Abstract
Diabetic retinopathy has long been recognized as a vascular disease that develops in most patients, and it was believed that the visual dysfunction that develops in some diabetics was due to the vascular lesions used to characterize the disease. It is becoming increasingly clear that neuronal cells of the retina also are affected by diabetes, resulting in dysfunction and even degeneration of some neuronal cells. Retinal ganglion cells (RGCs) are the best studied of the retinal neurons with respect to the effect of diabetes. Although investigations are providing new information about RGCs in diabetes, including therapies to inhibit the neurodegeneration, critical information about the function, anatomy and response properties of these cells is yet needed to understand the relationship between RGC changes and visual dysfunction in diabetes.
Diabetic retinopathy remains a major cause of morbidity in diabetic patients. To date, the retinopathy has been defined based on lesions that are clinically demonstrable, and all of those have been vascular in nature. Thus, lesions indicative of the early stages of retinopathy include capillary degeneration (which, if it later becomes extensive, can contribute to retinal ischaemia and subsequent neovascularization) as well as retinal oedema, cotton wool spots, haemorrhage, and hard exudates. Available clinical evidence strongly suggests that the late, clinically meaningful stages of the retinopathy are a direct consequence of the earlier changes. Development and progress of retinopathy can be slowed by intensive insulin therapy if administered from the onset of diabetes (Engerman & Kern, 1987; Diabetes Control and Complications Trial Research Group, 1993), but it remains very difficult for many patients to achieve and maintain intensive glycaemic control. More recent clinical studies have also demonstrated that blood pressure medications significantly slow the progression to the late, proliferative stages of diabetic retinopathy (Chaturvedi et al. 1998; UK Prospective Diabetes Study Group, 1998a,b; Chaturvedi, 2000).
The clinically demonstrable changes to the retinal vasculature in diabetes have led to the general assumption that the retinopathy is solely a microvascular disease. Nevertheless, diabetes can also damage non-vascular cells of the retina, resulting in alterations in function (Shirao & Kawasaki, 1998; Li et al. 2002; Hancock & Kraft, 2004; Barile et al. 2005; Phipps et al. 2006; Kern et al. 2007) and in loss of ganglion cells, horizontal cells, amacrine cells and photoreceptors (Sima et al. 1992; Kamijo et al. 1993; Hammes et al. 1995; Barber et al. 1998; Lieth et al. 2000; Zeng et al. 2000; Aizu et al. 2002; Asnaghi et al. 2003; Park et al. 2003; Kusner et al. 2004; Martin et al. 2004; Ning et al. 2004; Seki et al. 2004; Gastinger et al. 2006). Thus, diabetic retinopathy can include changes to the neural retina. This review will focus on the effects of diabetes on retinal ganglion cells.
Diabetes increases cell death of retinal ganglion cells: humans
Several studies of histological material have demonstrated that retinal ganglion cells seem to be lost in diabetic patients (Table 1). Moreover, in vivo use of scanning laser polarimetry and other techniques found a thinning of the thickness of the nerve fibre layer in diabetes, further consistent with loss of RGCs and their axons in diabetes. In many of these studies, the type of diabetes was not stipulated, but this omission seems not to be critical, since clinical evidence to date suggests that the retinopathy that develops in type 1 diabetes is indistinguishable from that which develops in type 2 diabetes.
Table 1.
Evidence suggesting diabetes-induced degeneration of RGCs in humans
| Method | Observation | Reference |
|---|---|---|
| Histology of autopsy samples | Atrophy of RGC, degeneration of NFL | Wolter 1961; Bloodworth, 1962; Kerrigan et al. 1997 |
| Immunohistochemistry of autopsy samples | Apoptosis of RGC, overexpression of Bax, and activated caspase-9 and -3 | Barber et al. 1998; Abu-El-Asrar et al. 2004; Abu El-Asrar et al. 2007; Oshitari et al. 2008 |
| NFL defects detected by red-free photography | Detectable in 20% of diabetics without microaneurysms, and 57% with microaneurysms | Chihara et al. 1993 |
| NFL ‘thickness’ from scanning laser polarimetry | Decreased in diabetic patients, and related to severity of retinopathy | Chihara & Zhang, 1998; Ozdek et al. 2002; Takahashi et al. 2006 |
| NFL ‘thickness’ from scanning laser polarimetry | Decreased in superior retina of diabetic patients | Lopes de Faria et al. 2002 |
Immunohistochemical studies of cross-sections of human retinas demonstrated an increase in expression of Bax, caspase-3 and caspase-9 in RGCs from diabetic patients (Oshitari et al. 2008), thus suggesting that at least some retinal ganglion cells might die via apoptosis. In addition, RGCs and occasional cells in the inner nuclear layer showed increased immunostaining for Bad, cytochrome c, and AIF in retinas from diabetic patients. Expression of Cox-2, Akt, and Mcl-1 was not altered in the diabetic retinas (Abu El-Asrar et al. 2007). Together these studies show that RGCs undergo apoptosis in humans with diabetes, leading to a reduction in the thickness of the nerve fibre layer.
Experimental diabetes causes degeneration of retinal ganglion cells in rats
The majority of studies of retinal ganglion cells in rats have utilized a chemical (streptozotocin; STZ) that is toxic to pancreatic β cells, thus causing insulin-deficient diabetes that resembles type 1 diabetes. The insulin deficiency that results after STZ in rats can be profound, so small doses of exogenous insulin are often administered to avoid diabetes-induced weight loss, dysmetabolism, severe polyuria and hyperglycaemia. The BB/W rat likewise is a model of type 1 diabetes, but develops diabetes spontaneously. Models of type 2 diabetes have not yet been used to examine changes in RGC physiology and survival.
