Abstract
NMDA receptors are of particular importance in the control of synaptic strength and integration of synaptic activity. Dopamine receptor modulation of NMDA receptors in neonatal striatum may influence the efficacy of synaptic transmission in the cortico-striatal pathway and if so, this modulation will affect the behaviour of the basal ganglia network. Here, we show that in acute brain slices of neonatal (P7) rat striatum the dopamine D1 receptor agonist SKF-82958 significantly decreases NMDA receptor currents in patch-clamp whole-cell recordings. This inhibition is not abolished by application of a G protein inhibitor (GDP-β-S) or irreversible G protein activator (GTP-γ-S) suggesting a G protein-independent mechanism. In addition, intracellular application of protein tyrosine kinase inhibitors (lavendustin A or PP2) abolished D1 inhibition of NMDA currents. In contrast, in older animals (P28) D1 receptor activation produces a potentiation of the NMDA response which suggests there is a developmental switch in D1 modulation of striatal NMDA receptors. Single-channel recordings show that direct D1 receptor inhibition of NMDA receptors cannot be observed in isolated membrane patches. We hypothesize that D1 inhibition in whole-cell recordings from neonatal rats may be mediated by a change in NMDA receptor trafficking. Consistent with this hypothesis, intracellular application of a dynamin inhibitory peptide (QVPSRPNRAP) abolished D1 inhibition of NMDA receptor currents. We therefore conclude that a tyrosine kinase-dependent alteration of NMDA receptor trafficking underlies D1 dopamine receptor-mediated down-regulation of NMDA receptor currents in medium spiny neurons of neonatal rat striatum.
N-Methyl-d-aspartate (NMDA) receptors play a central role in excitatory synaptic transmission, plasticity and excitotoxicity in the brain. NMDA receptor activity can be regulated by protein kinases (PKA, PKC and tyrosine kinases) (Blank et al. 1997; Lu et al. 1999; Xiong et al. 1999; Lei et al. 2002) and protein phosphatases (PP1 and calcineurin) (Lieberman & Mody, 1994; Morishita et al. 2001; Krupp et al. 2002; Rycroft & Gibb, 2004). There is substantial evidence showing that G protein-coupled receptors such as dopamine receptors modulate NMDA receptor activity (Blank et al. 1997; Chen et al. 2004; Cepeda & Levine, 2006; Surmeier et al. 2007). NMDA receptors and dopamine receptors are colocalized (Fiorentini et al. 2003; Scott et al. 2006; Cepeda & Levine, 2006) in striatal medium spiny neurons and the interaction between glutamatergic and dopaminergic input in the striatum is crucial for movement and behavioural control (Hallett & Standaert, 2004; Calabresi et al. 2007; Surmeier et al. 2007).
In prefrontal cortex, dopamine D1 receptor activation has been shown to potentiate NMDA receptor synaptic currents (Seamans et al. 2001; Chen et al. 2004). In the striatum, dopamine D1 receptors couple to Gs G proteins with stimulation of the classical adenylate cyclase pathway resulting in phosphorylation of DARPP-32 and inhibition of protein phosphatase-1 (Greengard, 2001). Some studies have shown that the classical pathway contributes to D1 enhancement of NMDA receptor currents; however, they have also shown different downstream effectors (Blank et al. 1997; Cepeda et al. 1998a; Flores-Hernandez et al. 2002). In addition Dunah & Standaert (2001) have shown that D1 receptor activation enhances the abundance of NR1, NR2A and NR2B subunits in the synaptosomal membrane fraction of striatal homogenates while Dunah et al. (2004) have shown that deletion of the gene for the protein tyrosine kinase, Fyn, inhibits this D1 receptor-induced enhancement.
On the other hand, several studies presented evidence that dopamine can attenuate NMDA-mediated currents (Lee et al. 2002; Lin et al. 2003). In particular Lee et al. (2002) demonstrated inhibition of NMDA responses by a direct protein–protein interaction between the dopamine D1 receptor and NR2A subunit C-termini. One possible hypothesis is that these apparently conflicting results of D1 inhibition or potentiation could be due to a developmental switch in D1 modulation that follows the increasing expressing of NR2A subunits with development.
In this study, we have used striatal medium spiny neurons from 7-day-old rats as a model system to investigate D1 modulation of NMDA receptors. At this developmental stage, D1 receptor activation caused a decrease of NMDA receptor whole cell currents. This decrease was not G protein dependent but was abolished by intracellular application of both a general inhibitor of tyrosine kinases (lavendustin A) and by the selective Src tyrosine kinase inhibitor, PP2. Furthermore, intracellular application of a dynamin inhibitory peptide prevented D1 inhibition of NMDA currents. Based on these results, we conclude that G protein-independent D1 inhibition of NMDA responses in whole-cell recordings is mediated by a tyrosine kinase-induced change in NMDA receptor trafficking.
