Abstract
We investigated the role of the vestibular commissural inhibitory system in vestibular compensation (VC, the behavioural recovery that follows unilateral vestibular loss), using in vivo microdialysis to measure GABA levels in the bilateral medial vestibular nucleus (MVN) at various times after unilateral labyrinthectomy (UL). Immediately after UL, in close correlation with the appearance of the characteristic oculomotor and postural symptoms, there is a marked increase in GABA release in the ipsi-lesional MVN. This is not prevented by bilateral flocculectomy, indicating that it is due to hyperactivity of vestibular commissural inhibitory neurones. Over the following 96 h, as VC occurs and the behavioural symptoms ameliorate, the ipsi-lesional GABA levels return to near-normal. Contra-lesional GABA levels do not change significantly in the initial stages of VC, but decrease at late stages so that when static symptoms have abated there remains a significant difference between the MVNs of the two sides. We also investigated the role of the commissural inhibition in Bechterew's phenomenon, by reversibly inactivating the intact contra-lesional labyrinth in compensating animals through superfusion of local anaesthetic on the round window. Transient inactivation of the intact labyrinth elicited the lateralized behaviour described by Bechterew, but did not alter the GABA levels in either MVN, suggesting the involvement of distinct cellular mechanisms. These findings indicate that an imbalanced commissural inhibitory system is a root cause of the severe oculomotor and postural symptoms of unilateral vestibular loss, and that re-balancing of commissural inhibition occurs in parallel with the subsequent behavioural recovery during VC.
Vestibular compensation (VC), the behavioural recovery that takes place after damage to the vestibular receptors of the inner ear, is an attractive model of deafferentation-induced plasticity in the adult brain. Ablation of one inner ear (e.g. through unilateral labyrinthectomy, UL), or unilateral vestibular neurectomy, causes severe oculomotor and postural symptoms including spontaneous ocular nystagmus, roll and yaw head tilt, contralateral limb extension and ipsilateral limb flexion, barrel rolling and circular walking (for reviews, see Smith & Curthoys, 1989; Dieringer, 1995; Curthoys & Halmagyi, 1999; Straka et al. 2005). Remarkably, most of these initial symptoms ameliorate rapidly so that barrel rolling and circular walking cease within hours, and ocular nystagmus gradually subsides over 2–3 days, as VC proceeds. This initial rapid recovery is followed by a much slower and usually incomplete recovery of dynamic oculomotor and postural function over the following weeks and months. Since there is no regeneration of the lesioned labyrinth or nerve, VC is attributed to neuronal and synaptic plasticity in the brainstem vestibular nuclei, cerebellum and related structures (Dieringer, 1995; Curthoys & Halmagyi, 1999; Straka et al. 2005).
The neuronal mechanisms underlying VC have been extensively investigated. The majority of studies have focused on neuronal and synaptic plasticity in the medial vestibular nucleus (MVN), the largest of the nuclei comprising the brainstem vestibular complex. In vivo studies have shown that immediately following UL, the normally high resting activity of the ipsi-lesional MVN neurons is virtually abolished while that of contra-lesional neurons either remains unchanged or is higher than normal (Precht et al. 1966; Smith & Curthoys, 1988; Ris et al. 1995; Ris & Godaux, 1998). This marked asymmetry in activity between the bilateral MVN is believed to be responsible for the oculomotor and postural symptoms induced by UL, since MVN neurons provide the major premotor drive to oculomotor, abducens and cervical spinal motoneurons that mediate the vestibulo-ocular and vestibulo-collic reflexes. During VC, there is a gradual restoration of resting activity in ipsi-lesional MVN neurons, and a ‘re-balancing’ of the resting firing rates on the two sides, approximately in parallel with the behavioural recovery (Ris et al. 1997; Straka et al. 2005).
Most current theories of VC assume that the initial drastic asymmetry in the firing activity of MVN neurons is due to a large imbalance in the reciprocal commissural inhibitory system that links the MVN of the two sides (review, Paterson et al. 2006). Thus, the silencing of the ipsi-lesional MVN neurons is caused not only by their disfacilitation after the loss of the excitatory synaptic input from the lesioned primary vestibular afferents, but also by an enhanced inhibitory drive from contra-lesional MVN neurons via the reciprocal commissural inhibitory system. Indeed the increased commissural inhibitory drive may be an important cause of the silencing of these neurons since bilateral labyrinthectomy, which equally disfacilitates the MVN of both sides, does not silence the resting activity of neurons in either MVN (Ris & Godaux, 1998). Several putative cellular mechanisms of VC have been proposed which may promote the recovery of resting activity in the deafferented ipsi-lesional MVN neurons at least partly by counteracting this increased commissural inhibition after UL (Straka et al. 2005; Paterson et al. 2006). However, there has been no direct investigation of the presumed imbalance in commissural inhibitory drive after UL, and there is no direct evidence to support the involvement of the commissural system in VC (Gliddon et al. 2005). In this study therefore we have used microdialysis in alert animals at various stages of compensation after UL, to directly measure the release of GABA in the bilateral MVN and determine the role of the commissural inhibitory system in VC.
Methods
Animals
Experimental design and procedures were carried out in compliance with the UK Animals (Scientific Procedures) Act 1986. A total of 44 male Lister–Hooded rats (120–150 g, age 5–6 weeks, Charles River Ltd, UK) were housed four to six animals per cage under standard controlled environmental conditions. After implantation of microdialysis guide cannulae, they were single-caged for the remainder of the experiment.
Implantation of microdialysis guide cannulae
Under deep surgical anaesthesia (1–2.5% halothane, delivered in a mixture of 30% N2O–70% O2), the animal was placed in a stereotactic frame with the head fixed so as to bring bregma and lambda into the horizontal plane. The crown of the head and the area behind the ears were shaved and disinfected with VetaSept (50%) before lidocaine (1%) was infused subcutaneously for pre-emptive analgesia of the field of surgery. Carprofen (5 mg kg−1, s.c.) was administered for post surgical analgesia.
The skull was exposed and two holes were drilled over the cerebellum, −3.5 mm AP and ±1.2 mm lateral to lambda, for the placement of the microdialysis guide cannulae. Holes were also drilled in the parietal bones to fasten two stainless steel jewellers' screws. The dura was opened and to avoid subarachnoid bleeding when the guides were implanted, the underlying pial membrane was also opened by pulling it gently with fine forceps. Two microdialysis guide cannulae (MAB 4.15.IC, Microbiotech, Sweden) were glued together at the base and the guide cannulae were slowly implanted en bloc through the two holes to a depth of 5 mm from the cerebellar surface. The guide cannulae were secured to the skull with acrylic dental cement, which was anchored to the two jewellers' screws fixed in the parietal bones.
Implantation of round window catheters
Some animals were implanted with a thin polyimide catheter (i.d. 120 μm, Microlumen Inc., Tampa, FL, USA) over the right round window, in order to infuse a local anaesthetic to obtain a transient, reversible vestibular de-afferentation. The implantation was performed using a lateral approach to the round window niche as described in detail by Magnusson & Tham (2006), immediately after the microdialysis guide cannulae implantation and in the same anaesthetic session. The catheter was secured to the microdialysis guide cannulae assembly with acrylic cement.
