Abstract
Movement execution is speeded up when a startle auditory stimulus is applied with an imperative signal in a simple reaction time task experiment, a phenomenon described as StartReact. The effect has been recently observed in a step adjustment task requiring fast selection of specific movements in a choice reaction time task. Therefore, we hypothesized that inducing a StartReact effect may be beneficial in obstacle avoidance under time pressure, when subjects have to perform fast gait adjustments. Twelve healthy young adults walked on a treadmill and obstacles were released in specific moments of the step cycle. On average the EMG onset latency in the biceps femoris shortened by 20% while amplitude increased by 50%, in trials in which an auditory startle accompanied obstacle avoidance. The presentation of a startle increased the probability of using a long step strategy, enlarged stride length modifications and resulted in higher success rates, to avoid the obstacle. We also examined the effects of the startle in a condition in which the obstacle was not present in comparison to a condition in which the obstacle was visibly present but it did not fall. In the latter condition, the obstacle avoidance reaction occurred with a similar latency but smaller amplitude as in trials in which the obstacle was actually released. Our results suggest that the motor programmes used for obstacle avoidance are probably stored at subcortical structures. The release of these motor programmes by a startling auditory stimulus may combine intersensory facilitation and the StartReact effect.
When a startling auditory stimulus is applied at the same time as the imperative signal in a simple reaction time task experiment, subjects execute the required task significantly faster while maintaining the basic motor program undisturbed (Valls-Soléet al. 1995, 1999; Carlsen et al. 2004a,b). The underlying physiological mechanisms of this phenomenon, termed StartReact effect (Valldeoriola et al. 1998; Valls-Soléet al. 1999), are not completely clear yet. It is suggested that, during preparation, simple reaction time motor programmes become fully represented in subcortical motor structures, where they are accessible to activation by external stimuli (Valls-Soléet al. 1999; Carlsen et al. 2004a,b; Kumru & Valls-Solé, 2006; Castellote et al. 2007). This may also explain the observation of Carlsen et al. (2004a) who found no significant effect of an auditory startle in choice reaction time tasks, in which the preprogramming of a response may not be possible. However, this seems not always to be the case since many authors have reported on the speeding up of movements in some forms of choice reaction time tasks (Valls-Solé, 2004; Kumru et al. 2006; Reynolds & Day, 2007; Oude Nijhuis et al. 2007). Another possibility to explain the StartReact effect is that the energy of the stimulus used as imperative signal increases with the presence of the startle, inducing the so-called intersensory facilitation (Nickerson, 1973; Gielen et al. 1983; Schmidt et al. 1984) and attributing the responses to the joint stimulation of multiple sensory modalities.
A few studies have reported on the effects of an auditory startle on some complex automatic movements such as walking (Schepens & Delwaide, 1995; Nieuwenhuijzen et al. 2000), gait initiation (MacKinnon et al. 2007) and sit-to-stand (Queralt et al. 2008). In most instances, latency shortening was the only change observed in the patterned activity. This suggests again that the subcortical motor structures responsible for the execution of automatic or overlearned motor tasks were activated by a startling auditory stimulus. A recently published example of such effect is the startle-induced shortening of reaction time when adjusting a stepping movement to the right or to the left (Reynolds & Day, 2007). The direction of the adjustments was not known in advance and was guided by the visual stimulus. Reynolds & Day (2007) suggested that shortening of stepping reactions could be particularly relevant in situations such as obstacle avoidance, when fast stepping adjustments are of the utmost importance. Avoiding a suddenly appearing obstacle during walking is a reaction time task where subjects have to perform fast gait adjustments. The strategy for obstacle avoidance to be adopted, i.e. lengthening or shortening of the stride, is influenced by the ongoing gait phase. This is an important difference with respect to the results reported by Carlsen et al. (2004a) who used a choice reaction time protocol requiring simple ballistic movements. In our work and in that reported by Reynolds & Day (2007), a choice reaction time task was implemented in an ongoing movement. These obstacle avoidance reactions are faster than voluntary reactions (Weerdesteyn et al. 2004), suggesting that subcortical pathways might be involved. However, so far, nothing is known about gait adjustments to an obstacle when an auditory startle is given. Therefore, the present study was carried out to investigate the effects of a startling auditory stimulus on obstacle avoidance at different phases of the gait cycle. We aimed at expanding our knowledge regarding motor control during gait. Further specific goals were to assess if startling auditory stimulus speeds up the impending movement in a situation of choice reaction time task under the constraints of time and the functional implications of the presence of an auditory startle in obstacle avoidance tasks. Another goal of the present study was to study how perturbations can affect gait. From previous work it is known that startle responses can be integrated surprisingly well in normal gait (Nieuwenhuijzen et al. 2000). However, it is unknown how such responses affect more complex gait, such as occur when stepping over obstacles. Such questions are important, for example, for our understanding of how gait perturbations can lead to a fall.
A second related question was whether the actual observation of the obstacle movement was an absolute requirement for a StartReact effect on obstacle avoidance. Some studies reported that when the acoustic stimulus was delivered during the foreperiod of a reaction time experiment, the reaction was indistinguishable from the one observed when the startle was delivered together with the imperative signal (Valls-Solé, 2004; Kumru & Valls-Solé, 2006). Similarly, MacKinnon et al. (2007) found that subjects were already prepared for right leg step initiation even before the imperative stimulus for a choice reaction was given. These seemingly ‘inappropriate’ reactions indicate that a startling auditory stimulus releases involuntarily a subcortically prepared motor programme. Based on these observations we hypothesized that obstacle avoidance reactions could be elicited even in the absence of the obstacle actually falling. To test this idea, we used two conditions: one in which the obstacle was not present at all and another one in which the obstacle was visibly present but did not fall into the subject's path.
