurons. We have used patch clamp recording to explore the activation properties of recombinant NR1/NR2C receptors expressed in HEK293 cells. NR1/NR2C receptors activated by a maximally effective concentration of glutamate and glycine had two main conductance levels of 45 pS and 28 pS when the extracellular Ca2+ concentration was 0.5 mm and the holding potential was −80 mV. The occurrence of the lower subconductance state was reduced in the absence of extracellular Ca2+. The distribution of closed durations recorded from patches with a high probability of containing only one active channel were best fitted by five exponential functions; the apparent open duration histogram could be fitted by two exponential functions (n = 10 patches). The apparent mean open time of NR1/NR2C receptors was brief (0.52 ± 0.04 ms), suggesting that the stability of the open state of the NR1/NR2C receptors is lower than other NR2-containing receptors. NR1/NR2C open probability was exceptionally low, being 0.011 ± 0.002 in patches containing a single active receptor (n = 8). Fast agonist concentration jumps were performed on outside out patches with multiple NR1/NR2C channels, which activated with a 10–90% rise time of 3.9 ± 0.4 ms, faster than other NR2-containing receptors. The deactivation time constant after a brief (5–8 ms) application of a maximally effective concentration of agonists was 319 ± 34 ms. The majority of the patches also showed a modest level of desensitization that could be described by either a single or a double exponential time course with the fastest time constant between 15 and 47 ms. Conceptual models of activation were fitted using the maximum interval likelihood (MIL) method to the sequence of open and closed durations recorded from outside-out patches that contained one active NR1/NR2C channel. NR1/NR2C receptor properties including modest desensitization and low open probability could be described by gating schemes similar to those previously proposed for other NMDA receptor subunit combinations.
Glutamate, the major excitatory neurotransmitter in the mammalian CNS, activates three classes of ionotropic receptors classified on the basis of pharmacology and sequence similarity (Dingledine et al. 1999; Erreger et al. 2004). Among these receptor subtypes, NMDA receptors are involved in key physiological processes such as synaptic plasticity and development. NMDA receptors are formed by the assembly of two NR1 and two NR2 subunits, and exhibit different kinetic and pharmacological properties that are NR2-subunit dependent (Vicini et al. 1998; Erreger et al. 2004; Dravid et al. 2007; Erreger et al. 2007). Biophysical properties and gating of NR2A, NR2B and NR2D containing receptors have been previously studied in detail (e.g. Stern et al. 1992; Wyllie et al. 1998; Banke & Traynelis, 2003; Popescu & Auerbach, 2003; Erreger et al. 2005a). However, much less is known about the single channel properties of NR1/NR2C receptors. When expressed in Xenopus laevis oocytes, NR1/NR2C receptors appear similar to NR1/NR2D receptors, and show two conductance states at physiological levels of extracellular calcium (Stern et al. 1992). NR1/NR2C and NR1/NR2D receptors also exhibit lower voltage-dependent Mg2+ block compared to NR1/NR2A and NR1/NR2B receptors (Monyer et al. 1992; Clarke & Johnson, 2006). The deactivation time course of whole cell NR1/NR2C current responses is similar to that of NR1/NR2B receptors (Monyer et al. 1992; Vicini et al. 1998). Analysis of the macroscopic current response waveform has previously indicated that NR1/NR2C receptors do not exhibit any desensitization either in whole cell or outside out patch recordings (Monyer et al. 1992; Krupp et al. 1996; Casado et al. 1996; Chen et al. 2006). The open probability of NR1/NR2C receptors is unknown. However studies in cerebellar granule neurons from NR2C knockout animals show an increase in the NMDA receptor-mediated excitatory postsynaptic current (EPSC) responses, suggesting that NR1/NR2C receptors have low open probability (Ebralidze et al. 1996; Lu et al. 2006).
The NR1 subunit is expressed ubiquitously in the CNS (Watanabe et al. 1992; Brose et al. 1993; Laurie & Seeburg, 1994) whereas the NR2 subunits display unique regional distributions (Watanabe et al. 1992; Monyer et al. 1994; Petralia et al. 994). The NR2C subunit is abundant in adult cerebellum where it is expressed in rats after postnatal day 10 (Monyer et al. 1994; Farrant et al. 1994; Wenzel et al. 1997; Karavanova et al. 2007). NR2C-containing receptors are also located in adult olfactory bulb, thalamus (Wenzel et al. 1997), and certain interneurons in the cerebral cortex and hippocampus (Monyer et al. 1994). Recent studies have shown the presence of NR2C receptors in oligodendrocytes and layer 4 spiny stellate cells of barrel cortex (Karadottir et al. 2005; Salter & Fern, 2005; Micu et al. 2006; Binshtok et al. 2006). Activation of NR2C-containing receptors in oligodendrocytes during ischaemia has been proposed to contribute to white matter injury (Karadottir et al. 2005; Salter & Fern, 2005). Novel sites of NR2C expression such as retrosplenial cortex, and pontine and vestibular nuclei have further been identified using knock-in mice with β-galactosidase reporter inserted at the NR2C subunit translation initiation site (Karavanova et al. 2007). Mice engineered to lack the NR2C gene show potentiation of NMDA receptor-mediated synaptic current amplitude and an accelerated rate of decay (Ebralidze et al. 1996). Simultaneous disruption of NR2A and NR2C receptors leads to motor discoordination (Kadotani et al. 1996).
We have studied the activation properties of recombinant NR1/NR2C receptors in voltage-clamp recordings of outside out patches excised from HEK293 cells. NR1/NR2C receptors have submillisecond apparent mean open duration, very low open probability, modest desensitization, and an activation time course that is slightly faster than other NR2-subunit containing receptors. Hypothetical gating schemes have been developed that are able to predict the exceptionally low open probability and the single channel and macroscopic current properties.
Methods
HEK293 cells (ATCC 1573) were transiently transfected using the calcium phosphate method (Chen & Okayama, 1987) or Fugene transfection reagent (Roche Diagnostics) with rat NR1-1a (GenBank U11418, U08261; pCIneo vector; hereafter NR1, provided by Dr Stephen Heinemann), rat NR2C (Q00961; pRK vector, provided by Dr Peter Seeburg) and green fluorescent protein (GFP) at a ratio of 1 : 2 : 0.5, as previously described (Banke & Traynelis, 2003). Current recordings from outside out patches held under voltage clamp at potentials (Vm) ranging between −60 and −80 mV were made as previously described (Erreger et al. 2005a); the holding potential was not corrected for the liquid junction potential, estimated to be +7 mV. Macroscopic current responses were recorded using an Axopatch 200B amplifier (Molecular Devices), filtered at 5 kHz (−3 dB, 8-pole Bessel), and digitized at 20–40 kHz with pCLAMP 9 software (Axon Instruments/Molecular Devices). The extracellular recording solution consisted of (in mm) 150 NaCl, 10 Hepes, 0.5 CaCl2, 3 KCl, with 0.5 mm glycine and 1 mm glutamate used to activate NR1/NR2C receptors unless otherwise noted (pH 7.4, 23°C). This solution was supplemented with 0.01 mm EDTA to chelate trace amounts of contaminant divalent ions, such as Zn2+. The internal solution consisted of (in mm) 110 caesium gluconate, 30 CsCl2, 5 Hepes, 4 NaCl, 0.5 CaCl2, 2 MgCl2, 5 BAPTA, 2 Na2ATP, and 0.3 Na2GTP (pH 7.35). Rapid solution exchange was achieved with a two-barrelled theta glass pipette controlled by a piezoelectric translator (Burleigh); junction currents were used to estimate speed of solution exchange after recordings. Exchange times for 10–90% solution were typically < 1 ms, approximately 4-fold faster than the current rise times in response to a saturating agonist concentration. Brief pulses for macroscopic experiments had a half-width of 5–8 ms.
