Abstract
Much of our present understanding about the mechanisms contributing to the activity-dependent refinement of sensory connections comes from experiments done in the retinogeniculate pathway. In recent years the mouse has emerged as a model system of study. This review outlines the major changes in connectivity that occur in this species and a potential mechanism that can account for such remodelling. During early postnatal life when spontaneous activity of retinal ganglion cells sweeps across the retina in waves, retinal projections from the two eyes to the dorsal lateral geniculate nucleus (LGN) segregate to form non-overlapping eye-specific domains. There is a loss of binocular innervation, a pruning of excitatory inputs from a dozen or more to one or two, and the emergence of inhibitory circuitry. Many of these changes underlie the development of precise eye-specific visual maps and receptive field structure of LGN neurons. Retinal activity plays a major role both in the induction and maintenance of this refinement. The activity-dependent influx of Ca2+ through L-type channels and associated activation of CREB signalling may underlie the pruning and stabilization of developing retinogeniculate connections.
The retinogeniculate pathway of the mouse undergoes extensive remodelling during early postnatal life (see Fig. 1; Torborg & Feller, 2005; Huberman, 2007) and with the advent of transgenic models this system has become a major experimental platform to study the mechanisms underlying the activity-dependent refinement of sensory connections. Thus, information about when and by what means retinal axons establish and then rearrange their patterns of connectivity in the lateral geniculate nucleus (LGN) is needed. Here I provide a brief review of my lab's work as well as others that pertain to the development and remodelling of retinogeniculate connections. Topics to be discussed include the development of eye-specific segregation in the LGN, the structural and functional remodelling of retinogeniculate connections, the role of retinal activity in shaping and maintaining patterns of connectivity, and the potential mechanisms underlying the remodelling of these connections.
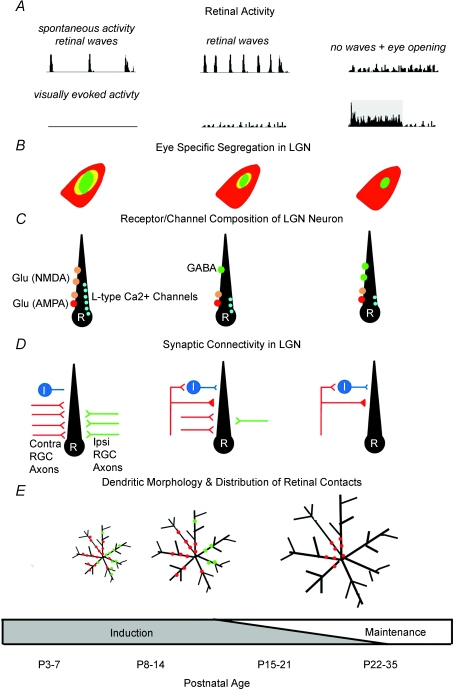
Figure 1. Summary of retinogeniculate refinement.
The major events and changes that occur along the retinogeniculate pathway during the first few weeks of postnatal life are shown. In A, spontaneous and visually evoked retinal activity, are displayed as poststimulus time histograms. The shaded region represents the response to a visual stimulus. In B, D and E, red and green represent retinal inputs arising from the contralateral and ipsilateral eye, respectively. In B, yellow depicts regions of overlap. In C and D, LGN neurons labelled I and R depict interneurons and relay cells, respectively. In D, weak and strong retinal inputs are displayed as opened and filled symbols, respectively.
The development of eye-specific segregation in the lateral geniculate nucleus
A cardinal feature of the mammalian retinogeinculate pathway is the segregation of retinal inputs from the two eyes (Fig. 1B). In the mouse there is no obvious lamination pattern that partitions projections by eye. Instead, projections from each eye are organized into non-overlapping territories called eye-specific domains that can only be visualized by the anterograde labelling of retinal ganglion cells. When cholera toxin β subunit conjugated to different Alexa fluorescent dyes are injected into one eye or the other, estimates of the spatial extent for projections arising from each eye as well as the degree of overlap that exists between them can be obtained (Muir-Robinson et al. 2002; Jaubert-Miazza et al. 2005).