RGC loss in diabetic rats
There is general agreement that all rat strains reported to date have shown RGC loss or damage in diabetes (Table 2). Consistent with a possible role of apoptosis in the death of retinal neurons, numerous initiator and effector caspases have been found to become activated in retinas of diabetic animals (Kowluru & Koppolu, 2002; Mohr et al. 2002), and neurons become TUNEL-positive or caspase 3-positive in retinas of diabetic rats (Barber et al. 1998; Lieth et al. 2000; Barber et al. 2005; Gastinger et al. 2006). In both neuronal and capillary cells, however, the number of cells dying at any given time is very small, perhaps contributing to the slow onset and progression of diabetic retinopathy.
Table 2.
RGC death or atrophy in diabetic rats
| Model | Duration | Method | Reference |
|---|---|---|---|
| STZ rats | 8–12 weeks | % of optic nerve axons | Scott et al. 1986 |
| BB/W rats | 12 months | Decrease in diameter but not density of optic nerve axons | Sima et al. 1992 |
| STZ rats | 7.5 months | TUNEL, RGC count | Barber et al. 1998 |
| STZ rats | 1–12 months | NeuN immunohistochemistry | Zeng et al. 2000 |
| STZ rats | 4 weeks | TUNEL | El-Remessy et al. 2006 |
| STZ rats | 12 weeks | TUNEL | Seigel et al. 2006 |
| STZ rats | 3 months | Thy-1 immunostain | Qin et al. 2006 |
| STZ rats | 4–5 weeks | Retrograde dye | Kusari et al. 2007 |
| STZ rats | 9 months | Count RGC | Zheng et al. 2007b |
Further circumstantial evidence of retinal ganglion cell loss or alteration in diabetic rats comes from histological studies of retinal thickness. There was a 22% decrease in the thickness of the inner plexiform layer in rats after 7.5 months of STZ-diabetes, suggesting a cumulative loss of neural dendrites and synapses in the inner retina (Barber et al. 1998). Studies by other investigators have reported similar losses of thickness in the plexiform layers due to experimental diabetes. In retinas of Sprague–Dawley rats there was a 10% reduction in the thickness of the inner plexiform layer reported after only 1 month of STZ-diabetes, while the reduction in the inner plexiform layer of STZ-diabetic Brown–Norway rats was nearly 16% (Aizu et al. 2002). While the rate of progression of degeneration appears to vary in different animal models, the data suggest that diabetes causes a progressive loss of the neuronal structures in the inner retina.
Other studies of the nerve fibre layer and optic nerve provide further evidence of RGC loss. The number or density of axons in the rat optic nerve was reduced by STZ-diabetes in some (Scott et al. 1986), but not all (Sima et al. 1992; Kamijo et al. 1993), rodent studies.
Effect of experimental diabetes on retinal ganglion cells in mice
The effect of diabetes on retinal ganglion cells has been studied using models in which diabetes was induced experimentally using streptozotocin to disrupt pancreatic β cells, in mice that spontaneously develop a type 1-like diabetes (Ins2Akita), and in the spontaneously diabetic KKAY strain (a model of Type 2 diabetes).
The spontaneous development of diabetes resulted in loss of cells in the ganglion cell layer in Ins2Akita mice (Barber et al. 2005). After 22 weeks of hyperglycaemia, there was a 23.4% reduction in the number of cell bodies in the retinal ganglion cell layer. This was accompanied by a significant reduction in the thickness of the inner plexiform layer in these animals. By crossing Ins2Akita with mice that express fluorescent proteins under the regulation of the Thy1 promoter, it was possible to quantify the specific loss of retinal ganglion cells (Gastinger et al. 2008). Cyan fluorescent protein (CFP) was expressed in the cell bodies of about 50% of all RGCs, and quantification of the number of fluorescent cells surviving after 3 months of diabetes revealed 16% depletion from the peripheral retina, but no significant loss in the central region. There was a 27% decrease in the thickness of the inner plexiform layer in Ins2Akita mice after 5.5 months of STZ-diabetes, suggesting a cumulative loss of neural dendrites and synapses (Barber et al. 2005).
Reductions in thickness of the inner and outer retina have been detected also in C57Bl/6 with diabetes induced using STZ (Martin et al. 2004; Zheng et al. 2007a). Surprisingly, however, there is controversy with regard to whether or not diabetes causes RGC death in this mouse strain. One group of investigators reported that STZ-diabetes in this strain rapidly resulted in extensive loss of RGCs, resulting in 20–25% fewer cells in the ganglion cell layer compared with age-matched control mice after only 14 weeks of diabetes (Martin et al. 2004), whereas other investigators using this same strain found no evidence of RGC degeneration even after 1 year of diabetes, based on counts of the number of cell bodies in the RGC layer of retinal cross-sections, retrograde labelling of retinal ganglion cells with fluorescent dye, and TUNEL staining of retinal cross-sections (Asnaghi et al. 2003; Feit-Leichman et al. 2005). A transient increase in TUNEL labelling and caspase-3 activity has been detected in retinas of C57Bl/6 mice shortly after injection of STZ (Feit-Leichman et al. 2005), but this did not result in increased TUNEL staining or loss of RGCs after a longer durations of diabetes. This discrepancy with respect to whether or not RGCs die in diabetic C57Bl/6 mice remains difficult to interpret.