Single channel analysis
Single channel records were idealized using SCAN (http://www.ucl.ac.uk/Pharmacology/dcpr95.html) and QUB software (http://www.qub.buffalo.edu). The amplitude distribution, apparent open and closed durations, correlation between open times and subconductance states and direct transitions were determined using SCAN and EKDIST (http://www.ucl.ac.uk/Pharmacology/dcpr95.html). A 50 μs resolution was imposed on the data record. Amplitudes in a range of 1.8–6 pA were analysed for amplitude distribution. A mixture of Gaussians were fitted to the amplitudes. QUB software was used for fitting gating models. Records were idealized with a segmental k-means algorithm (Qin, 2004) using QUB software (http://www.qub.buffalo.edu). All conductance levels were assumed to be equal for the analysis. Maximal interval likelihood fitting (MIL, Qin et al. 1996) was performed with an imposed dead time of 50 μs using QUB software. All of the loops in the gating schemes were constrained to obey microscopic reversibility. Kinetic schemes obtained with an imposed dead time of 100 μs for MIL fits generated similar macroscopic current profiles as those obtained with an imposed dead time of 50 μs (data not shown). Dwell-time histograms were generated and fitted using ChanneLab (http://www.synaptosoft.com) with imposed dead time of 50 μs. We calculated Tcrit values for determining the conditional distribution of dwell times using the shut time distribution from patches that appear to contain a single active channel (Jackson et al. 1983; Colquhoun & Sigworth, 1995; Erreger et al. 2005a). Tcrit values for NR1/NR2C channel records were 0.44, 12.6, 97 and 705 ms.
Analysis of macroscopic response time course
Macroscopic response waveforms obtained in outside-out patches were normalized, aligned and averaged among patches. The average macroscopic glutamate response waveform was then normalized to the measured open probability determined in one-channel patches. Aggregated Markov models were simultaneously fitted using a nonlinear least squares fitting algorithm (ChanneLab) to multiple macroscopic waveforms obtained for both short and long pulses of either high or low concentrations of glutamate. The results of the model were compared to all waveforms at each iteration of the fitting procedure. The same duration of macroscopic current response was fitted for each of the three experimental conditions (high glutamate long pulse, high glutamate short pulse and low glutamate long pulse) for each model. Fitting was performed using a Runga-Kutta numerical integrator to simulate each response waveform at each iteration using the same set of rate constants. Experimental deactivation rates were similar for short or long pulse of high glutamate, being 317 and 377 ms, respectively (Fig. 7B). Macroscopic currents from outside-out patches were recorded to determine the EC50 of glutamate at NR1/NR2C receptors. Glutamate was applied at 3, 5 and 1000 μm (with 0.5 mm glycine in the bath) and the peak amplitude was determined using Clampfit (pCLAMP 9). Concentration–response curves were fitted by the Hill equation using Prism 4.0 (GraphPad Software Inc.).
Figure 7. Scheme 3a can describe both single channel and macroscopic data.
A, MIL fit of single channel data with Scheme 3 is shown. The representative recording from one patch contained a total of 4765 closed and shut durations, an open probability of 0.008, and a apparent mean open time of 0.41 ms (imposed resolution of 50 μs). B, the results of macroscopic fitting of Scheme 3a to the data are shown. Three agonist application protocols were used to generate NR1/NR2C response waveforms in each patch: 1 mm glutamate for 2 s (HL), 1 mm glutamate for 5 ms (HS) and 5 μm glutamate for 2 s (LL), with 0.5 mm glycine always present in the bath. Averaged traces (n = 3) for each protocol were simultaneously fitted by Scheme 3a. The peak for 1 mm glutamate protocols was scaled to an open probability of 0.011. The EC50 of glutamate in outside-out patches was 4.2 ± 1.2 μm (n = 5). The agonist binding rates and the rates for the two desensitized states were allowed to vary during fitting, while all other rates in the gating scheme were fixed to the rate constants derived from the maximum interval likelihood fit of the single channel data by Scheme 3. Least squares fitting was used to fit the macroscopic currents (see Methods).
Statistics
All values are given as the mean ±s.e.m. We compared the differences of means using Student's t test, and values of P < 0.05 were considered significantly different. The power to detect a 20% difference at P < 0.05 ranged between 84 and 90%.
Results
NR1/NR2C receptors open to at least two conductance levels
The properties of recombinant NR1/NR2C receptors were studied in outside out patches excised from transfected HEK293 cells. For all steady state voltage clamp recordings, unitary NR1/NR2C currents were evoked by application of maximally effective concentrations of glutamate (1 mm) and glycine (0.5 mm); holding potential was −80 mV. We recorded from three outside-out patches under two different extracellular Ca2+ concentrations: (a) 0.5 mm and (b) no extracellular Ca2+ (10 μm EDTA added). Two conductance levels, with chord conductance of 45 ± 1 pS (74 ± 3%) and 28 ± 3 pS (26 ± 3%) (n = 3), were observed in the presence of 0.5 mm extracellular calcium (Fig. 1A). Two conductance states have also been reported at 1 mm extracellular Ca2+ for NR1/NR2C receptors expressed in Xenopus laevis oocytes (35 pS and 19 pS; Stern et al. 1992), native NMDA receptors expressed by large cerbellar neurons in culture (39 pS and 18 pS; Cull-Candy & Usowicz, 1989) and cerebellar granule neurons (33 pS and 20 pS; Farrant et al. 1994). Rat phaechromocytoma cells (PC12 cells) have also been reported to express NR1/NR2C receptors with two conductance states of 55 pS and 33 pS at 0.5 mm extracellular Ca2+ (Casado et al. 1996). We detected 106 transitions among 3536 open periods that were direct transitions between the subconductance levels. This sublevel frequency was much lower compared to Stern et al. (1992), which may partly be due to the lower extracellular Ca2+ (0.5 mm) in our experiments. We could detect asymmetry in direct transitions in all patches with a total of 36 transitions (32 ± 2%) from low to high conductance and 70 transitions (68 ± 2%) from high to low conductance. In contrast, symmetry in direct transitions between the subconductance states has been reported earlier for NR1/NR2C receptors (Stern et al. 1992); however, given the lower sublevel frequency in our experiments, there are not enough clear transitions in the data record to draw an unequivocal conclusion. The frequency of the lowest subconductance level was calcium dependent, being strongly reduced in the absence of extracellular calcium. The conductance levels in the nominal absence of extracellular calcium (10 μm EDTA added) were 57 ± 2 pS (93 ± 3%) and 48 ± 3 pS (7 ± 2%) (n = 3).
Figure 1. NR1/2C receptors have at least two conductance levels in the presence of 0.5 mm extracellular Ca2+.
A, representative steady state recordings of unitary currents from outside-out patch containing one active channel in the presence of 0.5 mm extracellular Ca2+ in response to maximally effective concentration of glutamate and glycine (1 mm glutamate, 0.5 mm glycine, Vm−80 mV, digitized at 20 kHz, filtered at 5 kHz). Amplitude histogram (right panel) for each opening determined using time course fitting could be fitted by the sum of two Gaussian components. NR1/NR2C receptors exhibit two conductance levels with the chord conductance of 48 pS (72%) and 35 pS (28%). B, representative unitary currents from the same outside-out patch in A recorded in the absence of nominal extracellular Ca2+ with 10 μm EDTA added to the external solution. The conductance of NR1/NR2C receptors is increased under these conditions, and the occurrence of lower subconductance state is reduced. Chord conductance values were 59 pS (95%) and 51 pS (5%).
The apparent mean open time of NR1/NR2C channels in the presence of 0.5 mm extracellular calcium was 0.44 ± 0.06 ms (n = 3). This is similar to the apparent mean open time of 0.42–0.85 ms reported previously for NR1/NR2C receptors expressed in PC12 cells, oocytes and cerebellar granule neurons (Stern et al. 1992; Farrant et al. 1994; Casado et al. 1996). The apparent mean open time was similar in the nominal absence of extracellular Ca2+ (0.37 ± 0.03 ms; n = 3; P > 0.4), suggesting that extracellular Ca2+ influences the configuration of the channel pore but not the stability of the open state. The apparent mean open time was independent of the conductance level, consistent with a previous report on NR1/NR2C receptors expressed in Xenopus laevis oocytes (Stern et al. 1992). At 0.5 mm extracellular Ca2+ the apparent mean open time of the lower conductance state was 0.39 ± 0.06 ms and that for the higher conductance state was 0.43 ± 0.09 ms (n = 3; P > 0.8). The apparent mean open time for NR1/NR2A and NR1/NR2B receptors has been reported to be sublevel dependent, with lower conductance openings having lower apparent mean open time (Gibb & Colquhoun, 1991; Traynelis & Cull-Candy, 1991; Stern et al. 1992).
