Abstract
Detection of the direction of image movement is accomplished first in the retina by an elegant neuronal circuit, which integrates multiple levels of spatially asymmetric synaptic interactions among subsets of bipolar, amacrine and ganglion cells. Central to these interactions is the asymmetric GABAergic inhibition exerted by the starburst amacrine cell (SAC), a cholinergic and GABAergic interneuron with a radially symmetric dendritic tree. SACs make reciprocal GABAergic synapses on each other to create a direct inhibitory receptive field surround, which suppresses the response of each SAC to centripetal image movement. Each radially projecting branch of a SAC responds to image movement with a centrifugal bias and, through directionally asymmetric synaptic connections with the dendrites of direction-selective ganglion cells (DSGCs), exerts a spatially offset inhibition that vetoes the response of DSGCs to image movement in a specific (null) direction. Recent physiological studies have greatly advanced our understanding of the mechanism of direction selectivity and also revealed a new level of complexity that remains to be understood.
The ability of the retina to detect the direction of image movement was discovered in the early 1960s by Barlow, Hill and Levick in a series of classic studies that showed that certain types of retinal output neurons respond vigorously to an image moving across their receptive fields in a particular (preferred) direction, but give little or no response to the same image moving in the reverse (null) direction (Barlow & Hill, 1963; Barlow et al. 1964; Barlow & Levick, 1965). The main synaptic circuit that performs this fundamental computation is now believed to reside in the inner plexiform layer (IPL) of the retina and consist of only a handful of cell types. This circuit is functionally well defined and experimentally approachable, making it an ideal model system for understanding neuronal interaction and network computation. Indeed, the mechanism underlying direction selectivity has been under intense investigation for the past four decades (Taylor & Vaney, 2003; Demb, 2007; Fried & Masland, 2007). However, even in this seemingly simple system (as compared to many other brain areas), the nature of cellular and synaptic interactions is far from simple. The wiring diagrams and signalling codes embedded in the direction-selective circuit have been enigmatic and have only recently begun to reveal some key mechanistic details.
Two types of DSGCs have been found in the rabbit retina (Barlow et al. 1964), the On and On–Off types, which can be further classified into three and four subtypes, respectively, based on their preferred directions in the retina (Oyster & Barlow, 1967; Oyster et al. 1972). DSGCs project to both the midbrain and the dorsal lateral geniculate nucleus of the thalamus (Buhl & Peichl, 1986; Pu & Amthor, 1990a,b; Dacey et al. 2003) and are believed to be involved in multiple functions, including the control of extraocular muscles for eye movement and optokinetic nystagmus (Oyster & Barlow, 1967; Oyster et al. 1972). Because most of the mechanistic properties of DSGCs are derived from On–Off DSGCs, which are more numerous than On DSGCs, the synaptic physiology discussed below is based mainly on studies of On-Off DSGCs, which will be referred to simply as DSGCs henceforth.
Synaptic physiology of direction-selective ganglion cells
Barlow & Levick (1965) suggested that the direction selectivity of ganglion cells is built from sequence-discriminating subunits. The primary mechanism for this discrimination was thought to result from a spatially offset lateral inhibition, which vetoes responses to sequences corresponding to stimulus movement in the null direction. Pharmacological experiments subsequently found that GABA receptor antagonists block direction selectivity by bringing out strong responses of DSGCs to stimulus movement in the null direction, suggesting that the spatially offset inhibition is mediated by GABA (Wyatt & Day, 1976; Caldwell et al. 1978); however, the site of this critical GABAergic inhibition had remained unclear for a long time. Direct measurement of the inhibitory input to DSGCs became possible when patch-clamp recordings were successfully applied to DSGCs in the whole-mount retina in the early 2000s (Taylor et al. 2000; Borg-Graham, 2001). Fried et al. (2002) subsequently concluded that (1) the direct inhibitory current input to a DSGS is larger for stimulus movement in the null direction than that in the preferred direction; (2) the inhibitory input precedes the excitatory input when the stimulus moves in the null, but not in the preferred direction; (3) the spatial extent of the inhibitory current input is offset from the DSGC dendritic field toward the null direction. The excitatory current input to a DSGC is also directionally asymmetric: larger for preferred direction movement than for null direction movement; however, the spatial extent of the excitatory input roughly coincides with the DSGC dendritic field (Fried et al. 2002; Taylor & Vaney, 2002). These findings establish that the synaptic inputs to a DSGC are already directionally asymmetric.
