Abstract
The visual system continually adjusts its sensitivity, or ‘adapts’, to the conditions of the immediate environment. Adaptation increases responses when input signals are weak, to improve the signal-to-noise ratio, and decreases responses when input signals are strong, to prevent response saturation. Retinal ganglion cells adapt primarily to two properties of light input: the mean intensity and the variance of intensity over time (contrast). This review focuses on cellular mechanisms for contrast adaptation in mammalian retina. High contrast over the ganglion cell's receptive field centre reduces the gain of spiking responses. The mechanism for gain control arises partly in presynaptic bipolar cell inputs and partly in the process of spike generation. Following strong contrast stimulation, ganglion cells exhibit a prolonged after-hyperpolarization, driven primarily by suppression of glutamate release from presynaptic bipolar cells. Ganglion cells also adapt to high contrast over their peripheral receptive field. Long-range adaptive signals are carried by amacrine cells that inhibit the ganglion cell directly, causing hyperpolarization, and inhibit presynaptic bipolar terminals, reducing gain of their synaptic output. Thus, contrast adaptation in ganglion cells involves multiple synaptic and intrinsic mechanisms for gain control and hyperpolarization. Several forms of adaptation in ganglion cells originate in presynaptic bipolar cells.
The visual system continually adjusts its sensitivity, or adapts, to efficiently encode the immediate lighting environment. Adaptation typically solves two problems: when input signals are weak, adaptation increases neuronal sensitivity, to improve the signal-to-noise ratio, whereas when input signals are strong, adaptation decreases neuronal sensitivity to prevent the response from saturating and thereby losing information (Gaudry & Reinagel, 2007a; Kohn 2007; Wark et al. 2007). At the earliest stages of the visual system, in the retina and lateral geniculate nucleus of the thalamus, neurons adapt primarily to two statistical properties of the input: the mean light intensity and the variance of light intensities over time, also called the ‘contrast’ (Shapley & Victor, 1978; Mante et al. 2005; Bonin et al. 2006). This short review is focused on contrast adaptation and will describe recent studies of the cellular mechanisms for adaptation in mammalian retina.
General mechanisms for adaptation
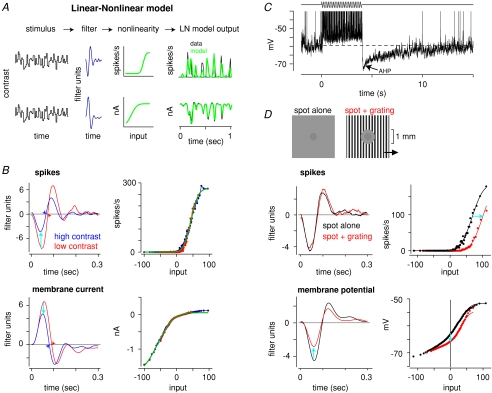
At the outset, it is worth considering what general classes of cellular mechanism could invoke contrast adaptation. As noted above, an adaptive mechanism should reduce sensitivity when contrast is high and increase sensitivity when contrast is low; Fig. 1 shows a simple representation of these principles. The stimulus (Fig. 1A) is a sinusoidal modulation of light (in arbitrary units), where the mean is constant but the contrast switches over time (low → high → low). The ‘responses’ (Fig. 1B–D) reflect the output of retinal ganglion cells with varying degrees and forms of adaptation. The traces represent the membrane potential (cyan) or firing rate (black) in a cell (in arbitrary units). The firing rate exhibits rectification (i.e. negative responses are set to zero) and saturation (i.e. responses cannot exceed 0.6), as in a real cell.
Figure 1. Two general mechanisms for contrast adaptation.
A, a sinusoidal stimulus has a constant mean but contrast switches over time (low → high → low). Stimulus is in arbitrary units (mean = 0, range =−1 to 1). B, a model response with no adaptation. Responses in B–D are plotted in arbitrary units. Responses are intended to be proportional to membrane potential or firing rate in a ganglion cell. The ‘firing rate’ is rectified (responses cannot go below zero) and saturates above 0.6, reflecting firing threshold and saturation in a real cell. C, response adapts by reducing gain at the switch to high contrast and then recovering gain at the switch back to low contrast. The gain variable (green line) is multiplied to the response. Over time, peak firing responses at high contrast are brought below the saturation point. Here and in D there is a slight delay (one-half stimulus cycle) in the onset of adaptation and recovery following a contrast switch. D, response adapts by hyperpolarizing during high contrast. Baseline change variable is added to the response. Over time, peak firing responses at high contrast are brought below the saturation point. At low contrast, the baseline change variable returns to zero, and the initial response level recovers.