Determination of the number of channels in the patch
Of the various recordings we made from outside out patches, we observed eight patches with no double openings. We used the approximation of Colquhoun & Hawkes (1990) to calculate the probability that only one active channel was present in the outside out patches without any double openings when exposed to saturating concentrations of agonists. This equation allows the calculation of the number of consecutive openings of a channel in a patch that contains two active channels without any double openings as a function of the open probability of the channel. Er is the mean number of consecutive openings in a run, and is related to Po2, the probability that a channel is open during an observed run of single openings that originates from two independent channels, i.e. Po2 is the fraction of time for which the channel is open during the run according to:
| (1) |
The low open probability (Po) of NR1/NR2C receptors makes it less intuitive that patches with only one channel can be identified, since two channels with low open probability might be expected to rarely open at the same time. However, eqn (1) takes into consideration the open probability of the receptor and the number of events to allow determination of the level of confidence that a particular patch only has one active channel. The equation assumes that the recordings are at steady state and to be of sufficient duration such that all receptor states will be visited numerous times. To test whether the open probability of NR1/NR2C receptors can be described by this equation, we used the single channel currents recorded from a patch in the presence of saturating agonist concentrations for which we had over 30 min of recording without any double openings (20 424 openings). MIL fitting of the single channel currents was done to a simple empirical linear model with four closed states and one open state. The rates derived from the MIL fit for this linear gating scheme were subsequently used in a Monte Carlo simulation of two channels activated by a maximal concentration of agonist. The open probability of the receptor was then systematically altered by changing the forward rate from the final closed state to the open state. Ten simulations of 50 s duration were performed for each of the four different open probability values (0.0044, 0.0132, 0.0392 and 0.113). These records were analysed to determine the number of single openings in runs between double openings. Figure 2A shows the number of openings per run of single openings from Monte Carlo simulations of the linear gating model (mean ±s.e.m., open squares). The approximation of Colquhoun & Hawkes (1990) (eqn (1), continuous line) accurately predicts the behaviour of channels with NR1/NR2C-like properties, and thus can be used as an analytical tool to assess probability that patches contain a single active channel. The three dashed lines indicate the upper limit for P = 0.0044, 0.0132 and 0.0392 calculated as 3.24Er, 4.33Er and 5.43Er, respectively (Colquhoun & Hawkes, 1990). We analysed experimental data from two patches in which we observed simultaneous double openings but never simultaneous triple openings. These two patches had a total of 7068 (current trace in Fig. 2B) and 6288 openings and a mean Po2 values of 0.011 (i.e. open probability was 0.0055). For these patches, we calculated the mean number ±s.e.m. of single openings before a double opening was observed. These analyses are plotted in Fig. 2A (filled squares) and show that the data lie close to the expected theoretical value. Thus, the approximation described by Colquhoun & Hawkes (1990) appears to be a useful tool to identify patches that contain a single NR1/NR2C receptor.
Figure 2. Determination of the number of active NR1/NR2C channels in a patch.
A, the sequence of NR1/NR2C receptor open and closed durations recorded from one patch in which we recorded 20 424 openings with no simultaneous double openings was fitted (MIL). Monte Carlo simulations (50 s duration, 100 kHz) were performed to determine the total number of single openings between two simultaneous double openings in a patch that contained two active channels using this model. The total number of single openings between two double openings was determined for 10 simulations. This was repeated 10 times for each of four different models with open probability altered by changes to the forward C4–O rate. The mean number of consecutive single openings (±s.e.m.) was plotted against two times the open probability (Po2, □). The continuous line shows the relationship between number of consecutive openings and open probability generated using the approximation described by Colquhoun & Hawkes (1990; eqn (1) above) and our measured Po2 value. The three dashed lines indicate upper confidence limits corresponding for P = 0.039, 0.013 and 0.004 calculated as 3.2Er, 4.3Er and 5.4Er, respectively. Experimental data from two patches that showed a limited number of double openings and thus contained at least two active channels are represented by ▪. B, outside-out patch containing two active NR1/2C receptors (Vm=−80 mV, digitized at 20 kHz, filtered at 5 kHz). Arrows indicate two simultaneous openings that occurred in this patch on average after every 276 ± 46 single openings (n = 5).
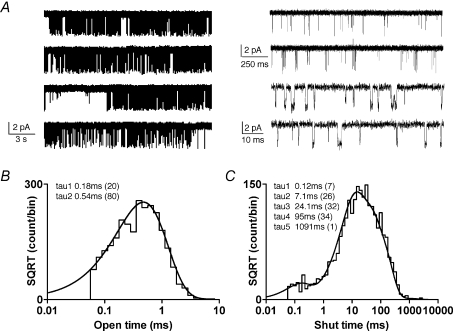
Properties of open and closed durations for NR1/NR2C receptors
Recordings were made from more than 60 outside out patches from HEK293 cells expressing NR1 and NR2C subunits in response to maximally active concentrations of glutamate and glycine in the presence of 0.5 mm extracellular Ca2+. We found that 8 of these 60 patches contained only one open active channel with a confidence greater than 99.9% based on eqn (1). The mean open probability for NR1/NR2C receptors determined from one channel patch was 0.011 ± 0.002 (n = 8 patches; 45 408 open periods). We also recorded two patches which started as two channel patches but after ∼1–2 min only opened as a one channel patch for the remainder of the recording (∼6–13 min). We used the last 3–4 min of recording from these patches for further analysis. The open and closed durations for these two patches were similar to those observed with patches containing only one active channel and were therefore included in the overall kinetic analysis. The open duration histograms could be fitted by the sum of two exponential components with time constants of 0.17 ms (46%) and 0.64 ms (54%) (n = 10 patches, 55 462 open periods). Because the concentrations of glutamate and glycine are more than 100-fold higher than EC50, we expect agonist rebinding to be rapid and closed times that reflect the delay to rebind exceptionally brief. Thus, most closed times in the data record reflect closure of agonist-bound receptors within an activation, not the delay for agonist to bind. The composite shut time histogram from 10 patches was fitted by five exponential functions with the time constants and areas of 0.22 ms (9%), 7.9 ms (35%), 33.8 ms (38%), 127 ms (17%) and 783 ms (1%) (n = 10 patches; 55 389 closed periods). A representative patch with fitted dwell-time histograms is shown in Fig. 3B and C. The fitted results were independent of imposed recording resolution between 50 and 100 μs (data not shown). These data suggest that NR1/NR2C, like other recombinant NMDA receptors, shows complex gating with multiple closed and open states visited within individual receptor activations. We did not observe an ultra-fast shut component as observed previously in NR1/NR2A receptors and NR1/NR2D receptors (time constant < 50 μs; Wyllie et al. 1998; Schorge et al. 2005). The briefest open and shut times can be influenced by the method of idealization (Schorge et al. 2005), and thus our digitization rate, filtering and idealization method may have prevented detection of the briefest events. However, the briefest shut durations for NR1/NR2C receptors had similar frequency when identified either using time course fitting (SCAN) or QUB software, suggesting the method of idealization was unlikely to fully explain the absence of a exceptionally brief shut time component (data not shown).
Figure 3. Steady-state NR1/NR2C unitary currents in outside out patches.
A, steady-state recordings of NR1/NR2C unitary currents from an outside-out patch that contained one active channel in the presence of maximally effective concentration of glutamate and glycine (1 mm glutamate, 0.5 mm glycine, Vm−80 mV, digitized at 20 kHz, filtered at 2–5 kHz) under three different time scales showing the range of shut time durations. B and C, open (B) and shut (C) duration histogram from a representative patch containing 2647 open and 2646 closed durations fitted with multiple exponential components; single channel currents were analysed using time course fitting (SCAN) and histograms fitted using the maximum likelihood method (EKDIST). The fitted time constants for multicomponent exponential functions are given in the inset with the percentage area for each component in parentheses. The fitted time constants for the pooled data from 10 patches are described in the text.
