Abstract
Previous studies have shown that low-frequency repetitive transcranial magnetic stimulation (rTMS) suppresses motor-evoked potentials (MEPs) evoked by single pulse TMS. The aim of the present paper was to investigate the central nervous system level at which rTMS produces a suppression of MEP amplitude. We recorded corticospinal volleys evoked by single pulse TMS of the motor cortex before and after 1 Hz rTMS in five conscious subjects who had an electrode implanted in the cervical epidural space for the control of pain. One of the patients had Parkinson's disease and was studied on medication. Repetitive TMS significantly suppressed the amplitude of later I-waves, and reduced the amplitude of concomitantly recorded MEPs. The earliest I-wave was not significantly modified by rTMS. The present results show that 1 Hz rTMS may decrease the amplitude of later descending waves, consistent with a cortical origin of the effect of 1 Hz rTMS on MEPs.
In recent years, several authors have attempted to produce changes in the effectiveness of synapses between cortical neurones in the human brain by using repeated pulses of transcranial magnetic stimulation (TMS) (Hallett, 2007). The hope is that rTMS-induced long-term depression (LTD) or long-term potentiation (LTP)-like changes of synaptic connections of the brain could promote plasticity in cortical circuits damaged by an acute or a chronic lesion (Ridding & Rothwell, 2007).
One of the first and still most commonly used protocols in human studies is low-frequency (around 1 Hz) rTMS. Chen et al. (1997) found that corticospinal excitability was reduced for 15 min after applying 0.9 Hz rTMS for the preceding 15 min, and speculated that this was due to long-term depression of synapses in the motor cortex. Later studies confirmed the result, but also showed that there was a high interindividual variability in response to this protocol (Maeda et al. 2000). Although most authors assume that the effects are due to changes in excitability of motor cortex, there are surprisingly few studies to test for possible effects on spinal cord excitability using F-waves, H-reflexes or responses to cervicomedullary stimulation. Indeed, the intensity of the pulses used in rTMS is often at or above resting motor threshold, with the result that they evoke considerable activation of spinal as well as cortical mechanisms. There are two conflicting reports of the effects of 1 Hz rTMS (90–95% resting motor threshold (RMT)) on forearm flexor H-reflexes: one found no change (Touge et al. 2001) whereas the other reported an increase in amplitude and decreased threshold (Valero-Cabre et al. 2001).
Although effects on spinal cord are still unclear, there is good evidence that there are at least some lasting effects on cortex. Imaging studies with PET and EEG show that 1 Hz rTMS causes long-lasting effects on cortical metabolism and resting activity (Rossi et al. 2000; Lee et al. 2003). However, these effects are not readily linked to changes in corticospinal excitability: 1 Hz rTMS over motor cortex increases activation at the site of stimulation whilst decreasing corticospinal excitability, while 1 Hz rTMS over premotor cortex reduces activation of motor cortex, yet has the same suppressive effect on corticospinal excitability (Rounis et al. 2005).
The aim of the present study was to evaluate directly the effects produced by low-frequency rTMS on motor cortical excitability. To this end, we recorded TMS-evoked corticospinal output, upstream of the spinal circuitry, before and after 1 Hz rTMS of the motor cortex in five patients who had an epidural stimulator implanted in the upper cervical cord. By monitoring the descending activity evoked by single test pulses we can obtain a direct indication of how 1 Hz conditioning alters cortical excitability uncontaminated by any change in spinal function that might complicate the interpretation of muscle responses.
Methods
Repetitive TMS
As described in previous publications (Di Lazzaro et al. 2004), we recorded descending corticospinal activity evoked by TMS of the motor cortex directly from the high cervical epidural space of five conscious subjects (aged 44, 38, 74, 90 and 57 years). These patients had cervical electrodes inserted for control of intractable dorso-lumbar pain. Four subjects (subjects 1, 2, 4 and 5) had no abnormality of the central nervous system, while subject 3 had early stage Parkinson's disease with symptoms completely controlled by l-DOPA.