In the first example, the response does not adapt (Fig. 1B). The membrane response is modulated in proportion to the stimulus, and at high contrast the firing rate saturates and becomes distorted. In the second example (Fig. 1C), the response adapts by reducing gain at high contrast, and therefore the firing rate no longer saturates. At the switch to low contrast, the gain returns to its initial level, and the response recovers its initial sensitivity. In the third example (Fig. 1D), the response adapts by hyperpolarizing (‘baseline change’), and therefore the firing rate no longer saturates. At the switch to low contrast, the baseline change returns to zero, and the low contrast response recovers its initial sensitivity.
The above example illustrates two ways that visual neurons can adapt and prevent response saturation at high contrast: a reduced gain or a membrane hyperpolarization. Both mechanisms reflect actual processes in neurons within the retina as well as at later stages in cortex (see Kohn, 2007). These two mechanisms need not occur independently, and indeed in the retina gain control and hyperpolarization co-occur (Baccus & Meister, 2002; Zaghloul et al. 2007; see also Lesica et al. 2007). Furthermore, time constants for adaptation and recovery in these simple examples are identical, but in reality these can vary over at least two orders of magnitude. For example, at a switch to high contrast, gain reduces in ∼100 ms, whereas at the switch to low contrast, membrane hyperpolarization can require ∼10 s for recovery (Victor, 1987; Smirnakis et al. 1997; Brown & Masland, 2001; Kim & Rieke, 2001; Baccus & Meister, 2002; Zaghloul et al. 2005; Manookin & Demb, 2006). Finally, Fig. 1 shows adaptation that occurs solely in the ‘membrane’ response, which is then reflected in the ‘firing rate’. However, in reality, adaptation in firing rate can exceed adaptation in the subthreshold response, indicating an intrinsic property for adaptation in the process of spike generation (Kim & Rieke, 2001, 2003; Zaghloul et al. 2005; Beaudoin et al. 2007).
Fast adaptation to contrast (gain control) over the receptive field centre
We studied contrast adaptation in ganglion cells in the in vitro guinea pig retina. The retina remains intact and functional for several hours and responds to light stimuli presented on a small computer monitor focused on the rods and cones (Demb et al. 1999; Manookin et al. 2008). Thus, retinal experiments performed in vitro use the same light stimulus protocols used in vivo. ON- and OFF-centre Y-type/α retinal ganglion cells, found in all mammals, are targeted for recording because this cell type shows strong adaptation to contrast in vivo (Peichl et al. 1987; Shapley & Victor, 1978; Benardete et al. 1992; Benardete & Kaplan, 1999; Solomon et al. 2004). Patch electrodes are used for both loose-patch recording of action potentials and whole-cell recording of membrane voltage or current.
In one set of experiments, we compared contrast adaptation in subthreshold and spiking responses of ganglion cells. A spot was presented over the ganglion cell's dendritic tree, corresponding to the receptive field centre, and spot intensity was updated at 60 Hz with values from a Gaussian distribution (approximating ‘white noise’). Every 10 s, the distribution s.d. switched between 10% (low contrast) and 30% of the mean (high contrast). At each contrast level, the cell's sensitivity was quantified using a linear–nonlinear (LN) cascade analysis (Fig. 2A). In this analysis, the ganglion cell is described as a linear temporal filter that emphasizes certain frequencies in the input (e.g. peak sensitivity at 8 Hz) (Chichilnisky, 2001; Demb, 2002; Carandini et al. 2005; Zaghloul et al. 2005). The filter is convolved with the stimulus to generate a linear model of the response; this linear model (i.e. filtered stimulus) serves as the ‘input’ passed through a static nonlinearity, which works like a ‘lookup table’, and translates linear model values into output values (nA, mV or spikes s−1). The nonlinearity accounts for rectification and saturation in the response. For example, the spike nonlinearity largely reflects the spike threshold (i.e. negative input values are set to zero); there is a similar degree of rectification in the excitatory membrane currents (Fig. 2A). For both the spiking and current measurements, the model accurately predicts the response (Fig. 2A). Thus the LN analysis provides a compact functional description of a cell's response for a given contrast level and output signal (for further quantification of model performance, see Zaghloul et al. 2003, 2007; Beaudoin et al. 2007). The LN analysis is a useful tool for quantifying gain control; a purely linear analysis could under- or overestimate gain change magnitudes due to nonlinearities in the output signal (Chander & Chichilnisky, 2001).
Figure 2. Ganglion cells adapt to contrast by reducing gain and hyperpolarizing.