The TUNEL technique in whole retinas also demonstrated that apoptosis of RGCs occurs in KKAY mice (Ning et al. 2004). While there are some discrepancies in the prevalence of RGC loss in different mouse models of diabetes, some of this may be due to the differential reaction of mice to STZ, which tend to require higher doses and repeated administration to induce hyperglycaemia that is comparable to the rat models.
Specific indications of retinal ganglion cell pathology
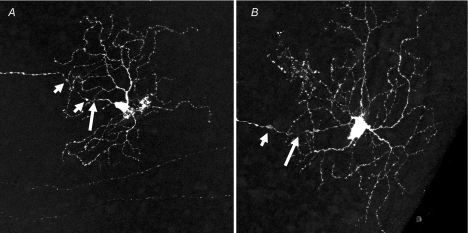
More recent data show that retinal ganglion cells in the Ins2Akita mouse retina develop structural alterations of RGCs within 3 months of diabetes. These features include swellings on axons, often associated with constriction close to the cell body (Fig. 1).
Figure 1. Retinal ganglion cell abnormalities in retinas from diabetic Ins2Akita mice.
Ins2Akita and Thy1-YFP mice were crossbred to produce spontaneously diabetic animals with endogenous expression of the yellow fluorescent protein in a subset of RGCs. Cells were imaged by confocal microcopy (Leica TCS SP2 AOBS) and rendered as maximum projections from z-stacks that included the axon and entire dendritic field. Abnormal features were noted in the ganglion cells of retinas from mice that were diabetic for three months. The abnormalities included axonal swellings (short arrows) and associated constriction (long arrows), as well as enlarged cell bodies and increased dendritic branches and terminals. A, medium ON-RGC; B, large ON-RGC (Gastinger et al. 2008).
Swellings similar to those in RGCs were noted on histamine immunoreactive fibres in the STZ-diabetic rat retina (Gastinger et al. 2001). In contrast, yellow fluorescent protein (expressed under the Thy1 promoter to restrict its expression to RGCs) was expressed throughout the RGC cell body and dendrites of a smaller number of RGCs, enabling quantitative analysis of the morphology of these cells (Gastinger et al. 2008). In diabetes, there was structural remodelling of dendrites, including an increase in the total length, density, and number of terminals. Interestingly, these changes were limited to the large ON-retinal ganglion cells and did not occur in any class of OFF-ganglion cell. Similar changes in morphology have been observed in human retinas and STZ-rats (Qin et al. 2006; Meyer-Rusenberg et al. 2007). These data suggest that the morphology of a subset of RGCs is altered by diabetes in such a way that could alter the functional output of certain subtypes of RGCs.
Diabetes has been reported to impair axonal retrograde transport in large- and medium-sized RGCs in type 1 diabetic rats, but not type 2 diabetic rats (Zhang et al. 1998, 2000). The defect in axoplasmic flow in RGC from type 1 diabetic rats was normalized using an aldose reductase inhibitor (Ino-Ue et al. 2000).
Loss of retinal function in diabetes
The onset of vision loss is insidious in diabetes, commonly beginning with a reduction in night vision or the ability to see details in low light conditions (Bailey & Sparrow, 2001). While clinical diagnosis of diabetic retinopathy requires detection of vascular pathology, the disease also includes deficits in the electroretinogram, and other measures of function such as contrast sensitivity (Della Sala et al. 1985; Sokol et al. 1985; Tzekov & Arden, 1999), suggesting that acquisition or processing of the visual signal is impaired (Lopes de Faria et al. 2001). Such functional changes can occur before the gross vascular defects become detectable clinically (Simonsen, 1980; Juen & Kieselbach, 1990). The oscillatory potentials of the electroretinogram, which are likely to be due to inner retinal neurotransmission (Dong et al. 2004), have prolonged peak latencies and/or decreased amplitudes in diabetic rats (Sakai et al. 1995; Hancock & Kraft, 2004; Phipps et al. 2006; Ramsey et al. 2006; Kern et al. 2007; Layton et al. 2007; Shinoda et al. 2007), suggesting abnormal inner retinal function. The origin of the electroretinogram anomalies is not known, but the source of oscillatory potentials may be from synaptic activity between amacrine neurons and bipolar or retinal ganglion cells (Wachtmeister, 1998). These deficits could be explained by degeneration in synaptic neurotransmission, or a combined loss of amacrine neurons and RGCs.
Apoptotic loss of RGCs combined with morphological changes in the surviving RGCs may account for some of the functional deficits in diabetes. Nevertheless, diabetes-induced deficits in RGC function might occur before morphologic changes. At present, there have been no detailed studies of RGC function in diabetes.
Experimental therapies resulting in preservation of retinal neurons in diabetes
There is still no consensus on the best pharmacological target for diabetic retinopathy, but apoptosis clearly has been shown to participate in the death of RGCs (Kerrigan et al. 1997; Barber et al. 1998, 2005; Abu-El-Asrar et al. 2004, 2007; Martin et al. 2004; Feit-Leichman et al. 2005; El-Remessy et al. 2006; Ali et al. 2008; Oshitari et al. 2008).