Methods
Participants
Twelve healthy adults (10 women, 2 men, mean age 25.67 ± 6.69 years) participated in the study. None of them suffered from any hearing, neurological or motor disorder that could interfere with the experiments. None had participated in previous experiments implying the methods used in this study, which was approved by the local medical ethics committee and was conducted in accordance with the Declaration of Helsinki. All subjects gave written informed consent to participate in the study.
Procedure
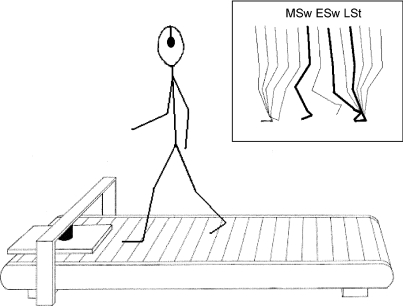
Two experiments were done in separate sessions. In the first experiment, participants walked on a treadmill at a fixed speed of 3 km h−1 wearing flexible gymnastic shoes and binaural earphones (Fig. 1). The obstacle, a wooden board measuring 40 cm × 30 cm × 1.5 cm, was suspended from a bridge via a small metallic piece attached to the middle part of the obstacle, held by a computer-operated electromagnet (Schillings et al. 1996, 1999, 2000; Weerdesteyn et al. 2003) that could be released by a trigger from the computer. It was placed in front of the subject at a distance of approximately 10 cm from the most anterior position reached by the toes in the swing phase.
Figure 1. Schematic diagram of the experimental setup.
The electromagnet is attached to a bridge over the front of the treadmill. The obstacle falls onto the treadmill in front of the subject's left foot after the electromagnet has been switched off by a trigger from the computer. The three obstacle release phases were late stance (LSt), early swing (ESw) and mid-swing (MSw).
After release, the obstacle always dropped in front of the left foot. Three reflective markers (diameter 14 mm) were attached to the left foot at heel, hallux and external maleolus. A fourth marker was placed on top of the obstacle. Marker positions were recorded by a 6-camera 3-D motion analysis system (Vicon) at a sample rate of 100 Hz. These marker positions were processed in real time in order to determine the moment of obstacle release related to gait phase. The obstacle was only released when a regular walking pattern was observed and after at least five unperturbed strides had been taken from the start of the trial. Stride regularity was defined as a maximum difference of 50 ms between two consecutive strides. The obstacle was dropped randomly at three different moments of the step cycle (Fig. 1): late stance (45–59% of the step cycle), early swing (60–69%) or mid-swing (70–85%). Note that the later the obstacle is released along the step cycle, the time allowed for the reaction is shorter and the condition becomes more challenging.
A custom-made noise generator delivered unexpected startling stimuli through binaural earphones, consisting of 50 ms white noise with an intensity of 110 dB. The experimental procedure consisted of 60 obstacle avoidance trials, 20 in each of the previously defined phases of the step cycle. The startling auditory stimulus was delivered in 5 trials (obstacle avoidance trials with startle), interspersed among the remaining 15 trials (obstacle avoidance trials without startle) for each step cycle. Startle was delivered at a latency of 40 ms after obstacle release.
The participants were requested to step over the obstacle, and stepping aside from it was specifically discouraged. Any contact with the obstacle was noted as a failure. Surface electromyography (EMG) data were collected from the biceps femoris, the rectus femoris, the tibialis anterior and the gastrocnemius medialis of the left leg. We also recorded the EMG activity from the sternocleidomastoid to check for the presence of a startle reaction. Self-adhesive Ag–AgCl electrodes (Tyco Arbo ECG) were placed approximately 2 cm apart and longitudinally on the belly of each muscle, according to European guidelines (Hermens et al. 1999). The EMG signals were sampled synchronously with the marker data at 1000 Hz.
The second experiment was conducted in 5 of the 12 subjects. The procedure was similar to the first experiment. Subjects performed trials in which the obstacle was released in the same three phases of the step cycle as in the first experiment. Randomly, we presented 21 trials in which an auditory startle was delivered at late stance. In five of them the obstacle was not present (no obstacle trials), in eight the obstacle was visibly present but it did not fall (stationary obstacle trials), and in the remaining eight trials startle was applied as in the first experiment, 40 ms after obstacle release.
In both experiments the number of trials in which the startling auditory stimulus was applied represented no more than 25% of the trials to ensure that subjects did not habituate to the stimulus (Siegmund et al. 2001; Carlsen et al. 2003; Queralt et al. 2008). To be aware of the type of interfering stimuli, subjects performed a few obstacle avoidance trials before beginning with the experiments and they also received a few isolated startling auditory stimuli.
Data analysis
EMG activity was full-wave rectified and low-pass filtered at 25 Hz (zero-lag, second order Butterworth filter). The EMG characteristics were determined for each of the selected muscles as the mean of 30 trials in the stride before obstacle release, which was used as the control stride. Onset latency of the EMG activity was determined by a combination of a computer algorithm and visual observation as the time between obstacle release and the instant at which EMG activity exceeded the average control stride plus 2 s.d.s For each muscle, we determined the rate of response occurrence as the percentage of trials in which an onset latency was detected. Average EMG amplitude was calculated over 100 ms following the muscle onset latency. Response amplitude was normalized with respect to the average activity of the control stride for each muscle. Averages and standard deviation of EMG onset latencies and EMG amplitudes were calculated for all subjects and phases of obstacle release.