GABAergic inputs from starburst amacrine cells to direction selective ganglion cells
The dendrites of DSGCs costratify with those of cholinergic (starburst) amacrine cells, which exist as two mirror-symmetric populations across the IPL (Masland & Mills, 1979; Vaney et al. 1981; Famiglietti, 1992). SACs have four to five primary dendrites, each branching out regularly into secondary and tertiary processes, with numerous varicosities imbedded in the distal dendritic zone (Famiglietti, 1983; Tauchi & Masland, 1984; Vaney, 1984). The dendritic tree emanates from the soma with a nearly perfect radial symmetry, rendering the cell a ‘starburst’ appearance and, hence, its popular name (Famiglietti, 1983). The dendrites of DSGCs also cofasciculate with starburst dendrites (Famiglietti, 1992; Vaney & Pow, 2000; Dong et al. 2004), suggesting intimate synaptic interactions between the two cell types, as evidenced by the electron-microscopic findings of direct synapses between them (Dacheux et al. 2003). Because SACs contain and release not only ACh, but also GABA (Brecha et al. 1988; Vaney & Young, 1988; O'Malley & Masland, 1989), they have long been proposed as a likely source of the critical lateral inhibition to DSGCs (Vaney, 1990; Borg-Graham & Grzywacz, 1992).
Functional evidence for a key role of SACs in direction selectivity came first from the findings that immunotoxin and neurotoxin ablation of SACs resulted in an apparent elimination of direction-selective responses in the retina, as well as a loss of optokinetic nystagmus (Yoshida et al. 2001; Amthor et al. 2002). Direct detection of a GABAergic input from SACs to DSGCs was accomplished first by Fried et al. (2002), who recorded simultaneously from pairs of SACs and DSGCs in the whole-mount rabbit retina and showed that DSGCs receive inhibitory synaptic inputs from SACs located on their null, but not their preferred, side. This important result, which was initially derived from a small number of paired recordings, was subsequently confirmed by many paired recordings (Lee et al. 2006), and it establishes that SACs exert a spatially offset GABAergic inhibition directly on DSGCs via directionally asymmetric hardwiring between SACs and DSGCs. This landmark finding forms the basis of the current model of direction selectivity of DSGCs (Fried et al. 2002; Taylor & Vaney, 2003) (Fig. 1). However, for this asymmetric wiring model of direction selectivity to work, the release of GABA from each dendritic branch of a SAC must also be directionally selective, as implicated by the finding that the inhibitory input to a DSGC is greatly reduced during either preferred-direction motion or two-spot flashes that simulate preferred-direction motion (Fried et al. 2002, 2005; Taylor & Vaney, 2002) (Fig. 2). Otherwise, SAC dendrites that make GABAergic synapses onto a DSGC from the null side will still suppress the DSGC when a light stimulus moves in the preferred direction, even though such a GABAergic inhibition is not expected to lead the glutamatergic excitation. Thus, a key component of the direction selective mechanism must reside upstream from DSGCs in SACs.
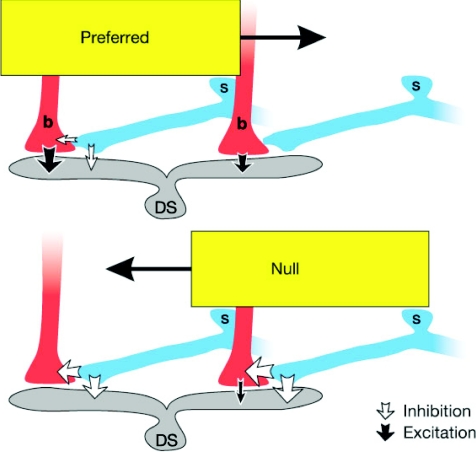
Figure 1. A current model of direction selectivity in DSGCs based on asymmetric wiring between SACs and DSGCs.