Correlations between the lengths of a channel open period and an adjacent closed period can provide information about the mechanism of channel activation. Correlations between open and closed durations have previously been described for recombinant NR1/NR2A receptors recorded in outside-out patches excised from Xenopus laevis oocytes, HEK293 cells and CA1 pyramidal neurons (Gibb & Colquhoun, 1991; Schorge et al. 2005; Wyllie et al. 2006; Erreger & Traynelis, 2008). We performed a runs test to evaluate whether correlation existed for NR1/NR2C receptors using a critical open time of 0.39 ms (Jackson et al. 1983). We found no significant correlation between the shut time duration and the apparent mean duration of the openings adjacent to them with the z-test statistics ranging from −0.971 to 0.966 (n = 10). The lack of correlation could reflect failure to detect the fastest shut events, which may be correlated with open times (Schorge et al. 2005). In addition, our steady state single channel recordings were made in the presence of maximally effective concentrations of glutamate and glycine. Correlations for glycine and nicotinic receptors are dependent on the agonist concentration, and are not detected when the receptor is fully liganded (Beato et al. 2002, 2004; Hatton et al. 2003; Burzomato et al. 2004). By contrast, NR1/NR2A receptor correlations appear to be independent of agonist concentration (Schorge et al. 2005). It seems possible that other NMDA receptors also show similar correlations independent of agonist concentration.
We subsequently constructed conditional distributions of adjacent intervals to further evaluate any potential correlations in the data record. Figure 4A shows conditional distributions of apparent intraburst open durations adjacent to brief (0.05–0.44 ms) or prolonged (97–705 ms) intraburst closed durations. The distribution of apparent open durations adjacent to brief closed durations shows similar fitted time constants and areas as the distribution of apparent open durations adjacent to prolonged closures. We also did not observe any trend in the analysis of the apparent mean open duration adjacent to closed duration in a specified range (Fig. 4B). The shut time ranges used were (in ms) 0.05–0.44, 0.44–12.6, 12.6–97, 97–705 and 705–10 000.
Figure 4. Lack of correlation between open and closed durations of NR1/NR2C channels in outside-out patches from HEK293 cells.
A, the closed duration histogram from a patch with single active channel recorded in the presence of maximally effective concentration of agonist was used to determine critical closed times to separate five fitted shut time components as described in Methods (Jackson et al. 1983). The critical times were 0.44, 12.6, 97, 705 ms. Conditional distributions were constructed from pooled data from four patches for intraburst apparent open durations adjacent to a brief closed durations in the range of 0.05–0.44 ms (continuous black line) or adjacent to longer duration closed times in the range of 97–705 ms (continuous grey line). The distributions and respective fitted exponential components for openings adjacent to brief closures were scaled to contain the same number of open periods as distributions for apparent open times adjacent to long closed times. The time constants for exponential fits to open periods were 0.18 ms (27%) and 0.52 ms (73%) for opening adjacent to brief closed durations and 0.15 ms (22%) and 0.45 ms (78%) for openings adjacent to long closed durations. B, the mean of the conditional apparent intraburst open durations either preceding (▪) or following (□) the specified shut time range are pooled from 10 patches and plotted against the fitted time constants describing the closed time distribution (Fig. 3). The closed duration ranges were (in ms) 0.05–0.44, 0.44–12.6, 12.6–97, 97–705 and 705–10 000. The dashed line in grey indicates the average of the mean open times from 10 patches (0.52 ms).
Macroscopic time course of NR1/NR2C current responses
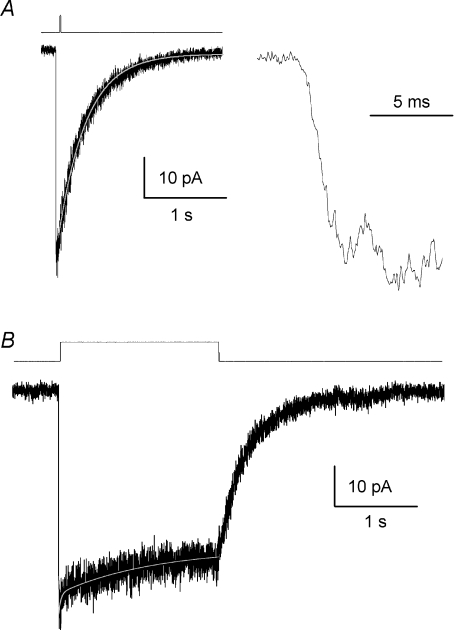
We performed fast agonist concentration jumps on outside out patches excised from NR1/NR2C-expressing HEK293 cells to estimate the macroscopic time course and properties of NR1/NR2C receptor current responses. A maximally effective concentration of glycine (0.5 mm) was present in all solutions. Outside-out patches were exposed to 1 mm glutamate for either a short duration of 5–8 ms or a longer duration of 2–4 s. The rise time of the macroscopic currents was 3.9 ± 0.4 ms (n = 11). For 5–8 ms duration concentration jump experiments, the current deactivation time course could be described by a single exponential function with a time constant of 319 ± 34 ms (n = 5). The deactivation time constant for currents in response to agonist application for 2–4 s was 417 ± 40 ms (n = 6) (Fig. 5). The deactivation time course was similar to that previously reported from whole cell recordings from HEK293 cells expressing rat or human NR1/NR2C receptors (382–481 ms) (Monyer et al. 1992; Vicini et al. 1998; Daggett et al. 1998). Although NR1/NR2C receptors are typically considered to be non-desensitizing (Kohr et al. 1994; Monyer et al. 1994; Krupp et al. 1996; Villarroel et al. 1998) we observed a modest level of desensitization during rapid agonist application in 9 out of 12 outside out patches activated by prolonged application of a saturating agonist concentration. The steady-state to peak level estimated at the end of a 2–4 s pulse of agonist was 0.78 ± 0.04 (n = 9). This ratio is much higher in comparison to that observed in excised outside-out patches containing NR1/NR2A (0.20–0.28, Wyllie et al. 1998; Erreger et al. 2005a) and NR1/NR2B (0.27, Banke & Traynelis, 2003) receptors. The time course for the onset of desensitization could be fitted with a single exponential function for 5 out of 9 patches, but required two exponential components for the other 4 patches. The mean time constant for those patches fitted with a single exponential was 235 ± 95 ms (n = 5); macroscopic current responses that were fitted with two exponentials had desensitization time constants of 59 ± 16 ms and 719 ± 174 ms (n = 4). Figure 5B depicts an average of three independent recordings with the desensitization time course fitted with two exponential functions. Interestingly, no desensitization was observed in whole-cell recordings (data not shown). This is consistent with the general finding that patch excision and dialysis of the intracellular compartment appears to accelerate NMDA receptor desensitization (e.g. Sather et al. 1990, 1992).
Figure 5. Macroscopic NR1/NR2C currents from outside-out patches.
A, the average response time course is shown for NR1/NR2C channels in outside-out patches exposed to fast agonist concentration jumps of 5 ms duration (1 mm glutamate, 0.5 mm glycine present in all solutions); the trace is the average of three independent patches. The decay time constant for the macroscopic current response waveform was 306 ms. As depicted in the inset, NR1/NR2C receptors exhibit fast activation with a 10–90% rise time constant of 4.6 ms. B, the average current waveform in response to rapid agonist application of 2 s duration is shown. The desensitization is fitted to the sum of two exponentials with time constants of 26 ms and 1113 ms
Mechanism of NR1/NR2C receptor activation
NR1/NR2C receptors exhibit some striking functional differences compared to NR1/NR2A and NR1/NR2B receptors, such as low open probability, short apparent mean open time, and relatively fast receptor activation. Reconciling divergent features such as rapid channel opening but low overall open probability is virtually impossible in the absence of a quantitative framework of receptor activation. A number of recent studies have presented conceptual models for the activation of NR1/NR2A and NR1/NR2B receptors that can accurately describe the single channel and macroscopic properties of these receptors (Popescu & Auerbach, 2003; Banke & Traynelis, 2003; Erreger et al. 2005a,b; Auerbach & Zhou, 2005; Schorge et al. 2005). These models now provide context in which to interpret synaptic function, pharmacological regulation, and structural data. In order to further understand the mechanism underlying activation of NR1/NR2C receptors and to assess its similarity to NR1/NR2A and NR1/NR2B receptors, we have fitted the sequence of single channel openings with a series of explicit models of receptor activation using the maximum interval likelihood (MIL) method (see Methods). In addition we also fitted conceptual models of activation that include the agonist binding and unbinding steps to macroscopic currents. All models were derived from previously described gating schemes for NMDA receptors (Fig. 6).
Figure 6. Conceptual models of NR1/2C receptor activation.