Microdialysis and unilateral labyrinthectomy
Two days after implanting the microdialysis guide cannulae, the animals were briefly anaesthetized with halothane to insert bilateral microdialysis probes (MAB 4.15, cuprophane membrane extending 1 mm below guide, diameter ˜0.2 mm, Microbiotech, Sweden). The probes were continuously perfused with a Ringer solution containing 140 mm NaCl, 3.0 mm KCl, 1.2 mm CaCl2 and 1.0 mm MgCl2, with or without a GABA reuptake inhibitor, NNC 711 (30 μm, Tocris Cookson Ltd, UK). The animals were allowed to recover and were then kept in a standard microdialysis bowl, with the fluid lines from the microdialysis probes connected to the external equipment via a swivelled balance arm. The perfusion rate was kept at 2.0 μl min−1 for 1 h and was then lowered to 1.0 μl min−1. Baseline sampling commenced after 2 h of perfusion. Microdialysate samples were collected every 10 min and frozen at −20°C until HPLC analysis, which was carried out within 3 days. After four baseline samples, the animal was anaesthetized again and a left-sided labyrinthectomy was performed by drilling into the vestibule and removing the contents by aspiration, as previously described (Bergquist et al. 2006). Microdialysis perfusion was continued during the UL procedure. Some animals were given a surgical sham treatment identical to the UL procedure, but without damage to the inner ear. In one group of animals the unilateral labyrinthectomy was preceded by a bilateral flocculectomy, which was accomplished by opening the lateral aspect of each floccular bone pocket and aspirating the flocculi (as described by Johnston et al. 2002). The unilateral labyrinthectomy was performed just after bilateral flocculectomy.
Following surgical treatment, the animals were returned to the microdialysis bowl and allowed to recover. Microdialysis perfusion was continued for up to 4 h, while vestibular deficits were observed and scored every 10 min. When microdialysis was performed on consecutive days after a UL, the microdialysis probe was removed at the end of each day's session, rinsed and stored wet until it was reintroduced in the same guide on the next day. In some experiments, the implanted round window catheter was used to obtain a reversible functional de-afferentation of the contra-lesional labyrinth. A bolus dose of 15–20 μl 3% ropivacain HCl (a gift from Astra-Zeneca, UK) was followed by 0.5 μl min−1 infusion for up to 1 h.
At the end of the experiment, the animal was killed and the brain was harvested for verification of probe locations. The brain was fixed in 4% paraformaldehyde and the cerebellum and brainstem were cut coronally into 200 μm sections that were examined macroscopically and photographed with a digital camera. The brainstem structures were identified by reference to a rat brain atlas (Paxinos & Watson, 1998).
Behavioural scoring
Vestibular deficits were scored for three components of the behavioural syndrome after unilateral vestibular de-afferentation: nystagmus, head roll tilt and postural asymmetry. Each component was given a maximum score of 10. Symptoms of left-sided de-afferentation were given positive scores, and symptoms of right-sided de-afferentation were given negative scores. Spontaneously occurring nystagmus was visually observed with the animal recumbent in the cage, sometimes after gently depressing the lower eyelid to facilitate observation. Spontaneous nystagmus was scored with 6–10 points, with 1 point for every 60 beats per minute (bpm). When nystagmus was not present at rest, the animal was either picked up or given a gentle airpuff over the head. Nystagmus evoked during these conditions was scored 1–5 points, with 1 point for every 60 bpm. Spontaneous head roll tilt was scored by estimating the angle between the jaw plane and the horizontal, with 10 points given either for a 90 deg angle or if the animal was recumbent on the de-afferented side or showed barrel-rolling towards that side. Postural deficits were scored as follows: spontaneous barrel rolling, 10 points; barrel rolling evoked by light touch or air-puff, 9 points; recumbent position on de-afferented side without leg support 8 points; some ipsi-lesional leg support 7 points; moving around on one side or using ipsi-lesional legs for recumbent support 6 points; moving around with bilateral leg support 5 points; moving around with occasional falls to the ipsi-lesional side 4 points; moving around leaning towards the ipsi-lesional side 3 points; hardly noticeable asymmetry 2 points, postural asymmetry only noticeable when picked up 1 point.
Vestibular de-afferentation behaviour was scored once every 10 min, so that a score was obtained for each microdialysis sampling period. In experiments with local inactivation of the contralesional labyrinth, some scores were also obtained without concomitant microdialysis sampling as the animals recovered after UL or the round window anaesthetic infusions.
Analysis of amino acid concentrations in microdialysate samples
The microdialysates were thawed and analysed for aspartate, glutamate (GLU), glutamine (GLN), glycine (GLY), taurine and GABA as previously described (Bergquist et al. 2006). In short the samples were derivatized with o-phtaldialdehyde (OPA) and separated on monolithic reverse phase columns (Chromolith Speedrod column, 50 × 4.6 mm in series with a Chromolith Performance column, 100 × 4.6 mm, Merck KGaA, Germany) using a ternary gradient with pH 5.7 and acetonitrile and MeOH as organic solvents. The OPA signal was detected with a fluorescence detector, excitation and emission wavelengths 340 nm and 455 nm, respectively (FD-2020Plus, Jasco Ltd, Great Dunmow, UK); recorded on a desktop PC; and analysed with the Chrompass Chromatography software package (Jasco Inc, Japan). The system was calibrated before each run and monitored regularly with external standards.
Calculations and statistics
Data are given as means ± s.e.m. The measurements of amino acids other than GABA are only reported when the interventions induced significant changes, or to describe baseline concentrations. To eliminate differences in probe recovery, microdialysate concentrations were transformed to percentage of baseline before statistical calculations and graphing. Line graphs depict full data sets, but statistics were calculated using means over 30 min or 60 min (Fig. 5) to avoid the problem of missing values. GraphPad Prism software (v. 4.03 for Windows, GraphPad Software, USA) was used for statistical tests. When appropriate, the influence of side and of time after UL on amino acid release was evaluated by two-way ANOVA. When significant effects were observed, the release from ipsi- and contralesional MVNs was compared at each time point using Student's t test with Bonferroni's correction to compensate for multiple comparisons. One-factor comparisons were made with one-way ANOVA or t tests as appropriate.
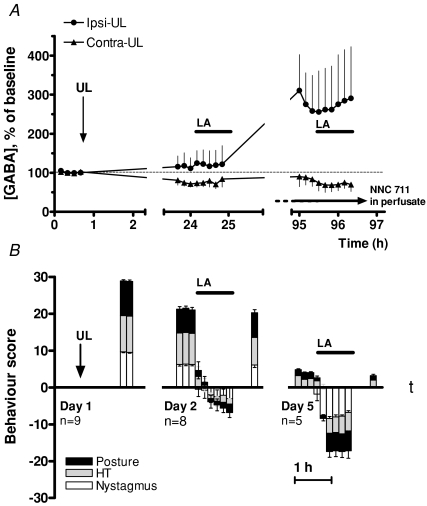
Figure 5. GABA release in the bilateral MVN during Bechterew's phenomenon.