The processes of starburst cells (‘s’, blue) that point in the null direction provide inhibition to DS cell dendrites (‘DS’, grey). Starburst processes respond best to movement away from the cell body, which makes the inhibitory input delivered to DS cells larger for movement in the null direction (bottom panel) than for movement in the preferred direction (top panel). An additional inhibition acts presynaptically to reduce excitation for null direction movement. Although this presynaptic inhibition is depicted as coming from starburst cells, the existence of another type of cell cannot be ruled out. The excitatory input to DS cells probably comes from bipolar cells (‘b’, red) and may also have a cholinergic component from other starburst cells. For movement in the null direction, the inhibitory input reaches each subregion of the DS cell ahead of the stimulus edge and therefore before excitation. For movement in the preferred direction, inhibition lags behind excitation. Reproduced from Fried et al. 2002, Nature 420, 411–414; reprinted by permission from Macmillan Publishers Ltd.
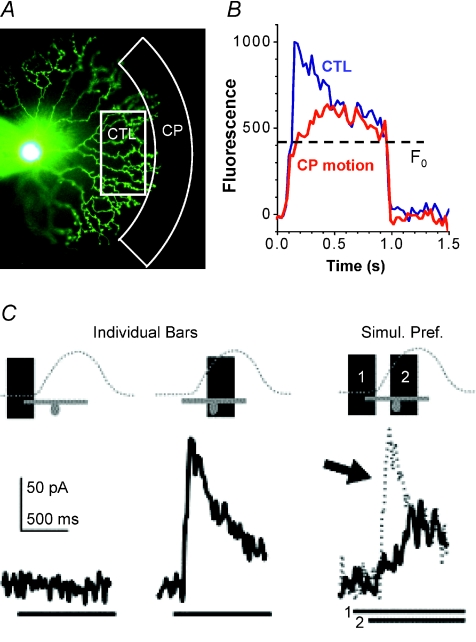
Figure 2. Directional suppression of calcium responses in SAC distal dendrites and of inhibitory inputs to DSGCs by two-flash apparent motion.
A, photomicrograph of a SAC under whole-cell current clamp with a pipette filled with Oregon Green 488 BAPTA-1. The rectangle (marked CTL) indicates the area in which a control light is flashed both to elicit a light response and to measure the Ca2+ response from the dendrites. Simulated centripetal motion is generated by flashing another light in the surround area (CP) 200 ms prior to the onset of the control (CTL) flash. Scale bar: 50 μm. B, the average fluorescence response of eight varicosities in area CTL to a CTL flash (blue trace) is strongly suppressed by a preceding CP flash that simulates a centripetal motion (red trace). Fo indicates the basal fluorescence intensity immediately prior to the light response. C, inhibitory synaptic inputs to a DSGC in response to flashed bars (100 × 300 μm). Simulated preferred-direction motion greatly suppresses the inhibitory input to the DSGC, showing a similarity to the suppression of the calcium response in SAC dendrites by CP motion. Adapted from from Lee & Zhou (2006), Neuron 51, 787–799 and from Fried et al. (2005), Neuron 46, 117–127, with permission from Elsevier.
Direction-selective GABA release from SACs
At the first glance, it may seem paradoxical that a morphologically symmetric cell like the starburst would produce the strong spatial asymmetry required for direction-selective GABA release. However, despite on the radial symmetry of starburst dendrites, there is a profound polar asymmetry in synaptic organization along each dendrite. Input synapses on starburst dendrites distribute roughly uniformly along the entire dendritic length, whereas output synapses are localized in the distal varicose zones, where synaptic vesicles are concentrated (Famiglietti, 1991). These distal varicose zones are believed to be electrotonically semi-isolated, due to the thin diameter of the dendrites and the heavy expression of KV3 voltage-gated potassium channels on the proximal dendrites (Miller & Bloomfield, 1983; Ozaita et al. 2004). This polar asymmetry in input–output relation, combined with the short electrotonic length of the dendrites, is thought to enable the distal dendrites to process directional signals independently. Computational models based on these properties predict that centrifugal stimulus movement activates the distal dendrites more strongly than does centripetal movement (Poznanski, 1992; Borg-Graham, 2001; Tukker et al. 2004). In a groundbreaking Ca2+-imaging experiment, Euler et al. (2002) used two-photon microscopy to show that distal starburst dendrites indeed respond to spot illumination with intracellular Ca2+ transients that are restricted to distal dendrites, and that these local Ca2+ responses tend to be directionally selective: stronger for centrifugal than for centripetal stimulus movement (Euler et al. 2002). Fourier analysis of somatic voltage responses of SACs and compartmental modelling further predict that nonlinear interactions between voltage-gated Ca2+ channel activation and a voltage gradient along the dendrites can produce directional asymmetry in the distal dendrites without synaptic inhibition (Hausselt et al. 2007).