Metabolic pathways that cause this apoptotic response are largely unknown. Common theories suggest that inflammation, oxidative stress or exposure to advanced glycation end products might contribute to retinal pathologies, including RGC apoptosis. Several experimental therapies have been found to inhibit RGC loss and some other neural changes in animal models of diabetes. Tests with anti-inflammatory drugs such as minocycline and the non-steroidal cycloxygenase inhibitor nepafenac have yielded positive results (Krady et al. 2005; Kern et al. 2007; Vincent & Mohr, 2007). Several salicylates, including some that have little effect on cycloxygenase, also inhibited the diabetes-induced degeneration of RGCs by a mechanism that seems to involve inhibiting the activation of the proinflammatory NF-κB (Zheng et al. 2007b). Another potential cause of RGC loss is excitotoxicity due to excessive synaptic glutamate activity. There is evidence that diabetes elevates the total amount of glutamate in humans and rats (Ambati et al. 1997; Lieth et al. 1998; Kowluru et al. 2001), while glutamate uptake and processing is also altered (Lieth et al. 1998; Li & Puro, 2002; Puro, 2002). The glutamate NMDA receptor antagonist, memantine, is regarded as a neuroprotective drug, and has been shown to reduce the amount of RGC loss in diabetes (Kusari et al. 2007). Cannabidiol has been suggested as a neuroprotective therapy for RGC loss in glaucoma (El-Remessy et al. 2003), and has also been shown to prevent neural cell death and vascular permeability after a short period of diabetes (El-Remessy et al. 2006). In addition, nerve growth factor (Hammes et al. 1995), IGF-1 (Seigel et al. 2006), aldose reductase inhibitors (Asnaghi et al. 2003; Cheung et al. 2005), erythropoietin (Zhang et al. 2008), and the peroxynitrite decomposition catalyst, FeTTPS (El-Remessy et al. 2005), all have been found to inhibit RGC degeneration in diabetes. Aminoguanidine and aldose reductase inhibitors have been found to inhibit atrophy of optic nerve axons (i.e. from the RGCs) in diabetes (Ino-ue et al. 1998a,b). Currently these studies are too few to clearly indicate the biochemical mechanism(s) leading to RGC death in diabetes, but do provide important information to initiate further work.
It is important to recognize that most of these studies have focused on preventing RGC degeneration, but effects of these and other therapies on dendritic field morphology and other features important for RGC function have not been assessed. Further studies using neuroprotective drugs should broaden their scope beyond neuronal death to include ganglion cell morphology and function, as well as effects on vascular permeability and structure. Such studies will determine if there is a causal relationship between the vascular and neural changes in diabetic retinopathy.
Conclusions
There is general agreement (with the exception of C57Bl/6 mice) that some RGCs die in diabetes, and some show alterations in structure. The molecular mechanism by which the cells die or develop other structural abnormalities is not yet clear, but inflammation, excitotoxicity, and oxidative/nitrative stress might be implicated based on the limited number of studies done to date. Importantly, the focus pertaining to RGSs in diabetes should not be restricted to degeneration. Information about diabetes-induced dysfunction, anatomy and response properties is expected to provide considerable insight into the visual dysfunction that develops in some diabetic patients. To accomplish this, more experts and studies on retinal ganglion cell structure, function and physiology are needed to investigate the response of the retina to diabetes. Ultimately, these studies are likely to shed light on the potential role of altered RGC function and survival in diabetes-induced vision loss.
Acknowledgments
The work of T.S.K. was supported by grants to T.S.K. from the Medical Research Service of the Department of Veteran Affairs and NIH (R01EY00300). A.J.B. gratefully acknowledges financial support from the American Diabetes Association, the Juvenile Diabetes Research Foundation, and The PA Lions Sight Conservation and Eye Research Foundation, and would like to thank Dr Matthew Gastinger for confocal images.
References
- Abu El-Asrar AM, Dralands L, Missotten L, Geboes K. Expression of antiapoptotic and proapoptotic molecules in diabetic retinas. Eye. 2007;21:238–245. doi: 10.1038/sj.eye.6702225. [DOI] [PubMed] [Google Scholar]
- Abu-El-Asrar AM, Dralands L, Missotten L, Al-Jadaan IA, Geboes K. Expression of apoptosis markers in the retinas of human subjects with diabetes. Invest Ophthalmol Vis Sci. 2004;45:2760–2766. doi: 10.1167/iovs.03-1392. [DOI] [PubMed] [Google Scholar]
