Abstract
The 100% oxygen (O2) technique has been used to detect and quantify right-to-left shunt for more than 50 years. The goal of this study was to determine if breathing 100% O2 affected intrapulmonary arteriovenous pathways during exercise. Seven healthy subjects (3 females) performed two exercise protocols. In Protocol I subjects performed an incremental cycle ergometer test (60 W + 30 W/2 min; breathing room air, 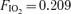 ) and arteriovenous shunting was evaluated using saline contrast echocardiography at each stage. Once significant arteriovenous shunting was documented (bubble score = 2), workload was held constant for the remainder of the protocol and
) and arteriovenous shunting was evaluated using saline contrast echocardiography at each stage. Once significant arteriovenous shunting was documented (bubble score = 2), workload was held constant for the remainder of the protocol and 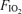 was alternated between 1.0 (hyperoxia) and 0.209 (normoxia) as follows: hyperoxia for 180 s, normoxia for 120 s, hyperoxia for 120 s, normoxia for 120 s, hyperoxia for 60 s and normoxia for 120 s. For Protocol II, subjects performed an incremental cycle ergometer test until volitional exhaustion while continuously breathing 100% O2. In Protocol I, shunting was seen in all subjects at 120–300 W. Breathing oxygen for 1 min reduced shunting, and breathing oxygen for 2 min eliminated shunting in all subjects. Shunting promptly resumed upon breathing room air. Similarly, in Protocol II, breathing 100% O2 substantially decreased or eliminated exercise-induced arteriovenous shunting in all subjects at submaximal and in 4/7 subjects at maximal exercise intensities. Our results suggest that alveolar hyperoxia prevents or reduces blood flow through arteriovenous shunt pathways.
was alternated between 1.0 (hyperoxia) and 0.209 (normoxia) as follows: hyperoxia for 180 s, normoxia for 120 s, hyperoxia for 120 s, normoxia for 120 s, hyperoxia for 60 s and normoxia for 120 s. For Protocol II, subjects performed an incremental cycle ergometer test until volitional exhaustion while continuously breathing 100% O2. In Protocol I, shunting was seen in all subjects at 120–300 W. Breathing oxygen for 1 min reduced shunting, and breathing oxygen for 2 min eliminated shunting in all subjects. Shunting promptly resumed upon breathing room air. Similarly, in Protocol II, breathing 100% O2 substantially decreased or eliminated exercise-induced arteriovenous shunting in all subjects at submaximal and in 4/7 subjects at maximal exercise intensities. Our results suggest that alveolar hyperoxia prevents or reduces blood flow through arteriovenous shunt pathways.
A recent series of studies using anatomically based approaches to study the pulmonary circulation and gas exchange during exercise have concluded that exercise-induced intrapulmonary arteriovenous shunting via large diameter pathways occurs in healthy humans and dogs (Eldridge et al. 2004; Stickland et al. 2004, 2007; Lovering et al. 2008). Notwithstanding these recent contributions, intrapulmonary shunt has long been considered to be trivial during exercise in healthy human subjects (Dempsey & Wagner, 1999).
Breathing 100% O2 would eliminate any diffusion limitation as well as the contribution of poorly ventilated alveoli to gas exchange inefficiency making it possible to quantify venous admixture (Riley & Cournand, 1949). Accordingly, large diameter arteriovenous pathways that would channel venous admixture through the lungs, circumventing the alveoli, should be detected using the 100% O2 technique. However, previous studies using either the multiple inert gas elimination technique (MIGET) (Wagner et al. 1986; Hammond et al. 1986) or the 100% O2 technique (Vogiatzis et al. 2008) have not detected significant intrapulmonary shunting during exercise.
An important fundamental assumption of the 100% O2 technique is that the elevated level of inspired oxygen does not have an effect on the pulmonary microcirculation. We have recently shown that reducing alveolar O2 tension 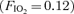 affects arteriovenous shunt recruitment, causing shunting to occur at lower absolute exercise intensities in some subjects and causing shunting to continue to occur in all subjects breathing hypoxic gas
affects arteriovenous shunt recruitment, causing shunting to occur at lower absolute exercise intensities in some subjects and causing shunting to continue to occur in all subjects breathing hypoxic gas 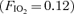 for 3–5 min post-exercise (Lovering et al. 2008). Thus we hypothesized that high alveolar O2 may modulate arteriovenous pathways in the opposite manner to low alveolar O2 by preventing or eliminating arteriovenous shunting.
for 3–5 min post-exercise (Lovering et al. 2008). Thus we hypothesized that high alveolar O2 may modulate arteriovenous pathways in the opposite manner to low alveolar O2 by preventing or eliminating arteriovenous shunting.
Methods
The study received approval from the University of Wisconsin-Madison Human Subjects Committee, and each subject gave written, informed consent before participation. All studies were performed according to the Declaration of Helsinki.