In adults, axons from nasal and most of temporal retina cross at the optic chiasm and project to the LGN, occupying as much as much as 85–90% of its total area. A much smaller group of retinal ganglion cells (5%) from the ventro-temporal region have axons that do not cross at the optic chiasm, but instead project ipsilaterally to terminate in the antero-medial region of LGN. Uncrossed projections form an irregularly shaped cylinder that runs rostral to caudal through LGN, occupying about 10–12% of the total area, but sharing little (< 1–2%) if any territory with crossed projections. While a coarse visuoptopic map is established shortly after retinal axons innervate the LGN, eye-specific patterning is not apparent at birth but emerges just after the first postnatal week. Initially, crossed and uncrossed axons innervate the LGN at slightly different times, with crossed projections arriving earlier (E15–16) than uncrossed ones (P0–2) (Godement et al. 1984; W. Guido unpublished observations). At these ages, crossed projections span almost the entire LGN. As uncrossed axons innervate the LGN, they too are diffusely organized but by P2 they begin to establish a rudimentary patch of terminal arbors in the dorso-medial sector. Between P2 and P5 the inputs from the two eyes share a substantial amount of terminal space in LGN. Our estimates of spatial extent reveal that at P3 uncrossed projections occupy about 60% of the LGN and overlap with crossed ones by as much as 57%. Between P3 and P7 there is a rapid retraction of uncrossed terminal arbors. Nonetheless, even at P7 uncrossed projections still occupy about 25% of LGN and share close to 20% with crossed projections. By P10, retinal projections from the two eyes show clear signs of segregation, and by natural eye opening (P14), they are well segregated and resemble the pattern found in the adult.
Patterns of synaptic connectivity in the developing LGN
The anatomical segregation of retinal projections into eye-specific domains is accompanied by major modifications in the pattern of synaptic connectivity (Fig. 1D). In vitro intracellular recordings of the synaptic responses of LGN cells illustrate the presence of functional retinogeniculate connections at very early postnatal ages (Mooney et al. 1996; Chen & Regehr, 2000; Jaubert-Miazza et al. 2005). Using an explant preparation that that preserves large segments of each optic nerve, we have shown that many developing LGN cells receive direct binocular excitatory input (Jaubert-Miazza et al. 2005; Ziburkus & Guido, 2006). Additionally, estimates of retinal convergence indicate that a single LGN cell can receive weak synaptic input from as many as one to two dozen retinal ganglion cells (Chen & Regehr, 2000; Jaubert-Miazza et al. 2005). After the first postnatal week, as retinal projections recede and clear signs of eye-specific segregation emerge, the degree of retinal convergence begins to diminish. By the second postnatal week, LGN cells receive far fewer retinal inputs that arise either from one eye or the other. During the following weeks, additional pruning occurs so that eventually LGN cells receive strong monocular input from as few as 1–3 retinal ganglion cells. We have begun to examine where and how retinal inputs are distributed on LGN relay cells by combining our anterograde labelling of retinal axons with 3-D confocal reconstructions of relay cells filled with biocytin (Fig. 1E). Preliminary findings have shown that at early ages (P7–9) retinal contacts can occupy as much as 50% of the surface area of relay cells. Retinal contacts are widespread and located on somata as well as proximal and distal regions of dendrites. We have also found that retinal contacts arise from both eyes, but the bulk is dominated by just one eye. This seems to be consistent with our electrophysiological experiments showing that while cells receive multiple retinal inputs from both eyes, the inputs from one outweigh those of the other (Ziburkus & Guido, 2006). There is also progressive elimination of contacts with age so that by the third postnatal week they arise from one eye and occupy only 1–5% of the total surface area of LGN cells. While the dendritic fields of relay cells show moderate expansion, to our surprise, we find that many aspects of dendritic complexity (e.g. number of primary dendrites, branch order, and number of branch points) remain relatively constant between postnatal weeks 1 and 3 (Fig. 1E). Taken together, these data suggest that the developmental plan of relay cells is established quite early and the dendritic scaffold for synaptic remodelling is in place by the first postnatal week.