- Aizu Y, Oyanagi K, Hu J, Nakagawa H. Degeneration of retinal neuronal processes and pigment epithelium in the early stage of the streptozotocin-diabetic rats. Neuropathology. 2002;22:161–170. doi: 10.1046/j.1440-1789.2002.00439.x. [DOI] [PubMed] [Google Scholar]
- Ali TK, Matragoon S, Pillai BA, Liou GI, El-Remessy AB. Peroxynitrite mediates retinal neurodegeneration by inhibiting nerve growth factor survival signaling in experimental and human diabetes. Diabetes. 2008;57:889–898. doi: 10.2337/db07-1669. [DOI] [PubMed] [Google Scholar]
- Ambati J, Chalam KV, Chawla DK, D'Angio CT, Guillet EG, Rose SJ, Vanderlinde RE, Ambati BK. Elevated gamma-aminobutyric acid, glutamate, and vascular endothelial growth factor levels in the vitreous of patients with proliferative diabetic retinopathy. Arch Ophthalmol. 1997;115:1161–1166. doi: 10.1001/archopht.1997.01100160331011. [DOI] [PubMed] [Google Scholar]
- Asnaghi V, Gerhardinger C, Hoehn T, Adeboje A, Lorenzi M. A role for the polyol pathway in the early neuroretinal apoptosis and glial changes induced by diabetes in the rat. Diabetes. 2003;52:506–511. doi: 10.2337/diabetes.52.2.506. [DOI] [PubMed] [Google Scholar]
- Bailey CC, Sparrow JM. Visual symptomatology in patients with sight-threatening diabetic retinopathy. Diabet Med. 2001;18:883–888. doi: 10.1046/j.1464-5491.2001.00589.x. [DOI] [PubMed] [Google Scholar]
- Barber AJ, Antonetti DA, Kern TS, Reiter CE, Soans RS, Krady JK, Levison SW, Gardner TW, Bronson SK. The Ins2Akita mouse as a model of early retinal complications in diabetes. Invest Ophthalmol Vis Sci. 2005;46:2210–2218. doi: 10.1167/iovs.04-1340. [DOI] [PubMed] [Google Scholar]
- Barber AJ, Lieth E, Khin SA, Antonetti DA, Buchanan AG, Gardner TW. Neural apoptosis in the retina during experimental and human diabetes. Early onset and effect of insulin. J Clin Invest. 1998;102:783–791. doi: 10.1172/JCI2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barile GR, Pachydaki SI, Tari SR, Lee SE, Donmoyer CM, Ma W, Rong LL, Buciarelli LG, Wendt T, Horig H, Hudson BI, Qu W, Weinberg AD, Yan SF, Schmidt AM. The RAGE axis in early diabetic retinopathy. Invest Ophthalmol Vis Sci. 2005;46:2916–2924. doi: 10.1167/iovs.04-1409. [DOI] [PubMed] [Google Scholar]
- Bloodworth JM., Jr Diabetic retinopathy. Diabetes. 1962;11:1–22. [PubMed] [Google Scholar]
- Chaturvedi N. Modulation of the renin-angiotensin system and retinopathy. Heart. 2000;84(Suppl. 1):i29–31. doi: 10.1136/heart.84.suppl_1.i29. discussion i50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi N, Sjolie AK, Stephenson JM, Abrahamian H, Keipes M, Castellarin A, Rogulja-Pepeonik Z, Fuller JH. Effect of lisinopril on progression of retinopathy in normotensive people with type 1 diabetes. The EUCLID Study Group. EURODIAB Controlled Trial Lisinopril Insulin-Dependent Diabetes Mellitus. Lancet. 1998;351:28–31. doi: 10.1016/s0140-6736(97)06209-0. [DOI] [PubMed] [Google Scholar]
- Cheung AK, Fung MK, Lo AC, Lam TT, So KF, Chung SS, Chung SK. Aldose reductase deficiency prevents diabetes-induced blood–retinal barrier breakdown, apoptosis, and glial reactivation in the retina of db/db mice. Diabetes. 2005;54:3119–3125. doi: 10.2337/diabetes.54.11.3119. [DOI] [PubMed] [Google Scholar]
- Chihara E, Matsuoka T, Ogura Y, Matsumura M. Retinal nerve fiber layer defect as an early manifestation of diabetic retinopathy. Ophthalmology. 1993;100:1147–1151. doi: 10.1016/s0161-6420(93)31513-7. [DOI] [PubMed] [Google Scholar]
- Chihara E, Zhang S. Analysis of diabetic optic neuropathy with a topographic laser scanning system. Nippon Ganka Gakkai Zasshi. 1998;102:431–435. [PubMed] [Google Scholar]
- Della Sala S, Bertoni G, Somazzi L, Stubbe F, Wilkins AJ. Impaired contrast sensitivity in diabetic patients with and without retinopathy: a new technique for rapid assessment. Br J Ophthalmol. 1985;69:136–142. doi: 10.1136/bjo.69.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- Dong CJ, Agey P, Hare WA. Origins of the electroretinogram oscillatory potentials in the rabbit retina. Vis Neurosci. 2004;21:533–543. doi: 10.1017/S0952523804214043. [DOI] [PubMed] [Google Scholar]
- El-Remessy AB, Al-Shabrawey M, Khalifa Y, Tsai NT, Caldwell RB, Liou GI. Neuroprotective and blood–retinal barrier-preserving effects of cannabidiol in experimental diabetes. Am J Pathol. 2006;168:235–244. doi: 10.2353/ajpath.2006.050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Remessy AB, Bartoli M, Platt DH, Fulton D, Caldwell RB. Oxidative stress inactivates VEGF survival signaling in retinal endothelial cells via PI 3-kinase tyrosine nitration. J Cell Sci. 2005;118:243–252. doi: 10.1242/jcs.01612. [DOI] [PubMed] [Google Scholar]