A, effects of the reversible inactivation of the intact, contra-lesional labyrinth on GABA concentrations in microdialysis perfusates from ipsi- and contra-lesional MVNs at 24 h and 95 h post-UL. B, simultaneous behavioural scores. The GAT-1 inhibitor NNC 711 was only used in the final sampling session (horizontal arrow). Horizontal bars (LA) indicate the time during which the contra-lesional labyrinth was inactivated by infusion of ropivacain onto the round window. Note that the ropivacain infusion began after the first 30 min in each recording session, allowing three microdialysis samples to be collected before the induction of the Bechterew phenomenon. As in the previous experiment, at 24 h post UL higher GABA concentrations were found in the ipsi-lesional than the contra-lesional MVN even in the absence of NNC 711, but this difference was not significant for the three samples collected before labyrinth inactivation. On day 5 (95 h post UL), in the presence of NNC 711, ipsi-lesional GABA levels before inactivation were significantly higher than contra-lesional levels (two-way ANOVA, F = 22, d.f. = 1, P = 0.0002). Inactivation of the contra-lesional labyrinth caused a complete reversal of the direction of the lateralized symptoms on day 2, and emergence of a vestibular deafferentation syndrome directed towards the inactivated labyrinth on day 5 (Bechterew's phenomenon). The behavioural reversal was not associated with changes in GABA concentrations in the MVN on either side, with or without GAT inhibition (A).
Results
Acute changes in GABA release in the MVN after unilateral labyrinthectomy
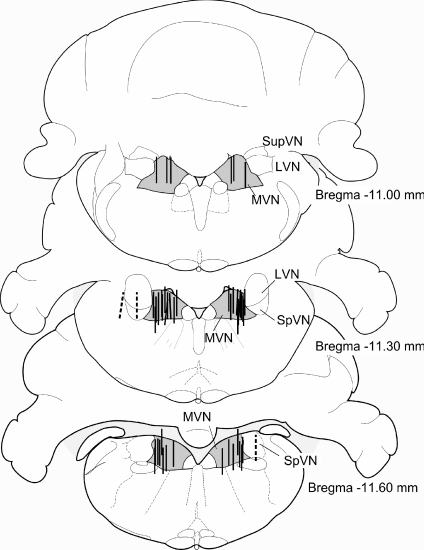
Figure 1 illustrates the locations of the microdialysis probes that were included (continuous lines) and excluded (dashed lines) in the present study. In preliminary experiments in animals anaesthetized with urethane, UL was found to have little effect on GABA concentrations in the ipsi- and contra-lesional MVNs over the first 2 h post-UL (data not shown). Because changes in GABA release are difficult to detect with microdialysis due to highly effective GABA re-uptake mechanisms (Timmerman & Westerink, 1997), in most subsequent experiments in alert animals the selective GAT1 inhibitor NNC 711 was included in the dialysate to inhibit GABA re-uptake. This caused not only the expected increase in measured GABA concentrations, but also a trend toward higher concentrations of glutamate in the microdialysate samples (Table 1). When the results from experiments with NNC 711 present were pooled and compared with those without GAT inhibition, glutamate concentrations were significantly higher in the NNC 711 treated group (1570 ± 293 versus 728 ± 130 nm, P = 0.0124, unpaired t test with Welch's correction). This is likely to be explained by either a decreased conversion of glutamate to GABA, or an increase in the formation of glutamate through the breakdown of GABA via ketoglutaric acid, during the inhibition of GABA uptake by NNC 711. The baseline concentrations of glycine and glutamine in the MVN were not affected by NNC 711 (Table 1).
Figure 1. Locations of microdialysis probes.
The locations of the active membrane parts of the microdialysis probes, reconstructed from photographs of brain sections at the end of each experiment, are indicated by lines. Measurements from microdialysis probes where at least 2/3 of the microdialysis membrane was located within the MVN were included in the analysis. Excluded probe locations are shown as dashed lines.
Table 1.
Mean concentrations of glutamine (GLN), GABA, glutamate (GLU) and glycine in the first four microdialysate samples taken in different experimental conditions (nm, means ± s.e.m.)
| NNC 711 Experiment | + A, n = 18 |
+ B, n = 12 |
− C, n = 12 |
− D, n = 15 |
ANOVA (F, d.f) |
|---|---|---|---|---|---|
| GLN | 19351 ± 2809 | 17966 ± 1986 | 20325 ± 1770 | 27418 ± 3375 | (2.4, 3) N.S. |
| GABA | 55.9 ± 8.7 | 66.4 ± 12.6 | 22.5 ± 3.1*† | 20.2 ± 1.1*† | (8.6, 3) P < 0.0001 |
| GLU | 1661 ± 378 | 1018 ± 222 | 944 ± 278 | 555 ± 58* | (3.8, 3) P = 0.0152 |
| GLY | 2416 ± 417 | 2316 ± 471 | 1464 ± 147 | 2979 ± 481 | (2.0, 3) N.S. |
A, before unilateral labyrinthectomy (UL) with the GAT1 inhibitor NNC 711 present in the perfusate. B, before bilateral flocculectomy and UL, in the presence of NNC 711. C, before UL, without NNC 711 present. D, before UL with contralateral round window catheter preimplanted, without NNC 711. Measurements from microdialysis probes in the left and right MVN are pooled as paired t tests indicated no significant differences between sides. N.S., non-significant. Bonferroni post hoc test,
P < 0.05 versus A,
P < 0.01 versus B.
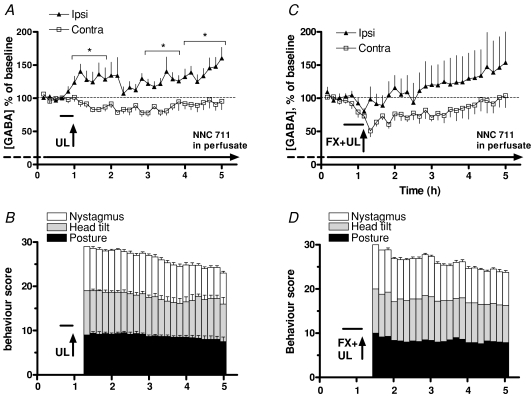
In the presence of NNC 711, a marked increase in GABA concentration was detected in the ipsi-lesional MVN immediately after UL, and a significant difference in GABA release between the ipsi- and contra-lesional MVNs developed within the first hour post-UL (Fig. 2A). The ipsilesional GABA levels started to increase even before the animals had recovered from the halothane anaesthesia induced for the UL procedure, and the lateral difference was sustained throughout the subsequent 4 h duration of the experiment. Once they had recovered from the halothane anaesthesia, the animals displayed pronounced symptoms of unilateral vestibular de-afferentation (Fig. 2B). Over the 4 h duration of the experiment the nystagmus score decreased from a mean of 9.5 ± 0.3 to a mean of 6.3 ± 1.1 (paired t test, n = 4, P = 0.026), while the postural and head tilt symptom scores remained high.
Figure 2. GABA release in ipsi-lesional and contra-lesional MVN after unilateral labyrinthectomy (A and B), and after unilateral labyrinthectomy preceded by a bilateral flocculectomy (C and D).