During this pruning process, there are corresponding changes in synaptic strength and in the receptor composition of excitatory glutamate-mediated responses (Fig. 1C–D; Chen & Regehr 2000; Liu & Chen, 2008). Initially, excitatory postsynaptic currents are relatively small (weak) and mediated almost exclusively by NMDA receptor activation. As pruning proceeds and the remaining excitatory responses increase in amplitude (strength), AMPA-mediated responses emerge. At a time when cells receive just a few retinal inputs, there is a 50-fold increase in synaptic strength and a 4-fold increase in the AMPA to NMDA receptor current ratio.
In addition to the remodelling of excitatory connections, inhibitory circuits that involve GABAeric interneurons of the LGN appear (Fig. 1C–D). In the adult, retinal axons possess collateral branches that make excitatory connections with intrinsic interneurons, which in turn form feedforward inhibitory connections with relay cells. Our anatomical and electrophysiological experiments suggest that inhibitory responses in LGN relay cells are not fully developed at early postnatal ages (Ziburkus et al. 2003; Jaubert-Miazza et al. 2005; Slusarczyk et al. 2006). They start to appear around postnatal day 5–7 but do not fully mature until near the time of natural eye opening (P14). While the balance of excitatory and inhibitory responses seem to develop at different times, the functional significance of this sequence remains unexplored. Perhaps the delayed maturation of inhibitory circuitry allows for an increased level of membrane depolarization which is needed to activate events implicated in synaptic remodelling (see below).
Retinal activity mediates the induction and maintenance retinogeniculate refinement
The refinement of connections in LGN has been attributed to the coordinated firing patterns of spontaneous retinal activity (Torborg & Feller, 2005). Even before photoreceptors are equipped to activate bipolar cells, neighbouring retinal ganglion cells fire spontaneously in rhythmic bursts that spread across the retina in a wave-like fashion (Fig. 1A; Wong, 1999; Demas et al. 2003). When these retinal waves are disrupted or completely eliminated, retinal axon arbors in LGN remain diffuse and fail to segregate properly into eye-specific domains (Torborg & Feller, 2005). Most notable are the observations made in two transgenic mice lines. Animals missing the β2 subunit of nicotinic acetylcholine (nACh) receptor lack an early phase of retinal wave activity, one that is mediated largely by cholinergic synaptic transmission (Bansal et al. 2000). As a result, retinal projections remain diffuse through early postnatal life and fail to segregate properly into eye-specific domains (Muir-Robinson et al. 2002). In the no-b wave (nob1) mouse, a mutant that lacks nyctalopin, a protein essential for photoreceptor and ON-bipolar cell synaptic transmission (Gregg et al. 2003), abnormally frequent and persistent retinal waves occur at the time when axon segregation is complete and retinal waves are normally replaced by visually evoked activity (Demas et al. 2006). These persistent high frequency waves cause newly established eye-specific domains in LGN to de-segregate and return to a diffuse state. In addition to the abnormal retinal wave activity, these mice exhibit a severe loss of visual sensitivity, lacking an ERG b-wave and discernable on- or off-stimulus evoked visual responses (Gregg et al. 2003, 2007). These combined deficits raise the possibility that the persistence of abnormal spontaneous retinal activity, perhaps at the expense of visually driven activity, disrupts retinogeniculate axon segregation. Studies employing a delayed dark rearing protocol further suggest that the strength and final patterns of connectivity in LGN requires patterned visually evoked activity (Hooks & Chen, 2006; Hooks & Chen, 2008). Taken together, these studies suggest that some aspect of spontaneous retinal waves and/or visually evoked activity is important for the continued maintenance of newly established connections. Thus, activity-dependent refinement in LGN comprises two somewhat overlapping phases (Fig. 1, bottom panel): an initial inductive phase where eye-specific domains are established and the bulk of synaptic pruning takes place, and a maintenance phase where newly refined connections require some form of retinal activity in order for them to stabilize, increase in strength, and eventually consolidate to form an adult-like pattern of connectivity.
