Abstract
Experimental reduction in placental growth often leads to increased placental efficiency measured as grams of fetus produced per gram of placenta, although little is known about the mechanisms involved. This study tested the hypothesis that the smallest placenta within a litter is the most efficient at supporting fetal growth by examining the natural intra-litter variation in placental nutrient transfer capacity in normal pregnant mice. The morphology, nutrient transfer and expression of key growth and nutrient supply genes (Igf2P0, Grb10, Slc2a1, Slc2a3, Slc38a1, Slc38a2 and Slc38a4) were compared in the lightest and heaviest placentas of a litter at days 16 and 19 of pregnancy, when mouse fetuses are growing most rapidly in absolute terms. The data show that there are morphological and functional adaptations in the lightest placenta within a litter, which increase active transport of amino acids per gram of placenta and maintain normal fetal growth close to term, despite the reduced placental mass. The specific placental adaptations differ with age. At E16, they are primarily morphological with an increase in the volume fraction of the labyrinthine zone responsible for nutrient exchange, whereas at E19 they are more functional with up-regulated placental expression of the glucose transporter gene, Slc2a1/GLUT1 and one isoform the System A family of amino acid transporters, Slc38a2/SNAT2. Thus, this adaptability in placental phenotype provides a functional reserve capacity for maximizing fetal growth during late gestation when placental growth is compromised.
Size at birth is critical in determining life expectancy. It affects not only neonatal viability but also adult rates of mortality and morbidity (Barker, 1994). Human epidemiological observations show that the smaller the neonate the less likely it is to survive at birth and the greater its risk of developing adult-onset degenerative diseases, such as hypertension, coronary heart disease and type 2 diabetes (Fowden et al. 2005; McMillen & Robinson, 2005; Hanson & Gluckman, 2008). In mammals, the major determinant of intrauterine growth is the placental supply of nutrients to the fetus (Harding & Johnston, 1995), which occurs primarily by diffusion and transporter-mediated transport (Sibley et al. 1997). In turn, these processes depend upon the size, morphology, blood flow and transporter abundance of the placenta (Fowden et al. 2006b). Therefore, fetal body weight in late gestation correlates positively with placental weight in many species, both during normal conditions and when placental weight is reduced experimentally either by direct placental manipulations or by indirect alterations of environmental conditions during development (Waldor et al. 1957; McLaren, 1965; Molteni et al. 1978; Allen et al. 2002). Most techniques aimed at reducing placental weight in experimental animals are associated with postnatal abnormalities in physiological function consistent with the human epidemiological data linking impaired intrauterine growth with an increased disease risk in later life (Fowden et al. 2008).
When placental growth is compromised experimentally, often more fetus is produced per gram of placenta than in normal circumstances (Fowden et al. 2006b). In pregnant sheep and rats, placental efficiency, measured as gram fetus per gram placenta, is increased in late gestation when fetal and placental weight are reduced by maternal heat stress, glucocorticoid administration, under- and overnutrition and by restriction of placentation or uterine blood flow (Owens et al. 1987; Ross et al. 1996; Wallace et al. 2003; Ain et al. 2005; Jansson et al. 2006). Similar increases in placental efficiency are also seen in pregnant mice when placental growth is restricted genetically by deletion of the placental-specific P0 transcript of the Igf2 gene (Constancia et al. 2002). In some of these studies, the increased placental efficiency is associated with enhanced transplacental transfer of nutrients per gram of placenta (Owens et al. 1987; Ross et al. 1996; Constancia et al. 2005). For example, in the small Igf2P0 mutant placenta, there is increased expression of amino acid transporters and enhanced amino acid transfer compared with the wild-type placenta (Constancia et al. 2005). Conversely, large placentas appear to be less efficient, irrespective of whether overgrowth is produced genetically or by environmental manipulations early in development (Kelly, 1992; Angiolini et al. 2006). Taken together, these studies suggest that the placenta can adapt its nutrient transfer capacity to help meet the fetal nutrient demands for growth, even when its own growth is compromised. However, little is known about the effects of natural variations in placental size on the placental capacity to supply nutrients to the fetus during normal conditions.
This study was designed to investigate how differences in placental size relate to the efficiency of the placenta to supply nutrients for the growing fetus. The intra-litter variation in placental nutrient transfer capacity in normal pregnant mice was examined by comparing the placentas of highest and lowest weight within a litter. The morphology and expression of key growth and nutrient supply genes (Igf2P0, Grb10, Slc2a1, Slc2a3, Slc38a1, Slc38a2 and Slc38a4) were determined in the lightest and heaviest placenta of a litter at days 16 and 19 of pregnancy when mouse fetuses are normally growing most rapidly in absolute terms (McLaren, 1965; Constancia et al. 2002).
Methods
Mice
Seventy-two virgin 6- to 8-week-old C57BL/6J female mice were used. They were housed under 12 h dark : 12 h light conditions and had free access to water and food (Special Dietary Services Rat and Mouse breeding diet no. 3, Dietex International). They were mated overnight with C57BL/6J males on selected days to produce pregnancies for study at specific gestational ages. The presence of a copulatory plug was designated as embryonic day (E) 1. All procedures were carried out in accordance with the UK Home Office regulations under the Animal (Scientific Procedures) Act 1986.
Experimental procedure
Unidirectional materno-fetal transfer of non-metabolizable radioactive tracers were measured in the pregnant mice at E16 (n = 31 litters) and E19 (n = 28 litters). Briefly, mice were anaesthetized with an intraperitoneal injection of 10 μl g−1 of fentanyl fluanisone and midazolam solutions in distilled water (1 : 1 : 2 water, Janseen Animal Health). After exposure of the maternal jugular vein, 1.3 × 104 Bq of one of the following tracers was injected intravenously in 100 μl of saline (0.9% w/v); [14C]inulin (Amersham CFA399; specfic activity 2.0 GBq mmol−1), [14C]methyl-d-glucose (NEN NEC-377; specific activity 2.1 GBq mmol−1) or [14C]methyl amino-isobutyrate (MeAIB) (NEN NEC-671; specific activity 1868.5 MBq mmol−1). These tracers were chosen to provide a measure of transplacental transport by simple diffusion, facilitated diffusion and active transport, respectively. At 2 min after tracer injection, a maternal blood sample was taken and the animal was killed by cervical dislocation. Conceptuses were dissected out and killed by decapitation. Whole fetuses were minced and lysed overnight at 55°C in 2 ml (E16) or 4 ml (E19) of Biosol (National Diagnostics). Aliquots of the fetal lysate were counted in a β counter (Packard Tri-Carb 1900). Fetally accumulated radioactivity was used to calculate the amount of tracer transferred per gram of placenta (d.p.m. (g placenta)−1) or per gram of fetus (d.p.m. (g fetus)−1) for each feto-placental unit in the litter.