Three previously described gating schemes for NMDA receptors were fitted to single channel data to evaluate gating properties of NR1/NR2C receptors. Scheme 1 has been previously described by Popescu et al. (2004) for activation of NR1/NR2A receptors. Scheme 1a is the extension of Scheme 1 to include the agonist binding steps. Scheme 2 is similar to the gating mechanisms previously described for NR1/NR2A and NR1/NR2B receptors (Erreger et al. 2005a, b). Scheme 3 is a modification of Scheme 2 to include an extra closed state and has been used to describe NR1/NR2A receptor function (Auerbach & Zhou, 2005; Schorge et al. 2005). The rate constants obtained from MIL fitting and least squares fitting of models to single channel data and macroscopic waveforms are provided in Table 1 and Table 2.
We studied individual receptor activations in response to maximally effective concentrations of coagonists glutamate and glycine. We limited analysis to patches for which there was greater than 99% confidence (eqn (1)) that they contain a single active channel. We first globally fitted the sequence of single channel openings and closings with a simple linear gating scheme that contained three closed and two open states (not shown, see Popescu et al. 2004). None of these three closed states are intended to reproduce the time-dependent fade of the response in the presence of continued glutamate, which is defined operationally as desensitization (Popescu et al. 2004; Erreger et al. 2005a). This simple linear scheme was insufficient to account for the single channel data, most likely because it did not contain enough closed states to accurately model the multiple shut duration components observed in the data record. Because we observed a reduction of macroscopic current responses in the continued presence of agonist, we subsequently assumed that the longer shut time intervals observed for the NR1/NR2C receptor represent some degree of time-dependent desensitization, similar to NR1/NR2A and NR1/NR2B receptors (Banke & Traynelis, 2003; Popescu et al. 2004; Erreger et al. 2005a). We therefore added two additional closed states accessible from the agonist bound gateway state to the model (D1 and D2) that could improve the ability of the model to show time-dependent relaxations after agonist concentration jumps. These states also provided a means to represent more kinetically distinct shut time intervals.
We first tested the kinetic model described as Scheme 1, which is similar to the linear model that was previously reported to describe the single channel and macroscopic data for NR1/NR2A receptors (Fig. 6; Popescu et al. 2004; Erreger et al. 2005a). The rates derived from the global MIL fits of Scheme 1 to steady-state single channel records from 10 patches that contained one channel (total of 55 462 openings; imposed resolution of 50 μs) are presented in Table 1. The single channel data could be described reasonably well by this model. A model similar to Scheme 1 but containing only a single open state had a lower quality fit with a log likelihood of 510 403 compared to 512 017 for Scheme 1. This is consistent with an open time histogram that can be described by at least two exponential components, suggestive of at least two open states (Fig. 3B). The lack of correlations between open periods and shut durations (Fig. 4) suggests that the open states are connected through a single gateway to the shut state, consistent with Scheme 1.
Table 1.
Maximum interval likelihood fitting of the sequence of open and closed durations
| Rates (s−1) | Scheme 1 | Scheme 2 | Scheme 3 | Scheme 4 |
|---|---|---|---|---|
| d1+ | 7.5 ± 3.0 | 120 ± 41 | 8.5 ± 3.3 | 7.0 ± 2.7 |
| d1− | 15 ± 4.2 | 49 ± 14 | 13 ± 4.2 | 15 ± 4.0 |
| d2+ | 1.2 ± 0.8 | 11 ± 4.7 | 1.3 ± 0.9 | 0.8 ± 0.5 |
| d2− | 3.0 ± 1.0 | 5.5 ± 1.9 | 3.1 ± 1.0 | 3.0 ± 0.9 |
| k1+ | 66 ± 19 | 100 ± 18 | 65 ± 18 | 17 ± 8 |
| k1− | 48 ± 10 | 2300 ± 230 | 43 ± 9 | 110 ± 46 |
| k2+ | 560 ± 73 | 1900 ± 550 | 570 ± 74 | 220 ± 94 |
| k2− | 3200 ± 470 | 1100 ± 320 | 3300 ± 490 | 1800 ± 420 |
| k3+ | 580 ± 120 | 1300 ± 620 | 590 ± 120 | 150 ± 76 |
| k3− | 3500 ± 420 | 2500 ± 360 | 3400 ± 420 | 2900 ± 430 |
| k4+ | 1400 ± 600 | — | 1300 ± 610 | 1300 ± 360 |
| k4− | 2600 ± 370 | — | 2500 ± 360 | 1600 ± 200 |
| k5+ | — | — | — | 1300 ± 610 |
| k5− | — | — | — | 2500 ± 360 |
| LL | 51201 | 511833 | 512017 | 512017 |
Idealized current records were fitted to the four gating schemes as described in Figs 6 and 8. All rates have units of s−1. Data are mean ±s.e.m. from 10 one-channel patches with a total of 55 462 open periods and 55 389 closed periods fitted individually. All loops were constrained to follow the property of microscopic reversibility. Rates are given to two significant digits.
We further tested whether the macroscopic current properties can be adequately described by this model. Scheme 1a is identical to Scheme 1 except that two glutamate binding steps have been added; we assume that the glycine site is saturated in all simulations. We subsequently recorded macroscopic current responses to the application for different duration of a maximally effective concentration of glutamate as well as submaximal concentration of glutamate in the same patch (5 μm). Our responses suggested an EC50 value of 4.2 ± 1.2 μm in excised membrane patches from HEK cells, similar to that observed for NR1/NR2C receptors in Xenopus laevis oocytes (1 μm, Ishii et al. 1993; 1.68 μm, Erreger et al. 2007). All responses in each patch were normalized to the response to prolonged (2–4 s) application of 1 mm glutamate, and averaged across patches (n = 3 for each condition). Three macroscopic waveforms were generated from this experiment. We obtained a mean waveform response to (a) maximally effective concentration of glutamate applied for prolonged duration, (b) maximally effective concentration of glutamate applied for brief duration, and (c) submaximally effective concentration of glutamate applied for prolonged duration. These three mean waveforms were then scaled to the mean peak open probability (0.011) and fitted simultaneously with Scheme 1a by evaluating the summed sum-of-squares difference between theory and experiment for all three concentration profiles at each iteration within the nonlinear least squared fitting algorithm. Only binding and desensitization rates were allowed to vary; gating steps were fixed to values determined from MIL analysis. Scheme 1a was able to predict the low open probability, modest degree of desensitization, deactivation kinetics, as well as the concentration dependence of the amplitude and rise time. However, the predicted rise time in response to a long pulse of 1 mm glutamate was approximately 2.5-fold slower than the experimental rise time (Table 2). Although not considerably different from the experimental result, the slower rise time may reflect a paradox in the model, in that low open probability necessitates slow opening rates relative to closing rates, which conversely slows the rise time to a level below that observed experimentally. Differences exist in the desensitization rates estimated by MIL fits to the single channel data and the least squares fitting of the macroscopic data. This is not surprising considering different methods and models were applied for these estimations. The desensitization rates obtained from the least square fitting to Scheme 1a are, however, more relevant since they include the unbinding step that is missing in the MIL fits to Scheme 1.
Table 2.
Fitting of the macroscopic NR1/2C current response time course
| Rates | Experiment | Scheme 1a | Scheme 2a | Scheme 3a | Scheme 4a |
|---|---|---|---|---|---|
| b+ | — | 1.3 | 1.5 | 1.4 | 0.97 |
| b− | — | 7.5 | 14 | 8.8 | 1.9 |
| d1+ | — | 75 | 850 | 97 | 7 |
| d1− | — | 31 | 160 | 22 | 14 |
| d2+ | — | 7.8 | 53 | 0.12 | 0.8 |
| d2− | — | 4.5 | 11 | 1.7 | 23 |
| Residual | — | 1.99 × 10−7 | 3.75 × 10−7 | 2.28 × 10−7 | 2.52 × 10−7 |
| Kd (μm) | — | 6 | 9 | 7 | 2 |
| EC50 (μm) | 4.2 | 3.4 | 3.5 | 3.4 | 3.1 |
| Po | 0.011 | 0.012 | 0.022 | 0.012 | 0.012 |
| Rise time | 3.9 | 9.7 | 0.9 | 8.9 | 6.6 |
| Deactivation | 320 | 360 | 340 | 390 | 390 |
| Desensitization (τ1) | 59 | 31 | 3 | 33 | 48 |
| Desensitization (τ2) | 720 | 170 | 58 | 750 | 150 |
The rise times and normalized residual sum of squares (indicated as Residual) were derived from the macroscopic fitting with the gating rates fixed to those derived from MIL fits (Table 1); the desensitization and agonist binding and unbinding rates were set as free parameters. The agonist binding rates are in μm−1 s−1 and all other rate constants are s−1. The rise time, deactivation and desensitization are in ms.