The patients gave their written informed consent. The study was performed according to the Declaration of Helsinki and was approved by the ethics committee of the Medical Faculty of the Catholic University of Rome.
Patients 1, 2, 4 and 5 were taking no centrally acting medication at the time of the experiments; patient 3 was taking l-DOPA and the electrophysiological test was performed about 2 h after administration of 100 mg of l-DOPA when the patient was completely asymptomatic with a UPDRS (Unified Parkinson's Disease Rating Scale) motor subscale score of 0. Magnetic stimulation was performed with a high power Magstim 200 (Magstim Co., Whitland, UK). A figure-of-eight coil with external loop diameters of 9 cm was held over the right motor cortex at the optimum scalp position to elicit motor-evoked potentials (MEPs) in the contralateral first dorsal interosseous muscle (FDI). Resting motor threshold (RMT) was defined according to the recommendations of the IFCN Committee (Rossini et al. 1994) as the minimum stimulus intensity that produced a liminal MEP (> 50 μV in 50% of 10 trials) with the tested muscle at rest.
Two different orientations of the stimulating coil over the motor strip were used, with the induced current flowing either in a latero-medial (LM) or in a posterior–anterior (PA) direction. RMT was determined separately for LM and PA stimulation. LM magnetic stimulation was used to identify the latency of the earliest (D-wave) descending volley (Di Lazzaro et al. 2004). The responses to 20 stimuli at an intensity of 150% RMT were averaged at rest.
Epidural recordings were made between the most proximal and distal of the four electrode contacts on the epidural electrode. These had a surface area of 2.54 mm2 and were 30 mm apart. The distal contact was connected to the reference input of the amplifier. MEPs and epidural activity were band-pass filtered (bandwidth 3Hz–3 kHz) (Digitimer D360 amplifiers) and each single trial was recorded on computer for later analysis using a CED 1401 A/D converter (Cambridge Electronic Design, Cambridge, UK) and associated software. Amplitude of the volleys was measured peak-to-peak between the negative peak and the following positive peak.
Repetitive TMS was delivered over the motor cortex ‘hot spot’ for MEPs in the contralateral FDI muscle using a MagStim SuperRapid (Magstim). This stimulator produces a biphasic pulse. A figure-of-eight coil with external loop diameters of 9 cm, was held over the motor cortex at the optimum scalp position to elicit motor responses in the contralateral FDI with the handle pointing posteriorly and approximately perpendicular to the central sulcus. The initial direction of the current induced in the brain was posterior–anterior. One Hertz rTMS was performed at 110% RMT, and 900 stimuli were delivered in a single train.
The responses to 20 stimuli at an intensity of 150% RMT were averaged at rest. Since the mechanism of the first I-wave (I1) is different from that of the later I-waves as suggested by the differential behaviour of the I1- and later I-waves in several TMS protocols and in inhibitory protocols in particular (Di Lazzaro et al. 2004), the effect of 1Hz rTMS on the amplitude of the I1- and of the later I-waves (the sum of the amplitude of all the individual waves after the I1-wave) were analysed separately.
Statistics
The aim of this study was to evaluate the effects of 1 Hz rTMS on the amplitude of the epidural volley evoked by a standard TMS pulse. The most robust design to do this would be to have two patient groups, one treated with real rTMS and the other treated with some form of sham rTMS. The data from both groups could then be inserted into a mixed model ANOVA to test whether the effect of real rTMS differs from that of sham. Unfortunately the number of patients with implanted epidural leads available to do these experiments is small. Indeed, it took some time to recruit the five patients included in this study.
To overcome this limitation, we analysed the data in the following way: we first tested whether there was an effect of rTMS on MEPs, I1-waves and later I-waves in a 2-factor ANOVA (main factors of Time (before and after rTMS) and Component). Where necessary, a Greenhouse–Geisser adjustment was used to correct for non-sphericity and in the case of significant main effects or interactions, post hoc analyses with the Tukey test for honest significant differences were applied.