- El-Remessy AB, Khalil IE, Matragoon S, Abou-Mohamed G, Tsai NJ, Roon P, Caldwell RB, Caldwell RW, Green K, Liou GI. Neuroprotective effect of (–)D9-tetrahydrocannabinol and cannabidiol in N-methyl-D-aspartate-induced retinal neurotoxicity: involvement of peroxynitrite. Am J Pathol. 2003;163:1997–2008. doi: 10.1016/s0002-9440(10)63558-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engerman RL, Kern TS. Progression of incipient diabetic retinopathy during good glycemic control. Diabetes. 1987;36:808–812. doi: 10.2337/diab.36.7.808. [DOI] [PubMed] [Google Scholar]
- Feit-Leichman RA, Kinouchi R, Takeda M, Fan Z, Mohr S, Kern TS, Chen DF. Vascular damage in a mouse model of diabetic retinopathy: relation to neuronal and glial changes. Invest Ophthalmol Vis Sci. 2005;46:4281–4287. doi: 10.1167/iovs.04-1361. [DOI] [PubMed] [Google Scholar]
- Gastinger MJ, Barber AJ, Khin SA, McRill CS, Gardner TW, Marshak DW. Abnormal centrifugal axons in streptozotocin-diabetic rat retinas. Invest Ophthalmol Vis Sci. 2001;42:2679–2685. [PMC free article] [PubMed] [Google Scholar]
- Gastinger MJ, Kunselman AR, Conboy EE, Bronson SK, Barber AJ. Dendrite remodeling and other abnormalities in the retinal ganglion cells of Ins2Akita diabetic mice. Invest Ophthalmol Vis Sci. 2008;49:2635–2642. doi: 10.1167/iovs.07-0683. [DOI] [PubMed] [Google Scholar]
- Gastinger MJ, Singh RS, Barber AJ. Loss of cholinergic and dopaminergic amacrine cells in streptozotocin-diabetic rat and Ins2Akita-diabetic mouse retinas. Invest Ophthalmol Vis Sci. 2006;47:3143–3150. doi: 10.1167/iovs.05-1376. [DOI] [PubMed] [Google Scholar]
- Hammes H-P, Federoff HJ, Brownlee M. Nerve growth factor prevents both neuroretinal programmed cell death and capillary pathology in experimental diabetes. Mol Med. 1995;1:527–534. [PMC free article] [PubMed] [Google Scholar]
- Hancock HA, Kraft TW. Oscillatory potential analysis and ERGs of normal and diabetic rats. Invest Ophthalmol Vis Sci. 2004;45:1002–1008. doi: 10.1167/iovs.03-1080. [DOI] [PubMed] [Google Scholar]
- Ino-Ue M, Ohgiya N, Yamamoto M. Effect of aminoguanidine on optic nerve involvement in experimental diabetic rats. Brain Res. 1998a;800:319–322. doi: 10.1016/s0006-8993(98)00512-5. [DOI] [PubMed] [Google Scholar]
- Ino-ue M, Yokogawa H, Yamamoto M, Naka H, Kuriyama H. Structural impairments in optic nerve of diabetic rats ameliorated with the aldose reductase inhibitor. Exp Eye Res. 1998b;66:397–401. doi: 10.1006/exer.1997.0426. [DOI] [PubMed] [Google Scholar]
- Ino-ue M, Zhang L, Naka H, Kuriyama H, Yamamoto M. Polyol metabolism of retrograde axonal transport in diabetic rat large optic nerve fiber. Invest Ophthalmol Vis Sci. 2000;41:4055–4058. [PubMed] [Google Scholar]
- Juen S, Kieselbach GF. Electrophysiological changes in juvenile diabetics without retinopathy. Arch Ophthalmol. 1990;108:372–375. doi: 10.1001/archopht.1990.01070050070033. [DOI] [PubMed] [Google Scholar]
- Kamijo M, Cherian PV, Sima AAF. The preventive effect of aldose reductase inhibition on diabetic optic neuropathy in the BB/W rat. Diabetologia. 1993;36:893–898. doi: 10.1007/BF02374469. [DOI] [PubMed] [Google Scholar]
- Kern TS, Miller CM, Du Y, Zheng L, Mohr S, Ball SL, Kim M, Jamison JA, Bingaman DP. Topical administration of nepafenac inhibits diabetes-induced retinal microvascular disease and underlying abnormalities of retinal metabolism and physiology. Diabetes. 2007;56:373–379. doi: 10.2337/db05-1621. [DOI] [PubMed] [Google Scholar]
- Kerrigan LA, Zack DJ, Quigley HA, Smith SD, Pease ME. TUNEL-positive ganglion cells in human primary open-angle glaucoma. Arch Ophthalmol. 1997;115:1031–1035. doi: 10.1001/archopht.1997.01100160201010. [DOI] [PubMed] [Google Scholar]
- Kowluru RA, Engerman RL, Case GL, Kern TS. Retinal glutamate in diabetes and effect of antioxidants. Neurochem Int. 2001;38:385–390. doi: 10.1016/s0197-0186(00)00112-1. [DOI] [PubMed] [Google Scholar]
- Kowluru RA, Koppolu P. Diabetes-induced activation of caspase-3 in retina: effect of antioxidant therapy. Free Radic Res. 2002;36:993–999. doi: 10.1080/1071576021000006572. [DOI] [PubMed] [Google Scholar]
- Krady JK, Basu A, Allen CM, Xu Y, LaNoue KF, Gardner TW, Levison SW. Minocycline reduces proinflammatory cytokine expression, microglial activation, and caspase-3 activation in a rodent model of diabetic retinopathy. Diabetes. 2005;54:1559–1565. doi: 10.2337/diabetes.54.5.1559. [DOI] [PubMed] [Google Scholar]
- Kusari J, Zhou S, Padillo E, Clarke KG, Gil DW. Effect of memantine on neuroretinal function and retinal vascular changes of streptozotocin-induced diabetic rats. Invest Ophthalmol Vis Sci. 2007;48:5152–5159. doi: 10.1167/iovs.07-0427. [DOI] [PubMed] [Google Scholar]