During the experiment we noted whether subjects selected a long step strategy or a short step strategy (Chen et al. 1994; Weerdesteyn et al. 2004, 2005) in avoiding the obstacle and the corresponding percentage of trials for each category was calculated. However, as in the second experiment there were trials in which the obstacle did not fall or trials without obstacle, the percentage of stride shortening or lengthening was calculated with respect to the previous step. We also noted whether the trial was successful or unsuccessful and avoidance success rates were calculated for each obstacle avoidance condition.
In order to analyse whether EMG onset latencies, amplitudes and proportions of avoidance strategies were different between both obstacle avoidance conditions (presence or absence of startle) and gait phases, repeated-measures ANOVA was conducted in the first experiment. Differences in stride length modifications between trials with and without startle were tested by means of paired t tests. Wilcoxon Signed Ranks test was also conducted to compare success rates between both conditions because these were not normally distributed due to frequently reaching 100% of success. For the second experiment, due to the small sample size, a Friedman test on the three startle conditions (no obstacle, stationary obstacle and obstacle avoidance) was performed and, if appropriate, post hoc Wilcoxon Signed Ranks test was conducted to determine differences among conditions. Statistical significance was chosen at P = 0.05.
Results
The first muscle activated in all obstacle avoidance trials was biceps femoris. This muscle had the highest rate of response occurrence (75.7%). In line with previous studies (Weerdesteyn et al. 2007), the biceps femoris was considered the prime mover of the obstacle avoidance task. After biceps femoris, there was no consistent patterned activation of other muscles which we recorded from. The rates of response occurrence in other muscles were 53.8% for rectus femoris, 64.9% for tibialis anterior and 47.2% for gastrocnemius medialis. Mean values revealed earlier responses in all subjects when an auditory startle was delivered together with the imperative signal. EMG responses in sternocleidomastoid were present in 77.2% of the auditory startle trials and the average onset latency was 56.1 ± 7.4 ms. Startle habituation was not observed.
The effect of startling auditory stimulus on obstacle avoidance
Onset latencies
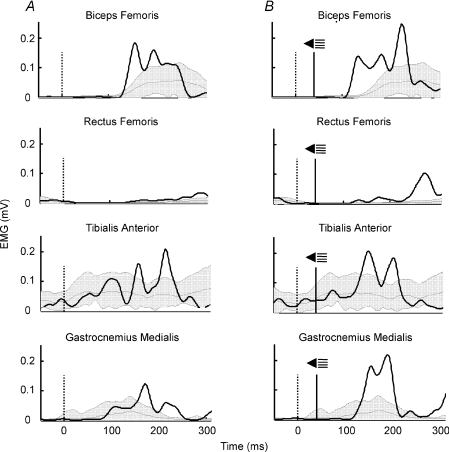
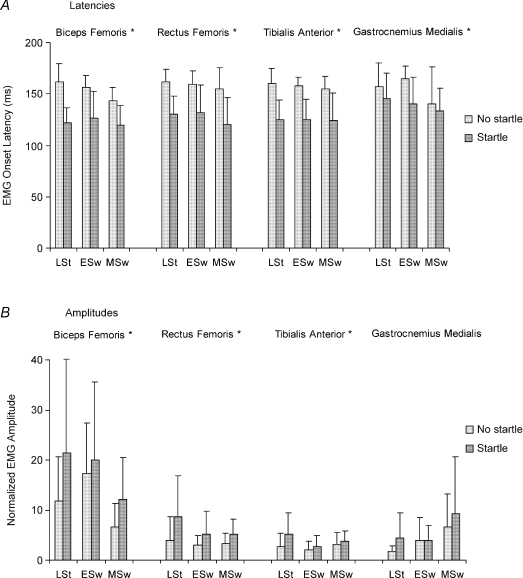
A noticeable shortening of the response onset was observed in all subjects when an auditory startle was delivered with the imperative signal. Two single representative mid-swing trials are shown in Fig. 2. This effect was seen for all muscles and conditions (Fig. 3A). The percentage of shortening taking all obstacle release conditions together was 20.0% for biceps femoris, 19.6% for rectus femoris, 20.9% for tibialis anterior and 9.4% for gastrocnemius medialis, values which correspond to earlier responses of 30.7, 31.1, 33.0 and 14.4 ms, respectively. Differences in EMG onset latencies between trials with and without startle were also seen in the second experiment. The percentage of shortening was 17.7% for biceps femoris, 15.4% for rectus femoris, 13.0% for tibialis anterior and 10.7% for gastrocnemius medialis, with earlier responses of 28.6, 25.1, 19.9 and 19.1 ms, respectively. Statistical analysis for the first experiment showed that the effect of startle was significant for all muscles (F1,11= 32.83, P < 0.001 for biceps femoris; F1,9= 24.50, P < 0.005 for rectus femoris; F1,10= 44.52, P < 0.001 for tibialis anterior; F1,9= 6.60, P < 0.05 for gastrocnemius medialis). There was no significant effect of phase (P > 0.05), except for biceps femoris (F2,22= 5.71, P < 0.05), with earlier responses in mid-swing condition followed by early swing and late stance conditions.
Figure 2. Examples of EMG responses for obstacle avoidance.