A, linear–nonlinear cascade analysis used to quantify contrast gain control. A contrast stimulus (random Gaussian flicker) is convolved with a linear temporal filter to generate a linear model of the response. This serves as the ‘input’ to the static nonlinearity, which translates linear model values into LN model output, in real response units (spikes s−1 or nA). Two models are shown for spiking (upper row) and membrane current responses (lower row) at high contrast. Data (averaged over repeats) are shown with the model (for additional details, see Beaudoin et al. 2007). B, contrast gain control can be modelled as a change in the linear filter, with a contrast-independent nonlinearity. (Gain control can be modelled equivalently, after normalizing the filter heights, as a change in the slope of the nonlinear function; Baccus & Meister, 2002; Zaghloul et al. 2007; Kerschensteiner et al. 2008.) Tripling contrast reduces spike filter height (cyan arrows) by ∼40% and membrane filter height by ∼20%. Thus, adaptation occurs both in synaptic inputs to the cell and in the process of spike generation. Integration time (time to zero-crossing) is decreased at high contrast (arrowheads). Spike response was recorded with loose-patch configuration; currents were recorded with whole-cell configuration (Vhold=−73 mV). Green lines indicate a cumulative Gaussian fit to the nonlinearity used to generate the LN model output shown in A. A and B were adapted from Beaudoin et al. (2007) with permission from the Society for Neuroscience. C, strong stimulation of the receptive field centre causes prolonged membrane after-hyperpolarization (AHP). Stimulus was a drifting grating over the receptive field centre (spatial frequency, 6.7 cycles mm−1; drift rate, 6 Hz; contrast, 100%). Spike height is truncated at −40 mV. Adapted from Manookin & Demb (2006) with permission from Elsevier. D, adaptation to contrast in the receptive field periphery of an OFF ganglion cell. Spiking and subthreshold voltage responses, recorded simultaneously, are described by the LN analysis. Adding a high contrast grating to the periphery shifts the spike nonlinearity rightward, indicating an increased threshold for firing. In the subthreshold membrane potential, the grating reduces the gain of the centre response, as represented by reduced filter height (after normalizing the slope of the depolarizing response in the nonlinearities), and causes a steady membrane hyperpolarization, as represented by a downward shift in the nonlinearity. Data are modified from Zaghloul et al. (2007) with permission from The American Physiological Society.
The effect of increasing contrast on the spiking response could be modelled as a change in the linear filter stage, with a contrast-independent nonlinearity (Fig. 2B). Thus, tripling contrast reduced the filter height by ∼40%, reflecting decreased response gain (Figs 1C and 2B) (Zaghloul et al. 2005; Beaudoin et al. 2007). Furthermore, tripling contrast reduced the integration time of the OFF cell filter (i.e. reduced the time to ‘zero-crossing’; Fig. 2B) by up to ∼10%, which reflects an increased sensitivity to high temporal frequencies (Zaghloul et al. 2005; Beaudoin et al. 2007). In the same cells, the gain change could be quantified in the membrane currents using the LN analysis (Fig. 2B). In these responses, tripling contrast reduced gain by only ∼20% (Beaudoin et al. 2007). A similar discrepancy was observed between gain control in spiking and subthreshold voltage responses recorded simultaneously (Zaghloul et al. 2005). Thus, spiking output adapted more than did the synaptic input, indicating that gain control arises partly in the process of spike generation. Similar results were observed previously in salamander ganglion cells (Kim & Rieke, 2001).
Further experiments in guinea pig ganglion cells showed a similar degree of contrast gain control in membrane voltage and current responses and in recordings with low or high calcium buffering. Thus, the mechanism for gain control in the subthreshold response did not depend on an intrinsic voltage- or calcium-dependent mechanism. Furthermore, gain control persisted while minimizing or blocking inhibitory neurotransmitter-gated conductances with voltage-clamp protocols or pharmacology. These results rule out a critical role for inhibitory amacrine cells in driving gain control; the results do not rule out a role for excitatory (e.g. cholinergic, dopaminergic) amacrine cells in driving gain control, but this possibility seems unlikely to us. Furthermore, gain control was absent in retinal horizontal cells, which receive input from cone photoreceptors (Beaudoin et al. 2007). Therefore, gain control in ganglion cell synaptic inputs can apparently be explained by mechanisms at the level of bipolar cells, the excitatory interneurons that relay cone signals to ganglion cells (Fig. 3). An adaptive mechanism in the bipolar cell may be localized to the dendrites, as suggested by recordings in salamander (Rieke, 2001), or at the bipolar cell synapse with the ganglion cell. We measured contrast adaptation in inhibitory currents of an OFF ganglion cell while blocking all ionotropic glutamate receptors (iGluRs) with receptor antagonists. The only known pathway that operates under these conditions (cone → ON cone bipolar cell → AII amacrine cell → OFF ganglion cell) uses metabotropic glutamate receptors (mGluRs), gap junctions and glycine receptors, respectively (Bloomfield & Dacheux, 2001; Manookin et al. 2008). Gain control in OFF ganglion cells persisted with iGluRs blocked and actually increased relatively to baseline conditions (Beaudoin et al. submitted). Thus, at least for this ON bipolar cell-mediated inhibitory circuit, gain control occurs independently of iGluRs, ruling out a critical mechanism at the bipolar cell ribbon synapse. Gain control apparently arises in the ON bipolar cell and is then reflected in the AII amacrine cell input to the ganglion cell.