We additionally evaluated a cyclic model previously described in a series of reports on NR1/NR2A and NR1/NR2B receptor gating (Banke & Traynelis, 2003; Erreger et al. 2005a,b; Auerbach & Zhou, 2005). Scheme 2 includes two independent pregating steps previously suggested to represent subunit-dependent conformational changes (Banke & Traynelis, 2003). The single channel properties could be described reasonably well by fitting Scheme 2 to the sequence of channel open and closed durations (Table 1). However, the fit of Scheme 2 and Scheme 2a to the single channel and macroscopic data, respectively, is of considerably lower quality than Scheme 1 and Scheme 1a, partly due to the constraints imposed by the cycle (Table 1, Table 2 and Fig. 8B). Scheme 3 is an expansion of Scheme 2 to include the idea that a conformational state exists in which both pregating steps are complete but the channel has yet to open, and channels can rapidly open from this conformation (e.g. Auerbach & Zhou, 2005; Schorge et al. 2005) (Fig. 6). This adds an additional closed state, allowing a better fit of the data. Scheme 3 can describe the single channel data with similar quality of fit as Scheme 1. Scheme 3 was expanded to Scheme 3a by adding two sequential glutamate binding steps. This model was subsequently fitted to mean macroscopic NR1/NR2C receptor current response waveforms. Similar to Scheme 1a, Scheme 3a was able to describe all features of the macroscopic current time course (Fig. 7). The rise time predicted by Scheme 3a for long pulse of 1 mm glutamate, similar to Scheme 1a, was 2-fold slower than experimental rise time (Table 2).
Figure 8. Conceptual model to describe the fast rise time of NR1/NR2C receptors.
A, the ability of a gating scheme similar to that described for NR1/NR2A receptors (Auerbach & Zhou, 2005) to account for fast rise time of the NR1/NR2C receptors was examined. Scheme 4 and Scheme 4a adequately described the single channel and macroscopic data, respectively. The rate constants are described in Table 1 and Table 2. B, least square fitting of Scheme 2a, Scheme 3a and Scheme 4a to the rising phase of NR1/NR2C current responses evoked by 1 mm glutamate for 5 ms in outside-out patches is shown. Similar to macroscopic fitting in Fig. 7, only the desensitization and agonist binding rates were free parameters. Scheme 4a describes a relatively faster rise time compared to Scheme 3a.
Although Scheme 1a and Scheme 3a satisfactorily predicted all of the key features of single channel and macroscopic current responses, we further tested Scheme 4, which has been previously described by Auerbach & Zhou (2005) for activation of NR1/NR2A receptors (Fig. 8). While microscopic reversibility is maintained, the rates within the loop in Scheme 4 are not symmetrically constrained as in Schemes 2 and 3. Consequently, this model has an extra degree of freedom. Fitting Scheme 4a to the data showed that these models described the single channel and macroscopic data reasonably well, respectively. The predicted rise time by Scheme 4a was 1.5-fold slower than experimental rise time (Table 2). The normalized residual of the fit to the rising phase for short and long pulse of high glutamate was lower for Scheme 4a compared to Scheme 3a. Although the rise time predicted by Scheme 4a was closer to the experimental rise time than that predicted by Scheme 3a, the normalized residual sum of squares indicates that overall the macroscopic data were better fitted by Scheme 3a than Scheme 4a (Fig. 8B; Table 2).
Discussion
We have described the single channel properties of recombinant NR1/NR2C receptors expressed in mammalian cells, and tested whether a variety of the gating schemes that have been previously proposed to account for activation of NR1/NR2A and NR1/NR2B receptors could appropriately describe the single channel and macroscopic properties of NR1/NR2C receptors. There are four noteworthy findings of this study. First, NR1/2C receptors have an unusually low open probability (0.011) in steady-state recording from outside out patches that contain one active channel exposed to maximally effective concentrations of glutamate and glycine. This is approximately 44-fold and 10-fold less than the peak open probability determined for NR1/NR2A and NR1/NR2B receptors, respectively (Banke & Traynelis, 2003; Erreger et al. 2005a). This open probability is closer to that of NR1/NR2D receptors, which have a super-cluster open probability of 0.04 (Wyllie et al. 1998). Second, NR1/NR2C receptors have very short apparent mean open time (0.52 ms at 23°C), which suggests a lower stability of the open conformation of the NR1/NR2C receptors compared to NR2A- or NR2B-containing receptors. Third, NR1/NR2C receptors have a relatively rapid rise time (10–90% rise 3.9 ms), compared to NR1/NR2A and NR1/NR2B receptors. This is particularly interesting since their low open probability suggests that the rates of channel opening are slow relative to the channel closing rate, although this may not always be the case in more complex models, for example the intermediate fully liganded states for NR1/NR2A receptors and glycine receptors (Burzomato et al. 2004; Schorge et al. 2005). Fourth, the NR1/NR2C receptors exhibit a complex shut duration histogram including long shut periods exceeding 100 ms. Our interpretation of these long-lived shut states is that they represent some degree of desensitization of NR1/NR2C receptors. This is consistent with the modest desensitization we observe in the macroscopic currents recorded from outside out patches. Our recordings of one NR1/NR2C channel isolated in an excised outside-out patch revealed a pattern of closed time durations within an activation that could prolong the deactivation time course (e.g. Jones & Westbrook, 1995). One of the distinct differences in the shut durations of NR1/NR2C receptors compared to NR1/NR2A and NR1/NR2B receptors is the clear separation between the time constant describing the fastest shut time component and the time constants describing other shut time components. Interestingly, these single channel properties further subdivide NR2A/NR2B and NR2C/NR2D receptors into two classes each of which share similar properties.
The NR2 subunit controls NMDA receptor gating
One important conclusion from this and our previous studies is that similar conceptual models are capable of describing NMDA receptors with a wide range of properties. That is, despite differences in open probability of over 40-fold, as well as differences in the desensitization rate, agonist potency, and single channel open duration, similar models can accurately reproduce the single channel and macroscopic response properties for NMDA receptors that contain the NR2A, NR2B, or NR2C subunits. For example, Scheme 1 and Scheme 3, which are similar to the gating schemes proposed for NR1/NR2A and NR1/NR2B receptors (Popescu et al. 2004; Erreger et al. 2005a; Auerbach & Zhou, 2005), were able to adequately describe most of the single channel and macroscopic properties of NR1/NR2C receptors. We interpret this to suggest that the basic mechanisms of channel gating are conserved across the NMDA receptor family, despite striking differences in micro- and macroscopic receptor response properties. Interestingly, all of the schemes we evaluated in this study modestly overestimated the rise time observed for the NR1/NR2C receptors. This seems to reflect the slow opening rates or slow pregating steps relative to channel closing rates, which keep open probability low but at the same time slow the time to reach peak response following agonist application. In contrast to NR1/NR2A and NR1/NR2B receptors, Scheme 2 was unable to satisfactorily describe NR1/NR2C receptor activation, suggesting the requirement of a gateway state before the channel opens. Thus, although the gating scheme for NR1/NR2C appears to have similar attributes to that of NR1/NR2A and NR1/NR2B receptors, there must also be some subtle differences that can allow fast response rise time yet keep the open probability low.
Shaping of synaptic currents by NR1/NR2C receptors
The NR2C-containing receptors are expressed most abundantly in the cerebellum; however, recent studies have suggested their expression and function in other cells and brain regions such as oligodendrocytes and layer-4 of barrel cortex (Karadottir et al. 2005; Salter & Fern, 2005; Micu et al. 2006; Binshtok et al. 2006). At the mossy fibre–granule cell synapse there is a developmental change in the kinetic profile of NMDA receptor mediated EPSCs due to replacement of NR2B subunit with NR2A and NR2C subunits. Cerebellar NR2C receptors are targeted to both synaptic and extrasynaptic locations in granule neurons (Rumbaugh & Vicini, 1999; Cathala et al. 2000). In adult rodent the NMDA receptor-mediated component of the mossy fibre–granule cell EPSC has slow decay kinetics characteristic of NR1/NR2C receptors (Ebralidze et al. 1996; Cathala et al. 2000). The amplitude of evoked and spontaneous EPSCs in NR2C−/− mice are approximately two-fold higher compared to wild-type mice (Ebralidze et al. 1996), consistent with a higher open probability of NR2A and NR2B receptors than NR2C-containing receptors. Moreover, the decay time is twofold faster in NR2C−/− mice, consistent with replacement of slowly deactivating NR2C containing NMDA receptors with fast deactivating NR2A-containing receptors in NR2C−/− mice. Because effects of low open probability on the charge transfer are qualitatively offset by slower deactivation of NR2C containing receptors, the charge transfer during an EPSC is expected to be (and is) similar between NR2C−/− and wild-type animals. Thus although NR1/NR2C receptors have low open probability in mammalian cells, they still contribute significantly to the synaptic profile at the cerebellar granule synapses. The single channel and macroscopic properties we describe in a mammalian expression system are similar to the NMDA receptor activation properties described at the cerebellar synapses. Thus the conceptual models we describe for NR1/NR2C receptor activation are likely to be relevant to native NR1/NR2C activation and may allow a better understanding of the synaptic NR1/NR2C mediated currents.