EMG activity of biceps femoris, rectus femoris, tibialis anterior and gastrocnemius medialis in response to an obstacle release at mid-swing. Representative trials from one subject. A, no startle trial. B, startle trial. The vertical dotted line indicates the obstacle release moment. The vertical continuous line shows when the startle was given. The shaded area represents mean and ±.2 s.d.s of EMG activity of the control stride. Superimposed (continuous line) is the trace of the representative trial. The obstacle was released at 72.8% of the step cycle in A and at 71.1% of the step cycle in B, which accounts for the slight delay of the control stride in B with respect to A (difference of 20 ms).
Figure 3. EMG effects of startling auditory stimulus on obstacle avoidance.
Mean values and standard deviation of onset latencies (A) and amplitudes (B) of EMG activity in biceps femoris, rectus femoris, tibialis anterior and gastrocnemius medialis muscles in response to an obstacle for no startle and startle trials. Obstacle release phases were late stance (LSt), early swing (ESw) and mid-swing (MSw). *P < 0.05 between startle and no startle conditions.
Amplitude of EMG bursts
The response to the approaching obstacle in trials with startle was also characterized by larger amplitudes of EMG bursts in comparison to those without startle (Fig. 3B). Differences in response amplitudes were significant in biceps femoris (F1,11= 10.98, P < 0.05), in rectus femoris (F1,9= 14.31, P < 0.005) and in tibialis anterior (F1,10= 6.61, P < 0.05). Overall, there was no consistent phase dependency in the startle-related change of EMG amplitudes. As expected, EMG amplitudes of trials with startle were also significantly larger than those without startle in the second experiment.
Stride modifications and success rates
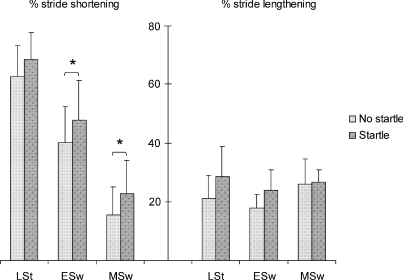
Obstacle avoidance strategies differed according to gait phase. Mean percentages of long step strategy are presented in Table 1. Generally, long step strategy was less often used if the time pressure increased (main effect of phase, F2,10= 11.34, P < 0.005). The presentation of an auditory startle together with the imperative signal caused a significant change in strategy, increasing the use of long step strategy by 13.7% (late stance trials), 19.5% (early swing trials) and 2.5% (mid-swing trials) (main effect of startle, F1,11= 13.97, P < 0.005). There was no interaction effect between phase and startle. In the second experiment, in which only late stance trials were performed together with startle, long step strategy was used in 45.6% of trials without startle and in 62.1% of those with startle. Therefore, the results were similar to experiment 1, where increased incidence of long step strategy was observed when an auditory startle was given. Within each strategy the amount of stride shortening or lengthening of the obstacle avoidance stride was affected by startle as well. Both percentages of stride shortening (in case of short step strategy) and lengthening (in case of long step strategy) were higher when the obstacle was presented together with an auditory startle in any of the obstacle release conditions (Fig. 4). These changes were significant in stride shortening for early swing and mid-swing conditions (P = 0.015 for early swing, P = 0.002 for mid-swing).
Table 1.
Mean percentages of long step strategy for obstacle avoidance
| Late stance | Early swing | Mid-swing | |
|---|---|---|---|
| Startle trials | 48.0 (44.2) | 15.6 (28.4) | 9.0 (28.8) |
| No startle trials | 61.7 (38.3) | 35.1 (44.0) | 11.5 (29.5) |
Data are the mean (s.d.) for each phase of the step cycle (Late stance, Early swing and Mid-swing).
Figure 4. Stride modification effects of startling auditory stimulus on obstacle avoidance.
Mean percentages and standard deviation of stride shortening or lengthening for no startle and startle trials. Obstacle release phases were late stance (LSt), early swing (ESw) and mid-swing (MSw). *P < 0.05. Note: the number of trials in each condition was different (see Table 1) since there were few late stance trials in which subjects performed a short step strategy (or early swing and mid-swing trials in which subjects performed a long step strategy). This may partly explain why significance was only obtained for early swing and mid-swing in stride shortening.
Success rates in obstacle avoidance trials without startle were high, for all phases (late stance 99.4%; early swing 99.5%; mid-swing 92.7%). However, when a startling auditory stimulus was presented along with obstacle release, success rate was 100% at all phases (late stance, early swing, mid-swing). Wilcoxon Signed Ranks test revealed that success rates in obstacle avoidance trials with startle were significantly higher (P = 0.025) than in those without startle.