Stereological analysis
The heaviest and lightest placentas in a litter irrespective of actual weight (E16, 5 litters; E19, 8 litters) were bisected and each half reweighed. One half was fixed in 4% paraformaldehyde, the other half was fixed in 4% glutaraldehyde in Pipes buffer. Following fixation, the paraformadehyde-fixed half was dehydrated, embedded in paraffin wax and sectioned completely at 7 μm. The corresponding glutaraldehyde-fixed half was dehydrated and embedded in Spurr epoxy resin (Taab, Aldermaston, UK) and a 1 μm vertical section cut close to the placental midline. The Computer Assisted Stereological Toolbox (CAST v2.0) was employed to superimpose grids and generate random fields of view within systematic random paraffin sections or resin sections. Placenta volumes densities were measured using a point grid and the following equation:
used to convert volume densities into absolute values, where V(obj) is the estimated placental volume, t is the total thickness of the placenta, a(p) is the area associated with each point and ΣP is the mean number of points per section. The estimate was adjusted for shrinkage using a shrinkage factor derived from comparing the mean diameter of 100 fresh and fixed maternal erythrocytes (Coan et al. 2004). The volumes of placental compartments were determined by point counting and then converting the volume densities into absolute values by using the previously determined placental volumes (Coan et al. 2004). The volume fractions of these compartments are the percentage of the whole placenta occupied by the labyrinthine zone, junctional zone and decidua.
Detailed structural analysis of the labyrinthine zone was performed using the resin sections. To determine labyrinthine zone component volume densities, a point grid was superimposed onto random fields of view and the total number of points hitting each component (fetal capillaries, maternal blood spaces, labyrinthine trophoblast) was counted. The densities were referred back to the absolute volume of the labyrinthine zone to obtain absolute component volumes. The component volume densities were converted to volume fractions in order to find the percentage of the labyrinthine zone occupied by each component.
Surface densities were determined by superimposing a cycloid arc grid on random fields of view within the labyrinthine zone and recording the number of points of intersection between cycloid arcs and the maternal and fetal surfaces of the interhaemal membrane. These were converted to absolute surface areas using the following equation:
where ΣI(struct) is the total number of intersections of the cycloid arcs with the structure, ΣP(ref) total number of points that hit the reference space, I(p) the length of the test line associated with each point in the grid, and V(ref) the volume of labyrinthine zone (Baddeley et al. 1986).
The harmonic mean interhaemal membrane thickness was determined by measuring the shortest distance across the membrane at random starting locations identified by superimposing a line grid on random fields of view within the labyrinthine zone. Per labyrinthine zone, 150 measurements were made and the harmonic mean thickness calculated from the reciprocal of the mean of the reciprocal distances as previously described (Coan et al. 2004). The theoretical diffusion capacity (TDC) of the interhaemal membrane was calculated using the following equation:
where K is a constant; in this study, Krogh's constant for oxygen diffusion was used.
Gene expression analysis
At both gestational ages, Northern blotting was used to analyse expression levels of (i) two labyrinthine-specific, growth regulatory genes, the growth inhibitory Grb10 and the growth stimulatory P0 transcript of Igf2 gene, and (ii) several nutrient transporter genes including the two main placental glucose transporters, Slc2a1/GLUT1 and Slc2a3/GLUT3, and the three known isoforms of the System A family of amino acid transporters, Slc38a1, Slc38a2 and Slc38a4, responsible for transplacental transport of neutral amino acids and MeAIB (Sibley et al. 1997; Constancia et al. 2002; Charalambous et al. 2003) in six litters per gestational age. RNAs were prepared from whole placentas using RNeasy kits (Qiagen). Northern blots were hybridized with RNA probes produced by in vitro transcription from linearized plasmids of cloned PCR products as previously described (Constancia et al. 2002). The Igf2P0 probe was from 1368 to 1954 bases in NM_010345; the Grb10 probe was from 10 164 to 10 849 bases in U71085; the Slc38a1 probe was from 63 to 121 bases in ENSMUST00000023937; the Slc38a2 probe was from 108 to 633 bases in NM_175121; the Slc38a4 probe that detects all transcripts was from bases 200–900 in NM_027052; the Slc2a1 probe was from 354 to 934 bases in NM_011400 and the Slc2a3 probe was from 776 to 1177 bases in NM_011401. The hybridized blots were visualized by phosphorimaging (Fuji) and densitometry was performed using the Aida Image Data Analyser v.3.27 Software.
Statistical analyses
Values for all data are expressed as mean ± s.e.m. The relationship between fetal and placental weight at both gestational ages was determined by Pearson's bivariate correlation. The effect of litter size on the relationship between fetal and placental weight was assessed by partial correlation analysis. For radioactive counts per gram placenta, the differences between the lightest and heaviest placentas were assessed by analysing whether the paired difference between counts was significantly different from zero. For stereological analysis, differences in absolute values between the lightest and heaviest placenta in a litter were assessed by paired t test while differences with age were assessed by unpaired t test. Compartment and component volume fractions (%) were arc sine transformed in order to perform a paired t test between the lightest and heaviest or an unpaired t test between gestational ages. Gene expression levels of lightest and heaviest placentas within a litter were normalized to their respective glyceraldehyde 3-phosphate dehydrogenase (Gapdh) levels and assessed for statistical significance by paired t test.