Subjects
Fifteen (5 female) healthy, non-smoking subjects aged 19–52 years volunteered to participate in the study. A screening cardiopulmonary history and physical examination were performed. A patent foramen ovale (PFO) determination was based on saline contrast bubbles appearing in the left heart in less than three heart beats with or without a Valsalva manoeuvre. The screening saline contrast echocardiogram suggested a patent foramen ovale (PFO) in 8/15 subjects. These eight subjects were excluded from study. However, three of the subjects with a PFO participated in a subset of studies examining the effect of breathing 100% O2 on saline contrast bubble dynamics (see ‘Saline contrast echocardiography’ below). The remaining seven subjects (3 female) aged 23–36 years were free of cardiopulmonary disease.
Pulmonary function and lung diffusion capacity for carbon monoxide testing
Baseline pulmonary function was determined using computerized spirometry (Pulmonizer model PFT 3000, Medical Science, St Louis, MO, USA) according to American Thoracic Society Standards (Miller et al. 2005). Single-breath diffusion capacity for carbon monoxide (DLCO) was determined according to American Thoracic Society standards (Macintyre et al. 2005) and compared to predicted values (Knudson et al. 1983, 1987). Measured and predicted values are reported in Table 1.
Table 1.
Anthropometric, pulmonary function and V̇O2max data
| Parameter | Mean ±s.d. |
|---|---|
| Age (years) | 29.7 ± 5.1 |
| Height (cm) | 173.5 ± 11.7 |
| Weight (kg) | 73.2 ± 17.7 |
| FVC (l) | 4.9 ± 1.3 (103 ± 7) |
| FEV1 (l) | 3.9 ± 0.7 (101 ± 8) |
| FEV1/FVC | 0.83 ± 0.07 (98 ± 7) |
| FEF25–75 (l s−1) | 4.1 ± 0.7 (98 ± 27) |
| DLCO (ml min−1 Torr−1) | 36.8 ± 8.7 (117 ± 15.0) |
| V02 (ml kg−1 min−1) |
50.7 ± 8.2 (140 ± 27) |
Values in parentheses are percentage predicted. FVC, forced vital capacity; FEV1, forced expiratory volume in 1 s; FEF25–75, forced expiratory flow of mid-expiratory volume; DLCO, diffusion capacity for carbon monoxide; V̇O2max relative maximal oxygen uptake.
Exercise protocols
For all exercise tests, subjects breathed through a low-resistance two-way non-rebreathing valve (model 2400, Hans Rudolph, KS, USA). Metabolic rate was determined at rest and during exercise using a Medgraphics CPX-D VO2 System (Medgraphics, MN, USA). Heart rate was measured with a three-lead ECG and recorded continuously. Subjects completed an incremental exercise test (30 W every 120 s starting from 60 W) to volitional exhaustion on a magnetically braked cycle ergometer (Medifit MFE 400L, the Netherlands) to determine the subject's respective maximal oxygen consumption (V̇O2max). Percentage predicted V̇O2max was calculated as previously described (Hansen et al. 1984) and reported in Table 1.
For Protocol I, subjects performed a progressive cycle ergometer test breathing room air 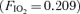 . The initial workload was set at 60 W and was increased by 30 W every 2 min. During the last minute of each workload contrast echocardiography was performed as described below. Once significant intrapulmonary shunt was observed (bubble score = 2; see bubble score criteria below under ‘Data analysis’), the subject continued to pedal at that workload for the duration of the protocol. Subsequently, the
. The initial workload was set at 60 W and was increased by 30 W every 2 min. During the last minute of each workload contrast echocardiography was performed as described below. Once significant intrapulmonary shunt was observed (bubble score = 2; see bubble score criteria below under ‘Data analysis’), the subject continued to pedal at that workload for the duration of the protocol. Subsequently, the 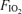 was alternated between 0.209 (normoxia) and 1.0 (hyperoxia) as follows: hyperoxia for 180 s, normoxia for 120 s, hyperoxia for 120 s, normoxia for 120 s, hyperoxia for 60 s and normoxia for 120 s (Fig. 1). Intrapulmonary arteriovenous shunting was evaluated using saline contrast echocardiography during the last minute of each stage longer than 60 s and during the last 30 s of hyperoxia for 60 s. Thus, the effect of breathing 100% O2 for 120 s, 60 s and 30 s during submaximal exercise was determined using Protocol I.
was alternated between 0.209 (normoxia) and 1.0 (hyperoxia) as follows: hyperoxia for 180 s, normoxia for 120 s, hyperoxia for 120 s, normoxia for 120 s, hyperoxia for 60 s and normoxia for 120 s (Fig. 1). Intrapulmonary arteriovenous shunting was evaluated using saline contrast echocardiography during the last minute of each stage longer than 60 s and during the last 30 s of hyperoxia for 60 s. Thus, the effect of breathing 100% O2 for 120 s, 60 s and 30 s during submaximal exercise was determined using Protocol I.