Responses to startling auditory stimulus in obstacle conditions
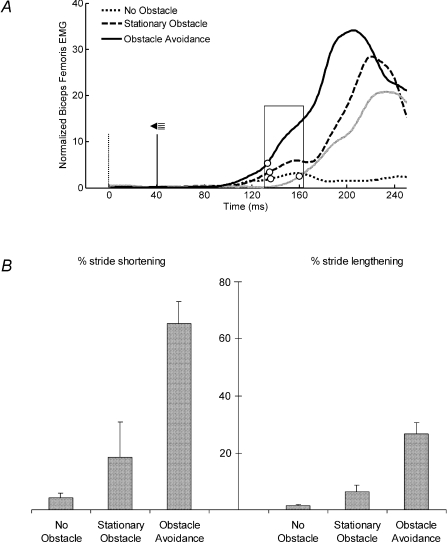
The average normalized EMG responses in the three startle conditions are shown in Fig. 5A together with the obstacle avoidance condition without startle (added for comparison). Onset latencies were clearly similar in conditions in which an auditory startle was delivered. Statistical analysis showed that differences in EMG onset latencies were not significant for any muscle (P > 0.05). Therefore, the mean onset latencies when a startle was applied together with the obstacle were not significantly different with respect to mean onset latencies measured when the stimulus was applied but the obstacle did not fall or it was not present (Fig. 5A). However, the rate of response occurrence was different for each condition. For instance, activation of biceps femoris, prime mover of the obstacle avoidance task, was observed in 52.0% of no obstacle trials, in 100.0% of stationary obstacle trials and in 90.0% of obstacle avoidance trials. Also, the mean amplitude of EMG activity was different in the three startle conditions. The largest EMG amplitude was observed in obstacle avoidance trials, followed by stationary obstacle trials, while the smallest amplitude was observed in no obstacle trials. These amplitudes were significantly different in biceps femoris (Friedman's statistic [2]= 10.00, P < 0.05) and rectus femoris (Friedman's statistic [2]= 6.63, P < 0.05). Post hoc analysis revealed that the amplitudes of biceps femoris in any of the three conditions were significantly different from each other. Furthermore, if we observed the time window indicated by a box in Fig. 5A, it is clear that the obstacle avoidance response when a startle was given was not just a summation of startle and obstacle avoidance separately. For this area, EMG amplitude of the obstacle avoidance condition with startle was 76.1% higher than the sum of stationary obstacle and obstacle avoidance without startle conditions.
Figure 5. Responses to startling auditory stimulus in obstacle conditions.
A, averaged EMG data of all subjects for biceps femoris in three different startle conditions (no obstacle, stationary obstacle and obstacle avoidance). Obstacle avoidance condition without startle (grey trace) is added for comparison. Open circles in the traces indicate mean onset latency of each condition. The vertical dotted line indicates the obstacle release moment. The vertical continuous line shows when the startle was given. The time window used to compare the amplitudes of the various conditions is indicated by a box. B, mean percentages and standard deviation of stride shortening or lengthening for startle conditions.
The percentages of stride shortening and lengthening are shown in Fig. 5B. In the obstacle avoidance condition the stride was clearly shortened (when a short step strategy was performed) or lengthened (when a long step strategy was performed). Barely perceptible modifications were observed in the no obstacle condition. However, the tendency to shorten and lengthen the stride in stationary obstacle condition was present. Both percentages of stride shortening and lengthening were significantly different among conditions (Friedman's statistic [2]= 10.00, P < 0.05).
Discussion
To our knowledge, this is the first study to investigate the effects of an auditory startle on the response to an obstacle avoidance task. Our main results are that when the startling auditory stimulus was applied together with the obstacle, subjects not only reacted faster but also had a more effective performance with fewer errors.
Response latencies and amplitudes
The onset latency of the EMG bursts recorded in startle trials requiring obstacle avoidance was shorter than in those without startle, an effect that was most strongly seen in the biceps femoris. The biceps femoris is also the most consistently activated muscle in avoidance responses following obstacle release (Weerdesteyn et al. 2007). This is in line with the speeding up of a reaction without its perturbation, as seen in the StartReact effect. The fact that in obstacle avoidance tasks, gait adjustments are done faster than other voluntary reactions (Weerdesteyn et al. 2004) led to the suggestion that subcortical motor structures may already be prepared to react with a patterned program to the presentation of an external visual stimulus. The present data are in line with this suggestion. Not only onset latencies benefited from the startle but also response amplitudes. Enhancement of amplitudes to a startle has been found in preparation for movements in simple reaction time tasks and is assumed to result from a sustained level of enhanced excitability in startle pathways preceding the onset of movement (Kumru & Valls-Solé, 2006).
In a separate experiment, we investigated whether such startle-induced effects on latencies and amplitudes were also present in the absence of the imperative stimulus. Subjects were given a startle when the obstacle was either not present, or was present but did not fall. The results clearly showed that the rate of response occurrence in biceps femoris was low when the obstacle was not present. However, in the presence of the obstacle subjects expect it to fall and engage in preparation of the appropriate motor program. In this situation, the startling auditory stimulus would trigger the prepared subcortical response without the obstacle actually falling, as it has been observed in simple reaction time experiments when the auditory startle was presented before the imperative signal (Valls-Solé, 2004; Kumru & Valls-Solé, 2006). In our experiment, the reaction to the startle, when subjects were expecting the obstacle to fall, had the same latency as the reaction to the obstacle combined with startle. Furthermore, we also observed a tendency of shortening or lengthening the stride in the trials in which subjects did not have an obstacle to avoid but the obstacle was present. In contrast, when the obstacle was not present, subjects did not make any gait adjustments when an auditory startle was given (Fig. 5B). These findings further strengthen the hypothesis of subcortically prepared responses triggered by an auditory startle.
It should be emphasized, nevertheless, that the response to a startle in trials with a stationary obstacle was smaller in amplitude than in trials that required obstacle avoidance. Such difference could be explained by assuming that the actual observation of the obstacle moving is a potent visual stimulus that provides a powerful extra input to the neural structure involved in generating the response. This may well be evidence of an intersensory facilitation hypothesis, in which facilitation occurs when inputs from various modalities (auditory startle and visual input, in this case) are added (Nickerson, 1973; Terao et al. 1997; Siegmund et al. 2001). Intersensory facilitation can only explain a small part of the StartReact effect (Valls-Soléet al. 1995, 1999; Sanegre et al. 2004). The data gathered in the present study suggest that the two effects may be complementary to each other. When a startle was applied with a stationary obstacle, latencies of the reaction were shortened to a similar extent as in trials in which an obstacle had to be avoided, suggesting a StartReact effect. However, the amplitude of the EMG activity became larger when avoiding the obstacle, which suggests a further role of visual inputs in augmentation of the response, a feature compatible with intersensory facilitation.