Results
Fetal and placental wet masses
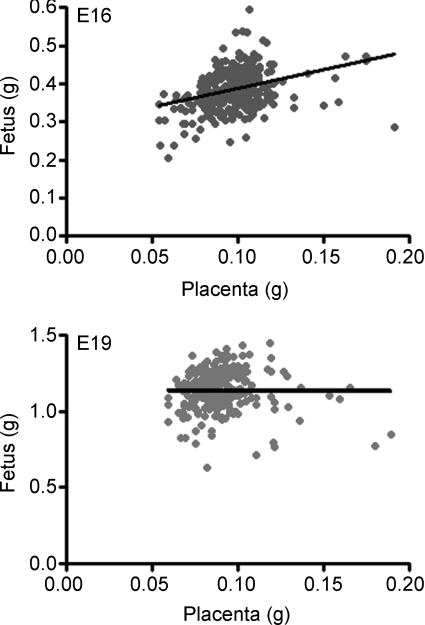
The lightest placenta was 65–75% of the weight of heaviest placenta within a litter at both gestational ages (Table 1). At E16, the fetuses with the lightest placenta weighed less than the fetuses with the heaviest placenta. However, at E19 fetal weight was similar in the two groups (Table 1). There was therefore a significant positive correlation between fetal and placental weight at E16 but not at E19 (Fig. 1). However, in part, the association between fetal and placental weight may have been due to litter size as both these weights were inversely correlated with litter size at E16 (fetal weight, r =−0.221; placental weight, r = −0.281, n = 283 pups from 36 litters, P < 0.01, both cases). Partial correlation analyses of the three variables showed that, at E16, both placental weight and litter size were significant influences on fetal weight but that placental weight had the significant effect (placental weight, r = 0.360, n = 283 pups, P < 0.001; litter size, r =−0.184, n = 283 pups, P < 0.02). At E19, neither placental weight nor litter size were significant influences on fetal weight using partial correlation analyses (P > 0.05, both cases). Placental efficiency in the lightest placenta, measured as the feto-placental weight ratio, was 30–45% greater than in the heaviest placenta at both gestational ages (Table 1). The lightest placenta therefore supported more grams of fetus per gram of placenta than the heaviest placenta within a litter. The lightest and heaviest placentas in a litter were randomly positioned within the uterine horn at both ages. Litter size ranged from 4 to 11 pups at both ages and did not differ in mean value with age (Mean litter size: E16, 7.92 ± 0.29; E19, 7.37 ± 0.31, P > 0.05).
Table 1.
Placental and fetal weights and fetal to placental weight ratio of the heaviest and lightest placentas in a litter at days 16 and 19 of pregnancy
| E16 (36 litters) | E19 (36 litters) | |||
|---|---|---|---|---|
| H | L | H | L | |
| Placenta (g) | 114 ± 2 | 80 ± 2* | 107 ± 4 | 78 ± 2* |
| Fetus (g) | 405 ± 7 | 356 ± 8* | 1149 ± 28 | 1105 ± 26 |
| Feto-placental weight ratio |
3.59 ± 0.1 |
4.54 ± 0.1* |
11.05 ± 0.4 |
14.51 ± 0.4* |
Mean (± s.e.m.). Abbreviations: H, heaviest placenta; L, lightest placenta. Analysis was performed on 36 H and 36 L placentas and their respective fetuses from 36 litters at E16 and E19.
Significant differences between the heaviest and lightest placenta in the same litter P < 0.0001 (paired t test).
Figure 1.
The relationship between individual placental and fetal weights at E16 (y = 0.98x + 0.29, r = 0.331, n = 283 fetuses from 36 litters, P < 0.0001) and E19 (y = −0.07x + 1.15, r =−0.009, n = 260 fetuses from 36 litters, P > 0.05).
Placental transport
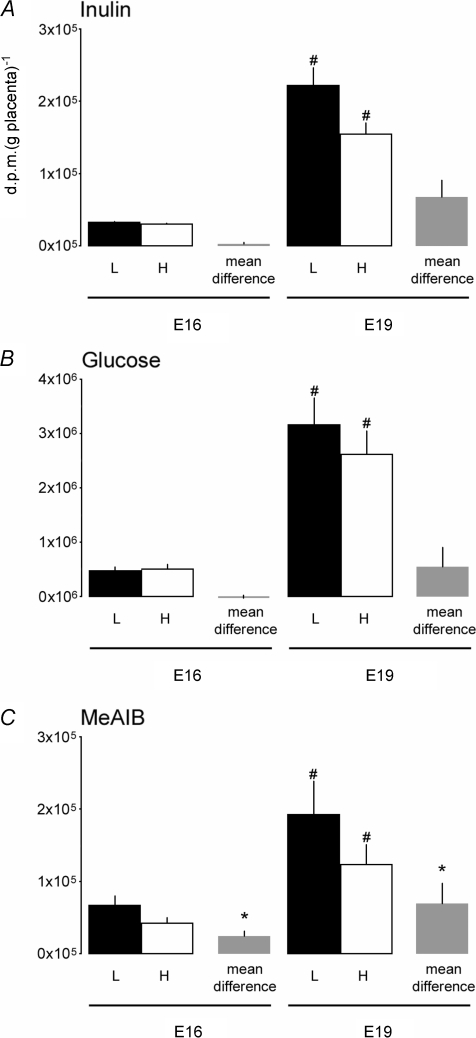
Measurements of transplacental transfer of the three tracers showed there were differences in the passive and active mechanisms of transplacental transport with placental weight within a litter. Unidirectional materno-fetal transfer of [14C]inulin and [14C]glucose per gram placenta were not significantly different between the lightest and heaviest placenta in the litter at either gestational age (Fig. 2A and B). In contrast, active transport of [14C]MeAIB was significantly greater per gram placenta in the lightest than heaviest placenta in the litter at both gestational ages (Fig. 2C). No significant differences in the transfer of any of the tracers were observed per gram of fetus at either E16 or E19 (data not shown). Transfer of all three tracers was greater at E19 than E16 (Fig. 2).