A, GABA concentrations in microdialysate samples from the ipsi- and contra-lesional MVNs during a unilateral labyrinthectomy (UL, vertical arrow) and the following 4 h. Two-way ANOVA of data stratified in 1 h bins showed a significant effect of side (F = 15.13, d.f. = 1, P = 0.0016; Bonferroni corrected t tests, ipsi versus contra *P < 0.05). B, simultaneous scoring of behavioural symptoms. No vestibular symptoms were observed before UL, but pronounced symptoms of unilateral vestibular loss occurred immediately after the animals recovered from the anaesthesia induced for the UL procedure. C, GABA concentrations in microdialysates from the ipsi- and contra-lesional MVNs over the first 4 h, when the UL was immediately preceded by a bilateral flocculectomy (FX). Two-way ANOVA of data stratified in 1 h bins showed a significant effect of side (F = 5.03, d.f. = 1, P = 0.0306; Bonferroni corrected t tests did not show significant differences in GABA release between sides for any given time point). D, simultaneous scoring of behavioural symptoms. The average durations of the UL and UL + FX procedures are indicated by horizontal bars. The GAT1 inhibitor (NNC 711) was present in the perfusion fluid to inhibit GABA re-uptake and enhance the detection of synaptically released GABA (horizontal arrow).
Origin of the acute increase in GABA levels in the ipsi-lesional MVN after UL
The major GABA-ergic afferents to the MVN derive from inhibitory neurons in the vestibular commissural system and from cerebellar Purkinje neurons, particularly in the ipsilateral flocculus. To determine whether the cerebellar flocculus was responsible for the increased release of GABA in the ipsi-lesional MVN after UL, in six animals a bilateral flocculectomy was performed at the same time as the UL. Bilateral flocculectomy caused an immediate fall in baseline GABA concentrations in the MVN of both sides (Fig. 2C, vertical arrow). Subsequently nonetheless, there was a sustained higher release of GABA in the ipsi-lesional MVN compared to the contra-lateral side, similar to that seen after UL alone (Fig. 2C). The variability in measured GABA levels on the ipsi-lesional side was, however, greater, and although there was a significant difference between ipsi-lesional and contra-lesional GABA levels (two-way ANOVA, F = 5.03, d.f. = 1, P = 0.0306), post hoc tests did not reveal a significant difference in GABA levels between the two sides at any of the individual time points.
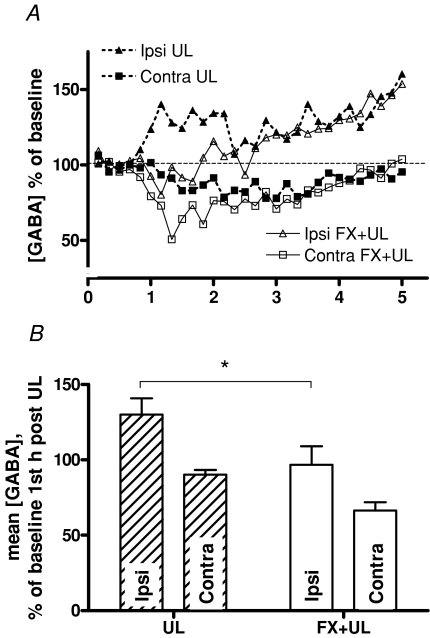
When the GABA release in flocculectomised UL animals was compared to that after UL alone, the GABA levels were very similar at the later time points (Fig. 3A). However, over the first hour post-UL the ipsi-lesional GABA release was significantly lower in flocculectomised UL animals than after UL alone (Fig. 3B, one-way ANOVA, F = 9.96, P = 0.0002; P < 0.05 for GABAipsi UL versus GABAipsi FX+UL). A similar but non-significant difference was found for contra-lesional GABA release (Fig. 3B). The behavioural symptoms in flocculectomised UL animals (Fig. 2D) were very pronounced in some animals, and were qualitatively somewhat different from those seen after UL alone. Nystagmus was often dissociated and the direction of the fast phase of the eye movement was more difficult to determine. Some animals also displayed pronounced axial torsion, which was rarely seen after UL alone. The behavioural symptom scores were however, similar to those after UL, with nystagmus being the only symptom that abated somewhat from a mean of 9.4 ± 0.3 in the first hour to 7.9 ± 0.3 in the fourth hour (paired t test, n = 6, P = 0.027).
Figure 3. The influence of the flocculus on GABA release after a unilateral labyrinthectomy.
A, comparison of the data shown in Fig. 2A and C. Except for the first 1–1.5 h after UL, the GABA release in MVNs of UL-animals and MVNs of animals labyrinthectomised after a bilateral flocculectomy was very similar. Error bars have been omitted for clarity. B, mean GABA release during the first hour post-UL in the ipsi-lesional and contra-lesional MVNs in UL animals and FX + UL animals. GABA release in the ipsi-lesional MVN post-UL was significantly lower after flocculectomy (one-way ANOVA, F = 9.96, P = 0.0002; *P < 0.05 Bonferroni corrected t test for GABAipsi ULversus GABAipsi FX+UL).
GABA release in the MVN during vestibular compensation
To determine the changes in GABA release levels in the MVN over the course of the behavioural recovery that occurs during VC, GABA release in the bilateral MVN was monitored on three consecutive days in UL animals (n = 6) and after sham surgery (n = 4 animals). In these experiments the microdialysis probes were re-inserted for sampling on days 2 and 3 post-UL, during brief periods of halothane anaesthesia (see Methods). Since prolonged GAT inhibition is known to alter GABA-ergic neurotransmission per se (Yu et al. 2007), the GABA re-uptake inhibitor NNC 711 was not used in these experiments.
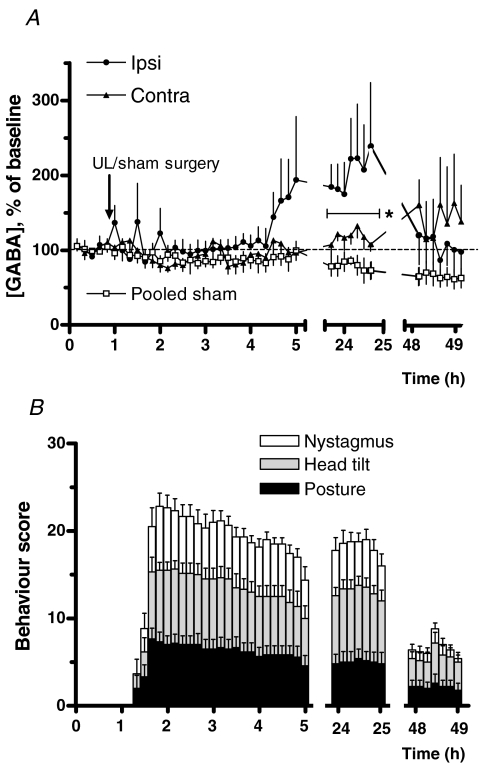
As expected from our preliminary experiments in anaesthetized animals, in the absence of NNC 711 no change in ipsi-lesional GABA concentrations was detected immediately post-UL, despite the strong vestibular deafferentation symptoms shown by the animals (Fig. 4A). After a delay of 3 h, however, the measured ipsi-lesional GABA concentrations began to increase, presumably because the continued elevated release of GABA in the ipsi-lesional MVN eventually saturated and overcame the endogenous re-uptake mechanisms. By 24 h post-UL the measured ipsi-lesional GABA levels were significantly higher than those on the contra-lesional side, even in the absence of NNC 711 (Fig. 4, 207 ± 46 versus 118 ± 11%, respectively, F = 10.38, d.f. = 1, P = 0.0023, *P < 0.05 Bonferroni corrected t test). This asymmetry in GABA levels in the two MVNs was accompanied by continuing strong vestibular deafferentation symptoms (Fig. 4B). At 48 h post-UL, ipsi-lesional GABA levels had decreased to near-normal, and there was no longer a significant difference between the ipsi- and contra-lesional MVNs (Fig. 4A). In parallel, there was a substantial improvement in behavioural deficit scores with an almost complete subsidence in the occurrence of nystagmus in particular (Fig. 4B).