Startle and stride modifications
If the obstacle was released in the late stance phase our subjects selected more frequently the long step strategy than in early swing and mid-swing phases. The finding that the proportion of long step strategies increases when the obstacle is presented earlier in the step cycle is in line with the studies of Chen et al. (1994) and Weerdesteyn et al. (2005). Patla et al. (1999) proposed that the main criterion for this selection of alternate foot placement is the minimal displacement of the foot from its original landing position. Despite that, inter-individual differences have been reported (Weerdesteyn et al. 2004, 2005). In our subjects, the startling auditory stimulus resulted in a more frequent use of the long step strategy. One explanation for this tendency could be related to the startle-induced shortening of the response onset latencies that shifts the response to a slightly earlier phase in the step cycle. This would increase the likelihood of using a long step strategy. Furthermore, some authors described the startle as a generalized motor response where flexor activity dominates (Landis & Hunt, 1939; Rossignol, 1975; Davis, 1984). In addition, a characteristic of the StartReact effect is that muscles highly prepared to react are indeed those that are activated first when an auditory startle is presented together with the imperative signal (Valls-Soléet al. 1999). The biceps femoris, which is one of the main knee flexor muscles, is also a prime mover for the obstacle avoidance task (Weerdesteyn et al. 2007). Furthermore, the startling auditory stimulus preferentially activates upper leg muscles such as biceps femoris (Nieuwenhuijzen et al. 2000). This predicts that the biceps femoris would be more rapidly and strongly activated in trials with startle, as was indeed shown by the present study. The result would be a faster and stronger knee flexion, which may more easily lead to a long than to a short step strategy.
Functional significance
In the present obstacle avoidance task, quick and large activation of the prime movers may be a determining factor to achieve success. In fact, in a study of Weerdesteyn et al. (2007), shorter latencies and larger response amplitudes were significantly associated with higher success rates. The shortening in latency and the increase in amplitude in trials with a startling auditory stimulus could provide a functional advantage in avoiding obstacles during gait. In addition, slow reaction times in choice stepping tasks have been identified as an excellent predictor of falls (Lord & Fitzpatrick, 2001). As a consequence, some authors have used step training to improve the speed of voluntary step initiations in both young and old subjects (Rogers et al. 2003). In their study, step initiation times could be reduced up to 17% but elderly consistently took longer steps than young subjects, presumably to extend their stability margin. The reduced step times were linked to the potentially startling waist pulls used for the training. Hence it is conceivable that an auditory StartReact effect would yield similar results if incorporated in a training program (see also Valls-Soléet al. 1999). The success of such intervention will probably be linked to the ability to solve the problem of an increased threat to stability in the populations concerned. For example, it can be predicted that vestibular loss patients will have greater difficulty with such training because they probably are less equipped to perceive the increased risk of instability linked to a quickened stepping response.
Regarding balance, it should be mentioned that speeding up stepping responses may also have consequences for stability. As pointed out by Reynolds & Day (2005), the stability during gait depends on predictive mechanisms, which result in a pre-step throw of the body (Lyon & Day, 2005; see also MacKinnon et al. 2007). Rapid changes in foot trajectory (because of on-line adjustments after seeing an obstacle) have the potential to disturb this process. This may be especially hazardous for elderly people. For example, using a choice stepping response over an obstacle, St George et al. (2007) showed that elderly were much more likely to contact the obstacle when asked to quickly step over it. Furthermore, it was shown that elderly subjects with a history of falls were much more likely to perform slowly on this type of task than non-falling elderly (Lord & Fitzpatrick, 2001). In addition, one should take into account that startle stimuli have the potential to inhibit the motor cortex (Kühn et al. 2004), thereby further increasing the risk of suppressing potentially important cortical reactions aimed at restoring stability. Whether startle stimuli may indeed override these critical balance recovery reactions in those cases where people reach their limits of stability needs to be established in further research, preferentially including groups of patients with balance disorders. Particularly neurological patients of whom the localization of the disorder is well described would be of great interest, as a loss of balance induced by conditions with a startle could provide insight into the brain areas involved in the weighting of task-induced and balance demands. In this respect, the vestibular system would be a good candidate to be considered.
In conclusion, our study shows that a startling auditory stimulus induces a speeded up activation of the main muscle executors used for obstacle avoidance tasks. This finding, along with the observation that obstacle avoidance can be triggered in the absence of an imperative signal (moving obstacle), strengthens the hypothesis that the motor programmes used for obstacle avoidance tasks are fully represented at a subcortical level, where they are readily accessible to a startling auditory stimulus. Intersensory facilitation may play a role in the execution of the entire motor program. There are also clear behavioural effects of an auditory startle on obstacle avoidance tasks. The improvement of success rate, the favouring of long step strategy and the increase of stride shortening (in the case of short step strategy) or lengthening (in the case of long step strategy) are all elements that may be related to a more effective activation of the prime movers leading to a biologically relevant advantage.
Acknowledgments
This study was supported by grants to A.Q. from the Spanish Ministry of Education and Science (AP2003–3658) and to J.M.C. from the Instituto de Salud Carlos III (TPY 1115/07 and TPY 1529/07). The authors gratefully acknowledge Roland Loeffen for technical support.