- Kusner LL, Sarthy VP, Mohr S. Nuclear translocation of glyceraldehyde-3-phosphate dehydrogenase: a role in high glucose-induced apoptosis in retinal Muller cells. Invest Ophthalmol Vis Sci. 2004;45:1553–1561. [PubMed] [Google Scholar]
- Layton CJ, Safa R, Osborne NN. Oscillatory potentials and the b-wave: partial masking and interdependence in dark adaptation and diabetes in the rat. Graefes Arch Clin Exp Ophthalmol. 2007;245:1335–1345. doi: 10.1007/s00417-006-0506-0. [DOI] [PubMed] [Google Scholar]
- Li Q, Puro DG. Diabetes-induced dysfunction of the glutamate transporter in retinal Muller cells. Invest Ophthalmol Vis Sci. 2002;43:3109–3116. [PubMed] [Google Scholar]
- Li Q, Zemel E, Miller B, Perlman I. Early retinal damage in experimental diabetes: electroretinographical and morphological observations. Exp Eye Res. 2002;74:615–625. doi: 10.1006/exer.2002.1170. [DOI] [PubMed] [Google Scholar]
- Lieth E, Barber AJ, Xu B, Dice C, Ratz MJ, Tanase D, Strother JM. Glial reactivity and impaired glutamate metabolism in short-term experimental diabetic retinopathy. Penn State Retina Research Group. Diabetes. 1998;47:815–820. doi: 10.2337/diabetes.47.5.815. [DOI] [PubMed] [Google Scholar]
- Lieth E, Gardner TW, Barber AJ, Antonetti DA. Retinal neurodegeneration: early pathology in diabetes. Clin Experiment Ophthalmol. 2000;28:3–8. doi: 10.1046/j.1442-9071.2000.00222.x. [DOI] [PubMed] [Google Scholar]
- Lopes De Faria JM, Katsumi O, Cagliero E, Nathan D, Hirose T. Neurovisual abnormalities preceding the retinopathy in patients with long-term type 1 diabetes mellitus. Graefes Arch Clin Exp Ophthalmol. 2001;239:643–648. doi: 10.1007/s004170100268. [DOI] [PubMed] [Google Scholar]
- Lopes De Faria JM, Russ H, Costa VP. Retinal nerve fibre layer loss in patients with type 1 diabetes mellitus without retinopathy. Br J Ophthalmol. 2002;86:725–728. doi: 10.1136/bjo.86.7.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PM, Roon P, Van Ells TK, Ganapathy V, Smith SB. Death of retinal neurons in streptozotocin-induced diabetic mice. Invest Ophthalmol Vis Sci. 2004;45:3330–3336. doi: 10.1167/iovs.04-0247. [DOI] [PubMed] [Google Scholar]
- Meyer-Rusenberg B, Pavlidis M, Stupp T, Thanos S. Pathological changes in human retinal ganglion cells associated with diabetic and hypertensive retinopathy. Graefes Arch Clin Exp Ophthalmol. 2007;245:1009–1018. doi: 10.1007/s00417-006-0489-x. [DOI] [PubMed] [Google Scholar]
- Mohr S, Tang J, Kern TS. Caspase activation in retinas of diabetic and galactosemic mice and diabetic patients. Diabetes. 2002;51:1172–1179. doi: 10.2337/diabetes.51.4.1172. [DOI] [PubMed] [Google Scholar]
- Ning X, Baoyu Q, Yuzhen L, Shuli S, Reed E, Li QQ. Neuro-optic cell apoptosis and microangiopathy in KKAY mouse retina. Int J Mol Med. 2004;13:87–92. [PubMed] [Google Scholar]
- Oshitari T, Yamamoto S, Hata N, Roy S. Mitochondria- and caspase-dependent cell death pathway involved in neuronal degeneration in diabetic retinopathy. Br J Ophthalmol. 2008;92:552–556. doi: 10.1136/bjo.2007.132308. [DOI] [PubMed] [Google Scholar]
- Ozdek S, Lonneville YH, Onol M, Yetkin I, Hasanreisoglu BB. Assessment of nerve fiber layer in diabetic patients with scanning laser polarimetry. Eye. 2002;16:761–765. doi: 10.1038/sj.eye.6700207. [DOI] [PubMed] [Google Scholar]
- Park SH, Park JW, Park SJ, Kim KY, Chung JW, Chun MH, Oh SJ. Apoptotic death of photoreceptors in the streptozotocin-induced diabetic rat retina. Diabetologia. 2003;46:1260–1268. doi: 10.1007/s00125-003-1177-6. [DOI] [PubMed] [Google Scholar]
- Phipps JA, Yee P, Fletcher EL, Vingrys AJ. Rod photoreceptor dysfunction in diabetes: activation, deactivation, and dark adaptation. Invest Ophthalmol Vis Sci. 2006;47:3187–3194. doi: 10.1167/iovs.05-1493. [DOI] [PubMed] [Google Scholar]
- Puro DG. Diabetes-induced dysfunction of retinal Muller cells. Trans Am Ophthalmol Soc. 2002;100:339–352. [PMC free article] [PubMed] [Google Scholar]
- Qin Y, Xu G, Wang W. Dendritic abnormalities in retinal ganglion cells of three-month diabetic rats. Curr Eye Res. 2006;31:967–974. doi: 10.1080/02713680600987674. [DOI] [PubMed] [Google Scholar]
- Ramsey DJ, Ripps H, Qian H. An electrophysiological study of retinal function in the diabetic female rat. Invest Ophthalmol Vis Sci. 2006;47:5116–5124. doi: 10.1167/iovs.06-0364. [DOI] [PubMed] [Google Scholar]
- Sakai H, Tani Y, Shirasawa E, Shirabo Y, Kawasaki K. Development of elecrtoretinographic alterations in streptozotocin-induced diabetes in rats. Opththalmic Res. 1995;27:57–63. doi: 10.1159/000267571. [DOI] [PubMed] [Google Scholar]
- Scott TM, Foote J, Peat B, Galway G. Vascular and neural changes in the rat optic nerve following induction of diabetes with streptozotocin. J Anat. 1986;144:145–152. [PMC free article] [PubMed] [Google Scholar]