In a second stage, we determined whether any effects involving Time were likely to be significantly different from measures on an untreated group of patients. To do this we estimated the approximate variation in size of repeated measurements of MEPs and I-waves in the absence of rTMS. In the present five patients we split the 20 control MEPs/I-waves evoked prior to rTMS into two consecutive groups of 10. The data were averaged and measured so that we could calculate the absolute percentage variation between MEPs and I-waves in the two blocks for each subject. We also evaluated the spontaneous variability of epidural volleys and MEP amplitudes in 10 previously studied subjects with an implanted cervical epidural electrode (mean age 43 ±8.1 years, mean ±s.d.) who did not receive rTMS. Again, the responses to 20 stimuli at an intensity of 150% RMT were recorded at rest and averaged in two blocks of 10 consecutive trials each. The grand mean percentage change and s.d. for the I1-, later I-waves and MEP amplitudes were calculated for the 15 subjects (the 5 subjects who underwent rTMS and the 10 subjects who did not undergo rTMS) and the upper limit of spontaneous variability was defined as the mean change plus two s.d.s. It was then possible to compare the data from each of our five patients before and after rTMS and ask whether the change in amplitude was outside this range. Note that our population estimate was based on averages of 10 trials whilst in the present patients we had averaged 20 trials before and after rTMS. Since we should obtain a better estimate of the mean from a larger number of trials, this implies that our criterion for change is more conservative than it might appear.
Finally, we used Spearman tests to evaluate correlations between the percentage changes in MEP amplitude and the percentage changes observed in I1- and later I-waves. Values are given as the mean ±s.d.
Results
Epidural volleys
LM magnetic stimulation evoked the earliest negative potential. It had a latency of 2.9 ms in subjects 1 and 4, 3.7 ms in subject 2, 2.7 ms in subject 3, and 2.3 ms in subject 5. The short latency of this wave is consistent with direct activation of corticospinal axons. We have therefore termed this volley D-wave (Di Lazzaro et al. 2004). Supra-threshold PA magnetic stimulation (150% RMT) evoked four descending waves in subjects 1 and 4 and five waves in subjects 2, 3 and 5 (Figs 1 and 2). The earliest of these waves had a latency which was 1–1.3 ms longer than the volley recruited by LM magnetic stimulation in subjects 1, 2 and 4, while it had the same latency of the earliest volley evoked by LM magnetic stimulation in subjects 3 and 5. Since the earliest volley elicited by LM magnetic stimulation is probably a D-wave, we have termed the volleys recruited by PA magnetic stimulation in subjects 1, 2 and 4 and the later volleys evoked by PA magnetic stimulation in subjects 3 and 5 as I-waves, numbered in order of their appearance (Di Lazzaro et al. 2004).
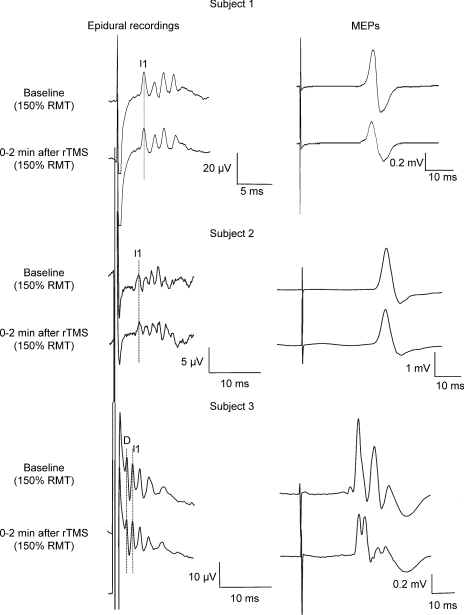
Figure 1. Corticospinal volleys and motor-evoked potentials evoked by single pulse magnetic stimulation in baseline conditions and after 1 Hz rTMS (900 stimuli) in subjects 1, 2 and 3.