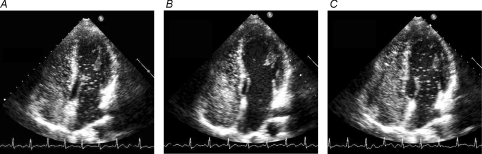
Figure 1. Saline contrast echocardiograms from a 36-year-old male subject (no. 6) during normoxic 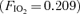 and hyperoxic
and hyperoxic 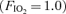 exercise at 180 W
exercise at 180 W 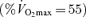 .
.
A, echocardiogram during normoxic exercise for 1 min at 180 W. Note saline contrast bubbles in the left heart indicating arteriovenous shunting. Bubble score = 3. B, echocardiogram during hyperoxic exercise for 120 s at 180 W. Note absence of saline contrast bubbles in the left heart indicating no arteriovenous shunting. Bubble score = 1. C, echocardiogram upon returning to normoxic exercise for 60 s at 180 W. Note appearance of saline contrast bubbles in the left heart recommenced indicating arteriovenous shunting. Bubble score = 3.
For Protocol II, subjects breathed hyperoxic gas 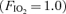 continuously during an incremental cycle ergometer exercise test (30 W every 120 s, as above) to volitional exhaustion. Hyperoxic gas for both protocols was breathed pressure free from a Mylar non-diffusing bag.
continuously during an incremental cycle ergometer exercise test (30 W every 120 s, as above) to volitional exhaustion. Hyperoxic gas for both protocols was breathed pressure free from a Mylar non-diffusing bag.
Five subjects completed the two exercise protocols on the same day with at least a 1 h break between the two protocols. The other two subjects (5 and 6) completed the protocols on separate days. In all cases, Protocol I was completed before Protocol II. Because long-term exposure to 100% O2 may have had a long-lasting effect on the pulmonary circulation, we decided to perform Protocol I, with brief exposures to 100% O2, before performing Protocol II to minimize any potential effects of the first protocol on the second protocol.
Saline contrast echocardiography
Saline contrast echocardiography with harmonic imaging was performed as described previously (Eldridge et al. 2004; Lovering et al. 2008). Briefly, a 20-gauge intravenous catheter was placed in the median basilic vein. A three-way stopcock was attached, and two 10 ml syringes were attached to the other two ports. One syringe contained 1 ml of air, and the other contained 3–4 ml of sterile saline. The contrast bubbles were created by flushing the saline–air solution from one syringe to another. A forceful hand injection of the agitated saline–air solution was performed while images were obtained simultaneously in the apical four-chamber view (iE33 echocardiography system, Philips). Without right-to-left shunting, saline contrast bubbles are visualized as a cloud of echoes in the right heart and then gradually disappear as the bubbles become trapped and eliminated in the pulmonary microcirculation (Butler & Hills, 1979; Meltzer et al. 1981). If the contrast bubbles pass through the lungs, they will appear in the left heart after a delay of at least three cardiac cycles. Because subjects with a PFO were excluded from our study, delayed appearance of bubbles in the left heart indicates transpulmonary passage of contrast bubbles either through excessively distended pulmonary capillaries (Rodriguez-Roisin et al. 1992) or through arteriovenous shunts (Barzilai et al. 1991; Gudavalli et al. 2002). All contrast echocardiograms were performed with the subject seated on the cycle ergometer in the forward leaning ‘Aerobar’ position with the mouthpiece and nose clip in place. Limitations of this technique have been previous reported (Eldridge et al. 2004; Lovering et al. 2008).
In a subset of preparatory studies, we examined the effect of breathing 100% O2 on saline contrast bubble dynamics using two different approaches. First, in two subjects without PFO we used both room air and 100% O2 to make the saline contrast bubble suspension during hyperoxic exercise and we found that both bubble suspensions resulted in identical results, i.e. no shunt during hyperoxic exercise. Second, we examined saline contrast bubbles in three subjects with a PFO that were excluded from the present study. In these subjects with a PFO, immediate, atrial level shunting via the PFO persisted while breathing 100% O2 at rest and during exercise, despite intrapulmonary shunt being prevented, and thus the degree of overall shunting was reduced.
Data analysis
Descriptive and physiological data are presented as means ±s.d. Workload comparisons in normoxia and hyperoxia were made using a paired t test. Echocardiograms were digitally recorded at ≥ 30 frames s−1 by the same echo technician and analysed offline frame by frame (Camtronics Medical System, Hartland, WI, USA). Two cardiologists who were blinded to the condition read all echocardiograms independently. There was 100% agreement for the onset of shunting between the two readers. Shunt onset was defined as the appearance of individual bubbles (> 3 bubbles in the left heart after at least 3 cardiac cycles) giving a bubble score of 2 or more. Our qualitative bubble scoring system is based on quantity and spatial distribution of the contrast bubbles in the left ventricle in any single frame. No bubbles in any frame received a score of 0, trace amounts of bubbles (≤ 3 in any frame) received a score of 1, mild amounts of bubbles (3–12 bubbles in any frame) received a score of 2, multiple isolated bubbles (> 12 bubbles in any frame) received a score of 3, many bubbles heterogeneously distributed within the left ventricle received a score of 4, and many bubbles homogeneously distributed within the left ventricle received a score of 5. Analysis of bubbles scores was made using a Friedman's test with Dunn's multiple comparison post test. Subjects without complete data (n = 2) were excluded from analysis. Significance was set to P < 0.05.