While there is some debate about whether retinal waves play an instructional role in retinogeniculate axon segregation (Chalupa, 2007), the nearest-neighbour, same-eye relations underlying the spatiotemporal patterning of waves make them a prime candidate for promoting Hebbian synaptic plasticity. In Hebbian models, temporally correlated activity between pre- and postsynaptic elements leads to a strengthening and consolidation of synapses, whereas asynchronous activity or the absence of activity results in synapse weakening and elimination. Hebbian-based changes in synaptic strength have been noted in several developing vertebrate sensory structures and linked to intracellular signalling cascades that promote the structural refinement of connections. Long-term modifications in synaptic strength may also embody the synaptic rearrangements occurring in LGN. Bidirectional changes in synaptic strength have been reported at the retinogeniculate synapse (Butts et al. 2007). These modifications seem to adhere to a Hebbian learning rule in which the relative timing between presynaptic high frequency stimulation (HFS) of retinal fibres (similar to waves) and postsynaptic depolarization (via current injection) of an LGN cell proves to be the defining feature. Cells exhibit LTP when optic tract stimulation and LGN depolarization are coincident or overlap, but show mild LTD when pairing is non-overlapping. Using a rodent explant preparation that preserves retinal connections from the two eyes, we also show that that HFS of retinal afferents leads to changes in synaptic strength (Guido, 2006; Ziburkus et al. submitted). The polarity and magnitude of these changes are both age and pathway specific. In one set of intriguing experiments involving binocularly innervated LGN cells, heightened activity along the tetanized pathway leads to an increase in synaptic strength (homosynaptic LTP) as well as a reduction in synaptic strength (heterosynaptic LTD) along the untetanized, less active pathway.
The role of L-type Ca2+ channels in the developmental remodelling of retinogeniculate connections
A critical issue to resolve is the identification of neural elements and signalling events that mediate changes in retinogeniculate connectivity. A likely candidate involves the activity-dependent influx of Ca2+ associated with NMDA receptors and/or voltage-dependent Ca2+ channels. While the role of NMDA receptors figures prominently in many models of activity-dependent refinement, their involvement at the retinogeniculate synapse seems far less important. Correlated firing between retinal ganglion cells and LGN cells persist in the absence of NMDA receptors (Mooney et al. 1996) and NMDA receptor blockade does not seem to interfere with eye-specific segregation, at least in the ferret (Smetters et al. 1994). Instead, NMDA receptors in immature LGN cells seem to promote sustained levels of membrane depolarization that lead to spike firing (Liu & Chen, 2008), perhaps to ensure that retinal wave activity is relayed to the developing visual cortex (Hanganu et al. 2006) and utilized for the refinement of topographic maps (Cang et al. 2005). Their voltage dependency and long decay times also contribute to the spatial and temporal summation of EPSPs and the activation of voltage gated L-type Ca2+ channels (Lo et al. 2002), an element that may represent a more likely substrate for activity-dependent plasticity in LGN.