Methods
All animal experiments were carried out in accordance with the UK Animals (Scientific Procedures) Act 1986. Every effort was made to minimize animal suffering and the number of animals used. Seven-day-old Sprague–Dawley rats were killed by decapitation and horizontal striatal slices (300 μm thick) were made using a vibroslicer (Dosaka DTK 1000, Ted Pella Inc., Reading, CA, USA) by cutting the brain in an ice-cold (< 4°C) oxygenated slicing solution of composition (in mm): sucrose, 206; KCl, 2.5; CaCl2, 1.0; MgCl2, 1.0; NaH2PO4, 1.25; NaHCO3, 26; glucose, 25; pH 7.4. Slices were maintained for 1–8 h at room temperature (20–24°C) in Krebs solution containing (in mm): NaCl, 125; KCl, 2.5; CaCl2, 1.0; MgCl2, 2.0; NaH2PO4, 1.25; NaHCO3, 26; glucose, 25; TTX 0.0001; pH 7.4. Slices were viewed on the stage of an upright microscope (Zeiss Axioscope FS) using Normaski differential interference contrast optics (Edwards et al. 1989). Healthy striatal medium spiny neurons suitable for patch-clamping were identified in the slice by their location, size and morphology (Gotz et al. 1997).
During recording, slices were bathed in Mg-free Krebs solution containing (in mm): NaCl, 125; KCl, 2.5; CaCl2, 1.0; NaH2PO4, 1.25; NaHCO3, 26; glucose, 25; TTX 0.0001; pH 7.4 continuously gassed with a mixture of O2 (95%) and CO2 (5%). Recordings were made with patch pipettes filled with pipette solution containing (in mm): CsCl2, 140; EGTA, 10; Hepes, 10; NaCl, 10; MgCl2, 1; ATP, 1; GTP, 1 (or GDP-β-S, 0.5 or GTP-γ-S, 0.5); adjusted to pH 7.4 with NaOH.
Patch pipettes were pulled from thick-walled borosilicate glass capillaries (GC150F-7.5, Harvard Apparatus Ltd) and fire polished on a microforge (Narishige MF-83) to a final resistance of 6–15 MΩ. For single channel recordings, pipettes were coated with an insulating silicone resin (Sylgard 184, Dow Corning, USA). Both whole cell and single channel currents were recorded at a membrane potential of −60 mV at room temperature (20–24°C). In whole-cell recordings series resistance compensation between 75 and 90% was used. NMDA currents were evoked by 2 min applications of NMDA (10 μm) and glycine (10 μm) followed by a 5 min application of the dopamine D1 receptor agonist SKF-82958 (20 nm) in the presence of the D2 receptor antagonist spiperone (2 nm). Finally we applied SKF-82958 (20 nm) and spiperone (2 nm) accompanied by NMDA (10 μm) and glycine (10 μm) for 2 min. These concentrations of SKF-82958 and spiperone were chosen to give 83% D1 receptor occupancy by SKF-82958 and more than 95% D2 receptor block by spiperone based on equilibrium constants of 4 nm and 73 nm for SKF-82958 binding to D1 and D2 receptors, respectively, and 220 nm and 0.08 nm for spiperone binding to D1 and D2 receptors (Mottola et al. 1996; Kebabian et al. 1997). At these concentrations of dopamine receptor ligands, the possibility of direct effects on the NMDA receptor that occur at much higher concentrations is minimized (Castro et al. 1999; Cui et al. 2006). In outside-out patch experiments, the patch was first exposed to NMDA (1 μm) and glycine (10 μm) for 2 min, followed by SKF-82958 (20 nm) and spiperone (2 nm) with NMDA and glycine for 2–3 min following an equilibration period of 1 min.
Whole cell and single channel currents were recorded using an Axopatch 200A patch-clamp amplifier (Axon Instruments). Signals were amplified and filtered at 2 kHz (8 pole Bessel) and digitized at 20 kHz using an analog-to-digital converter (CED 1401plus, Cambridge Electronic Design, UK), and stored on computer using the program WinEDR (V2.2.3 available at http://spider.science.strath.ac.uk/PhysPharm/showPage.php?pageName=software_ses). Single channel analysis software was designed by Prof D. Colquhoun (can be requested at http://www.ucl.ac.uk/Pharmacology/dc.html).