However, while intrinsic electric properties of SACs can produce a centrifugally biased response to image movement, they are not sufficient to account fully for the direction-selective responses observed in distal starburst dendrites, especially when the stimulus moves centripetally from outside the starburst dendritic field. Two-spot apparent motion experiments show that direction selectivity in distal starburst dendrites involves both centrifugal excitation and centripetal inhibition (Lee & Zhou, 2006). It is important to note that stationary spot illumination, without centrifugal motion, already evokes a significant Ca2+ response at distal starburst dendrites (Euler et al. 2002; Lee & Zhou, 2006), which is suppressed by two-spot stimulation simulating centripetal motion (Lee & Zhou, 2006) (Fig. 2). Similarly, a stationary spot illumination readily evokes a large GABAergic input to preferred direction motion (Fried et al. 2005), which can be suppressed by two-spot stimulation simulating preferred direction motion (Fried et al. 2005). These results suggest that a spatially offset lateral inhibition is critical and represents a common mechanism of direction selectivity in both DSGCs and SACs.
How does the asymmetric inhibition to a SAC come about? An important clue to the underlying synaptic circuitry comes from dual patch clamp recordings from neighbouring starburst cells (Zheng et al. 2004; Lee & Zhou, 2006). Overlapping SACs, including those that contact each other only by their distal dendrites, mutually inhibit each other via reciprocal, GABAA receptor-mediated synapses. The spatial extent of this lateral inhibition matches closely that of the direct inhibitory receptive field surround, which is also mediated mainly by GABAA receptors. The characteristically prolonged nature of the GABAergic transmission between SACs is also consistent with the kinetics of the surround inhibition to SACs. Together, these results indicate that the synaptic mechanism for direction selectivity in distal starburst dendrites is built primarily from a classic centre–surround receptive field, with the centre receiving glutamatergic inputs from bipolar cells, and the surround receiving a direct GABAergic input from neighbouring SACs (Fig. 3). Such a concentric receptive field structure would normally be directionally symmetric for a neuron that makes the output decision at the soma or axon hillock. However, because the output decisions in a SAC are made independently in individual distal dendrites, the concentric receptive field structure produces a profound directional asymmetry at distal dendrites, where synaptic inputs from the centre (centrifugal direction) are dominated by glutamatergic excitation from bipolar cells, but synaptic inputs from the surround (centripetal direction) are mediated predominantly by GABAergic inhibition. This synaptic mechanism, together with a centrifugal facilitation produced, in part, by intrinsic properties of SACs, would produce robust direction-selective GABA release from SACs (Lee & Zhou, 2006).
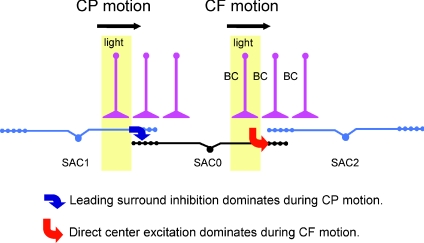
Figure 3. A synaptic model of direction selectivity at the distal processes of SACs.