Each trace is the average of 20 sweeps. Magnetic stimulation evokes four descending waves in patient 1. In this patient the peak of the earliest (I1) I-wave is indicated by the vertical line. Two minutes after rTMS, the size of the later I-waves is decreased, the amplitude of the I1-wave is unchanged. The suppression is more pronounced for the last I-wave (I4). The amplitude of MEP is also decreased after rTMS. Magnetic stimulation evokes five descending waves in subject 2. The peak of the earliest (I1) I-wave is indicated by the vertical line. Two minutes after rTMS, the size of the later I-waves and of MEPs is unchanged. Magnetic stimulation evokes five descending waves in subject 3. The peaks of the D-wave and of the earliest (I1) I-wave are indicated by the vertical line. Two minutes after rTMS, the size of the later I-waves is decreased, the amplitude of the I1-wave is unchanged. The suppression is more pronounced for the I3-wave. The amplitude of MEP is also decreased after rTMS.
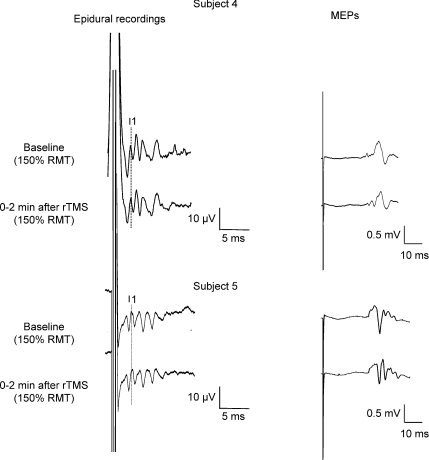
Figure 2. Corticospinal volleys and motor-evoked potentials evoked by single pulse magnetic stimulation in baseline conditions and after 1 Hz rTMS (900 stimuli) in subjects 4 and 5.
Each trace is the average of 20 sweeps. Magnetic stimulation evokes four descending waves in patient 4. The peak of the earliest (I1) I-wave is indicated by the vertical line. Two minutes after rTMS, the size of the later I-waves is decreased, the amplitude of the I1-wave is unchanged. The suppression is more pronounced for the last I-wave (I4). The amplitude of MEP is also decreased after rTMS. Magnetic stimulation evokes five descending waves in subject 5. The peak of the earliest (I1) I-wave is indicated by the vertical line. Two minutes after rTMS, the size of the later I-waves and of MEPs is unchanged.
Repetitive TMS
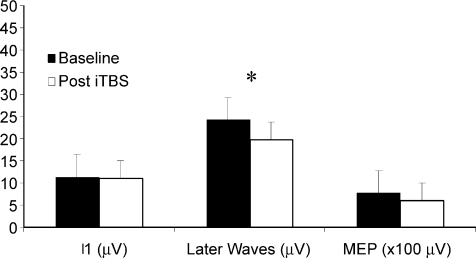
Figure 3 shows the effect of rTMS on the amplitudes of MEPs and on the I1- or later I-waves (the sum of the amplitudes of all the waves following the I1-wave) in the five subjects. A repeated measures ANOVA with Component (three levels: I1-wave amplitude, later I-waves, and MEP amplitude) and Time as main factors showed a significant effect of time (F1,4 = 11.168, P = 0.029) and of the interaction Component × Time (F2,8 = 4.923, P = 0.040). Post hoc analysis showed that only later I-waves were significantly reduced after 1 Hz rTMS (baseline 24.3 ±10.7 μV versus 19.6 ±7.3 μV after stimulation, P = 0.013; a reduction of 19%). I1-wave amplitude was unchanged (baseline 11.3 ±5.7 μV versus 11 ±6.2 μV after stimulation; P = 0.993). MEPs showed a tendency toward significant suppression (baseline 0.77 ±0.21 mV versus 0.61 ±0.20 mV after stimulation; P = 0.092). In fact, if we had measured only MEPs, as in most rTMS experiments, without including I-wave data (which required correction for multiple comparisons) then there is a significant decline of about 20% using a paired t test (P = 0.036).