Each digitized single channel record was analysed using ‘SCAN’, an interactive computer program. Display and analysis of single channel data distributions was done using the program ‘EKDIST’ (Colquhoun & Sigworth, 1995). Before analysis, a fixed resolution for open times and closed times that gave a false event rate less than 10−11 events per second was imposed. Before a patch was accepted for detailed analysis, the long-term stability of the data records was checked by making stability plots for amplitudes (using the amplitude of openings longer than 2.0 filter rise times: 332 μs), open times, shut times and Popen (Colquhoun & Sigworth, 1995). Stability plots for open and shut times were made by calculating a moving average of 50 consecutive open or shut time intervals with an overlap of 25 events and plotting this average against the interval number at the centre of the averaged values. Stability plots for open probability were made by calculating a Popen value for each set of 50 open and shut times. Once the stability of the record had been confirmed, amplitude distributions were made containing individual channel amplitudes longer than 332 μs (2.0 filter rise times). Distributions of channel amplitudes were fitted with the sum of three Gaussian components with the standard deviation constrained to be the same for each component (Colquhoun & Sigworth, 1995). Direct transitions between channel conductance levels were identified using an amplitude-based separation of unitary currents using critical amplitude values (Acrit) calculated from the Gaussian components fitted to the amplitude distribution (Colquhoun & Sigworth, 1995). Each amplitude level had a minimum duration longer than 2.5 filter rise times (415 μs), without intervening closures longer than the shut time resolution (120 μs). Distributions of channel open times or closed times were fitted using the maximum likelihood method (Colquhoun & Sigworth, 1995) with probability density functions that were a mixture of three exponential components for open times and five exponential components for closed times (Gibb & Colquhoun, 1992).
For statistical comparisons, Student's t tests and a randomization test was used that does not involve making any assumptions about the shape of the distribution of observations (this can be requested at http://www.ucl.ac.uk/Pharmacology/dcpr95.html). Statistical significance was set at P < 0.05.
Results
Experiments were carried out upon medium spiny neurons in striatal brain slices from 7-day-old rats. Medium spiny neurons constitute about 90% of the striatal neuron population (Jain et al. 2001; Rymar et al. 2004), and their size means that when viewed under Normarski optics in acute brain slices they are easy to distinguish from the much rarer, large interneurons (Gotz et al. 1997).
Medium spiny neurons were voltage clamped at −60 mV in the presence of TTX (100 nm) to block voltage-gated Na+ currents. NMDA (10 μm) and glycine (10 μm) were applied for 2 min to measure the steady-state whole-cell NMDA current. SKF-82958 (20 nm), a D1 receptor selective agonist, was used to activate D1 receptors in the presence of the D2 receptor antagonist spiperone (2 nm) to avoid D2 receptor activation. SKF-82958 and spiperone were applied for 5 min in the absence of NMDA and then for 2 min in the presence of NMDA and glycine (Fig. 1A). In each slice only one whole cell recording was made in the presence of SKF-82958.
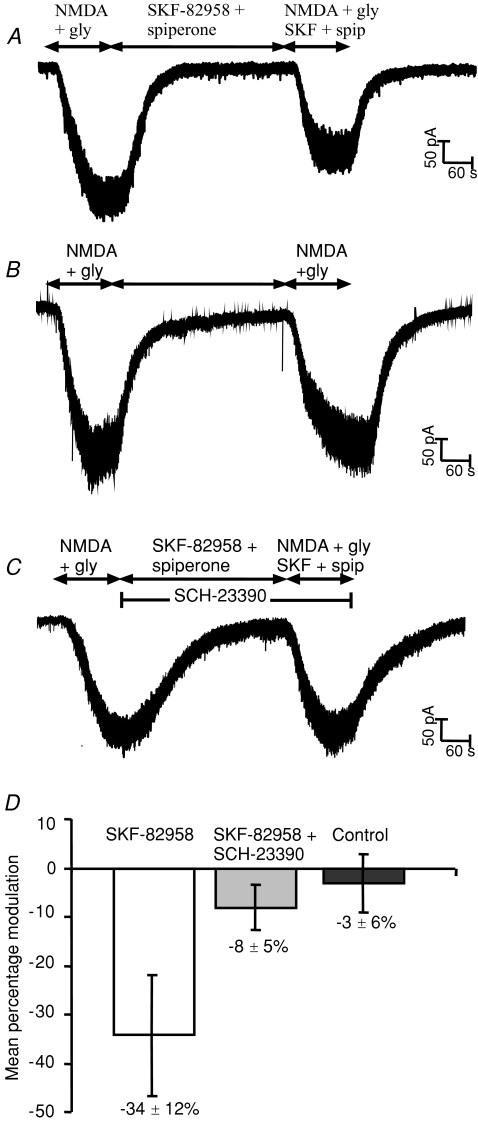
Figure 1. D1 receptor activation inhibits whole cell NMDA receptor currents recorded from 7 day-old rat striatal neurons.