Figure 2.
Mean (± s.e.m.) transfer and mean difference in transfer in d.p.m. (g placenta)−1 of [14C]inulin (A), [14C]methyl-d-glucose (B) and [14C]methyl aminoisobutyric acid (MeAIB) (C) across the lightest and heaviest placentas within a litter. H, heaviest placenta; L, lightest placenta. E16: inulin, n = 7 H and 7 L placentas from 7 litters; glucose, n = 9 H and 9 L placentas from 9 litters; MeAIB, n = 15 H and 15 L placentas from 15 litters. E19: inulin, n = 6 H and 6 L placentas from 6 litters; glucose, n = 10 H and 10 L placentas from 10 litters; MeAIB, n = 12 H and 12 L placentas from 12 litters. *Significantly different from zero (P < 0.05, significance of a single mean). #Significantly different from the corresponding weight category values at E16 (P < 0.001, t test).
Placental structure
Morphological differences between lightest and heaviest placenta
Morphological differences between the lightest and heaviest placenta within a litter were assessed by stereological analysis of their three-dimensional structure. The absolute volume of the lightest and heaviest placenta in a litter corresponded to their weights and was significantly less in the lightest than heaviest placenta at both gestational ages (Tables 1 and 2). At E16, this difference in total volume was accompanied by significant reductions in the absolute volume of the junctional zone but not in the absolute volume of the labyrinthine zone of the lightest placenta compared to the heaviest. Consequently, when expressed as volume fractions of the whole placenta, the junctional zone occupied less and the labyrinthine zone more, of the total volume in the lightest compared to the heaviest placenta (Table 2). Within the labyrinthine zone of the lightest placenta, there was a significantly reduced fetal capillary volume in the lightest compared to heaviest placenta (Table 2). However, there were no differences in the absolute volume of the trophoblast or of the maternal blood spaces, or in the volume fractions of all three labyrinthine components, compared to the heaviest placenta in the litter at this age (Table 2). At E19, the smaller total volume of the lightest placenta was associated with reductions in the absolute volume of the labyrinthine zone and of the trophoblast, fetal capillaries and maternal blood spaces within this zone (Table 2). However, the absolute volume of the junctional zone did not differ significantly between the lightest and heaviest placentas in a litter, nor did the volume fractions of this compartment (Table 2). The theoretical capacity for simple diffusion, calculated from the surface area and thickness of the labyrinthine interhaemal membrane, was similar in the lightest and heaviest placentas at both E16 and E19 (Table 2). However, when surface area was expressed per gram of placenta, the lightest placenta had significantly greater trophoblast surface area per gram than the heaviest placenta at E16, but not at E19 (Table 2). The maternal compartment of the placenta, decidua basalis, was significantly reduced in volume at E16 but not at E19 in the lightest compared to the heaviest placenta (Table 2).
Table 2.
Morphological comparisons of the heaviest and lightest placentas in a litter at days 16 and 19 of pregnancy
| E16 (5 litters) | E19 (8 litters) | |||
|---|---|---|---|---|
| H | L | H | L | |
| Volume of placental compartments (mm3) | ||||
| Placenta | 116.0 ± 2.6 | 80.4 ± 2.5* | 103.7 ± 5.7 | 76.9 ± 5.8* |
| Lz | 50.0 ± 2.5 | 44.9 ± 0.9 | 58.9 ± 1.8† | 45.3 ± 3.0* |
| Jz | 42.3 ± 0.9 | 24.8 ± 0.9* | 30.5 ± 4.4 | 21.1 ± 2.2 |
| Db | 23.7 ± 3.1 | 10.8 ± 1.7* | 14.3 ± 1.4 | 10.4 ± 1.4 |
| Volume of labyrinthine zone components (mm3) | ||||
| FC | 9.7 ± 1.2 | 5.5 ± 0.5* | 12.2 ± 0.9 | 8.5 ± 1.6* |
| MBS | 10.7 ± 0.7 | 10.0 ± 0.8 | 12.9 ± 0.9 | 11.0 ± 1.1* |
| LT | 29.7 ± 2.7 | 29.2 ± 0.6 | 33.0 ± 2.3 | 25.0 ± 2.0* |
| Placental compartment volume fraction (%) | ||||
| Lz | 41.1 ± 1.3 | 48.4 ± 0.7* | 49.4 ± 1.6† | 50.4 ± 0.4† |
| Jz | 37.2 ± 0.6 | 33.7 ± 0.7* | 32.2 ± 1.6† | 31.4 ± 1.1 |
| Db | 26.6 ± 1.8 | 21.1 ± 1.8 | 21.6 ± 0.9 | 21.1 ± 1.5 |
| Labyrinthine zone component volume fraction (%) | ||||
| LT | 50.2 ± 1.7 | 53.9 ± 0.9 | 48.8 ± 1.6 | 48.7 ± 1.4† |
| MBS | 27.5 ± 0.9 | 28.2 ± 1.0 | 28.0 ± 0.9 | 29.9 ± 1.0 |
| FC | 26.1 ± 2.0 | 20.5 ± 1.0 | 27.4 ± 1.4 | 25.2 ± 2.2 |
| Surface area of the trophoblast membrane (cm2) | ||||
| Fetal side | 13.3 ± 1.4 | 8.9 ± 1.2* | 22.1 ± 2.2† | 16.2 ± 2.4*† |
| Maternal side | 13.8 ± 1.7 | 17.3 ± 1.8 | 31.6 ± 3.2† | 26.4 ± 3.4† |
| Mean | 13.6 ± 0.5 | 13.1 ± 0.9 | 23.5 ± 4.0† | 18.6 ± 3.4† |
| Harmonic mean thickness (μm) | 3.78 ± 0.2 | 3.95 ± 0.3 | 2.95 ± 0.1† | 3.05 ± 0.1† |
| Theoretical diffusion capacity (mm2 min−1 kPa−1) | 6.3 ± 0.4 | 5.8 ± 0.6 | 17.2 ± 1.4† | 16.5 ± 1.1† |
| Mean surface area/gram placenta (cm2 g−1) | 124 ± 4 | 170 ± 10* | 257 ± 18† | 289 ± 22† |
Mean (± s.e.m.). Abbreviations: Db, decidua basalis; FC, fetal capillaries; H, heaviest placenta; Jz, junctional zone; L, lightest placenta; LT, labyrinthine trophoblast; Lz, labyrinthine zone; MBS, maternal blood spaces. Analysis was performed on 5 H and 5 L placentas from 5 litters at E16 and from 8 H and 8 L placentas from 8 litters at E19.