Results
Lung function and maximal oxygen uptake
Anthropometric, pulmonary function, DLCO, and exercise data for the seven subjects that completed the exercise protocols were within normal limits and are presented in Table 1.
Protocol I, arteriovenous shunting during normoxic and hyperoxic exercise
In all subjects, arteriovenous shunting did not occur at rest in normoxic conditions but occurred during submaximal exercise. Once shunting began to occur, it continued at all subsequent submaximal exercise intensities while breathing room air (Fig. 1A).
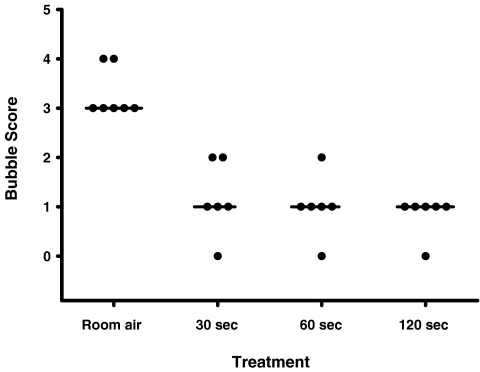
In Protocol I, significant shunt (bubble score = 2) was detected during exercise at a mean workload of 210 ± 62 W (70 ± 8% V̇O2max) (Table 2). While maintaining a constant workload, arteriovenous shunting was again evaluated after breathing 100% O2 for 120, 60 and 30 s, with 120 s of breathing normoxia in between hyperoxia bouts. In all subjects tested, breathing 100% O2 for 120 s eliminated arteriovenous shunting at the same workload where significant shunting occurred while breathing room air (Table 3, Figs 1B and 2). Breathing 100% O2 for 60 and 30 s prevented shunting in 5/6 and 4/6 subjects, respectively (Table 3, Fig. 2). In all cases of breathing 100% O2, bubble scores were reduced compared to submaximal exercise in normoxic conditions (Table 3, Figs 1B and 2). Shunting always resumed upon return to breathing room air for 60 s (Fig. 1C).
Table 2.
Maximal normoxic and hyperoxic workload and workload at significant shunt onset in normoxic exercise
| Workload (W) | |||
|---|---|---|---|
| Subject | Normoxia max | Shunt onsetaV̇O2max | Hyperoxia max |
| 1 | 210 | 150 (79) | 240 |
| 2 | 300 | 240 (81) | 360 |
| 3 | 240 | 120 (66) | 270 |
| 4 | 390 | 300 (68) | 390 |
| 5 | 390 | 240 (66) | 420 |
| 6 | 300 | 180 (61) | 330 |
| 7 | 300 | 240 (81) | 330 |
| Mean | 304 | 210 (70) | 334* |
| s.d. | 68 | 62 (8) | 63 |
Bubble score = 2. *Significantly different from normoxia, P < 0.05, paired t test.
Table 3.
Bubble scores in each subject in all conditions
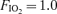 |
|||||
|---|---|---|---|---|---|
| Subject |
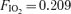 Submax Submax |
Submax (2 min) | Submax (1 min) | Submax (30 s) | Max |
| 1 | 3 | 1 | 1 | 1 | 1 |
| 2 | 3 | — | 1 | 1 | 1 |
| 3 | 4 | 1 | 1 | 2 | 2 |
| 4 | 3 | 1 | 0 | 1 | 2 |
| 5 | 4 | 1 | — | — | 1 |
| 6 | 3 | 1 | 2 | 2 | 3 |
| 7 | 3 | 0 | 1 | 0 | 1 |
| Mean | 3.3 | 0.8* | 1.0 | 1.2 | 1.6 |
| s.d. | 0.5 | 0.4 | 0.6 | 0.8 | 0.8 |
Significantly different compared to bubble score at submaximal workload in exercise with 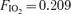 , P < 0.05, Friedman's test with a Dunn's post test. ‘No data obtained’ is represented by —.
, P < 0.05, Friedman's test with a Dunn's post test. ‘No data obtained’ is represented by —.
Figure 2. Bubble score as a function of exposure to hyperoxia during exercise.
Horizontal lines represent the mode. Bubble scores were significantly reduced with 120 s of exposure to 100% O2, Friedman's test with a Dunn's post test.
Protocol II, arteriovenous shunting during hyperoxic exercise
In a subsequent incremental cycle ergometer exercise test, subjects breathed 100% O2 from rest through maximal exercise. Maximal workload achieved in hyperoxic exercise was 9.4 ± 5% greater than that achieved during normoxia (Table 2). During this test, arteriovenous shunting did not occur in four of the seven subjects while breathing 100% O2 for the duration of the test (Table 3). In the three subjects that shunted in hyperoxia, bubble scores were lower during maximal hyperoxic exercise than during submaximal normoxic exercise in two of the three subjects (subjects 3 and 4 in Table 3).