References
- Carlsen AN, Chua R, Inglis JT, Sanderson DJ, Franks IM. Startle response is dishabituated during a reaction time task. Exp Brain Res. 2003;152:510–518. doi: 10.1007/s00221-003-1575-5. [DOI] [PubMed] [Google Scholar]
- Carlsen AN, Chua R, Inglis JT, Sanderson DJ, Franks IM. Can prepared responses be stored subcortically? Exp Brain Res. 2004a;159:301–309. doi: 10.1007/s00221-004-1924-z. [DOI] [PubMed] [Google Scholar]
- Carlsen AN, Chua R, Inglis JT, Sanderson DJ, Franks IM. Prepared movements are elicited by startle. J Mot Behav. 2004b;36:253–264. doi: 10.3200/JMBR.36.3.253-264. [DOI] [PubMed] [Google Scholar]
- Castellote JM, Kumru H, Queralt A, Valls-Solé J. A startle speeds up the execution of externally guided saccades. Exp Brain Res. 2007;177:129–136. doi: 10.1007/s00221-006-0659-4. [DOI] [PubMed] [Google Scholar]
- Chen HC, Ashton-Miller JA, Alexander NB, Schultz AB. Age effects on strategies to avoid obstacles. Gait Posture. 1994;2:139–146. [Google Scholar]
- Davis M. The mammalian startle response. In: Eaton RC, editor. Neural Mechanism of Startle Behavior. New York: Plenum Press; 1984. pp. 287–351. [Google Scholar]
- Gielen SC, Schmidt RA, Van Den Heuvel PJ. On the nature of intersensory facilitation of reaction time. Percept Psychophys. 1983;34:161–168. doi: 10.3758/bf03211343. [DOI] [PubMed] [Google Scholar]
- Hermens HJ, Freriks B, Merletti R, et al. SENIAM 8: European Recommendations for Surface ElectroMyoGraphy. Enschede The Netherlands: Roessingh Research and Development b.v.; 1999. [Google Scholar]
- Kühn AA, Sharott A, Trottenberg T, Kupsch A, Brown P. Motor cortex inhibition induced by acoustic stimulation. Exp Brain Res. 2004;158:120–124. doi: 10.1007/s00221-004-1883-4. [DOI] [PubMed] [Google Scholar]
- Kumru H, Urra X, Compta Y, Castellote JM, Turbau J, Valls-Solé J. Excitability of subcortical motor circuits in Go/noGo and forced choice reaction time tasks. Neurosci Lett. 2006;406:66–70. doi: 10.1016/j.neulet.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Kumru H, Valls-Solé J. Excitability of the pathways mediating the startle reaction before execution of a voluntary movement. Exp Brain Res. 2006;169:427–432. doi: 10.1007/s00221-005-0156-1. [DOI] [PubMed] [Google Scholar]
- Landis C, Hunt WA. The Startle Pattern. New York: Farrar and Rinehart; 1939. [Google Scholar]
- Lord SR, Fitzpatrick RC. Choice stepping reaction time: a composite measure of falls risk in older people. J Gerontol A Biol Sci Med Sci. 2001;56:M627–M632. doi: 10.1093/gerona/56.10.m627. [DOI] [PubMed] [Google Scholar]
- Lyon IN, Day BL. Predictive control of body mass trajectory in a two-step sequence. Exp Brain Res. 2005;161:193–200. doi: 10.1007/s00221-004-2058-z. [DOI] [PubMed] [Google Scholar]
- MacKinnon CD, Bissig D, Chiusano J, Miller E, Rudnick L, Jager C, Zhang Y, Mille ML, Rogers MW. Preparation of anticipatory postural adjustments prior to stepping. J Neurophysiol. 2007;97:4368–4379. doi: 10.1152/jn.01136.2006. [DOI] [PubMed] [Google Scholar]
- Nickerson RS. Intersensory facilitation of reaction time: energy summation or preparation enhancement? Psychol Rev. 1973;80:489–509. doi: 10.1037/h0035437. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuijzen PHJA, Schillings AM, Van Galen GP, Duysens J. Modulation of the startle response during human gait. J Neurophysiol. 2000;84:65–74. doi: 10.1152/jn.2000.84.1.65. [DOI] [PubMed] [Google Scholar]
- Oude Nijhuis LB, Janssen L, Bloem BR, van Dijk JG, Gielen SC, Borm GF, Overeem S. Choice reaction times for human head rotations are shortened by startling acoustic stimuli, irrespective of stimulus direction. J Physiol. 2007;584:97–109. doi: 10.1113/jphysiol.2007.136291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patla A, Prentice SD, Rietdyk S, Allard F, Martin C. What guides the selection of alternate foot placement during locomotion in humans? Exp Brain Res. 1999;128:441–450. doi: 10.1007/s002210050867. [DOI] [PubMed] [Google Scholar]
- Queralt A, Valls-Solé J, Castellote JM. The effects of a startle on the sit-to-stand manoeuvre. Exp Brain Res. 2008;185:603–609. doi: 10.1007/s00221-007-1185-8. [DOI] [PubMed] [Google Scholar]
- Reynolds RF, Day BL. Rapid visuo-motor processes drive the leg regardless of balance constraints. Curr Biol. 2005;15:48–49. doi: 10.1016/j.cub.2004.12.051. [DOI] [PubMed] [Google Scholar]