Figure 4. GABA release in the ipsi-lesional and contra-lesional MVN during vestibular compensation.
A, GABA concentrations, in the absence of NNC 711, in microdialysates from the ipsi- and contra-lesional MVNs sampled over 48 h post-UL (n = 6 day 1 post UL, n = 5 days 2–3 post UL). Without GAT1 inhibition, an increase in ipsilesional GABA was only detectable 3–4 h post-UL (compare Fig. 2A and C). A significant effect of side was found in two-way ANOVA (F = 10.38, d.f. = 1, P = 0.0023, *P < 0.05 Bonferroni corrected t test ipsi versus contra). The corresponding microdialysates measurements after a sham surgery procedure (n = 5) showed no difference between sides (two-way ANOVA, F = 0.443, d.f. = 1, P = 0.51). Pooled measurements after sham surgery are indicated by open squares. B, simultaneous behavioural scores for each sampling time. Note the reduction in nystagmus and improvement in postural and head-tilt symptoms on day 2, indicating the progress of vestibular compensation.
Animals subjected to a surgical sham procedure showed no difference in GABA concentrations between the two MVNs, and the measured GABA concentrations remained stable over the entire experiment (Fig. 4A). Glutamate and glycine concentrations in the ipsi-lesional MVN also increased after UL, in particular on days 2 and 3, but very similar ipsi-lesional increases were observed in the sham operated animals (data not shown). When the sham effects were subtracted from the UL effects, no lateral difference in glutamate and glycine release remained (two-way ANOVA, glutamate: Pside = 0.58; glycine: Pside = 0.066).
Role of the commissural inhibitory system in the Bechterew phenomenon
Animals that have both labyrinths simultaneously removed show a general ataxia and spatial disorientation, but no lateralized oculomotor or postural symptoms, presumably because the brainstem vestibular nuclei on both sides are equally disfacilitated. Intriguingly, however, an animal that has successfully compensated after a unilateral labyrinthectomy will again show the typical lateralized symptoms of UL if the remaining labyrinth is subsequently removed, even though it now possesses no labyrinths. This is known as Bechterew's phenomenon (Bechterew, 1883; see also Vibert et al. 1999; Straka et al. 2005), and it indicates the re-appearance of an imbalance in bilateral MVN neuron activity either directly due to the disfacilitation of the vestibular nuclei on the side of the second labyrinthectomy, or to an imbalance in the commissural inhibitory system similar to that after UL. To determine if the commissural inhibitory system is also implicated in Bechterew's phenomenon, nine animals were allowed to compensate for 4 days after UL (Fig. 5). On day 2 (24 h post-UL), and on day 4 (95 h post-UL), the remaining intact labyrinth was reversibly inactivated by the infusion of a local anaesthetic through an implanted catheter adjacent to the round window, while GABA concentrations were monitored with microdialysis probes in both MVNs (Methods). In each of the experimental sessions the inactivation infusion began after 30 min of sampling, so that the first three microdialysis samples were obtained before the inactivation of the contra-lesional labyrinth (Fig. 5A). The GAT inhibitor NNC 711 was used during the final microdialysis sampling session on day 5 (Fig. 5A, horizontal arrow), but not during the microdialysis sampling on day 1 and 2, again to avoid any long-term effects of re-uptake inhibition on GABA-ergic neurotransmission in the MVN.
The animals had pronounced behavioural symptoms on day 2 (Fig. 5B), and as observed also in the experiment illustrated in Fig. 4, the measured GABA levels in the ipsi-lesional MVN were higher than those in the contra-lesional MVN even in the absence of NNC 711 (Fig. 5A). Within a few minutes of the inactivation of the intact labyrinth, however, the behavioural symptoms were abolished (Fig. 5B). With continued infusion to maintain the functional inactivation of the intact labyrinth for 1 h, the animals developed clear but relatively weak symptoms in the opposite direction. However, the suppression of the initial symptoms and the partial, weak expression of Bechterew's phenomenon were not accompanied by any detectable change in GABA levels in the ipsi-lateral or contra-lateral MVNs (Fig. 5A). Following the end of the infusion, the vestibular deafferentation symptoms reappeared as the blockade of the intact labyrinth was reversed, and 1 h after the end of the middle ear infusion the animals again showed the characteristic oculomotor and postural symptoms (Fig. 5B).
By day 4, the static behavioural symptoms had largely subsided and in particular there was no ocular nystagmus (Fig. 5B). In these animals, functional inactivation of the intact labyrinth with local anaesthetic (LA, horizontal bar) produced a marked Bechterew's phenomenon with the reappearance of a strong nystagmus and postural symptoms in the opposite direction. These symptoms appeared within a few minutes after the start of the round window infusion, and were maintained for the duration of the anaesthetic infusion and for some 30 min after its termination. Since this was the final experimental session for this group of animals, and because at 24–25 h the Bechterew's symptoms had not been accompanied by any detectable change in GABA release, the re-uptake blocker NNC 711 was included in the microdialysis perfusate for this experimental session to maximize the likelihood of detecting any changes in GABA release that occurred. GABA re-uptake blockade significantly increased the measured GABA levels on the ipsi-lesional side as expected, but surprisingly the GABA levels in the contra-lesional MVN in the presence of the re-uptake inhibitor were no different than those seen at 24–25 h, without re-uptake blockade (Fig. 5A). Thus a persistent significant difference between GABA release levels on the ipsi- and contra-lesional side was observed. As on day 2, the functional inactivation of the intact labyrinth and the appearance of Bechterew's symptoms were not accompanied by any significant change in GABA levels in either the ipsi- or contra-lesional MVN.
GABA release levels in the MVN after the subsidence of the initial static symptoms in UL animals
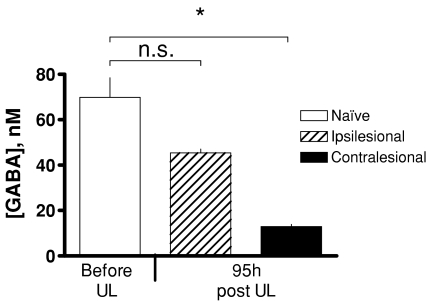
The final time point in the experiment exploring the mechanisms of the Bechterew phenomenon also allowed us to determine if the lateral imbalance in GABA release subsides completely in animals that have compensated for the initial static symptoms after UL. At 95 h post-UL nystagmus was absent, and the remaining head tilt and postural symptoms had also ameliorated so that the behavioural symptoms score had decreased from 28.8 ± 0.7 at 1 h post-UL, to 4.3 ± 0.6. However, the GABA concentrations in the microdialysis samples collected in the first 30 min of the recording session, before the round window anaesthetic infustion was started, showed that the GABA levels in the ipsi-lesional MVN were still higher than those in the contra-lesional MVN (Fig. 6). A closer examination of absolute GABA concentrations at this time point showed that ipsi-lesional GABA concentrations were not significantly different from those found in naïve animals in the presence of NNC 711, whereas GABA concentrations in the contra-lesional MVN were significantly lower (Fig. 6). The lateral difference in GABA release levels observed at 95 h post-UL was therefore due to a decrease in contra-lesional GABA concentrations, while ipsi-lesional GABA levels were not different from normal.