Figure 3. Histograms showing grand mean amplitudes of the I1-wave, of the later I-waves (the sum of the amplitudes of waves following I1) and of motor-evoked potentials in baseline conditions and after 1 Hz rTMS in the five studied subjects.
The amplitude of later I-waves is significantly reduced after rTMS (* P < 0.05). The amplitude of MEPs is also reduced by about 20% after rTMS. The amplitude of the I1-wave is unchanged.
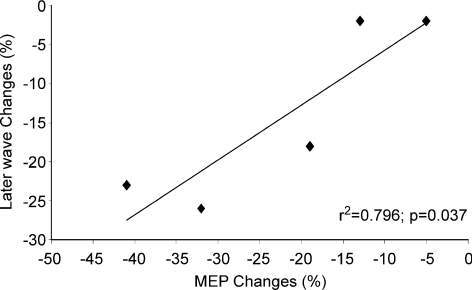
There was a significant linear correlation between later I-wave and MEP amplitudes (Spearman test, r2 = 0.796; P = 0.037) (Fig. 4) but not between I1-wave and MEP amplitude (Spearman test, r2 = 0.23; P = 0.285).
Figure 4. Correlation between the change in later I-wave amplitude and change in MEP amplitude after rTMS in the five subjects studied.
There is a significant linear correlation between later I-wave and MEP amplitudes (Spearman test, r2 = 0.796; P = 0.037).
Spontaneous variability of the amplitude of epidural volleys and of MEPs
Figures 1 and 2 show the epidural recordings before and after rTMS in the five subjects. There is a substantial variation in the size of the effect produced by rTMS. In order to evaluate whether the changes observed in individual patients were larger than the spontaneous variability of epidural volleys, we compared them with the spontaneous variability of epidural volley amplitudes observed in the same patients before rTMS together with data from a larger population of 10 subjects with cervical epidural electrodes who did not undergo rTMS.
In the total group of 15 subjects, the mean amplitude of the later I-waves was 16.1 ± 9.4 μV (mean ± s.d.) in the first trial and 16.2 ± 9.1 μV in the second trial (P = 0.64, paired t test). The change in the amplitude of the later volleys between the two trials ranged from 2.7% to 13.9% in these subjects with a mean absolute change of 7.2 ± 4.45%. Therefore, a change greater than 16.1% of the amplitude of later volleys (mean + 2 s.d.s in the 15 subjects) was considered outside the expected range of natural variation. The mean amplitude of the I1-wave was 8.1 ± 4.8 μV in the first trial and 8.05 ± 4.4 μV in the second trial (P = 0.75, paired t test) with an interindividual range from 0% to 11.4% (mean 6.5 ± 4%) in the 15 subjects. Therefore, a change greater than 14.4% of the amplitude of the I1-wave (mean + 2 s.d.s in the 15 subjects) was considered outside the expected range of natural variation. The reduction of later I-waves was larger than expected in subject 1 (–26%), in subject 3 (–23%), and in subject 4 (–18%), while subject 2 (–2%) and subject 5 (–2%) showed changes within this range. None of the subjects showed a change in I1-wave amplitude larger than the spontaneous variability observed without rTMS (subject 1, +4%; subject 2, –0.5%; subject 3, –14%; subject 4, –6%; subject 5, –5%).
Discussion
Low-frequency rTMS at 1 Hz led to a pronounced decrease in the excitability of cortical circuits generating the later I-waves whilst the I1-wave was unaffected. The suppression of later I-wave amplitude was strongly correlated with the inhibition of MEPs consistent with the idea that suppression of these waves was responsible for the reduction in amplitude of MEPs. We argue that this is good evidence to support the conclusion that at least some of the reduction in corticospinal excitability after 1 Hz rTMS occurs because of after-effects on cortical circuitry.