Discussion
The purpose of this study was to determine if breathing 100% O2 had an effect on exercise-induced arteriovenous shunt vessel recruitment. Breathing 100% O2 for 120 s eliminated exercise-induced arteriovenous shunting during a given absolute submaximal workload. Moreover, breathing 100% O2 during maximal incremental exercise prevented arteriovenous shunting in 4/7 subjects, and appeared to greatly reduce shunt magnitude in 2 of the 3 other subjects. These results raise a question concerning the use of the 100% O2 technique as a valid method for assessing inducible arteriovenous shunt in normoxia, particularly as it pertains to exercise. The mechanism of action of hyperoxia on these pulmonary pathways is unknown.
Methodological considerations
We have previously described extensively the limitations of saline contrast echocardiography in several peer-reviewed papers and refer the reader to those for more detailed technical considerations (Eldridge et al. 2004; Lovering et al. 2008). An important concern is whether oxygen tension influenced the saline contrast echocardiography technique per se. The observation that atrial level shunting persisted at rest in subjects with PFO clearly demonstrates that the viability of the contrast under hyperoxic conditions was not significantly altered. Furthermore, we performed saline contrast echocardiography on two subjects using 100% O2 as opposed to room air to create the contrast. Importantly, the type of gas used to make contrast did not affect either shunt onset, or the removal of arteriovenous shunt while breathing hyperoxia. Thus, the modulation of arteriovenous shunts with hyperoxia does not appear related to the concentration of O2 within the contrast.
Effect of hyperoxia on exercise-induced intrapulmonary arteriovenous vessels
Hyperoxia has been shown to reduce pulmonary artery pressure and cardiac output during constant work exercise at altitude; however, data obtained at sea level indicate no significant decrease in pulmonary artery pressure with oxygen resulting and no change in pulmonary vascular resistance (Groves et al. 1987) (Table 5 of that paper). Oxygen breathing does not affect ventilation/perfusion matching in normoxia (Hammond et al. 1986), suggesting that hypoxic pulmonary vasoconstriction is not active during sea level exercise and thus explaining why 100% O2 would not affect pulmonary vascular resistance during sea level exercise. Short-term hyperoxia has been shown to reduce sympathetic nervous activity (Stickland et al. 2008), which may reduce translocation of blood to the thorax and thus pulmonary vascular pressures (Flamm et al. 1990); however, it is unlikely that this would have a substantial effect on pulmonary vascular pressures. Thus it appears that 100% O2 is unlikely to influence gross pulmonary haemodynamics, particularly during exercise. Breathing 100% O2 during moderate-intensity exercise increases 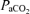 by ∼4 mmHg compared with normoxic exercise (Hammond et al. 1986). An increase in
by ∼4 mmHg compared with normoxic exercise (Hammond et al. 1986). An increase in 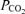 per se in the mixed venous blood perfusing the pulmonary arterial vasculature may also modulate arteriovenous shunts. The control of intrapulmonary arteriovenous pathways requires more study.
per se in the mixed venous blood perfusing the pulmonary arterial vasculature may also modulate arteriovenous shunts. The control of intrapulmonary arteriovenous pathways requires more study.
Unconventional vessels in the pulmonary circulation?
The pulmonary circulation differs from the systemic circulation in its response to oxygen tension. First, while the systemic circulation vasodilates in response to hypoxia, the pulmonary circulation vasoconstricts. Similarly, the pulmonary circulation vasodilates in response to hyperoxia whereas the systemic circulation vasoconstricts. Along these lines we have recently demonstrated that hypoxia 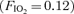 had a significant effect on exercise-induced arteriovenous shunting (Lovering et al. 2008). Specifically, shunting occurred at rest and at lower workloads in some individuals and persisted 3–5 min post-exercise while breathing hypoxic gas mixtures in all individuals tested. In the current study we have found that the opposite occurs, such that hyperoxia
had a significant effect on exercise-induced arteriovenous shunting (Lovering et al. 2008). Specifically, shunting occurred at rest and at lower workloads in some individuals and persisted 3–5 min post-exercise while breathing hypoxic gas mixtures in all individuals tested. In the current study we have found that the opposite occurs, such that hyperoxia 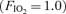 results in either the closure of exercise-induced arteriovenous vessels or significantly attenuates the degree of shunting at a given submaximal absolute workload. Accordingly, these arteriovenous vessels appear to behave more like systemic vessels than their conventional pulmonary counterparts, and more research into their regulation is warranted. Of note, pulmonary hypoxic vasoconstriction (Morrell et al. 1995) and cutaneous hyperoxic vasoconstriction (Yamazaki et al. 2007) occurs within the same time frame (˜120 s) as that in our study, suggesting a similar vasomotor control mechanism.
results in either the closure of exercise-induced arteriovenous vessels or significantly attenuates the degree of shunting at a given submaximal absolute workload. Accordingly, these arteriovenous vessels appear to behave more like systemic vessels than their conventional pulmonary counterparts, and more research into their regulation is warranted. Of note, pulmonary hypoxic vasoconstriction (Morrell et al. 1995) and cutaneous hyperoxic vasoconstriction (Yamazaki et al. 2007) occurs within the same time frame (˜120 s) as that in our study, suggesting a similar vasomotor control mechanism.