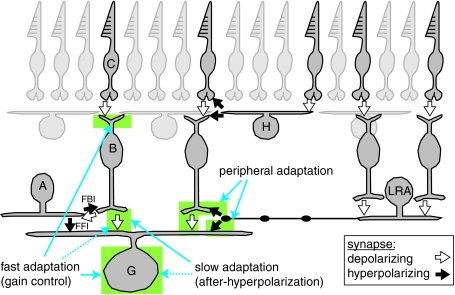
Figure 3. Multiple mechanisms for contrast adaptation in retinal ganglion cells.
Basic retinal organization is illustrated with pathways connecting to an OFF ganglion cell (G). Cones (C) synapse with OFF bipolar cells (B) which synapse with the ganglion cell. Inhibitory signals are transmitted laterally by horizontal cells (H) in the outer retina and by amacrine cells in the inner retina. A ‘long-range’ amacrine cell (LRA) is shown with an axon stretching across the retina. Amacrine cells are driven by bipolar cells, and are therefore sensitive to relatively high spatial frequencies. For clarity, only a subset of bipolar and amacrine cells are shown, but generally all cell types ‘tile’ the retina, providing complete coverage. Fast adaptation (gain control) is driven by an unknown mechanism in the bipolar cell dendrites, a potential synaptic mechanism at the level of bipolar cell output and by an intrinsic property of the ganglion cell. Strong stimulation of ganglion cells results in a slow form of adaptation: prolonged after-hyperpolarization (AHP). The AHP is driven primarily by suppression of bipolar cell glutamate release, with a minor contribution from an intrinsic property of the ganglion cell. Adaptation to contrast in the receptive field periphery is driven by amacrine cell inhibition of the bipolar cell terminal, to reduce gain of the centre response, and direct inhibition of the ganglion cell dendrite, to hyperpolarize the membrane.
An intrinsic mechanism for gain control in ganglion cell spike generation
The intrinsic mechanism for adaptation in ganglion cell spike generation has been studied in isolated salamander ganglion cells by injecting low or high amplitude current into cell bodies to mimic the response to low or high contrast (Kim & Rieke, 2003). In this case, adaptation to large amplitude modulations could be explained by slow inactivation of sodium channels; frequent spike bursts reduced the pool of available sodium channels, resulting in a persistent depression of spiking output. This type of mechanism is predicted by theoretical models of spiking neurons (Yu & Lee, 2003; Gaudry & Reinagel, 2007a,b). Sodium channel inactivation may explain the intrinsic property for adaptation in mammalian ganglion cells, although further studies of intact cells should investigate a potential role of ion channels in the dendrites.
Under some conditions, adaptation depends almost exclusively on an intrinsic property of ganglion cells: under dim light conditions where responses are driven by the rod bipolar pathway, ganglion cell firing showed gain control, whereas synaptic inputs showed almost none (Beaudoin et al. submitted). Furthermore, mice lacking two transcription factors normally expressed in OFF bipolar cells showed reduced contrast adaptation in OFF ganglion cell spiking responses (Kerschensteiner et al. 2008). One interpretation of these results is that adaptation in OFF bipolar cells was reduced or eliminated in these mice, and thus adaptation in ganglion cell spiking reflected solely the contribution of the ganglion cell's intrinsic mechanism; however, further measurements of synaptic inputs in these ganglion cells are required to confirm this interpretation. Some ganglion cell types recorded in vivo lacked contrast adaptation, and thus these cells apparently lack the intrinsic property for gain control found in other cell types (Benardete et al. 1992).