Figure 6. GABA levels in the MVN after 5 days of vestibular compensation.
Mean absolute concentrations of GABA (± s.e.m.) measured in the presence of NNC 711, in the MVN of naïve animals (before UL or FX + UL, first three microdialysis samples in Fig. 2A and C) and those measured in the ipsi-lesional and contra-lesional MVN after 95 h of compensation post-UL (data from the first three samples taken in the final recording session in Fig. 5). Ipsi-lesional GABA levels after 95 h of compensation are not significantly different from those in the naïve MVN, while those in the contra-lesional MVN are significantly lower.
Discussion
These findings provide the first direct experimental evidence to show that lateralized changes in GABAergic neurotransmission in the bilateral MVN underlie both the initial behavioural symptoms that follow unilateral vestibular deafferentation, and the subsequent behavioural recovery that takes place during vestibular compensation. Immediately after UL, in close correlation with the appearance of the oculomotor and postural symptoms of unilateral vestibular loss, there is a marked increase in GABA release in the ipsi-lesional MVN. This is not prevented by bilateral flocculectomy, strongly indicating that it is due to hyperactivity of the inhibitory neurones of the vestibular commissural system which mediate commissural inhibition from the contra-lesional MVN. With the development of vestibular compensation over 48–96 h post-UL and the amelioration of the behavioural symptoms, the elevated GABA levels in the ipsi-lesional MVN return to near-normal, indicating that the early behavioural deficits after UL are driven by increased commissural GABA release in the ipsilesional MVN.
By contrast GABA release levels in the contra-lesional MVN do not change significantly in the initial stages of compensation. However, at 95 h post-UL when the initial static symptoms have abated, the blockade of GABA re-uptake in the MVN by the GAT-1 inhibitor NNC 711 revealed a significant asymmetry in GABA release levels between the ipsi-lesional and contra-lesional sides. In the ipsi-lesional MVN, blocking GABA re-uptake caused the measured GABA concentrations to increase to the same levels as seen during re-uptake blockade in naïve, normal animals, while on the contra-lesional side the GABA concentrations achieved during re-uptake blockade were significantly lower. Thus GAT1-inhibition 4 days post-UL failed to increase GABA levels in the contra-lesional MVN as much as would be expected in normal animals, suggesting that there is a marked decrease in the level of on-going synaptic release of GABA on the contra-lesional side at this time point. It is tempting to speculate that this persistent lateral difference in GABA release pattern contributes to maintenance of the compensated state, as well as to the persistent dynamic vestibular deficits that remain at the later time points of vestibular compensation after UL.
These changes demonstrate, for the first time, that the vestibular commissural inhibitory system is fundamentally implicated in the drastic effects of UL on the firing rates of the MVN neurons and the ensuing severe symptoms of vestibular deafferentation. In most current theories of vestibular compensation, a role for an imbalanced commissural inhibitory system has been inferred from in vivo studies of the effects of UL on the activity of type I and type II MVN neurons and their known connections (Precht et al. 1966; Smith & Curthoys, 1989; Ris et al. 1995; Ris & Godaux, 1998). This has led many investigators to study changes in GABAergic neurotransmission in the MVN of normal and compensating animals (Dutia et al. 1992; Johnston et al. 2001; Tighilet & Lacour, 2001; Magnusson et al. 2002; Horii et al. 2003; Eleore et al. 2005; Gliddon et al. 2005; Zhang et al. 2005; Bergquist et al. 2006). In particular, it has been proposed that changes in the functional efficacy of inhibitory GABAA, GABAB and glycine receptors in the deafferented MVN neurons may help to promote the recovery of their resting activity early in compensation (Vibert et al. 2000; Yamanaka et al. 2000). The present results support this view, and indeed serve to emphasize the key role that the vestibular commissural system may have in generating the oculomotor and postural symptoms of the vestibular deafferentation syndrome. Bilateral labyrinthectomy, which disfacilitates the vestibular nuclei of both sides equally, does not induce nystagmus, head deviation or the postural asymmetries associated with UL, and nor does it cause the silencing of the firing rate of MVN neurons of either side (Ris & Godaux, 1998). Thus it would appear that the root cause of the silencing of the ipsi-lesional MVN neurons after UL, and the consequential severe behavioural symptoms, is the increased commissural inhibition from the hyperactive MVN neurons on the contra-lesional side and the sustained elevated levels of GABA release in the ipsi-lesional MVN, as demonstrated here. Understanding the factors which modulate GABA release in the commissural system (Bergquist et al. 2006), and the mechanisms of plasticity in the commissural system, would thus appear to be essential in elucidating the cellular mechanisms involved in the acute stage of VC.
Interestingly, the present results show that the effects of bilateral flocculectomy consist of a bilateral decrease in GABA release levels in the MVN. The role of the cerebellar flocculus in VC has been of some interest, but remains unclear. Flocculectomised animals have more severe vestibular deafferentation symptoms and appear to recover more slowly than normal, as confirmed by the behavioural observations in the present study. However VC is not abolished after flocculectomy (Courjon et al. 1982; Kitahara et al. 1997; Kitahara et al. 1998). It has been suggested that changes in the inhibitory inputs from the flocculi may help in reducing the asymmetry in firing rates of the MVN neurons on the lesioned and intact sides (Kitahara et al. 1997; Kitahara et al. 1998). In the present study, GABA release levels in flocculectomised animals were very similar to those seen in normal animals, except for the first hour post-UL. This implies that the cerebellar flocculi are not in a position to regulate or reduce the gross imbalance in the commissural system after UL. This does not, however, rule out an important role for the cerebellar input to the MVN in ‘motor learning’ and the re-calibration of the vestibulo-ocular and vestibulo-collic reflexes during VC, which may not be readily apparent in the tissue extracellular GABA concentrations that were measured in this study. It should also be borne in mind that the present experiments do not address the possible role of nodulus and uvula Purkinje neurons, which also project to the MVN (Bernard, 1987; Barmack et al. 2001).
While the present findings indicate that an imbalanced vestibular commissural inhibitory system is a root cause for the silencing of the ipsi-lesional MVN neurons after UL, the cellular mechanisms underlying Bechterew's phenomenon appear to be distinct. Thus, the middle ear infusion technique used here to produce transient, reversible inactivation of the intact contra-lesional labyrinth in compensating animals, was effective in eliciting the expected Bechterew's behavioural symptoms but without any measurable accompanying changes in GABA release levels in either MVN. Although it is possible that the inhibition of GABA re-uptake by NNC 711 would make it more difficult to detect a fall in ipsi-lesional GABA levels, this is unlikely as the GABA levels remained stable also without NNC 711 in the perfusate. The vestibular symptoms in Bechterew's phenomenon therefore are not associated with a significant imbalance in commissural inhibition, but instead appear to be due to the disfacilitation of the contra-lesional MVN neurons during labyrinth inactivation. Thus, although both UL and Bechterew's phenomenon give rise to essentially similar behavioural symptoms, the differences between the underlying cellular mechanisms are revealing. Following UL, in parallel with the changes in GABA-ergic and glycinergic synaptic efficacy associated with plasticity in the commissural inhibitory system, the ipsi- and contra-lesional MVN cells also show marked changes in their intrinsic electrophysiological properties (Cameron & Dutia, 1997; Him & Dutia, 2001; Beraneck et al. 2003; Beraneck et al. 2004). While no electrophysiological studies of MVN neurons during Bechterew's phenomenon have yet been carried out, it is possible that this may provide a useful paradigm to investigate the cellular and molecular basis of the activity-related changes in intrinsic properties induced by disfacilitation, without the accompanying imbalance in commissural inhibition. Such plasticity in intrinsic neuronal properties may also underlie the recovery of resting discharge rates after bilateral labyrinthectomy (Ris & Godaux, 1998).