- Reynolds RF, Day BL. Fast visuomotor processing made faster by sound. J Physiol. 2007;583:1107–1115. doi: 10.1113/jphysiol.2007.136192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers MW, Jonhson ME, Martinez KM, Mille ML, Hedman LD. Step training improves the speed of voluntary step initiation in aging. J Gerontol A Biol Sci Med Sci. 2003;58:46–51. doi: 10.1093/gerona/58.1.m46. [DOI] [PubMed] [Google Scholar]
- Rossignol S. Startle responses recorded in the leg of a man. Electroencephalogr Clin Neurophysiol. 1975;39:389–397. doi: 10.1016/0013-4694(75)90102-9. [DOI] [PubMed] [Google Scholar]
- St George RJ, Fitzpatrick RC, Rogers MW, Lord SR. Choice stepping response and transfer times: effects of age, fall risk, and secondary tasks. J Gerontol A Biol Sci Med Sci. 2007;62:537–542. doi: 10.1093/gerona/62.5.537. [DOI] [PubMed] [Google Scholar]
- Sanegre MT, Castellote JM, Haggard P, Valls-Solé J. The effects of a startle on awareness of action. Exp Brain Res. 2004;155:527–531. doi: 10.1007/s00221-004-1849-6. [DOI] [PubMed] [Google Scholar]
- Schepens B, Delwaide PJ. Modifications of audio-spinal facilitation during gait in normal man. Electroencephalog Clin Neurophysiol. 1995;97:424–431. doi: 10.1016/0924-980x(95)00137-a. [DOI] [PubMed] [Google Scholar]
- Schillings AM, Van Wezel BMH, Duysens J. Mechanically induced stumbling during human treadmill walking. J Neurosci Meth. 1996;67:11–17. doi: 10.1016/0165-0270(95)00149-2. [DOI] [PubMed] [Google Scholar]
- Schillings AM, Van Wezel BMH, Mulder TH, Duysens J. Widespread short-latency stretch reflexes and their modulation during stumbling over obstacles. Brain Res. 1999;816:480–486. doi: 10.1016/s0006-8993(98)01198-6. [DOI] [PubMed] [Google Scholar]
- Schillings AM, Van Wezel BMH, Mulder TH, Duysens J. Muscular responses and movement strategies during stumbling over obstacles. J Neuropysiol. 2000;83:2093–2102. doi: 10.1152/jn.2000.83.4.2093. [DOI] [PubMed] [Google Scholar]
- Schmidt RA, Gielen SC, Van Den Heuvel PJ. The locus of intersensory facilitation of reaction time. Acta Psychol (Amst) 1984;57:145–164. doi: 10.1016/0001-6918(84)90040-4. [DOI] [PubMed] [Google Scholar]
- Siegmund GP, Inglis JT, Sanderson DJ. Startle response of human neck muscles sculpted by readiness to perform ballistic head movements. J Physiol. 2001;535:289–300. doi: 10.1111/j.1469-7793.2001.00289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terao Y, Ugawa Y, Suzuki M, Sakai K, Hanajima R, Gemba-Shimizu K, Kanazawa I. Shortening of simple reaction time by peripheral electrical and submotor-threshold magnetic cortical stimulation. Exp Brain Res. 1997;115:541–545. doi: 10.1007/pl00005724. [DOI] [PubMed] [Google Scholar]
- Valldeoriola F, Valls-Solé J, Tolosa E, Ventura PJ, Nobbe FA, Martí MJ. The effects of a startling acoustic stimulus on reaction time in patients with different Parkinsonian syndromes. Neurology. 1998;51:1315–1320. doi: 10.1212/wnl.51.5.1315. [DOI] [PubMed] [Google Scholar]
- Valls-Solé J. Contribution of subcortical motor pathways to the execution of ballistic movements. Suppl Clin Neurophysiol. 2004;57:554–562. doi: 10.1016/s1567-424x(09)70394-0. [DOI] [PubMed] [Google Scholar]
- Valls-Solé J, Rothwell JC, Goulart F, Cossu G, Muñoz E. Patterned ballistic movements triggered by a startle in healthy humans. J Physiol. 1999;516:931–938. doi: 10.1111/j.1469-7793.1999.0931u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valls-Solé J, Solé A, Valldeoriola F, Muñoz E, González LE, Tolosa ES. Reaction time and acoustic startle in normal human subjects. Neurosci Lett. 1995;195:97–100. doi: 10.1016/0304-3940(94)11790-p. [DOI] [PubMed] [Google Scholar]
- Weerdesteyn V, Nienhuis B, Geurts ACH, Duysens J. Age-related deficits in early response characteristics of obstacle avoidance under time pressure. J Gerontol A Biol Sci Med Sci. 2007;62:1042–1047. doi: 10.1093/gerona/62.9.1042. [DOI] [PubMed] [Google Scholar]
- Weerdesteyn V, Nienhuis B, Hampsink B, Duysens J. Gait adjustments in response to an obstacle are faster than voluntary reactions. Hum Mov Sci. 2004;23:351–363. doi: 10.1016/j.humov.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Weerdesteyn V, Nienhuis B, Mulder T, Duysens J. Older women strongly prefer lengthening to shortening in avoiding obstacles. Exp Brain Res. 2005;161:39–46. doi: 10.1007/s00221-004-2043-6. [DOI] [PubMed] [Google Scholar]
- Weerdesteyn V, Schillings AM, Van Galen G, Duysens J. Distraction affects the performance of obstacle avoidance. J Mot Behav. 2003;35:53–63. doi: 10.1080/00222890309602121. [DOI] [PubMed] [Google Scholar]