The concentric centre–surround receptive field of a SAC (SCA0) is shaped largely by a centre excitatory input from bipolar cells (BC) and a surround inhibitory input from neighbouring SACs. Centripetal (CP) light movement evokes a leading GABAergic input (blue arrow) to the distal zone from the surround SACs (represented by SAC1), whereas centrifugal (CF) light movement evokes predominantly an excitatory input (red arrow) to the distal zone from the centre. Adapted from Lee & Zhou (2006, Neuron 51,787–799 with permission from Elsevier.
Summary and concluding remarks
The direction-selective circuit so far identified consists primarily of DSGCs, SACs and bipolar cells. At the DSGC level, direction selectivity is shaped largely by a spatially offset inhibition from SACs, whose distal dendrites both release GABA asymmetrically and synapse on DSGCs asymmetrically. Direction selectivity in DSGCs is further sharpened by directionally asymmetric excitatory inputs and by a postsynaptic mechanism, which involves local signal processing and spike generation in DSGC dendrites (Oesch et al. 2005). At the SAC level, direction selectivity is formed in the distal starburst dendrites by a profound polar asymmetry between centrifugal excitation and centripetal inhibition. GABAergic inhibition, mediated largely by reciprocal inhibition between SACs, plays a key role in suppressing SAC responses to centripetal stimulus motion. However, the nature of GABAergic and glycinergic inputs during centrifugal motion is currently unclear. A model based on a differential expression of two different chloride transporters proposes that the excitability of GABAergic input changes as the image moves centrifugally or centripetally along a SAC dendrite (Gavrikov et al. 2003, 2006). Further experiments are required to test directly the predictions of this model. In addition to synaptic interactions, intrinsic cellular properties also play an important role in shaping direction selectivity in SAC dendrites, predominantly by contributing to centrifugal facilitation (Hausselt et al. 2007). At the bipolar cell level, direction-selective release of glutamate is strongly implicated by the asymmetric excitatory inputs received by DSGCs, but the underlying mechanism is unclear. A key issue is whether local terminals of a bipolar cell axon can function independently to provide directionally biased glutamatergic inputs to several DSGCs. An intriguing possibility in this regard is that SACs asymmetrically inhibit bipolar cell axon terminals, which in turn synapse on DSGCs in a spatially selective manner.
While the critical GABAergic contribution of SACs to direction selectivity is well accepted, the cholinergic role of SACs remains mysterious. Various models of cholinergic contributions to direction selectivity and motion sensitivity have been proposed over the years (Masland et al. 1984; Vaney, 1990; Grzywacz et al. 1998; Chiao & Masland, 2002). However, the basic mode of nicotinic cholinergic action in the retina still remains obscured: does ACh mediate fast neurotransmission at precise synaptic sites between SACs and DSGCs, and/or does it play a diffuse, paracrine role in modulating the activity of many ganglion cells? This will be another interesting area of future study. A recent preliminary report indicates that detailed characterization of cholinergic synaptic physiology may help us gain new insights into the function of SACs (Lee et al. 2006).
Two revelations from the past four decades of studies of direction selectivity seem to stand out as particularly striking. First, from a functional point of view, direction selectivity illustrates an enormous amount of integration of multiple synergistic mechanisms even in a relatively simple system. Such integration may be important for network computation in general. Second, from an anatomical view point, the direction-selective circuit reveals a new level of synaptic selectivity and network specificity that was previously unappreciated. The developmental mechanisms underlying the establishment of the selective connectivity within this circuit will continue to intrigue neurobiologists, perhaps for another four decades.
Acknowledgments
This work was supported by NIH grants EY017353 (ZJZ) and EY10894 (Z.J.Z.).