Significant differences between H and L placentas at the same gestational age (P < 0.05, paired t test).
Significant difference between E16 and E19 within the same weight group (P < 0.05, unpaired t test).
Gestational differences in placental morphology
Between E16 and E19, the absolute volume of the labyrinthine zone increased significantly in the heaviest but not the lightest placenta in a litter (Table 2). This led to an increase in the volume fraction of the labyrinthine zone and a reduction in the volume fraction of the junctional zones with increasing gestational age in the heaviest placentas alone (Table 2). Within the labyrinthine zone, there was a significant decrease in volume fraction occupied by trophoblast in the lightest but not the heaviest placentas between E16 and E19. However, no changes in volume fractions of the maternal blood spaces or fetal capillaries were found with increasing age in either group of placentas. In contrast, there were significant decreases in the thickness of the interhaemal membrane and significant increases in its surface area on both the fetal and maternal sides with increasing gestational age in both the lightest and heaviest placentas, which resulted in similar ontogenic increases in the weight-specific exchange surface and theoretical diffusion capacity of the placenta in the two weight groups (Table 2). The decidua basalis of the heaviest placenta was significantly reduced between E16 and E19, whereas no change was found in the lightest placenta with gestation (Table 2).
Placental expression of growth and transport regulatory genes
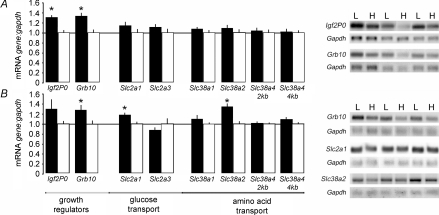
At E16, expression of both the Grb10 gene and P0 transcript of the Igf2 gene was significantly up-regulated in the lightest compared to the heaviest placenta (Fig. 3A). Expression of these two labyrinthine-specific genes was increased by 25–35% in line with the 15–20% increase in the volume fraction of the labyrinthine zone in the lightest placentas (Table 2). At E19, expression of Grb10, but not the Igf2P0 transcript, remained significantly elevated in the lightest compared to the heaviest placentas in the litter (Fig. 3B).
Figure 3.
Expression analysis of growth regulatory genes (Igf2P0 and Grb10) and of glucose and System A amino acid transporter genes (Slc2a1, Slc2a3, Slc38a1, Slc38a2 and Slc38a4) by Northern blotting in the lightest (filled columns) and heaviest (open columns) placenta within a litter at E16 (A; n = 7 litters) and E19 (B; n = 6 litters). Graphs of mean expression levels (± s.e.m.) are shown with the levels in the heaviest placenta in a litter normalized to 1 together with representative Northern blots of total RNA for each gene compared to Gapdh loading for 3 litters at each age. *Significantly different from the value in the heaviest placenta (P < 0.01, paired t test).
Expression of the glucose transporter genes, Slc2a1/GLUT1 and Slc2a3/GLUT3, and the three isoforms of the System A family of amino acid transporters, Slc38a1, Slc38a2 and Slc38a4, was not significantly different between the lightest and heaviest placenta in a litter at E16 (Fig. 3A). At E19, expression of Slc2a1/GLUT1 and the Slc38a2 isoform of the System A amino acid transporter was up-regulated by 20–40% in the lightest compared to the heaviest placentas in the litter (Fig. 3B). None of the other transporter genes analysed differed in expression with placental weight at this age (Fig. 3B).
Discussion
The data demonstrate that the morphological and functional characteristics of the normal mouse placenta differ with natural variations in placental size. The lightest placenta within a normal mouse litter is more efficient at supporting fetal growth than the largest placenta. At both E16 and E19, 30% more fetus was produced per gram of placenta by the lightest than heaviest placenta in a litter, and by E19, fetal weight was similar in the two groups, despite the significant difference in placental weight. These differences in placental efficiency were accompanied by morphological and functional adaptations in the placenta and by increased active transport of MeAIB across the lightest compared to heaviest placenta in a litter. The specific adaptations of the smallest compared to the largest placenta differed with gestational age. Thus at E16, they were primarily morphological, with a relative increase in the labyrinthine zone, whereas at E19, they were largely functional with up-regulated placental expression of two nutrient supply genes, Slc2a1/GLUT1 and Slc38a2/SNAT2 in the lightest placenta. Therefore, placental nutrient supply capacity varies over the normal range of placental weights in the mouse and appears to be responsive to the nutrient demands of the rapidly growing fetus during late gestation.
Placental size and fetal growth
Although longitudinal measurements of the growth of individual feto-placental units were not possible in the present study, the fetal growth trajectory during late gestation appeared to differ with placental size. At E19, the fetuses with the lightest placentas were similar in weight to those with the heaviest placentas in a litter, despite being smaller at E16. Therefore, the lightest placenta supported more growth than the heaviest placenta between E16 and E19. Furthermore, glucose and MeAIB transfer per gram of fetus were similar in the two groups at both ages. The positive correlation observed between placental and fetal weight at E16 was also lost by E19. These findings are consistent with previous studies in the same strain of mice that showed that fetal weight is positively correlated with placental weight at E17 but not later in gestation (McLaren, 1965; Sibley et al. 2004). Thus, the naturally small placenta is able to support the normal growth spurt of the mouse fetus during late gestation.