- Seigel GM, Lupien SB, Campbell LM, Ishii DN. Systemic IGF-I treatment inhibits cell death in diabetic rat retina. J Diabetes Complications. 2006;20:196–204. doi: 10.1016/j.jdiacomp.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Seki M, Tanaka T, Nawa H, Usui T, Fukuchi T, Ikeda K, Abe H, Takei N. Involvement of brain-derived neurotrophic factor in early retinal neuropathy of streptozotocin-induced diabetes in rats: therapeutic potential of brain-derived neurotrophic factor for dopaminergic amacrine cells. Diabetes. 2004;53:2412–2419. doi: 10.2337/diabetes.53.9.2412. [DOI] [PubMed] [Google Scholar]
- Shinoda K, Rejdak R, Schuettauf F, Blatsios G, Volker M, Tanimoto N, Olcay T, Gekeler F, Lehaci C, Naskar R, Zagorski Z, Zrenner E. Early electroretinographic features of streptozotocin-induced diabetic retinopathy. Clin Experiment Ophthalmol. 2007;35:847–854. doi: 10.1111/j.1442-9071.2007.01607.x. [DOI] [PubMed] [Google Scholar]
- Shirao Y, Kawasaki K. Electrical responses from diabetic retina. Prog Retin Eye Res. 1998;17:59–76. doi: 10.1016/s1350-9462(97)00005-0. [DOI] [PubMed] [Google Scholar]
- Sima AA, Zhang WX, Cherian PV, Chakrabarti S. Impaired visual evoked potential and primary axonopathy of the optic nerve in the diabetic BB/W-rat. Diabetologia. 1992;35:602–607. doi: 10.1007/BF00400249. [DOI] [PubMed] [Google Scholar]
- Simonsen SE. The value of the oscillatory potential in selecting juvenile diabetics at risk of developing proliferative retinopathy. Acta Ophthalmol (Copenh) 1980;58:865–878. doi: 10.1111/j.1755-3768.1980.tb08312.x. [DOI] [PubMed] [Google Scholar]
- Sokol S, Moskowitz A, Skarf B, Evans R, Molitch M, Senior B. Contrast sensitivity in diabetics with and without background retinopathy. Arch Ophthalmol. 1985;103:51–54. doi: 10.1001/archopht.1985.01050010055018. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Goto T, Shoji T, Tanito M, Park M, Chihara E. Diabetes-associated retinal nerve fiber damage evaluated with scanning laser polarimetry. Am J Ophthalmol. 2006;142:88–94. doi: 10.1016/j.ajo.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Tzekov R, Arden GB. The electroretinogram in diabetic retinopathy. Surv Ophthalmol. 1999;44:53–60. doi: 10.1016/s0039-6257(99)00063-6. [DOI] [PubMed] [Google Scholar]
- UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes. UKPDS 38. BMJ. 1998a;317:703–713. [PMC free article] [PubMed] [Google Scholar]
- UK Prospective Diabetes Study Group. Efficacy of atenolol and captopril in reducing risk of macrovascular and microvascular complications in type 2 diabetes. UKPDS 39. BMJ. 1998b;317:713–720. [PMC free article] [PubMed] [Google Scholar]
- Vincent JA, Mohr S. Inhibition of caspase-1/interleukin-1α signaling prevents degeneration of retinal capillaries in diabetes and galactosemia. Diabetes. 2007;56:224–230. doi: 10.2337/db06-0427. [DOI] [PubMed] [Google Scholar]
- Wachtmeister L. Oscillatory potentials in the retina: what do they reveal. Prog Retin Eye Res. 1998;17:485–521. doi: 10.1016/s1350-9462(98)00006-8. [DOI] [PubMed] [Google Scholar]
- Wolter JR. Diabetic retinopathy. Am J Ophthalmol. 1961;51:1123–1141. doi: 10.1016/0002-9394(61)91802-5. [DOI] [PubMed] [Google Scholar]
- Zeng XX, Ng YK, Ling EA. Neuronal and microglial response in the retina of streptozotocin-induced diabetic rats. Vis Neurosci. 2000;17:463–471. doi: 10.1017/s0952523800173122. [DOI] [PubMed] [Google Scholar]
- Zhang L, Inoue M, Dong K, Yamamoto M. Alterations in retrograde axonal transport in optic nerve of type I and type II diabetic rats. Kobe J Med Sci. 1998;44:205–215. [PubMed] [Google Scholar]
- Zhang L, Ino-Ue M, Dong K, Yamamoto M. Retrograde axonal transport impairment of large- and medium-sized retinal ganglion cells in diabetic rat. Curr Eye Res. 2000;20:131–136. [PubMed] [Google Scholar]
- Zhang J, Wu Y, Jin Y, Ji F, Sinclair SH, Luo Y, Xu G, Lu L, Dai W, Yanoff M, Li W, Xu GT. Intravitreal injection of erythropoietin protects both retinal vascular and neuronal cells in early diabetes. Invest Ophthalmol Vis Sci. 2008;49:732–742. doi: 10.1167/iovs.07-0721. [DOI] [PubMed] [Google Scholar]
- Zheng L, Du Y, Miller C, Gubitosi-Klug RA, Ball S, Berkowitz BA, Kern TS. Critical role of inducible nitric oxide synthase in degeneration of retinal capillaries in mice with streptozotocin-induced diabetes. Diabetologia. 2007a;50:1987–1996. doi: 10.1007/s00125-007-0734-9. [DOI] [PubMed] [Google Scholar]
- Zheng L, Howell SJ, Hatala DA, Huang K, Kern TS. Salicylate-based anti-inflammatory drugs inhibit the early lesion of diabetic retinopathy. Diabetes. 2007b;56:337–345. doi: 10.2337/db06-0789. [DOI] [PubMed] [Google Scholar]