In LGN, L-type channels are located primarily on somata and proximal dendrites (Budde et al. 1998; Jaubert-Miazza et al. 2005). These channels seem to reside close to retinal synapses (Rafols & Valverde, 1973; Slusarczyk et al. 2006), thus allowing for coordinated coactivation and amplification of retinally evoked postsynaptic events (Fig. 1C). In fact, strong and/or repetitive stimulation of retinal fibres evokes EPSPs that activate high-amplitude, long-lasting, slow-decaying depolarizations (Lo et al. 2002; Jaubert-Miazza et al. 2005; Liu & Chen, 2008). These so-called plateau potentials have a voltage dependency and pharmacology that resembles the activation of high-threshold L-type (long-lasting) Ca2+ channels (Kammermeier & Jones, 1997). Synaptically evoked plateau potentials in LGN are transient events. They are encountered far more frequently at early postnatal ages but then decline with age, so that after natural eye opening they are rarely observed (Jaubert-Miazza et al. 2005). A number of factors seem to contribute to this, including the density of L-type expression, emergence of inhibitory synaptic activity, and the maturation of intrinsic membrane properties. Perhaps, the most important factors seem to be the high degree of retinal convergence and heightened NMDA activity noted in immature LGN cells (Lo et al. 2002; Liu & Chen, 2008). These events lead to sustained levels of depolarization and thereby greatly increase the likelihood that high-threshold L-type channels are activated. In vivo, the driving force behind such activation appears to be retinal waves (Mooney et al. 1996). The episodic barrages of retinally evoked EPSPs brought about by retinal waves are of sufficient amplitude and duration to activate plateau potentials. In vitro recordings of synaptic responses reveal that repetitive stimulation of retinal afferents in a manner that approximates the high frequency discharge of retinal waves gives rise to robust plateau activity (Lo et al. 2002; Jaubert-Miazza et al. 2005; Butts et al. 2007). The Ca2+ influx through L-type channels has been linked to the activation of transcription factors that lead to the expression of genes associated with synaptic plasticity and remodelling (Lonze & Ginty, 2002). A likely candidate involves the cAMP response element (CRE/CREB) transcription pathway (Mermelstein et al. 2000; Dolmetsch et al. 2001). The CRE binding protein (CREB) is a Ca2+ and cAMP regulated transcriptional activating protein shown to be important for retinogeniculate axon segregation. In the mouse LGN, CRE-mediated gene expression peaks during early postnatal life (Pham et al. 2001) and mutant mice that show reduced levels of L-type Ca2+ channel activity or decreased levels of CRE fail to segregate properly (Cork et al. 2001; Pham et al. 2001). Thus, the Ca2+ influx through L-type channels may provide the basis for the pruning and stabilization of developing retinogeniculate connections.
Acknowledgments
This work was supported by EY12716. For their invaluable scientific contributions, I would like to thank past (Jokubas Ziburkus, Fu-Sun Lo, Erick Green, Lisa Jaubert-Miazza, Kim Bui, Jeremy Mills) and present (Thomas Krahe, Rana El-Danaf, Emily Dilger, Tania Seabrook) lab members, as well as the labs of Martha Bickford, Ron Gregg and Rachel Wong.
References
- Bansal A, Singer JH, Hwang BJ, Xu W, Beaudet A, Feller MB. Mice lacking specific nicotinic acetylcholine receptor subunits exhibit dramatically altered spontaneous activity patterns and reveal a limited role for retinal waves in forming ON and OFF circuits in the inner retina. J Neurosci. 2000;20:7672–7681. doi: 10.1523/JNEUROSCI.20-20-07672.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde T, Munsch T, Pape HC. Distribution of L-type calcium channels in rat thalamic neurones. Eur J Neurosci. 1998;10:586–597. doi: 10.1046/j.1460-9568.1998.00067.x. [DOI] [PubMed] [Google Scholar]