Acknowledgments
We thank Drs D. Colquhoun and K. Erreger for critical comments on the manuscript. We thank Dr K. B. Hansen for useful suggestions and help with single channel simulations. This work was supported by NIH-NINDS (NS036654, S.T.), the Michael J Fox Foundation (S.T.), and the American Epilepsy Foundation and Health Future Foundation (S.M.D.).
References
- Auerbach A, Zhou Y. Gating reaction mechanisms for NMDA receptor channels. J Neurosci. 2005;25:7914–7923. doi: 10.1523/JNEUROSCI.1471-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banke TG, Traynelis SF. Activation of NR1/NR2B NMDA receptors. Nat Neurosci. 2003;6:144–152. doi: 10.1038/nn1000. [DOI] [PubMed] [Google Scholar]
- Beato M, Groot-Kormelink PJ, Colquhoun D, Sivilotti LG. Openings of the rat recombinant a1 homomeric glycine receptor as a function of the number of agonist molecules bound. J Gen Physiol. 2002;119:443–466. doi: 10.1085/jgp.20028530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beato M, Groot-Kormelink PJ, Colquhoun D, Sivilotti LG. The activation mechanism of a1 homomeric glycine receptors. J Neurosci. 2004;24:895–906. doi: 10.1523/JNEUROSCI.4420-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binshtok AM, Fleidervish IA, Sprengel R, Gutnick MJ. NMDA receptors in layer 4 spiny stellate cells of the mouse barrel cortex contain the NR2C subunit. J Neurosci. 2006;26:708–715. doi: 10.1523/JNEUROSCI.4409-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brose N, Gasic GP, Vetter DE, Sullivan JM, Heinemann SF. Protein chemical characterization and immunocytochemical localization of the NMDA receptor subunit NMDA R1. J Biol Chem. 1993;268:22663–22671. [PubMed] [Google Scholar]
- Burzomato V, Beato M, Groot-Kormelink PJ, Colquhoun D, Sivilotti LG. Single-channel behavior of heteromeric α1β glycine receptors: an attempt to detect a conformational change before the channel opens. J Neurosci. 2004;24:10924–10940. doi: 10.1523/JNEUROSCI.3424-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casado M, Lopez-Guajardo A, Mellstrom B, Naranjo JR, Lerma J. Functional N-methyl-D-aspartate receptors in clonal rat phaeochromocytoma cells. J Physiol. 1996;490:391–404. doi: 10.1113/jphysiol.1996.sp021153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathala L, Misra C, Cull-Candy S. Developmental profile of the changing properties of NMDA receptors at cerebellar mossy fiber-granule cell synapses. J Neurosci. 2000;20:5899–5905. doi: 10.1523/JNEUROSCI.20-16-05899.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BS, Braud S, Badger JD, 2nd, Isaac JT, Roche KW. Regulation of NR1/NR2C N-methyl-D-aspartate (NMDA) receptors by phosphorylation. J Biol Chem. 2006;281:16583–16590. doi: 10.1074/jbc.M513029200. [DOI] [PubMed] [Google Scholar]
- Chen C, Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987;7:2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke RJ, Johnson JW. NMDA receptor NR2 subunit dependence of the slow component of magnesium unblock. J Neurosci. 2006;26:5825–5834. doi: 10.1523/JNEUROSCI.0577-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D, Hawkes AG. Stochastic properties of ion channel openings and bursts in a membrane patch that contains two channels: evidence concerning the number of channels present when a record containing only single openings is observed. Proc R Soc Lond B Biol Sci. 1990;240:453–477. doi: 10.1098/rspb.1990.0048. [DOI] [PubMed] [Google Scholar]
- Colquhoun D Sigworth. Fitting and statistical analysis of single-channel records. In: Sakmann B, Neher E, editors. Single-Channel Recording. New York: Plenum Press; 1995. [Google Scholar]
- Cull-Candy SG, Usowicz MM. On the multiple-conductance single channels activated by excitatory amino acids in large cerebellar neurones of the rat. J Physiol. 1989;415:555–582. doi: 10.1113/jphysiol.1989.sp017736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daggett LP, Johnson EC, Varney MA, Lin FF, Hess SD, Deal CR, Jachec C, Lu CC, Kerner JA, Landwehrmeyer GB, Standaert DG, Young AB, Harpold MM, Velicelebi G. The human N-methyl-D-aspartate receptor 2C subunit: genomic analysis, distribution in human brain, and functional expression. J Neurochem. 1998;71:1953–1968. doi: 10.1046/j.1471-4159.1998.71051953.x. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- Dravid SM, Erreger K, Yuan H, Nicholson K, Le P, Lyuboslavsky P, Almonte A, Murray E, Mosely C, Barber J, French A, Balster R, Murray TF, Traynelis SF. Subunit-specific mechanisms and proton sensitivity of NMDA receptor channel block. J Physiol. 2007;581:107–128. doi: 10.1113/jphysiol.2006.124958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebralidze AK, Rossi DJ, Tonegawa S, Slater NT. Modification of NMDA receptor channels and synaptic transmission by targeted disruption of the NR2C gene. J Neurosci. 1996;16:5014–5025. doi: 10.1523/JNEUROSCI.16-16-05014.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erreger K, Chen PE, Wyllie DJ, Traynelis SF. Glutamate receptor gating. Crit Rev Neurobiol. 2004;16:187–224. doi: 10.1615/critrevneurobiol.v16.i3.10. [DOI] [PubMed] [Google Scholar]
- Erreger K, Dravid SM, Banke TG, Wyllie DJ, Traynelis SF. Subunit-specific gating controls rat NR1/NR2A and NR1/NR2B NMDA channel kinetics and synaptic signalling profiles. J Physiol. 2005a;563:345–358. doi: 10.1113/jphysiol.2004.080028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erreger K, Geballe MT, Dravid SM, Snyder JP, Wyllie DJ, Traynelis SF. Mechanism of partial agonism at NMDA receptors for a conformationally restricted glutamate analog. J Neurosci. 2005b;25:7858–7866. doi: 10.1523/JNEUROSCI.1613-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erreger K, Geballe MT, Kristensen A, Chen PE, Hansen KB, Lee CJ, Yuan H, Le P, Lyuboslavsky PN, Micale N, Jorgensen L, Clausen RP, Wyllie DJ, Snyder JP, Traynelis SF. Subunit-specific agonist activity at NR2A-, NR2B-, NR2C-, and NR2D-containing N-methyl-D-aspartate glutamate receptors. Mol Pharmacol. 2007;72:907–920. doi: 10.1124/mol.107.037333. [DOI] [PubMed] [Google Scholar]
- Erreger K, Traynelis SF. Zinc inhibition of rat NR1/NR2A N-methyl-D-aspartate receptors. J Physiol. 2008;586:763–778. doi: 10.1113/jphysiol.2007.143941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrant M, Feldmeyer D, Takahashi T, Cull-Candy SG. NMDA-receptor channel diversity in the developing cerebellum. Nature. 1994;368:335–339. doi: 10.1038/368335a0. [DOI] [PubMed] [Google Scholar]
- Gibb AJ, Colquhoun D. Glutamate activation of a single NMDA receptor-channel produces a cluster of channel openings. Proc Biol Sci. 1991;243:39–45. doi: 10.1098/rspb.1991.0007. [DOI] [PubMed] [Google Scholar]
- Hatton CJ, Shelley C, Brydson M, Beeson D, Colquhoun D. Properties of the human muscle nicotinic receptor, and of the slow–channel myasthenic syndrome mutant eL221F, inferred from maximum likelihood fits. J Physiol. 2003;547:729–760. doi: 10.1113/jphysiol.2002.034173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii T, Moriyoshi K, Sugihara H, Sakurada K, Kadotani H, Yokoi M, Akazawa C, Shigemoto R, Mizuno N, Masu M, et al. Molecular characterization of the family of the N-methyl-D-aspartate receptor subunits. J Biol Chem. 1993;268:2836–2843. [PubMed] [Google Scholar]