Intrapulmonary arteriovenous shunt and gas exchange
Intrapulmonary shunt has previously been described as a negligible contributor to the reduction in gas exchange efficiency that occurs during exercise in healthy human subjects (Dempsey & Wagner, 1999). This conclusion is based largely upon experimental data collected using a combination of gas exchange-dependent techniques such as the MIGET (Wagner et al. 1986; Hammond et al. 1986) and, more recently, the 100% O2 technique (Vogiatzis et al. 2008). However, our work has relied exclusively on anatomically based approaches and therefore detects arteriovenous shunt via large diameter pathways (Eldridge et al. 2004; Stickland et al. 2004, 2007; Lovering et al. 2007, 2008) as opposed to physiological shunt (i.e. very low ventilation/perfusion units).
At submaximal exercise, increasing the 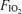 to 1.0 for 2 min eliminated arteriovenous shunting in all subjects. During maximal hyperoxic exercise, arteriovenous shunting was eliminated or significantly reduced as compared to normoxia. This demonstration that breathing 100% O2 alone has a direct effect on the physiological outcome being measured (i.e. arteriovenous shunt) provides important evidence as to why the 100% O2 technique may be unable to evaluate arteriovenous shunt during submaximal or maximal normoxic exercise. Indeed, recent work using the 100% O2 technique to specifically examine the contribution of arteriovenous shunting to the gas exchange inefficiency reported that breathing 100% O2 at near-maximal exercise reduced the mean calculated venous admixture from 3.5% of cardiac output in normoxia to 0.5% of cardiac output with 100% O2 (Vogiatzis et al. 2008). Based on these two seemingly contradictory studies, we hypothesize that arteriovenous shunt pathways may be contributing to the gas exchange inefficiency that occurs during normoxic exercise in multiple ways. One, as large diameter pathways, arteriovenous shunt may allow blood to bypass the conventional gas exchanging capillary alveolar interface, resulting in impairment in pulmonary gas exchange. These shunts, however, would not be ‘seen’ during 100% O2 breathing as oxygen itself eliminates arteriovenous shunt. Alternatively, or in addition, these large diameter vessels may participate in a limited degree of gas exchange such that equilibration of alveolar gas with the blood at the centre of these large diameter vessels may not be complete (Genovesi et al. 1976; Stickland & Lovering, 2006). As such, using 100% O2 during exercise would overcome the limitation of diffusion in these vessels that occurs in normoxic conditions. Either of these two possibilities would explain why venous admixture decreased from 3.5% to 0.5% of cardiac output at peak exercise when breathing 100% O2 (Vogiatzis et al. 2008). Future work determining the regulation of these arteriovenous pathways and their contribution to gas exchange during exercise is needed.
to 1.0 for 2 min eliminated arteriovenous shunting in all subjects. During maximal hyperoxic exercise, arteriovenous shunting was eliminated or significantly reduced as compared to normoxia. This demonstration that breathing 100% O2 alone has a direct effect on the physiological outcome being measured (i.e. arteriovenous shunt) provides important evidence as to why the 100% O2 technique may be unable to evaluate arteriovenous shunt during submaximal or maximal normoxic exercise. Indeed, recent work using the 100% O2 technique to specifically examine the contribution of arteriovenous shunting to the gas exchange inefficiency reported that breathing 100% O2 at near-maximal exercise reduced the mean calculated venous admixture from 3.5% of cardiac output in normoxia to 0.5% of cardiac output with 100% O2 (Vogiatzis et al. 2008). Based on these two seemingly contradictory studies, we hypothesize that arteriovenous shunt pathways may be contributing to the gas exchange inefficiency that occurs during normoxic exercise in multiple ways. One, as large diameter pathways, arteriovenous shunt may allow blood to bypass the conventional gas exchanging capillary alveolar interface, resulting in impairment in pulmonary gas exchange. These shunts, however, would not be ‘seen’ during 100% O2 breathing as oxygen itself eliminates arteriovenous shunt. Alternatively, or in addition, these large diameter vessels may participate in a limited degree of gas exchange such that equilibration of alveolar gas with the blood at the centre of these large diameter vessels may not be complete (Genovesi et al. 1976; Stickland & Lovering, 2006). As such, using 100% O2 during exercise would overcome the limitation of diffusion in these vessels that occurs in normoxic conditions. Either of these two possibilities would explain why venous admixture decreased from 3.5% to 0.5% of cardiac output at peak exercise when breathing 100% O2 (Vogiatzis et al. 2008). Future work determining the regulation of these arteriovenous pathways and their contribution to gas exchange during exercise is needed.