Slow adaptation to contrast over the receptive field centre
Under some conditions, strong stimulation of the receptive field centre is followed by prolonged suppression of firing, which corresponds to prolonged membrane after-hyperpolarization (AHP) (Baccus & Meister, 2002; Brown & Masland, 2001; Solomon et al. 2004; Manookin & Demb, 2006). Figure 2C shows the response of an OFF Y-type ganglion cell to a high contrast, drifting sine-wave grating. This stimulus drove large depolarization and spiking responses. When the stimulus switched back to the mean luminance (0% contrast), the membrane potential was hyperpolarized below rest and required up to ∼10 s to recover. The AHP was only partially explained by an intrinsic property of the cell: direct current injection evoked depolarization and spiking, but the subsequent AHP was much smaller than the visually evoked AHP in the same cells (Manookin & Demb, 2006). A relatively high spatial frequency stimulus was necessary to evoke a large AHP, presumably because the bipolar cells must be strongly stimulated. Pharmacological experiments suggested that the AHP did not depend on synaptic inhibition, Ca2+-activated K+ channels or mGluRs. Thus, the AHP was apparently driven by prolonged depression of glutamate release at bipolar cell synapses (Brown & Masland, 2001; Manookin & Demb, 2006). The mechanism for the AHP is not well understood but may involve vesicle depletion or some other form of short-term depression at the bipolar cell ribbon synapse (Palmer et al. 2003; Singer & Diamond, 2006). The AHP was commonly recorded in OFF Y-type ganglion cells and certain ON cells, but not in the ON Y-type cell (Manookin & Demb, 2006). Thus, a depressive mechanism apparently exists only in certain types of bipolar cell.
Adaptation to contrast over the receptive field periphery
Ganglion cells also adapt to contrast presented in the peripheral region of their receptive field (Werblin, 1972; Shapley & Victor, 1979; Enroth-Cugell & Jakiela, 1980; Solomon et al. 2006). We measured the sensitivity of the receptive field centre, using Gaussian flicker of a spot over the dendritic tree, while switching the contrast in the periphery every 10 s between 0% and 100%. The contrast pattern was a drifting grating, excluded from the centre region, with thin bars of ∼100 μm width (Fig. 2D). This high spatial frequency exceeded the resolution of horizontal cells in the outer retina, but matched the resolution of bipolar cells, which drive long-range amacrine (LRA) cells in the inner retina (Demb et al. 1999; Dacey et al. 2000; Volgyi et al. 2001; Roska & Werblin, 2003; Ölveczky et al. 2007; Zaghloul et al. 2007). These amacrine cells inhibit bipolar cell axon terminals and ganglion cell dendrites (Fig. 3). The effect of the peripheral grating on receptive field centre sensitivity was analysed in both extracellular and intracellular recordings, using the LN analysis described above. The peripheral grating had a relatively large suppressive effect in OFF cells, and so we focus on those cells below (Zaghloul et al. 2007).
The peripheral grating suppressed the spiking response to central contrast, and the effect could be modelled, using the LN analysis, by a rightward shift in the spike nonlinearity (Fig. 2D). In the subthreshold voltage response, the grating caused two effects. There was a ∼20–30% reduction in gain and a tonic membrane hyperpolarization of up to ∼3 mV (Fig. 2D). The reduced gain can be modelled by a reduced filter height (after normalizing the slopes of the nonlinear functions’ depolarizing responses across conditions), whereas the tonic hyperpolarization was quantified by the downward shift in the y-intercept of the nonlinear function (Fig. 2D). Thus, for OFF cells the combination of a gain change and a steady hyperpolarization corresponded to the shift in the spike nonlinearity. The mechanism for peripheral contrast adaptation could be explained by synaptic inhibition at two sites. Voltage clamp measurements showed that the peripheral grating evoked direct inhibition of the ganglion cell dendrite as well as inhibition of the presynaptic bipolar terminal (Zaghloul et al. 2007) (Fig. 3). Using the LN analysis, we showed that the peripheral grating reduced the gain of the centre response similarly in membrane voltage and excitatory synaptic currents, and thus the peripheral grating suppressed the gain of the centre response primarily by suppressing the bipolar terminal rather than by a ‘shunting’ mechanism at the ganglion cell dendrite. Therefore, direct synaptic inhibition of the ganglion cell dendrite partially explained the tonic membrane hyperpolarization, whereas inhibition of the presynaptic bipolar cell terminals primarily explained the gain change in the subthreshold response (Fig. 3).