Extracellular glutamate levels in the bilateral MVNs after unilateral labyrinthectomy have been measured in two previous studies (Inoue et al. 2003; Yu et al. 2007), Both studies reported an early, transient increase in contra-lesional glutamate levels, something we did not observe in the present study with or without the use of NNC 711. Differences in the perfusion protocol may in part explain this discrepancy, as the earlier studies used twice as high concentrations of Ca2+ as in our protocol. The lower Ca2+ concentrations are generally believed to better reflect the extracellular environment in the brain, and under such conditions glutamate concentrations are mainly regulated by release via the cystein–glutamate antiporter and do not reflect synaptic release alone (Baker et al. 2002). Although part of the commissural inhibitory pathways of the vestibular nuclei are glycinergic (Precht et al. 1973; Bagnall et al. 2007), we did not observe significant changes in GLY release in the bilateral MVNs after UL in this study (data not shown). This may in part be explained by the difficulty of separating GLY from citrulline and threonine chromatographically, and by the fact that we did not seek to optimize the experimental conditions for the detection of synaptic GLY for example by using a glycine uptake (GLyT1) inhibitor.
Conclusions
These findings provide the first direct experimental evidence demonstrating that the severe oculomotor and postural symptoms of unilateral vestibular loss are accompanied by a marked imbalance in the brainstem commissural inhibitory system. The rapid appearance of an imbalance in GABA release between the ipsi-lesional and contra-lesional MVN immediately after UL, and the reversal of this asymmetry in parallel with behavioural recovery indicate that plasticity in GABAergic neurotransmission through mechanisms that regulate GABA release, uptake or functional efficacy, and cellular plasticity in commissural neurons may play an important role in the acute stage of vestibular compensation. Such rapid-onset mechanisms are likely to be of particular relevance in conditions that involve partial and fluctuating vestibular loss, where compensation is typically poor and incomplete, since more delayed-onset compensatory mechanisms may not be able to operate effectively (for a discussion see Paterson et al. 2006). A fuller understanding of the mechanisms of plasticity in commissural GABAergic neurotransmission may therefore be potentially useful for therapeutic interventions in such cases. The cellular mechanisms underlying the vestibular syndrome that follows the loss of the remaining labyrinth in a compensated animal (Bechterew's phenomenon) are however, distinct, and appear to be more directly linked to the disfacilitation of the MVN neurons after deafferentation.
Acknowledgments
This work was supported by the Wellcome Trust, project grant 073041. F.B. was also supported by the Swedish Research Council for part of this work.
References
- Yu H, An Y, Jiang H, Jin Q, Jin Y. Changes of amino acid concentrations in the rat medial vestibular nucleus following unilateral labyrinthectomy. Sheng Li Xue Bao. 2007;59:71–78. [PubMed] [Google Scholar]
- Bagnall MW, Stevens RJ, du Lac S. Transgenic mouse lines subdivide medial vestibular nucleus neurons into discrete, neurochemically distinct populations. J Neurosci. 2007;27:2318–2330. doi: 10.1523/JNEUROSCI.4322-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker D, Xi Z, Shen H, Swanson C, Kalivas P. The origin and neuronal function of in vivo nonsynaptic glutamate. J Neurosci. 2002;22:9134–9141. doi: 10.1523/JNEUROSCI.22-20-09134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barmack NH, Qian ZY, Kim HJ, Yoshimura J. Activity-dependent distribution of protein kinase C-delta within rat cerebellar Purkinje cells following unilateral labyrinthectomy. Exp Brain Res. 2001;141:6–20. doi: 10.1007/s002210100855. [DOI] [PubMed] [Google Scholar]
- Beraneck M, Hachemaoui M, Idoux E, Ris L, Uno A, Godaux E, Vidal PP, Moore LE, Vibert N. Long-term plasticity of ipsilesional medial vestibular nucleus neurons after unilateral labyrinthectomy. J Neurophysiol. 2003;90:184–203. doi: 10.1152/jn.01140.2002. [DOI] [PubMed] [Google Scholar]
- Beraneck M, Idoux E, Uno A, Vidal PP, Moore LE, Vibert N. Unilateral labyrinthectomy modifies the membrane properties of contralesional vestibular neurons. J Neurophysiol. 2004;92:1668–1684. doi: 10.1152/jn.00158.2004. [DOI] [PubMed] [Google Scholar]
- Bergquist F, Ruthven A, Ludwig M, Dutia MB. Histaminergic and glycinergic modulation of GABA release in the vestibular nuclei of normal and labyrinthectomised rats. J Physiol. 2006;577:857–868. doi: 10.1113/jphysiol.2006.120493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JF. Topographical organization of olivocerebellar and corticonuclear conections in the rat – an WGA-HRP study. 1. Lobule-IX, Lobule-X and the flocculus. J Comp Neurol. 1987;263:241–258. doi: 10.1002/cne.902630207. [DOI] [PubMed] [Google Scholar]
- Cameron SA, Dutia MB. Cellular basis of vestibular compensation: changes in intrinsic excitability of MVN neurones. Neuroreport. 1997;8:2595–2599. doi: 10.1097/00001756-199707280-00035. [DOI] [PubMed] [Google Scholar]
- Courjon J, Flandrin J, Jeannerod M, Schmid R. The role of the flocculus in vestibular compensation after hemilabyrinthectomy. Brain Res. 1982;239:251–257. doi: 10.1016/0006-8993(82)90847-2. [DOI] [PubMed] [Google Scholar]
- Curthoys IS, Halmagyi GM. Vestibular compensation. Adv Otorhinolaryngol. 1999;55:82–110. doi: 10.1159/000059059. [DOI] [PubMed] [Google Scholar]
- Dieringer N. Vestibular compensation – neural plasticity and its relations to functional recovery after labyrinthine lesions in frogs and other vertebrates. Prog Neurobiol. 1995;46:97–129. [PubMed] [Google Scholar]
- Dutia MB, Johnston AR, McQueen DS. Tonic activity of rat medial vestibular nucleus neurons in vitro and its inhibition by GABA. Exp Brain Res. 1992;88:466–472. doi: 10.1007/BF00228176. [DOI] [PubMed] [Google Scholar]
- Eleore L, Vassias I, Bernat I, Vidal PP, de Waele C. An in situ hybridization and immunofluorescence study of GABAA and GABAB receptors in the vestibular nuclei of the intact and unilaterally labyrinthectomized rat. Exp Brain Res. 2005;160:166–179. doi: 10.1007/s00221-004-1997-8. [DOI] [PubMed] [Google Scholar]