References
- Amthor FR, Keyser KT, Dmitrieva NA. Effects of the destruction of starburst-cholinergic amacrine cells by the toxin AF64A on rabbit retinal directional selectivity. Vis Neurosci. 2002;19:495–509. doi: 10.1017/s0952523802194119. [DOI] [PubMed] [Google Scholar]
- Barlow HB, Hill RM. Selective sensitivity to direction of movement in ganglion cells of the rabbit retina. Science. 1963;139:412–414. doi: 10.1126/science.139.3553.412. [DOI] [PubMed] [Google Scholar]
- Barlow HB, Hill RM, Levick WR. Retinal ganglion cells responding selectively to direction and speed of image motion in the rabbit. J Physiol. 1964;173:377–407. doi: 10.1113/jphysiol.1964.sp007463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow HB, Levick WR. The mechanism of directionally selective units in rabbit's retina. J Physiol. 1965;178:477–504. doi: 10.1113/jphysiol.1965.sp007638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg-Graham LJ. The computation of directional selectivity in the retina occurs presynaptic to the ganglion cell. Nat Neurosci. 2001;4:176–183. doi: 10.1038/84007. [DOI] [PubMed] [Google Scholar]
- Borg-Graham L, Grzywacz NM. A model of the directional selectivity circuit in retina: transformations by neurons singly and in concert. In: McKenna T, Davis JL, Zoenetzer SF, editors. Single Neuron Computation. London: Academic Press; 1992. pp. 347–375. [Google Scholar]
- Brecha N, Johnson D, Peichl L, Wassle H. Cholinergic amacrine cells of the rabbit retina contain glutamate decarboxylase and g-aminobutyrate immunoreactivity. Proc Natl Acad Sci U S A. 1988;85:6187–6191. doi: 10.1073/pnas.85.16.6187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhl EH, Peichl L. Morphology of rabbit retinal ganglion cells projecting to the medial terminal nucleus of the accessory optic system. J Comp Neurol. 1986;253:163–174. doi: 10.1002/cne.902530204. [DOI] [PubMed] [Google Scholar]
- Caldwell JH, Daw NW, Wyatt HJ. Effects of picrotoxin and strychnine on rabbit retinal ganglion cells: lateral interactions for cells with more complex receptive fields. J Physiol. 1978;276:277–298. doi: 10.1113/jphysiol.1978.sp012233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiao CC, Masland RH. Starburst cells nondirectionally facilitate the responses of direction-selective retinal ganglion cells. J Neurosci. 2002;22:10509–10513. doi: 10.1523/JNEUROSCI.22-24-10509.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacey DM, Peterson BB, Robinson FR, Gamlin PD. Fireworks in the primate retina: in vitro photodynamics reveals diverse LGN-projecting ganglion cell types. Neuron. 2003;37:15–27. doi: 10.1016/s0896-6273(02)01143-1. [DOI] [PubMed] [Google Scholar]
- Dacheux RF, Chimento MF, Amthor FR. Synaptic input to the on-off directionally selective ganglion cell in the rabbit retina. J Comp Neurol. 2003;456:267–278. doi: 10.1002/cne.10521. [DOI] [PubMed] [Google Scholar]
- Demb JB. Cellular mechanisms for direction selectivity in the retina. Neuron. 2007;55:179–186. doi: 10.1016/j.neuron.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Dong W, Sun W, Zhang Y, Chen X, He S. Dendritic relationship between starburst amacrine cells and direction-selective ganglion cells in the rabbit retina. J Physiol. 2004;556:11–17. doi: 10.1113/jphysiol.2004.060715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euler T, Detwiler PB, Denk W. Directionally selective calcium signals in dendrites of starburst amacrine cells. Nature. 2002;418:845–852. doi: 10.1038/nature00931. [DOI] [PubMed] [Google Scholar]
- Famiglietti EV., Jr ‘Starburst’ amacrine cells and cholinergic neurons: mirror-symmetric on and off amacrine cells of rabbit retina. Brain Res. 1983;261:138–144. doi: 10.1016/0006-8993(83)91293-3. [DOI] [PubMed] [Google Scholar]