Conclusions
The retina provides an important model system for understanding how plastic mechanisms within a complex neural network, with a well-understood function, alter the responses of output neurons. Our studies suggest that contrast adaptation in mammalian retinal ganglion cells arises through multiple cellular mechanisms, including: the depression of bipolar cell output; synaptic inhibition of bipolar cell terminals and ganglion cell dendrites by amacrine cells; and intrinsic cellular properties of ganglion cells (Fig. 3). Several adaptive mechanisms occur at the level of bipolar cells and apparently do not require local synaptic inhibition. There are ∼30 types of amacrine cell in mammalian retina (Wässle, 2004), and the Y-type cells under study receive ∼60–80% of their synaptic input from amacrine cells (Freed & Sterling, 1988; Kolb & Nelson, 1993). Thus, the minimal role for local synaptic inhibition was somewhat surprising. For example, there are well-known circuit elements, involving narrow-field amacrine cells, for feedback inhibition (FBI) onto bipolar terminals and feed-forward inhibition (FFI) onto ganglion cells (Fig. 3), but the role of these circuit elements in adaptation remains unclear (Manookin & Demb, 2006; Beaudoin et al. 2007). One possibility is that narrow-field amacrine cells primarily contribute to adaptation to complex stimulus features, beyond the mean or variance of the input (Hosoya et al. 2005). Furthermore, future experiments will be required to test whether excitatory (e.g. cholinergic, dopaminergic) amacrine cells play any role in contrast adaptation.
This review focused on mechanisms for contrast adaptation, but recent studies also elucidated mechanisms for mean luminance adaptation, under rod- and cone-driven light levels (Dunn et al. 2006, 2007; Dunn & Rieke, 2008). Interestingly, the post-photoreceptor circuit mechanisms for mean luminance adaptation also were explained at the level of bipolar cells and did not seem to require synaptic inhibition. Thus, we need to understand further whether there are common or distinct mechanisms for mean luminance and contrast adaptation at the level of bipolar cells.
Acknowledgments
This work was supported by NIH grant EY14454. I thank Dr Daniel Green for comments on the manuscript.
References
- Baccus SA, Meister M. Fast and slow contrast adaptation in retinal circuitry. Neuron. 2002;36:909–919. doi: 10.1016/s0896-6273(02)01050-4. [DOI] [PubMed] [Google Scholar]
- Beaudoin DL, Borghuis BG, Demb JB. Cellular basis for contrast gain control over the receptive field center of mammalian retinal ganglion cells. J Neurosci. 2007;27:2636–2645. doi: 10.1523/JNEUROSCI.4610-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benardete EA, Kaplan E. The dynamics of primate M retinal ganglion cells. Vis Neurosci. 1999;16:355–368. doi: 10.1017/s0952523899162151. [DOI] [PubMed] [Google Scholar]
- Benardete EA, Kaplan E, Knight BW. Contrast gain control in the primate retina: P cells are not X-like, some M cells are. Vis Neurosci. 1992;8:483–486. doi: 10.1017/s0952523800004995. [DOI] [PubMed] [Google Scholar]
- Bloomfield SA, Dacheux RF. Rod vision: pathways and processing in the mammalian retina. Prog Retin Eye Res. 2001;20:351–384. doi: 10.1016/s1350-9462(00)00031-8. [DOI] [PubMed] [Google Scholar]
- Bonin V, Mante V, Carandini M. The statistical computation underlying contrast gain control. J Neurosci. 2006;26:6346–6353. doi: 10.1523/JNEUROSCI.0284-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SP, Masland RH. Spatial scale and cellular substrate of contrast adaptation by retinal ganglion cells. Nat Neurosci. 2001;4:44–51. doi: 10.1038/82888. [DOI] [PubMed] [Google Scholar]
- Carandini M, Demb JB, Mante V, Tolhurst DJ, Dan Y, Olshausen BA, Gallant JL, Rust NC. Do we know what the early visual system does? J Neurosci. 2005;25:10577–10597. doi: 10.1523/JNEUROSCI.3726-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chander D, Chichilnisky EJ. Adaptation to temporal contrast in primate and salamander retina. J Neurosci. 2001;21:9904–9916. doi: 10.1523/JNEUROSCI.21-24-09904.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chichilnisky EJ. A simple white noise analysis of neuronal light responses. Network. 2001;12:199–213. [PubMed] [Google Scholar]