- Butts DA, Kanold PO, Shatz CJ. A burst-based ‘hebbian’ learning rule at retinogeniculate synapses links retinal waves to activity-dependent refinement. Plos Biol. 2007;5:e61. doi: 10.1371/journal.pbio.0050061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cang J, Renteria RC, Kaneko M, Liu X, Copenhagen DR, Stryker MP. Development of precise maps in visual cortex requires patterned spontaneous activity in the retina. Neuron. 2005;48:797–809. doi: 10.1016/j.neuron.2005.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalupa LM. A reassessment of the role of activity in the formation of eye-specific retinogeniculate projections. Brain Res Rev. 2007;55:228–236. doi: 10.1016/j.brainresrev.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Chen C, Regehr WG. Developmental remodeling of the retinogeniculate synapse. Neuron. 2000;28:955–966. doi: 10.1016/s0896-6273(00)00166-5. [DOI] [PubMed] [Google Scholar]
- Cork RJ, Namkung Y, Shin HS, Mize RR. Development of the visual pathway is disrupted in mice with a targeted disruption of the calcium channel β3-subunit gene. J Comp Neurol. 2001;440:177–191. doi: 10.1002/cne.1378. [DOI] [PubMed] [Google Scholar]
- Demas J, Eglen SJ, Wong RO. Developmental loss of synchronous spontaneous activity in the mouse retina is independent of visual experience. J Neurosci. 2003;23:2851–2860. doi: 10.1523/JNEUROSCI.23-07-02851.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demas J, Sagdullaev BT, Green E, Jaubert-Miazza L, McCall MA, Gregg RG, Wong RO, Guido W. Failure to maintain eye-specific segregation in nob, a mutant with abnormally patterned retinal activity. Neuron. 2006;50:247–259. doi: 10.1016/j.neuron.2006.03.033. [DOI] [PubMed] [Google Scholar]
- Dolmetsch RE, Pajvani U, Fife K, Spotts JM, Greenberg ME. Signaling to the nucleus by an L-type calcium channel-calmodulin complex through the MAP kinase pathway. Science. 2001;294:333–339. doi: 10.1126/science.1063395. [DOI] [PubMed] [Google Scholar]
- Godement P, Salaun J, Imbert M. Prenatal and postnatal development of retinogeniculate and retinocollicular projections in the mouse. J Comp Neurol. 1984;230:552–575. doi: 10.1002/cne.902300406. [DOI] [PubMed] [Google Scholar]
- Gregg RG, Kamermans M, Klooster J, Lukasiewicz PD, Peachey NS, Vessey KA, McCall MA. Nyctalopin expression in retinal bipolar cells restores visual function in a mouse model of complete X-linked congenital stationary night blindness. J Neurophysiol. 2007;98:3023–3033. doi: 10.1152/jn.00608.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg RG, Mukhopadhyay S, Candille SI, Ball SL, Pardue MT, McCall MA, Peachey NS. Identification of the gene and the mutation responsible for the mouse nob phenotype. Invest Ophthalmol Vis Sci. 2003;44:378–384. doi: 10.1167/iovs.02-0501. [DOI] [PubMed] [Google Scholar]
- Guido W. Cellular mechanisms underlying the remodeling of retinogeniculate connections. In: Erzrurmulu R, Guido W, Molnar Z, editors. Development and Plasticity in Sensory Thalamus and Cortex. New York: Springer; 2006. pp. 208–227. [Google Scholar]
- Hanganu IL, Ben-Ari Y, Khazipov R. Retinal waves trigger spindle bursts in the neonatal rat visual cortex. J Neurosci. 2006;26:6728–6736. doi: 10.1523/JNEUROSCI.0752-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooks BM, Chen C. Distinct roles for spontaneous and visual activity in remodeling of the retinogeniculate synapse. Neuron. 2006;52:281–291. doi: 10.1016/j.neuron.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Hooks BM, Chen C. Vision triggers an experience-dependent sensitive period at the retinogeniculate synapse. J Neurosci. 2008;28:4807–4817. doi: 10.1523/JNEUROSCI.4667-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman AD. Mechanisms of eye-specific visual circuit development. Curr Opin Neurobiol. 2007;17:73–80. doi: 10.1016/j.conb.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Jaubert-Miazza L, Green E, Lo FS, Bui K, Mills J, Guido W. Structural and functional composition of the developing retinogeniculate pathway in the mouse. Vis Neurosci. 2005;22:661–676. doi: 10.1017/S0952523805225154. [DOI] [PubMed] [Google Scholar]