- Famiglietti EV. Synaptic organization of starburst amacrine cells in rabbit retina: analysis of serial thin sections by electron microscopy and graphic reconstruction. J Comp Neurol. 1991;309:40–70. doi: 10.1002/cne.903090105. [DOI] [PubMed] [Google Scholar]
- Famiglietti EV. Dendritic co-stratification of ON and ON-OFF directionally selective ganglion cells with starburst amacrine cells in rabbit retina. J Comp Neurol. 1992;324:322–335. doi: 10.1002/cne.903240303. [DOI] [PubMed] [Google Scholar]
- Fried SI, Masland RH. Image processing: how the retina detects the direction of image motion. Curr Biol. 2007;17:R63–R66. doi: 10.1016/j.cub.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Fried SI, Munch TA, Werblin FS. Mechanisms and circuitry underlying directional selectivity in the retina. Nature. 2002;420:411–414. doi: 10.1038/nature01179. [DOI] [PubMed] [Google Scholar]
- Fried SI, Munch TA, Werblin FS. Directional selectivity is formed at multiple levels by laterally offset inhibition in the rabbit retina. Neuron. 2005;46:117–127. doi: 10.1016/j.neuron.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Gavrikov KE, Dmitriev AV, Keyser KT, Mangel SC. Cation–chloride cotransporters mediate neural computation in the retina. Proc Natl Acad Sci U S A. 2003;100:16047–16052. doi: 10.1073/pnas.2637041100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrikov KE, Nilson JE, Dmitriev AV, Zucker CL, Mangel SC. Dendritic compartmentalization of chloride cotransporters underlies directional responses of starburst amacrine cells in retina. Proc Natl Acad Sci U S A. 2006;103:18793–18798. doi: 10.1073/pnas.0604551103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzywacz NM, Amthor FR, Merwine DK. Necessity of acetylcholine for retinal directionally selective responses to drifting gratings in rabbit. J Physiol. 1998;512:575–581. doi: 10.1111/j.1469-7793.1998.575be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausselt SE, Euler T, Detwiler PB, Denk W. A dendrite-autonomous mechanism for direction selectivity in retinal starburst amacrine cells. PLoS Biol. 2007;5:e185. doi: 10.1371/journal.pbio.0050185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Kim K, Zhou ZJ. Detection of functional cholinergic and GABAergic communications between starburst amacrine cell and direction selective ganglion cell. Invest Ophthalmol Vis Sci. 2006;47 ARVO E-Abstract 2676. [Google Scholar]
- Lee S, Zhou ZJ. The synaptic mechanism of direction selectivity in distal processes of starburst amacrine cells. Neuron. 2006;51:787–799. doi: 10.1016/j.neuron.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masland RH, Mills JW. Autoradiographic identification of acetylcholine in the rabbit retina. J Cell Biol. 1979;83:159–178. doi: 10.1083/jcb.83.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masland RH, Mills JW, Cassidy C. The functions of acetylcholine in the rabbit retina. Proc R Soc Lond B Biol Sci. 1984;223:121–139. doi: 10.1098/rspb.1984.0086. [DOI] [PubMed] [Google Scholar]
- Miller RF, Bloomfield SA. Electroanatomy of a unique amacrine cell in the rabbit retina. Proc Natl Acad Sci U S A. 1983;80:3069–3073. doi: 10.1073/pnas.80.10.3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Malley DM, Masland RH. Co-release of acetylcholine and γ-aminobutyric acid by a retinal neuron. Proc Natl Acad Sci U S A. 1989;86:3414–3418. doi: 10.1073/pnas.86.9.3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesch N, Euler T, Taylor WR. Direction-selective dendritic action potentials in rabbit retina. Neuron. 2005;47:739–750. doi: 10.1016/j.neuron.2005.06.036. [DOI] [PubMed] [Google Scholar]
- Oyster CW, Barlow HB. Direction-selective units in rabbit retina: distribution of preferred directions. Science. 1967;155:841–842. doi: 10.1126/science.155.3764.841. [DOI] [PubMed] [Google Scholar]