Summary
We have demonstrated that arteriovenous shunting during exercise can be eliminated by hyperoxia 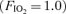 during submaximal exercise, and prevented or significantly reduced at maximal exercise in 6/7 subjects tested. The impact of preventing arteriovenous shunt during hyperoxic exercise remains unclear. What is evident, however, is that the 100% O2 technique may be inadequate for accurate detection of arteriovenous pathways that are inducible during normoxic exercise. This study may explain why methods which depend on gas exchange, such as 100% O2 breathing, do not detect shunting during exercise. Our results may reconcile discrepancies between studies of the pulmonary circulation in exercise where different methods have been used.
during submaximal exercise, and prevented or significantly reduced at maximal exercise in 6/7 subjects tested. The impact of preventing arteriovenous shunt during hyperoxic exercise remains unclear. What is evident, however, is that the 100% O2 technique may be inadequate for accurate detection of arteriovenous pathways that are inducible during normoxic exercise. This study may explain why methods which depend on gas exchange, such as 100% O2 breathing, do not detect shunting during exercise. Our results may reconcile discrepancies between studies of the pulmonary circulation in exercise where different methods have been used.
Acknowledgments
We thank Ms Jensena M. Carlson, Ms Sarah M. Otten, Mrs Peggy Zingler RRT, Dr Carter Ralphe and Mrs Jaime Beebe for excellent technical assistance. This work was supported by National Heart, Lung, and Blood Institute Grants HL-15469 and T32 HL07654 (A.T.L.), and Grant-In-Aid from the American Heart Association 0550176Z (M.W.E.) and 0625636Z (M.A.). M. K. Stickland was supported by the Natural Sciences and Engineering Council of Canada.
References
- Barzilai B, Waggoner AD, Spessert C, Picus D, Goodenberger D. Two-dimensional contrast echocardiography in the detection and follow-up of congenital pulmonary arteriovenous malformations. Am J Cardiol. 1991;68:1507–1510. doi: 10.1016/0002-9149(91)90287-u. [DOI] [PubMed] [Google Scholar]
- Butler BD, Hills BA. The lung as a filter for microbubbles. J Appl Physiol. 1979;47:537–543. doi: 10.1152/jappl.1979.47.3.537. [DOI] [PubMed] [Google Scholar]
- Dempsey JA, Wagner PD. Exercise-induced arterial hypoxemia. J Appl Physiol. 1999;87:1997–2006. doi: 10.1152/jappl.1999.87.6.1997. [DOI] [PubMed] [Google Scholar]
- Eldridge MW, Dempsey JA, Haverkamp HC, Lovering AT, Hokanson JS. Exercise-induced intrapulmonary arteriovenous shunting in healthy humans. J Appl Physiol. 2004;97:797–805. doi: 10.1152/japplphysiol.00137.2004. [DOI] [PubMed] [Google Scholar]
- Flamm SD, Taki J, Moore R, Lewis SF, Keech F, Maltais F, Ahmad M, Callahan R, Dragotakes S, Alpert N, et al. Redistribution of regional and organ blood volume and effect on cardiac function in relation to upright exercise intensity in healthy human subjects. Circulation. 1990;81:1550–1559. doi: 10.1161/01.cir.81.5.1550. [DOI] [PubMed] [Google Scholar]
- Genovesi MG, Tierney DF, Taplin GV, Eisenberg H. An intravenous radionuclide method to evaluate hypoxemia caused by abnormal alveolar vessels. Limitation of conventional techniques. Am Rev Respir Dis. 1976;114:59–65. doi: 10.1164/arrd.1976.114.1.59. [DOI] [PubMed] [Google Scholar]
- Groves BM, Reeves JT, Sutton JR, Wagner PD, Cymerman A, Malconian MK, Rock PB, Young PM, Houston CS. Operation Everest II: elevated high-altitude pulmonary resistance unresponsive to oxygen. J Appl Physiol. 1987;63:521–530. doi: 10.1152/jappl.1987.63.2.521. [DOI] [PubMed] [Google Scholar]
- Gudavalli A, Kalaria VG, Chen X, Schwarz KQ. Intrapulmonary arteriovenous shunt: diagnosis by saline contrast bubbles in the pulmonary veins. J Am Soc Echocardiogr. 2002;15:1012–1014. doi: 10.1067/mje.2002.121435. [DOI] [PubMed] [Google Scholar]
- Hammond MD, Gale GE, Kapitan KS, Ries A, Wagner PD. Pulmonary gas exchange in humans during exercise at sea level. J Appl Physiol. 1986;60:1590–1598. doi: 10.1152/jappl.1986.60.5.1590. [DOI] [PubMed] [Google Scholar]
- Hansen JE, Sue DY, Wasserman K. Predicted values for clinical exercise testing. Am Rev Respir Dis. 1984;129:S49–S55. doi: 10.1164/arrd.1984.129.2P2.S49. [DOI] [PubMed] [Google Scholar]