I-waves represent synchronous activity of corticospinal axons originating from trans-synaptic activation of corticospinal cells. Although their origin is still unclear, there is a good deal of evidence to suggest that the early and late I-waves are generated by independent cortical mechanisms (Ziemann & Rothwell, 2000; Di Lazzaro et al. 2004). Thus, our results suggest that low-frequency rTMS produces its effect by influencing the intrinsic circuitry of the motor cortex that generates later I-waves. The effect of low-frequency rTMS on the later I-waves is similar to that observed using other TMS protocols. For example, the suppression seen in short latency intracortical inhibition and the suppression produced via transcallosal inhibition both preferentially affect the later I-waves and leave the I1-wave virtually unchanged (Di Lazzaro et al. 2004). This specificity has been interpreted as indicating that these inhibitory effects do not change the overall excitability of the corticospinal neurones since that would lead to suppression of all I-wave inputs. Instead, the data are consistent with the idea that there can be specific inhibitory effects on the intracortical circuits that generate late I-waves. By analogy, it can be speculated that 1 Hz rTMS does not change the excitability of corticospinal cells but suppresses the excitability of cortico-cortical connections projecting upon the corticospinal cells.
The effect of 1 Hz rTMS on corticospinal volleys contrasts with the effects produced by a different inhibitory rTMS protocol, continuous theta burst stimulation (cTBS) (Huang et al. 2005). cTBS preferentially suppresses the I1-wave and leaves the later I-waves virtually unchanged (Di Lazzaro et al. 2005). Thus, the two inhibitory protocols seem to modulate different circuits of the motor cortex. Low-frequency rTMS probably suppresses the excitability of cortical interneurones or the multiple connections between these interneurones and the corticospinal cells. In contrast, cTBS may suppress the excitability of the monosynaptic input to corticospinal neurones that is thought to be responsible for the I1-wave (Di Lazzaro et al. 2004).
Interindividual variation in effects
There was a large variation in the effects of rTMS in the five patients that we studied. This is not surprising since previous studies have also commented on the high interindividual variability of the response to low-frequency rTMS (Maeda et al. 2000). However, since the changes in late I-waves correlated well with the changes in MEP, it seems likely that they represent real effects of rTMS rather than random variation. In fact, examined individually, 3 of the 5 subjects showed a greater change in late I-waves after rTMS than expected from random sampling in a cohort of 15 subjects who had not received any rTMS.
One of our patients had early stage Parkinson's disease which is known to affect several measures of motor cortical excitability (Priori & Lefaucheur, 2007). In addition, DOPA administration might have modified the response to rTMS because it is well known that the dopaminergic system is capable of modulating activity-dependent changes in synaptic strength (Jay, 2003). This has been confirmed even using TMS protocols investigating motor memory (Flöel et al. 2005). Indeed, patients off their normal therapy have a smaller response to another plasticity protocol commonly used on motor cortex, paired associative stimulation (Morgante et al. 2006), but this is normalized by treatment with l-DOPA. Our patient had only mild symptoms and was studied after taking her normal anti-parkinsonian medication when she was completely asymptomatic. In this state, her MEPs were suppressed after 1 Hz rTMS and her late I-waves were reduced in amplitude. Thus, her data support the link between rTMS-induced changes in cortex and MEP amplitude in the periphery independent of any possible Parkinson's Disease and/or DOPA-related change in cortical excitability.
In conclusion, we found that 1 Hz rTMS can reduce the excitability of cortical mechanisms generating later I-waves. This is the first direct demonstration of its physiological action on circuits of the motor cortex.