- Dacey D, Packer OS, Diller L, Brainard D, Peterson B, Lee B. Center surround receptive field structure of cone bipolar cells in primate retina. Vision Res. 2000;40:1801–1811. doi: 10.1016/s0042-6989(00)00039-0. [DOI] [PubMed] [Google Scholar]
- Demb JB. Multiple mechanisms for contrast adaptation in the retina. Neuron. 2002;36:781–783. doi: 10.1016/s0896-6273(02)01100-5. [DOI] [PubMed] [Google Scholar]
- Demb JB, Haarsma L, Freed MA, Sterling P. Functional circuitry of the retinal ganglion cell's nonlinear receptive field. J Neurosci. 1999;19:9756–9767. doi: 10.1523/JNEUROSCI.19-22-09756.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn FA, Doan T, Sampath AP, Rieke F. Controlling the gain of rod-mediated signals in the mammalian retina. J Neurosci. 2006;26:3959–3970. doi: 10.1523/JNEUROSCI.5148-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn FA, Lankheet MJ, Rieke F. Light adaptation in cone vision involves switching between receptor and post-receptor sites. Nature. 2007;449:603–606. doi: 10.1038/nature06150. [DOI] [PubMed] [Google Scholar]
- Dunn FA, Rieke F. Single-photon absorptions evoke synaptic depression in the retina to extend the operational range of rod vision. Neuron. 2008;57:894–904. doi: 10.1016/j.neuron.2008.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C, Jakiela HG. Suppression of cat retinal ganglion cell responses by moving patterns. J Physiol. 1980;302:49–72. doi: 10.1113/jphysiol.1980.sp013229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed MA, Sterling P. The ON-alpha ganglion cell of the cat retina and its presynaptic cell types. J Neurosci. 1988;8:2303–2320. doi: 10.1523/JNEUROSCI.08-07-02303.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudry KS, Reinagel P. Benefits of contrast normalization demonstrated in neurons and model cells. J Neurosci. 2007a;27:8071–8079. doi: 10.1523/JNEUROSCI.1093-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudry KS, Reinagel P. Contrast adaptation in a nonadapting LGN model. J Neurophysiol. 2007b;98:1287–1296. doi: 10.1152/jn.00618.2006. [DOI] [PubMed] [Google Scholar]
- Hosoya T, Baccus SA, Meister M. Dynamic predictive coding by the retina. Nature. 2005;436:71–77. doi: 10.1038/nature03689. [DOI] [PubMed] [Google Scholar]
- Kerschensteiner D, Liu H, Cheng CW, Demas J, Cheng SH, Hui CC, Chow RL, Wong RO. Genetic control of circuit function: Vsx1 and Irx5 transcription factors regulate contrast adaptation in the mouse retina. J Neurosci. 2008;28:2342–2352. doi: 10.1523/JNEUROSCI.4784-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KJ, Rieke F. Temporal contrast adaptation in the input and output signals of salamander retinal ganglion cells. J Neurosci. 2001;21:287–299. doi: 10.1523/JNEUROSCI.21-01-00287.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KJ, Rieke F. Slow Na+ inactivation and variance adaptation in salamander retinal ganglion cells. J Neurosci. 2003;23:1506–1516. doi: 10.1523/JNEUROSCI.23-04-01506.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn A. Visual adaptation: physiology, mechanisms, and functional benefits. J Neurophysiol. 2007;97:3155–3164. doi: 10.1152/jn.00086.2007. [DOI] [PubMed] [Google Scholar]
- Kolb H, Nelson R. OFF-alpha and OFF-beta ganglion cells in cat retina. II. Neural circuitry as revealed by electron microscopy of HRP stains. J Comp Neurol. 1993;329:85–110. doi: 10.1002/cne.903290107. [DOI] [PubMed] [Google Scholar]
- Lesica NA, Jin J, Weng C, Yeh CI, Butts DA, Stanley GB, Alonso JM. Adaptation to stimulus contrast and correlations during natural visual stimulation. Neuron. 2007;55:479–491. doi: 10.1016/j.neuron.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manookin MB, Beaudoin DL, Ernst ZR, Flagel LJ, Demb JB. Disinhibition combines with excitation to extend the operating range of the OFF visual pathway in daylight. J Neurosci. 2008;28:4136–4150. doi: 10.1523/JNEUROSCI.4274-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manookin MB, Demb JB. Presynaptic mechanism for slow contrast adaptation in mammalian retinal ganglion cells. Neuron. 2006;50:453–464. doi: 10.1016/j.neuron.2006.03.039. [DOI] [PubMed] [Google Scholar]
- Mante V, Frazor RA, Bonin V, Geisler WS, Carandini M. Independence of luminance and contrast in natural scenes and in the early visual system. Nat Neurosci. 2005;8:1690–1697. doi: 10.1038/nn1556. [DOI] [PubMed] [Google Scholar]