- Knudson RJ, Kaltenborn WT, Knudson DE, Burrows B. The single-breath carbon monoxide diffusing capacity. Reference equations derived from a healthy nonsmoking population and effects of hematocrit. Am Rev Respir Dis. 1987;135:805–811. doi: 10.1164/arrd.1987.135.4.805. [DOI] [PubMed] [Google Scholar]
- Knudson RJ, Lebowitz MD, Holberg CJ, Burrows B. Changes in the normal maximal expiratory flow-volume curve with growth and aging. Am Rev Respir Dis. 1983;127:725–734. doi: 10.1164/arrd.1983.127.6.725. [DOI] [PubMed] [Google Scholar]
- Lovering AT, Romer LM, Haverkamp HC, Pegelow DF, Hokanson JS, Eldridge MW. Intrapulmonary shunting and pulmonary gas exchange during normoxic and hypoxic exercise in healthy humans. J Appl Physiol. 2008;104:1418–1425. doi: 10.1152/japplphysiol.00208.2007. [DOI] [PubMed] [Google Scholar]
- Lovering AT, Stickland MK, Kelso AJ, Eldridge MW. Direct demonstration of 25- and 50-μm arteriovenous pathways in healthy human and baboon lungs. Am J Physiol Heart Circ Physiol. 2007;292:H1777–H1781. doi: 10.1152/ajpheart.01024.2006. [DOI] [PubMed] [Google Scholar]
- Macintyre N, Crapo RO, Viegi G, Johnson DC, van der Grinten CP, Brusasco V, Burgos F, Casaburi R, Coates A, Enright P, Gustafsson P, Hankinson J, Jensen R, McKay R, Miller MR, Navajas D, Pedersen OF, Pellegrino R, Wanger J. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005;26:720–735. doi: 10.1183/09031936.05.00034905. [DOI] [PubMed] [Google Scholar]
- Meltzer RS, Sartorius OE, Lancee CT, Serruys PW, Verdouw PD, Essed CE, Roelandt J. Transmission of ultrasonic contrast through the lungs. Ultrasound Med. 1981;7:377–384. doi: 10.1016/0301-5629(81)90048-x. [DOI] [PubMed] [Google Scholar]
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, Macintyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- Morrell NW, Nijran KS, Biggs T, Seed WA. Magnitude and time course of acute hypoxic pulmonary vasoconstriction in man. Respir Physiol. 1995;100:271–281. doi: 10.1016/0034-5687(95)00002-u. [DOI] [PubMed] [Google Scholar]
- Riley RL, Cournand A. Ideal alveolar air and the analysis of ventilation-perfusion relationships in the lungs. J Appl Physiol. 1949;1:825–847. doi: 10.1152/jappl.1949.1.12.825. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Roisin R, Agusti AG, Roca J. The hepatopulmonary syndrome: new name, old complexities. Thorax. 1992;47:897–902. doi: 10.1136/thx.47.11.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickland MK, Lovering AT. Exercise-induced intrapulmonary arteriovenous shunting and pulmonary gas exchange. Exerc Sport Sci Rev. 2006;34:99–106. doi: 10.1249/00003677-200607000-00003. [DOI] [PubMed] [Google Scholar]
- Stickland MK, Lovering AT, Eldridge MW. Exercise-induced arteriovenous intrapulmonary shunting in dogs. Am J Respir Crit Care Med. 2007;176:300–305. doi: 10.1164/rccm.200702-206OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickland MK, Morgan BJ, Dempsey JA. Carotid chemoreceptor modulation of sympathetic vasoconstrictor outflow during exercise in healthy humans. J Physiol. 2008;586:1743–1754. doi: 10.1113/jphysiol.2007.147421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickland MK, Welsh RC, Haykowsky MJ, Petersen SR, Anderson WD, Taylor DA, Bouffard M, Jones RL. Intra-pulmonary shunt and pulmonary gas exchange during exercise in humans. J Physiol. 2004;561:321–329. doi: 10.1113/jphysiol.2004.069302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogiatzis I, Zakynthinos S, Boushel R, Athanasopoulos D, Guenette JA, Wagner H, Roussos C, Wagner PD. The contribution of intrapulmonary shunts to the alveolar-to-arterial oxygen difference during exercise is very small. J Physiol. 2008;586:2381–2391. doi: 10.1113/jphysiol.2007.150128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner PD, Gale GE, Moon RE, Torre-Bueno JR, Stolp BW, Saltzman HA. Pulmonary gas exchange in humans exercising at sea level and simulated altitude. J Appl Physiol. 1986;61:260–270. doi: 10.1152/jappl.1986.61.1.260. [DOI] [PubMed] [Google Scholar]
- Yamazaki F, Takahara K, Sone R, Johnson JM. Influence of hyperoxia on skin vasomotor control in normothermic and heat-stressed humans. J Appl Physiol. 2007;103:2026–2033. doi: 10.1152/japplphysiol.00386.2007. [DOI] [PubMed] [Google Scholar]