- Kammermeier PJ, Jones SW. High-voltage-activated calcium currents in neurons acutely isolated from the ventrobasal nucleus of the rat thalamus. J Neurophysiol. 1997;77:465–475. doi: 10.1152/jn.1997.77.1.465. [DOI] [PubMed] [Google Scholar]
- Liu X, Chen C. Different Roles for AMPA and NMDA receptors in transmission at the immature retinogeniculate synapse. J Neurophysiol. 2008;99:629–643. doi: 10.1152/jn.01171.2007. [DOI] [PubMed] [Google Scholar]
- Lo FS, Ziburkus J, Guido W. Synaptic mechanisms regulating the activation of a Ca2+-mediated plateau potential in developing relay cells of the LGN. J Neurophysiol. 2002;87:1175–1185. doi: 10.1152/jn.00715.1999. [DOI] [PubMed] [Google Scholar]
- Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35:605–623. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- Mermelstein PG, Bito H, Deisseroth K, Tsien RW. Critical dependence of cAMP response element-binding protein phosphorylation on L-type calcium channels supports a selective response to EPSPs in preference to action potentials. J Neurosci. 2000;20:266–273. doi: 10.1523/JNEUROSCI.20-01-00266.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney R, Penn AA, Gallego R, Shatz CJ. Thalamic relay of spontaneous retinal activity prior to vision. Neuron. 1996;17:863–874. doi: 10.1016/s0896-6273(00)80218-4. [DOI] [PubMed] [Google Scholar]
- Muir-Robinson G, Hwang BJ, Feller MB. Retinogeniculate axons undergo eye-specific segregation in the absence of eye-specific layers. J Neurosci. 2002;22:5259–5264. doi: 10.1523/JNEUROSCI.22-13-05259.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham TA, Rubenstein JL, Silva AJ, Storm DR, Stryker MP. The CRE/CREB pathway is transiently expressed in thalamic circuit development and contributes to refinement of retinogeniculate axons. Neuron. 2001;31:409–420. doi: 10.1016/s0896-6273(01)00381-6. [DOI] [PubMed] [Google Scholar]
- Rafols JA, Valverde F. The structure of the dorsal lateral geniculate nucleus in the mouse. A Golgi and electron microscopic study. J Comp Neurol. 1973;150:303–332. doi: 10.1002/cne.901500305. [DOI] [PubMed] [Google Scholar]
- Slusarczyk S, Kucuk C, Chomsung R, Eisenback MA, Guido W, Bickford ME. 2006 Abstract Viewer/Itinerary Planner. Washington,DC: Society for Neuroscience; 2006. Synaptic organization of the adult and neonatal mouse lateral geniculate nucleus. Program No. 241.3.17. [Google Scholar]
- Smetters DK, Hahm J, Sur M. An N-methyl-D-aspartate receptor antagonist does not prevent eye-specific segregation in the ferret retinogeniculate pathway. Brain Res. 1994;658:168–178. doi: 10.1016/s0006-8993(09)90023-3. [DOI] [PubMed] [Google Scholar]
- Torborg CL, Feller MB. Spontaneous patterned retinal activity and the refinement of retinal projections. Prog Neurobiol. 2005;76:213–235. doi: 10.1016/j.pneurobio.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Wong RO. Retinal waves and visual system development. Annu Rev Neurosci. 1999;22:29–47. doi: 10.1146/annurev.neuro.22.1.29. [DOI] [PubMed] [Google Scholar]
- Ziburkus J, Guido W. Loss of binocular responses and reduced retinal convergence during the period of retinogeniculate axon segregation. J Neurophysiol. 2006;96:2775–2784. doi: 10.1152/jn.01321.2004. [DOI] [PubMed] [Google Scholar]
- Ziburkus J, Lo FS, Guido W. Nature of inhibitory postsynaptic activity in developing relay cells of the lateral geniculate nucleus. J Neurophysiol. 2003;90:1063–1070. doi: 10.1152/jn.00178.2003. [DOI] [PubMed] [Google Scholar]