- Oyster CW, Takahashi E, Collewijn H. Direction-selective retinal ganglion cells and control of optokinetic nystagmus in the rabbit. Vision Res. 1972;12:183–193. doi: 10.1016/0042-6989(72)90110-1. [DOI] [PubMed] [Google Scholar]
- Ozaita A, Petit-Jacques J, Volgyi B, Ho CS, Joho RH, Bloomfield SA, Rudy B. A unique role for Kv3 voltage-gated potassium channels in starburst amacrine cell signaling in mouse retina. J Neurosci. 2004;24:7335–7343. doi: 10.1523/JNEUROSCI.1275-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poznanski RR. Modelling the electrotonic structure of starburst amacrine cells in the rabbit retina: a functional interpretation of dendritic morphology. Bull Math Biol. 1992;54:905–928. doi: 10.1007/BF02460658. [DOI] [PubMed] [Google Scholar]
- Pu ML, Amthor FR. Dendritic morphologies of retinal ganglion cells projecting to the lateral geniculate nucleus in the rabbit. J Comp Neurol. 1990a;302:675–693. doi: 10.1002/cne.903020320. [DOI] [PubMed] [Google Scholar]
- Pu ML, Amthor FR. Dendritic morphologies of retinal ganglion cells projecting to the nucleus of the optic tract in the rabbit. J Comp Neurol. 1990b;302:657–674. doi: 10.1002/cne.903020319. [DOI] [PubMed] [Google Scholar]
- Tauchi M, Masland RH. The shape and arrangement of the cholinergic neurons in the rabbit retina. Proc R Soc Lond B Biol Sci. 1984;223:101–119. doi: 10.1098/rspb.1984.0085. [DOI] [PubMed] [Google Scholar]
- Taylor WR, He S, Levick WR, Vaney DI. Dendritic computation of direction selectivity by retinal ganglion cells. Science. 2000;289:2347–2350. doi: 10.1126/science.289.5488.2347. [DOI] [PubMed] [Google Scholar]
- Taylor WR, Vaney DI. Diverse synaptic mechanisms generate direction selectivity in the rabbit retina. J Neurosci. 2002;22:7712–7720. doi: 10.1523/JNEUROSCI.22-17-07712.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor WR, Vaney DI. New directions in retinal research. Trends Neurosci. 2003;26:379–385. doi: 10.1016/S0166-2236(03)00167-X. [DOI] [PubMed] [Google Scholar]
- Tukker JJ, Taylor WR, Smith RG. Direction selectivity in a model of the starburst amacrine cell. Vis Neurosci. 2004;21:611–625. doi: 10.1017/S0952523804214109. [DOI] [PubMed] [Google Scholar]
- Vaney DI. ‘Coronate’ amacrine cells in the rabbit retina have the ‘starburst’ dendritic morphology. Proc R Soc Lond B Biol Sci. 1984;220:501–508. doi: 10.1098/rspb.1984.0016. [DOI] [PubMed] [Google Scholar]
- Vaney DI. The mosaic of amacrine cells in the mammalian retina. Prog Retin Eye Res. 1990;9:49–100. [Google Scholar]
- Vaney DI, Peichi L, Boycott BB. Matching populations of amacrine cells in the inner nuclear and ganglion cell layers of the rabbit retina. J Comp Neurol. 1981;199:373–391. doi: 10.1002/cne.901990305. [DOI] [PubMed] [Google Scholar]
- Vaney DI, Pow DV. The dendritic architecture of the cholinergic plexus in the rabbit retina: selective labeling by glycine accumulation in the presence of sarcosine. J Comp Neurol. 2000;421:1–13. [PubMed] [Google Scholar]
- Vaney DI, Young HM. GABA-like immunoreactivity in cholinergic amacrine cells of the rabbit retina. Brain Res. 1988;438:369–373. doi: 10.1016/0006-8993(88)91366-2. [DOI] [PubMed] [Google Scholar]
- Wyatt HJ, Day NW. Specific effects of neurotransmitter antagonists on ganglion cells in rabbit retina. Science. 1976;191:204–205. doi: 10.1126/science.1857. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Watanabe D, Ishikane H, Tachibana M, Pastan I, Nakanishi S. A key role of starburst amacrine cells in originating retinal directional selectivity and optokinetic eye movement. Neuron. 2001;30:771–780. doi: 10.1016/s0896-6273(01)00316-6. [DOI] [PubMed] [Google Scholar]
- Zheng JJ, Lee S, Zhou ZJ. A developmental switch in the excitability and function of the starburst network in the mammalian retina. Neuron. 2004;44:851–864. doi: 10.1016/j.neuron.2004.11.015. [DOI] [PubMed] [Google Scholar]