Placental morphology
The mean volumes of total placenta, labyrinthine and junctional zones, trophoblast, fetal capillaries and maternal blood spaces observed in the current study were within the range of values reported previously for C57BL/6J mice during late gestation (Coan et al. 2004). The labyrinthine zone is the main site for nutrient transfer in the mouse placenta during late gestation and its absolute volume and volume fraction increase progressively during the second half of pregnancy (Coan et al. 2004). In the present study, development of the labyrinthine zone within the lightest placenta appeared to be preserved at E16, as both the volume of this zone and the trophoblast within it were similar in the lightest and heaviest placentas at this age. Indeed, at E16, the lightest placenta had a larger labyrinthine volume fraction and 40% more exchange surface per gram than the heaviest placenta. Between E16 and E19, the labyrinthine zone of the lightest placenta failed to expand in volume and, by E19, was smaller in total volume and contained less trophoblast and blood spaces than the heaviest placenta in the litter. However, the surface area of trophoblast for exchange did increase between E16 and E19 in both placental groups, resulting in a similar weight-specific exchange area in the lightest and heaviest placenta at E19. These morphological adaptations may explain, in part, the increased efficiency of the lightest placenta at E16 but would be expected to increase passive diffusion per gram tissue at this age since the theoretical diffusion capacity of the trophoblast membrane itself did not differ with placental size at either age. However, there were no differences in the unidirectional materno-fetal transfer of inulin per gram of placenta between the lightest and heaviest placentas at E16, which suggests that the smallest placenta may be less permeable per unit surface area than the largest placenta in the litter at this age. Passive diffusion across the trophoblast exchange barrier is believed to occur through extracellular water-filled pores which increase in size between E16 and E19 in the mouse placenta (Sibley et al. 2004). The current findings suggest that the number and/or size of these pores may be reduced in the lightest compared to the heaviest placenta at E16 but not at E19.
At E16, the proportionate increase in the labyrinthine zone in the lightest placentas occurred at the expense of the junctional zone. Both the absolute volume and the volume fraction of this zone were lower in the smallest relative to the largest placentas in a litter at E16. The function of the junctional zone in the mouse placenta is not fully understood. It contains glycogen cells and produces growth factors and pregnancy-related hormones, which may affect fetal growth indirectly (Watson & Cross, 2005). It is required for normal fetal development as its absence in the Ascl2−/− null mouse is associated with embryonic mortality by E13 (Tanaka et al. 1997). Therefore, reduction in the junctional zone volume may be an adaptive response to reduce placental nutrient utilization, thereby, partitioning more of the maternal resources to the fetus. Indeed, an inverse relationship between fetal weight and the volume of glycogen cells in the junctional zone has been observed previously in normal mice at E18 (Kurz et al. 1999). Alternatively, preferential differentiation down the labyrinthine trophoblast rather than the spongiotrophoblast route in the lightest compared to the heaviest placenta may reflect a primary developmental event during placentation rather than an adaptive response to fetal growth retardation occurring later in gestation.
Placental expression of growth and nutrient transporter genes
The changes in placental morphology in the lightest placenta were accompanied by increased expression of two imprinted genes, Grb10 and the placental specific P0 transcript of Igf2, which act through different mechanisms to control the growth of the mouse placenta (Charalambous et al. 2003; Fowden et al. 2006a). At E16, abundance of these two labyrinthine-specific transcripts was up-regulated in line with the increase in volume fraction of the labyrinthine zone, which indicates that their increase in expression may be the consequence not the cause of the proportionate increase in labyrinthine zone volume. At E19, only Grb10 expression remained elevated in the small placenta, even though there was no difference in volume fraction of the labyrinthine zone between the lightest and heaviest placenta in a litter at this age. Grb10 encodes an adaptor protein which influences tissue accretion and the metabolic fate of nutrients by altering signalling through a range of growth factor receptors (Charalambous et al. 2003). In the lightest placenta, inhibitory influences on its growth and metabolism may therefore predominate at E19, consistent with partitioning more nutrients to the rapidly growing fetus.
Relative expression of the nutrient transporter genes between the lightest and heaviest placentas depended on gestational age. At E16, there were no differences in expression of any of the transporter genes examined with placental size, whereas, at E19, expression of Slc2a1/GLUT1 and the Slc38a2/SNAT2 isoform of the System A amino acid transporters were up-regulated in the lightest compared to the heaviest placentas in a litter. The GLUT1 transporter is present in all three trophoblast layers of the labyrinthine zone in the mouse placenta and is believed to have an important role in transplacental glucose transfer as embryonic growth is restricted in the Slc2a1−/− null mouse (Wang et al. 2006). Similarly, the placental System A amino acid transporters are known to have an important role in fetal growth in rodents (Sibley et al. 2005). Up-regulation of System A gene expression has also been observed in the small human placenta and when the fetal nutrient demands exceed the supply capacity of the mouse placenta following deletion of either the Igf2P0 transcript or the Slc2a3 gene (Godfrey et al. 1998; Constancia et al. 2005; Ganguly et al. 2007). Furthermore, increased placental expression of the Slc38a2 isoform of the System A amino acid transporters occurs in association with changes in maternal nutrient partitioning in pregnant guinea pigs treated with IGF-I (Sferruzzi-Perri et al. 2007; Roberts et al. 2008). Placental expression of the Slc38a family of genes therefore appears to be responsive to the fetal growth potential in a number of species.