- Jackson MB, Wong BS, Morris CE, Lecar H, Christian CN. Successive openings of the same acetylcholine receptor channel are correlated in open time. Biophys J. 1983;42:109–114. doi: 10.1016/S0006-3495(83)84375-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MV, Westbrook GL. Desensitized states prolong GABAA channel responses to brief agonist pulses. Neuron. 1995;15:181–191. doi: 10.1016/0896-6273(95)90075-6. [DOI] [PubMed] [Google Scholar]
- Kadotani H, Hirano T, Masugi M, Nakamura K, Nakao K, Katsuki M, Nakanishi S. Motor discoordination results from combined gene disruption of the NMDA receptor NR2A and NR2C subunits, but not from single disruption of the NR2A or NR2C subunit. J Neurosci. 1996;16:7859–7867. doi: 10.1523/JNEUROSCI.16-24-07859.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karadottir R, Cavelier P, Bergersen LH, Attwell D. NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature. 2005;438:1162–1166. doi: 10.1038/nature04302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karavanova I, Vasudevan K, Cheng J, Buonanno A. Novel regional and developmental NMDA receptor expression patterns uncovered in NR2C subunit-β-galactosidase knock-in mice. Mol Cell Neurosci. 2007;34:468–480. doi: 10.1016/j.mcn.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohr G, Eckardt S, Luddens H, Monyer H, Seeburg PH. NMDA receptor channels: subunit-specific potentiation by reducing agents. Neuron. 1994;12:1031–1040. doi: 10.1016/0896-6273(94)90311-5. [DOI] [PubMed] [Google Scholar]
- Krupp JJ, Vissel B, Heinemann SF, Westbrook GL. Calcium-dependent inactivation of recombinant N-methyl-D-aspartate receptors is NR2 subunit specific. Mol Pharmacol. 1996;50:1680–1688. [PubMed] [Google Scholar]
- Laurie DJ, Seeburg PH. Regional and developmental heterogeneity in splicing of the rat brain NMDAR1 mRNA. J Neurosci. 1994;14:3180–3194. doi: 10.1523/JNEUROSCI.14-05-03180.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Fu Z, Karavanov I, Yasuda RP, Wolfe BB, Buonanno A, Vicini S. NMDA receptor subtypes at autaptic synapses of cerebellar granule neurons. J Neurophysiol. 2006;96:2282–2294. doi: 10.1152/jn.00078.2006. [DOI] [PubMed] [Google Scholar]
- Micu I, Jiang Q, Coderre E, Ridsdale A, Zhang L, Woulfe J, Yin X, Trapp BD, McRory JE, Rehak R, Zamponi GW, Wang W, Stys PK. NMDA receptors mediate calcium accumulation in myelin during chemical ischaemia. Nature. 2006;439:988–992. doi: 10.1038/nature04474. [DOI] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Monyer H, Sprengel R, Schoepfer R, Herb A, Higuchi M, Lomeli H, Burnashev N, Sakmann B, Seeburg PH. Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science. 1992;256:1217–1221. doi: 10.1126/science.256.5060.1217. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Wang YX, Wenthold RJ. The NMDA receptor subunits NR2A and NR2B show histological and ultrastructural localization patterns similar to those of NR1. J Neurosci. 1994;14:6102–6120. doi: 10.1523/JNEUROSCI.14-10-06102.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu G, Auerbach A. Modal gating of NMDA receptors and the shape of their synaptic response. Nat Neurosci. 2003;6:476–483. doi: 10.1038/nn1044. [DOI] [PubMed] [Google Scholar]
- Popescu G, Robert A, Howe JR, Auerbach A. Reaction mechanism determines NMDA receptor response to repetitive stimulation. Nature. 2004;430:790–793. doi: 10.1038/nature02775. [DOI] [PubMed] [Google Scholar]
- Qin F. Restoration of single-channel currents using the segmental k-means method based on hidden Markov modeling. Biophys J. 2004;86:1488–1501. doi: 10.1016/S0006-3495(04)74217-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin F, Auerbach A, Sachs F. Estimating single-channel kinetic parameters from idealized patch-clamp data containing missed events. Biophys J. 1996;70:264–280. doi: 10.1016/S0006-3495(96)79568-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumbaugh G, Vicini S. Distinct synaptic and extrasynaptic NMDA receptors in developing cerebellar granule neurons. J Neurosci. 1999;19:10603–10610. doi: 10.1523/JNEUROSCI.19-24-10603.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter MG, Fern R. NMDA receptors are expressed in developing oligodendrocyte processes and mediate injury. Nature. 2005;438:1167–1171. doi: 10.1038/nature04301. [DOI] [PubMed] [Google Scholar]
- Sather W, Dieudonne S, MacDonald JF, Ascher P. Activation and desensitization of N-methyl-D-aspartate receptors in nucleated outside-out patches from mouse neurones. J Physiol. 1992;450:643–672. doi: 10.1113/jphysiol.1992.sp019148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sather W, Johnson JW, Henderson G, Ascher P. Glycine-insensitive desensitization of NMDA responses in cultured mouse embryonic neurons. Neuron. 1990;4:725–731. doi: 10.1016/0896-6273(90)90198-o. [DOI] [PubMed] [Google Scholar]
- Schorge S, Elenes S, Colquhoun D. Maximum likelihood fitting of single channel NMDA activity with a mechanism composed of independent dimers of subunits. J Physiol. 2005;569:395–418. doi: 10.1113/jphysiol.2005.095349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern P, Behe P, Schoepfer R, Colquhoun D. Single-channel conductances of NMDA receptors expressed from cloned cDNAs: comparison with native receptors. Proc Biol Sci. 1992;250:271–277. doi: 10.1098/rspb.1992.0159. [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Cull-Candy SG. Pharmacological properties and H+ sensitivity of excitatory amino acid receptor channels in rat cerebellar granule neurones. J Physiol. 1991;433:727–763. doi: 10.1113/jphysiol.1991.sp018453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicini S, Wang JF, Li JH, Zhu WJ, Wang YH, Luo JH, Wolfe BB, Grayson DR. Functional and pharmacological differences between recombinant N-methyl-D-aspartate receptors. J Neurophysiol. 1998;79:555–566. doi: 10.1152/jn.1998.79.2.555. [DOI] [PubMed] [Google Scholar]
- Villarroel A, Regalado MP, Lerma J. Glycine-independent NMDA receptor desensitization: localization of structural determinants. Neuron. 1998;20:329–339. doi: 10.1016/s0896-6273(00)80460-2. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Inoue Y, Sakimura K, Mishina M. Developmental changes in distribution of NMDA receptor channel subunit mRNAs. Neuroreport. 1992;3:1138–1140. doi: 10.1097/00001756-199212000-00027. [DOI] [PubMed] [Google Scholar]
- Wenzel A, Fritschy JM, Mohler H, Benke D. NMDA receptor heterogeneity during postnatal development of the rat brain: differential expression of the NR2A, NR2B, and NR2C subunit proteins. J Neurochem. 1997;68:469–478. doi: 10.1046/j.1471-4159.1997.68020469.x. [DOI] [PubMed] [Google Scholar]
- Wyllie DJ, Behe P, Colquhoun D. Single-channel activations and concentration jumps: comparison of recombinant NR1a/NR2A and NR1a/NR2D NMDA receptors. J Physiol. 1998;510:1–18. doi: 10.1111/j.1469-7793.1998.001bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyllie DJ, Johnston AR, Lipscombe D, Chen PE. Single-channel analysis of a point mutation of a conserved serine residue in the S2 ligand-binding domain of the NR2A NMDA receptor subunit. J Physiol. 2006;574:477–489. doi: 10.1113/jphysiol.2006.112193. [DOI] [PMC free article] [PubMed] [Google Scholar]