References
- Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, Cohen LG. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology. 1997;48:1398–1403. doi: 10.1212/wnl.48.5.1398. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Dileone M, Mazzone P, Insola A, Tonali PA, Rothwell JC. The physiological basis of transcranial motor cortex stimulation in conscious humans. Clin Neurophysiol. 2004;115:255–266. doi: 10.1016/j.clinph.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Saturno E, Oliviero A, Dileone M, Mazzone P, Insola A, Tonali PA, Ranieri F, Huang YZ, Rothwell JC. Theta-burst repetitive transcranial magnetic stimulation suppresses specific excitatory circuits in the human motor cortex. J Physiol. 2005;565:945–950. doi: 10.1113/jphysiol.2005.087288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flöel A, Breitenstein C, Hummel F, Celnik P, Gingert C, Sawaki L, Knecht S, Cohen LG. Dopaminergic influences on formation of a motor memory. Ann Neurol. 2005;58:121–130. doi: 10.1002/ana.20536. [DOI] [PubMed] [Google Scholar]
- Hallett M. Transcranial magnetic stimulation: a primer. Neuron. 2007;55:187–199. doi: 10.1016/j.neuron.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Jay TM. Dopamine: a potential substrate for synaptic plasticity and memory mechanisms. Prog Neurobiol. 2003;69:375–390. doi: 10.1016/s0301-0082(03)00085-6. [DOI] [PubMed] [Google Scholar]
- Lee L, Siebner HR, Rowe JB, Rizzo V, Rothwell JC, Frackowiak RS, Friston KJ. Acute remapping within the motor system induced by low-frequency repetitive transcranial magnetic stimulation. J Neurosci. 2003;23:5308–5318. doi: 10.1523/JNEUROSCI.23-12-05308.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda F, Keenan JP, Tormos JM, Topka H, Pascual-Leone A. Interindividual variability of the modulatory effects of repetitive transcranial magnetic stimulation on cortical excitability. Exp Brain Res. 2000;133:425–430. doi: 10.1007/s002210000432. [DOI] [PubMed] [Google Scholar]
- Morgante F, Espay AJ, Gunraj C, Lang AE, Chen R. Motor cortex plasticity in Parkinson's disease and levodopa-induced dyskinesias. Brain. 2006;129:1059–1069. doi: 10.1093/brain/awl031. [DOI] [PubMed] [Google Scholar]
- Priori A, Lefaucheur JP. Chronic epidural motor cortical stimulation for movement disorders. Lancet Neurol. 2007;6:279–286. doi: 10.1016/S1474-4422(07)70056-X. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Rothwell JC. Is there a future for therapeutic use of transcranial magnetic stimulation? Nat Rev Neurosci. 2007;8:559–567. doi: 10.1038/nrn2169. [DOI] [PubMed] [Google Scholar]
- Rossi S, Pasqualetti P, Rossini PM, Feige B, Ulivelli M, Glocker FX, Battistini N, Lucking CH, Kristeva-Feige R. Effects of repetitive transcranial magnetic stimulation on movement-related cortical activity in humans. Cereb Cortex. 2000;10:802–808. doi: 10.1093/cercor/10.8.802. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Electroencephalogr Clin Neurophysiol. 1994;91:79–92. doi: 10.1016/0013-4694(94)90029-9. Report of an IFCN committee. [DOI] [PubMed] [Google Scholar]
- Rounis E, Lee L, Siebner HR, Rowe JB, Friston KJ, Rothwell JC, Frackowiak RS. Frequency specific changes in regional cerebral blood flow and motor system connectivity following rTMS to the primary motor cortex. Neuroimage. 2005;26:164–176. doi: 10.1016/j.neuroimage.2005.01.037. [DOI] [PubMed] [Google Scholar]
- Touge T, Gerschlager W, Brown P, Rothwell JC. Are the after-effects of low-frequency rTMS on motor cortex excitability due to changes in the efficacy of cortical synapses? Clin Neurophysiol. 2001;112:2138–2145. doi: 10.1016/s1388-2457(01)00651-4. [DOI] [PubMed] [Google Scholar]
- Valero-Cabré A, Oliveri M, Gangitano M, Pascual-Leone A. Modulation of spinal cord excitability by subthreshold repetitive transcranial magnetic stimulation of the primary motor cortex in humans. Neuroreport. 2001;12:3845–3848. doi: 10.1097/00001756-200112040-00048. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Rothwell JC. I-waves in motor cortex. J Clin Neurophysiol. 2000;17:397–405. doi: 10.1097/00004691-200007000-00005. [DOI] [PubMed] [Google Scholar]