Placental size and nutrient transport capacity
The cause of the increased active transport of MeAIB across the lightest compared with the heaviest placenta per gram of placental tissue appears to differ at the two gestational ages. At E16, it probably reflects the relative increase in exchange surface per gram of placenta as there were no changes in placental expression of the sodium-dependent System A family of amino acid transporters primarily responsible for transplacental MeAIB transport. At E19, the increased MeAIB transport occurred despite a significant reduction in trophoblast volume and fetal-facing surface area, and was related to increased placental expression of the Slc38a2/SNAT2 isoform of the System A family of amino acid transporters. At both gestational ages, other amino acid transporters could have contributed to the increased MeAIB transport across the lightest placenta as there are at least nine different amino acid transporters in the placenta and sodium-independent transport of MeAIB has been observed in human microvillous vesicles (Sibley et al. 1997, 2005; Godfrey et al. 1998). In contrast to MeAIB, there was no difference in the facilitated diffusion of glucose across the lightest and heaviest placenta in a litter, despite the increased volume fraction of the labyrinthine zone at E16 and increased placental expression of Slc2a1/GLUT1 at E19 in the lightest placenta. The absence of increased glucose transfer in the lightest placenta despite the morphological and functional adaptations may be due to low placental blood flow related to the 30–40% reduction in fetal capillary volume. Reducing placental blood flow affects facilitated diffusion more readily than active transport across the placenta and has been shown to decrease transplacental glucose transfer in sheep at blood flow rates 50% below normal (Wilkening et al. 1985; Sibley et al. 1997, 2005). The increased placental Slc2a1 expression in the lightest placenta at E19 therefore helps maintain transplacental glucose transfer at the values seen in the heaviest placenta in the litter despite the reduced fetal capillary volumes.
Taken together, the current findings indicate that large and small placentas adopt different strategies in supporting fetal growth during late gestation. Large placentas continue to adapt morphologically during late gestation increasing growth of the labyrinthine exchange area at the expense of the junctional zone. Small placentas, on the other hand, appear to have a more limited capacity for morphological adaptation between E16 and E19 but adapt functionally during this period to increase nutrient transfer relative to the large placenta. This adaptability in placental phenotype provides not only a functional reserve capacity to better match the placental nutrient supply with the fetal nutrient demands but also a potential mechanism of signalling environmental conditions to the fetus. However, by altering the relative proportions of different types of nutrients supplied to the fetus, these adaptive changes in placental nutrient transfer capacity and fetal growth trajectory may affect the development of individual fetal tissues with patho-physiological consequences long after birth. Placental phenotype may therefore be a better marker of disease risk in later life than many of the other indices, like birth weight, that are commonly used to indicate exposure to suboptimal conditions during intrauterine development (Sibley et al. 2005).
Acknowledgments
We would like to thank Nuala Daw and Gerrard Peck for their technical assistance and the BBSRC for their financial support of this work.
References
- Ain R, Canham LN, Soares MJ. Dexamethasone-induced intrauterine growth restriction impacts the placental prolactin family, insulin-like growth factor-II and the Akt signaling pathway. J Endocrinol. 2005;185:253–263. doi: 10.1677/joe.1.06039. [DOI] [PubMed] [Google Scholar]
- Allen WR, Wilsher S, Turnbull C, Stewart F, Ousey J, Rossdale PD, Fowden AL. Influence of maternal size on placental, fetal and postnatal growth in the horse. I. Development in utero. Reproduction. 2002;123:445–453. [PubMed] [Google Scholar]
- Angiolini E, Fowden A, Coan P, Sandovici I, Smith P, Dean W, Burton G, Tycko B, Reik W, Sibley C, Constancia M. Regulation of placental efficiency for nutrient transport by imprinted genes. Placenta. 2006;27(Suppl. A):S98–S102. doi: 10.1016/j.placenta.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Baddeley AJ, Gundersen HJ, Cruz-Orive LM. Estimation of surface area from vertical sections. J Microsc. 1986;142:259–276. doi: 10.1111/j.1365-2818.1986.tb04282.x. [DOI] [PubMed] [Google Scholar]
- Barker DJP. Mothers, Babies and Disease in Later Life. London: BMJ Publishing Group; 1994. [Google Scholar]
- Charalambous M, Smith FM, Bennett WR, Crew TE, Mackenzie F, Ward A. Disruption of the imprinted Grb10 gene leads to disproportionate overgrowth by an Igf2-independent mechanism. Proc Natl Acad Sci USA. 2003;100:8292–8297. doi: 10.1073/pnas.1532175100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coan PM, Ferguson-Smith AC, Burton GJ. Developmental dynamics of the definitive mouse placenta assessed by stereology. Biol Reprod. 2004;70:1806–1813. doi: 10.1095/biolreprod.103.024166. [DOI] [PubMed] [Google Scholar]
- Constancia M, Angiolini E, Sandovici I, Smith P, Smith R, Kelsey G, Dean W, Ferguson-Smith A, Sibley CP, Reik W, Fowden A. Adaptation of nutrient supply to fetal demand in the mouse involves interaction between the Igf2 gene and placental transporter systems. Proc Natl Acad Sci USA. 2005;102:19219–19224. doi: 10.1073/pnas.0504468103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constancia M, Hemberger M, Hughes J, Dean W, Ferguson-Smith A, Fundele R, Stewart F, Kelsey G, Fowden A, Sibley C, Reik W. Placental-specific IGF-II is a major modulator of placental and fetal growth. Nature. 2002;417:945–948. doi: 10.1038/nature00819. [DOI] [PubMed] [Google Scholar]
- Fowden AL, Forhead AJ, Coan PM, Burton GJ. The placenta and intrauterine programming. J Neuroendocrinol. 2008;20:439–450. doi: 10.1111/j.1365-2826.2008.01663.x. [DOI] [PubMed] [Google Scholar]