- Gliddon CM, Darlington CL, Smith PF. GABAergic systems in the vestibular nucleus and their contribution to vestibular compensation. Prog Neurobiol. 2005;75:53–81. doi: 10.1016/j.pneurobio.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Him A, Dutia MB. Intrinsic excitability changes in vestibular nucleus neurons after unilateral deafferentation. Brain Res. 2001;908:58–66. doi: 10.1016/s0006-8993(01)02600-2. [DOI] [PubMed] [Google Scholar]
- Horii A, Kitahara T, Smith PF, Darlington CL, Masumura C, Kubo T. Effects of unilateral labyrinthectomy on GAD, GATI and GABA receptor gene expression in the rat vestibular nucleus. Neuroreport. 2003;14:2359–2363. doi: 10.1097/00001756-200312190-00014. [DOI] [PubMed] [Google Scholar]
- Inoue S, Yamanaka T, Kita T, Nakashima T, Hosoi H. Glutamate release in the rat medial vestibular nucleus following unilateral labyrinthectomy using in vivo microdialysis. Brain Res. 2003;991:78–83. doi: 10.1016/j.brainres.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Johnston AR, Him A, Dutia MB. Differential regulation of GABAA and GABAB receptors during vestibular compensation. Neuroreport. 2001;12:597–600. doi: 10.1097/00001756-200103050-00033. [DOI] [PubMed] [Google Scholar]
- Johnston AR, Seckl JR, Dutia MB. Role of the flocculus in mediating vestibular nucleus neuron plasticity during vestibular compensation in the rat. J Physiol. 2002;545:903–911. doi: 10.1113/jphysiol.2002.024281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitahara T, Takeda N, Kiyama H, Kubo T. Molecular mechanisms of vestibular compensation in the central vestibular system. Acta Otolaryngol Suppl. 1998;539:19–27. [PubMed] [Google Scholar]
- Kitahara T, Takeda N, Saika T, Kubo T, Kiyama H. Role of the flocculus in the development of vestibular compensation: Immunohistochemical studies with retrograde tracing and flocculectomy using Fos expression as a marker in the rat brainstem. Neuroscience. 1997;76:571–580. doi: 10.1016/s0306-4522(96)00374-0. [DOI] [PubMed] [Google Scholar]
- Magnusson AK, Tham R. Reversible and controlled peripheral vestibular loss by continuous infusion of ropivacaine (Narop) into the round window niche of rats. Neurosci Lett. 2006;400:16–20. doi: 10.1016/j.neulet.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Magnusson AK, Ulfendahl M, Tham R. Early compensation of vestibulo-oculomotor symptoms after unilateral vestibular loss in rats is related to GABAB receptor function. Neuroscience. 2002;111:625–634. doi: 10.1016/s0306-4522(01)00618-2. [DOI] [PubMed] [Google Scholar]
- Paterson J, Menzies J, Bergquist F, Dutia MB. Cellular mechanisms of vestibular compensation. Neuroembryol Aging. 2006;3:183–193. [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic Press; 1998. [Google Scholar]
- Precht W, Schwindt P, Baker R. Removal of vestibular commissural inhibition by antagonists of GABA and glycine. Brain Res. 1973;62:222–226. doi: 10.1016/0006-8993(73)90631-8. [DOI] [PubMed] [Google Scholar]
- Precht W, Shimazu H, Markham C. A mechanism of central compensation of vestibular function following hemilabyrinthectomy. J Neurophysiol. 1966;29:996–1010. doi: 10.1152/jn.1966.29.6.996. [DOI] [PubMed] [Google Scholar]
- Ris L, Capron B, deWaele C, Vidal PP, Godaux E. Dissociations between behavioural recovery and restoration of vestibular activity in the unilabyrinthectomized guinea-pig. J Physiol. 1997;500:509–522. doi: 10.1113/jphysiol.1997.sp022037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ris L, Dewaele C, Serafin M, Vidal PP, Godaux E. Neuronal activity in the ipsilateral vestibular nucleus following unilateral labyrinthectomy in the alert guinea-pig. J Neurophysiol. 1995;74:2087–2099. doi: 10.1152/jn.1995.74.5.2087. [DOI] [PubMed] [Google Scholar]
- Ris L, Godaux E. Neuronal activity in the vestibular nuclei after contralateral or bilateral labyrinthectomy in the alert guinea pig. J Neurophysiol. 1998;80:2352–2367. doi: 10.1152/jn.1998.80.5.2352. [DOI] [PubMed] [Google Scholar]
- Smith P, Curthoys I. Neuronal activity in the ipsilateral medial vestibular nucleus of the guinea pig following unilateral labyrinthectomy. Brain Res. 1988;444:308–319. doi: 10.1016/0006-8993(88)90939-0. [DOI] [PubMed] [Google Scholar]
- Smith PF, Curthoys IS. Mechanisms of recovery following unilateral labyrinthectomy: a review. Brain Res Brain Res Rev. 1989;14:155–180. doi: 10.1016/0165-0173(89)90013-1. [DOI] [PubMed] [Google Scholar]
- Straka H, Vibert N, Vidal PP, Moore LE, Dutia MB. Intrinsic membrane properties of vertebrate vestibular neurons: Function, development and plasticity. Prog Neurobiol. 2005;76:349–392. doi: 10.1016/j.pneurobio.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Tighilet B, Lacour M. Gamma amino butyric acid (GABA) immunoreactivity in the vestibular nuclei of normal and unilateral vestibular neurectomized cats. Eur J Neurosci. 2001;13:2255–2267. doi: 10.1046/j.0953-816x.2001.01622.x. [DOI] [PubMed] [Google Scholar]
- Timmerman W, Westerink B. Brain microdialysis of GABA and glutamate: what does it signify? Synapse. 1997;27:242–261. doi: 10.1002/(SICI)1098-2396(199711)27:3<242::AID-SYN9>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Vibert N, Babalian A, Serafin M, Gasc JP, Muhlethaler M, Vidal PP. Plastic changes underlying vestibular compensation in the guinea-pig persist in isolated, in vitro whole brain preparations. Neuroscience. 1999;93:413–432. doi: 10.1016/s0306-4522(99)00172-4. [DOI] [PubMed] [Google Scholar]
- Vibert N, Beraneck M, Bantikyan A, Vidal PP. Vestibular compensation modifies the sensitivity of vestibular neurones to inhibitory amino acids. Neuroreport. 2000;11:1921–1927. doi: 10.1097/00001756-200006260-00023. [DOI] [PubMed] [Google Scholar]
- von Bechterew W. Ergebnisse der Durchschneidung des N. acusticus nebst Erörterung der Bedeutung der semicirculären Kanäle für das Körpergleichgewicht. Pflugers Arch Ges Physiol. 1883;30:312–347. [Google Scholar]
- Yamanaka T, Him A, Cameron SA, Dutia MB. Rapid compensatory changes in GABA receptor efficacy in rat vestibular neurones after unilateral labyrinthectomy. J Physiol. 2000;523:413–424. doi: 10.1111/j.1469-7793.2000.t01-1-00413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Ashton J, Horii A, Darlington CL, Smith PF. Immunocytochemical and stereological analysis of GABAB receptor subunit expression in the rat vestibular nucleus following unilateral vestibular deafferentation. Brain Res. 2005;1037:107–113. doi: 10.1016/j.brainres.2005.01.018. [DOI] [PubMed] [Google Scholar]