- Ölveczky BP, Baccus SA, Meister M. Retinal adaptation to object motion. Neuron. 2007;56:689–700. doi: 10.1016/j.neuron.2007.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer MJ, Hull C, Vigh J, von Gersdorff H. Synaptic cleft acidification and modulation of short-term depression by exocytosed protons in retinal bipolar cells. J Neurosci. 2003;23:11332–11341. doi: 10.1523/JNEUROSCI.23-36-11332.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peichl L, Ott H, Boycott BB. Alpha ganglion cells in mammalian retinae. Proc R Soc Lond B Biol Sci. 1987;231:169–197. doi: 10.1098/rspb.1987.0040. [DOI] [PubMed] [Google Scholar]
- Yu Y, Potetz B, Lee TS. The role of spiking nonlinearity in contrast gain control and information transmission. Vision Res. 2005;45:583–592. doi: 10.1016/j.visres.2004.09.024. [DOI] [PubMed] [Google Scholar]
- Rieke F. Temporal contrast adaptation in salamander bipolar cells. J Neurosci. 2001;21:9445–9454. doi: 10.1523/JNEUROSCI.21-23-09445.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roska B, Werblin F. Rapid global shifts in natural scenes block spiking in specific ganglion cell types. Nat Neurosci. 2003;6:600–608. doi: 10.1038/nn1061. [DOI] [PubMed] [Google Scholar]
- Shapley RM, Victor JD. The effect of contrast on the transfer properties of cat retinal ganglion cells. J Physiol. 1978;285:275–298. doi: 10.1113/jphysiol.1978.sp012571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapley RM, Victor JD. Nonlinear spatial summation and the contrast gain control of cat retinal ganglion cells. J Physiol. 1979;290:141–161. doi: 10.1113/jphysiol.1979.sp012765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JH, Diamond JS. Vesicle depletion and synaptic depression at a mammalian ribbon synapse. J Neurophysiol. 2006;95:3191–3198. doi: 10.1152/jn.01309.2005. [DOI] [PubMed] [Google Scholar]
- Smirnakis SM, Berry MJ, Warland DK, Bialek W, Meister M. Adaptation of retinal processing to image contrast and spatial scale. Nature. 1997;386:69–73. doi: 10.1038/386069a0. [DOI] [PubMed] [Google Scholar]
- Solomon SG, Lee BB, Sun H. Suppressive surrounds and contrast gain in magnocellular-pathway retinal ganglion cells of macaque. J Neurosci. 2006;26:8715–8726. doi: 10.1523/JNEUROSCI.0821-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon SG, Peirce JW, Dhruv NT, Lennie P. Profound contrast adaptation early in the visual pathway. Neuron. 2004;42:155–162. doi: 10.1016/s0896-6273(04)00178-3. [DOI] [PubMed] [Google Scholar]
- Victor JD. The dynamics of the cat retinal X cell centre. J Physiol. 1987;386:219–246. doi: 10.1113/jphysiol.1987.sp016531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volgyi B, Xin D, Amarillo Y, Bloomfield SA. Morphology and physiology of the polyaxonal amacrine cells in the rabbit retina. J Comp Neurol. 2001;440:109–125. doi: 10.1002/cne.1373. [DOI] [PubMed] [Google Scholar]
- Wark B, Lundstrom BN, Fairhall A. Sensory adaptation. Curr Opin Neurobiol. 2007;17:423–429. doi: 10.1016/j.conb.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wässle H. Parallel processing in the mammalian retina. Nat Rev Neurosci. 2004;5:747–757. doi: 10.1038/nrn1497. [DOI] [PubMed] [Google Scholar]
- Werblin FS. Lateral interactions at inner plexiform layer of vertebrate retina: antagonistic responses to change. Science. 1972;175:1008–1010. doi: 10.1126/science.175.4025.1008. [DOI] [PubMed] [Google Scholar]
- Yu Y, Lee TS. Dynamical mechanisms underlying contrast gain control in single neurons. Phys Rev E Stat Nonlin Soft Matter Phys. 2003;68:011901. doi: 10.1103/PhysRevE.68.011901. [DOI] [PubMed] [Google Scholar]
- Zaghloul KA, Boahen K, Demb JB. Different circuits for ON and OFF retinal ganglion cells cause different contrast sensitivities. J Neurosci. 2003;23:2645–2654. doi: 10.1523/JNEUROSCI.23-07-02645.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaghloul KA, Boahen K, Demb JB. Contrast adaptation in subthreshold and spiking responses of mammalian Y-type retinal ganglion cells. J Neurosci. 2005;25:860–868. doi: 10.1523/JNEUROSCI.2782-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaghloul KA, Manookin MB, Borghuis BG, Boahen K, Demb JB. Functional circuitry for peripheral suppression in mammalian Y-type retinal ganglion cells. J Neurophysiol. 2007;97:4327–4340. doi: 10.1152/jn.01091.2006. [DOI] [PubMed] [Google Scholar]