- Fowden AL, Giussani DA, Forhead AJ. Endocrine and metabolic programming during intrauterine development. Early Hum Dev. 2005;81:723–734. doi: 10.1016/j.earlhumdev.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Fowden AL, Sibley C, Reik W, Constancia M. Imprinted genes, placental development and fetal growth. Horm Res. 2006a;65(Suppl. 3):50–58. doi: 10.1159/000091506. [DOI] [PubMed] [Google Scholar]
- Fowden AL, Ward JW, Wooding FP, Forhead AJ, Constancia M. Programming placental nutrient transport capacity. J Physiol. 2006b;572:5–15. doi: 10.1113/jphysiol.2005.104141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly A, McKnight RA, Raychaudhuri S, Shin BC, Ma Z, Moley K, Devaskar SU. Glucose transporter isoform-3 mutations cause early pregnancy loss and fetal growth restriction. Am J Physiol Endocrinol Metab. 2007;292:E1241–E1255. doi: 10.1152/ajpendo.00344.2006. [DOI] [PubMed] [Google Scholar]
- Godfrey KM, Matthews N, Glazier J, Jackson A, Wilman C, Sibley CP. Neutral amino acid uptake by the microvillous plasma membrane of the human placenta is inversely related to fetal size at birth in normal pregnancy. J Clin Endocrinol Metab. 1998;83:3320–3326. doi: 10.1210/jcem.83.9.5132. [DOI] [PubMed] [Google Scholar]
- Hanson MA, Gluckman PD. Developmental origins of health and disease: new insights. Basic Clin Pharmacol Toxicol. 2008;102:90–93. doi: 10.1111/j.1742-7843.2007.00186.x. [DOI] [PubMed] [Google Scholar]
- Harding JE, Johnston BM. Nutrition and fetal growth. Reprod Fertil Dev. 1995;7:539–547. doi: 10.1071/rd9950539. [DOI] [PubMed] [Google Scholar]
- Jansson N, Pettersson J, Haafiz A, Ericsson A, Palmberg I, Tranberg M, Ganapathy V, Powell TL, Jansson T. Down-regulation of placental transport of amino acids precedes the development of intrauterine growth restriction in rats fed a low protein diet. J Physiol. 2006;576:935–946. doi: 10.1113/jphysiol.2006.116509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly RW. Nutrition and placental development. Proc Nutr Soc Aust. 1992;17:203–211. [Google Scholar]
- Kurz H, Zechner U, Orth A, Fundele R. Lack of correlation between placenta and offspring size in mouse interspecific crosses. Anat Embryol. 1999;200:335–343. doi: 10.1007/s004290050284. [DOI] [PubMed] [Google Scholar]
- McLaren A. Genetic and environmental effects on foetal and placental growth in mice. J Reprod Fertil. 1965;9:79–98. doi: 10.1530/jrf.0.0090079. [DOI] [PubMed] [Google Scholar]
- McMillen IC, Robinson JS. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev. 2005;85:571–633. doi: 10.1152/physrev.00053.2003. [DOI] [PubMed] [Google Scholar]
- Molteni RA, Stys SJ, Battaglia FC. Relationship of fetal and placental weight in human beings: fetal/placental weight ratios at various gestational ages and birth weight distributions. J Reprod Med. 1978;21:327–334. [PubMed] [Google Scholar]
- Owens JA, Falconer J, Robinson JS. Restriction of placental size in sheep enhances efficiency of placental transfer of antipyrine, 3-O-methyl-D-glucose but not of urea. J Dev Physiol. 1987;9:457–464. [PubMed] [Google Scholar]
- Roberts CT, Owens JA, Sferruzzi-Perri AN. Distinct actions of insulin-like growth factors (IGFs) on placental development and fetal growth: lessons from mice and guinea pigs. Placenta. 2008;29(Suppl. A):S42–S47. doi: 10.1016/j.placenta.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Ross JC, Fennessey PV, Wilkening RB, Battaglia FC, Meschia G. Placental transport and fetal utilization of leucine in a model of fetal growth retardation. Am J Physiol Endocrinol Metab. 1996;270:E491–E503. doi: 10.1152/ajpendo.1996.270.3.E491. [DOI] [PubMed] [Google Scholar]
- Sferruzzi-Perri AN, Owens JA, Standen P, Taylor RL, Heinemann GK, Robinson JS, Roberts CT. Early treatment of the pregnant guinea pig with IGFs promotes placental transport and nutrient partitioning near term. Am J Physiol Endocrinol Metab. 2007;292:E668–E676. doi: 10.1152/ajpendo.00320.2006. [DOI] [PubMed] [Google Scholar]
- Sibley CP, Coan PM, Ferguson-Smith AC, Dean W, Hughes J, Smith P, Reik W, Burton GJ, Fowden AL, Constancia M. Placental-specific insulin-like growth factor 2 (Igf2) regulates the diffusional exchange characteristics of the mouse placenta. Proc Natl Acad Sci USA. 2004;101:8204–8208. doi: 10.1073/pnas.0402508101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley C, Glazier J, D'Souza S. Placental transporter activity and expression in relation to fetal growth. Exp Physiol. 1997;82:389–402. doi: 10.1113/expphysiol.1997.sp004034. [DOI] [PubMed] [Google Scholar]
- Sibley CP, Turner MA, Cetin I, Ayuk P, Boyd CA, D'Souza SW, Glazier JD, Greenwood SL, Jansson T, Powell T. Placental phenotypes of intrauterine growth. Pediatr Res. 2005;58:827–832. doi: 10.1203/01.PDR.0000181381.82856.23. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Gertsenstein M, Rossant J, Nagy A. Mash2 acts cell autonomously in mouse spongiotrophoblast development. Dev Biol. 1997;190:55–65. doi: 10.1006/dbio.1997.8685. [DOI] [PubMed] [Google Scholar]
- Waldor DP, Foote WC, Self HL, Chapman AB, Casid LE. Factors affecting fetal pig weight late in gestation. J Anim Sci. 1957;16:976–983. [Google Scholar]
- Wallace JM, Bourke DA, Aitken RP, Milne JS, Hay WW., Jr Placental glucose transport in growth-restricted pregnancies induced by overnourishing adolescent sheep. J Physiol. 2003;547:85–94. doi: 10.1113/jphysiol.2002.023333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Pascual JM, Yang H, Engelstad K, Mao X, Cheng J, Yoo J, Noebels JL, De Vivo DC. A mouse model for Glut-1 haploinsufficiency. Hum Mol Genet. 2006;15:1169–1179. doi: 10.1093/hmg/ddl032. [DOI] [PubMed] [Google Scholar]
- Watson ED, Cross JC. Development of structures and transport functions in the mouse placenta. Physiology (Bethesda) 2005;20:180–193. doi: 10.1152/physiol.00001.2005. [DOI] [PubMed] [Google Scholar]
- Wilkening RB, Battaglia FC, Meschia G. The relationship of umbilical glucose uptake to uterine blood flow. J Dev Physiol. 1985;7:313–319. [PubMed] [Google Scholar]