A, response to NMDA (10 μm) and glycine (10 μm) applied for 2 min, followed by 5 min SKF-82958 (20 nm) and spiperone (2 nm), and finally NMDA (10 μm), glycine (10 μm), SKF-82958 (20 nm) and spiperone (2 nm) for 2 min. Brief downward deflections of the current trace show occasional miniature synaptic currents. B, response to NMDA and glycine applied for 2 min, followed by 5 min of control recording solution, followed by NMDA and glycine for 2 min. C, response to NMDA (10 μm) and glycine (10 μm) applied for 2 min, followed by 5 min SKF-82958 (20 nm), SCH-23390 (300 nm) and spiperone (2 nm), and finally NMDA (10 μm), glycine (10 μm), SKF-82958 (20 nm), SCH-23390 (300 nm) and spiperone (2 nm) for 2 min. D, mean percentage change (±s.e.m.) in NMDA current in the presence of SKF-82598 and spiperone (unpaired t test P < 0.05), in the presence of SKF-82598, spiperone and SCH-23390, and from control experiments involving two successive NMDA responses.
D1 receptor activation reduced NMDA receptor currents in striatal medium spiny neurons
The D1 receptor agonist SKF-82958 significantly (P < 0.05) decreased the NMDA whole-cell current from 249 ± 36 pA to 153 ± 31 pA (mean ±s.e.m., n= 10 neurons) (Fig. 1A). To confirm this inhibition, in a separate group of slices we made control experiments in 15 cells where two consecutive NMDA responses, 5 min apart, were recorded without application of SKF-82958 or spiperone (Fig. 1B). In these experiments there was no significant change (−3.1 ± 6.0%) in the second NMDA response (246 ± 61 pA) when compared with the first NMDA response (258 ± 58 pA), while in contrast SKF-82958 significantly decreased the second NMDA response by 34.2 ± 12.3% (P < 0.05) (Fig. 1D). Moreover, experiments in the presence of the D1 antagonist SCH-23390 (300 nm; Fig. 1C) blocked the SKF-82958 induced inhibition of the NMDA responses (control 173 ± 28 pA; SCH-23390 + SKF-82958 167 ± 34 pA; n= 10, P > 0.05). These results also demonstrate that the presence of the D2 receptor antagonist spiperone, and hence block of any constitutive D2 tone in the slice, did not significantly affect NMDA responses. It is clear that D1 receptor activation in neonatal striatal medium spiny neurons reduced by about one-third the NMDA receptor-mediated whole-cell currents.
D1 inhibition of NMDA receptor currents in neonatal rats changes to potentiation with development
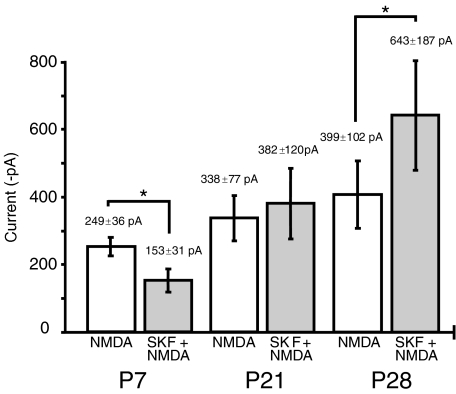
In order to investigate if the effect of D1 receptor activation changes with development, experiments equivalent to those made with P7 rats were made with rats aged P21 and P28. In these experiments at age P21 there was no significant change (+11.3 ± 21.7%, P > 0.05, n= 9) in the second NMDA response in the presence of SKF-82958 and spiperone (382 ± 120 pA) when compared with the first NMDA response (338 ± 77 pA). In contrast, at P28 SKF-82958 significantly increased by 45.6 ± 19% (P < 0.05, n= 6) the second NMDA response (643 ± 187 pA) compared to the first response (399 ± 102 pA) (Fig. 2). Moreover, experiments in the presence of the D1 antagonist, SCH-23390 (300 nm), blocked the SKF-82958 induced enhancement at P28 of the NMDA responses (control 562 ± 193 pA; SCH-23390 + SKF-82958 + spiperone 501 ± 184 pA; n= 6, P > 0.05). Although the size of the NMDA response increased between P7 (251 ± 27 pA, n= 32) and P28 (476 ± 102 pA, n= 12 cells, P < 0.05), cell capacitance did not significantly (P > 0.05) increase over this age range (P7 19.4 ± 1.0 pF, n= 92; P28 23.4 ± 3.4 pF, n= 10).
Figure 2. Developmental changes in D1 receptor modulation of whole cell NMDA receptor currents recorded in striatal neurons from 7-day-old (P7), 21-day-old (P21) and 28-day-old (P28) rats.
D1 receptor activation produced a significant (*P < 0.05, n= 10) inhibition of the NMDA current in P7 neurones, inconsistent effects at P21 (n= 9) and a significant (*P < 0.05, n= 6) potentiation of the NMDA response at P28.
Neither G proteins nor PKA are involved in D1 inhibition of neonatal NMDA receptor currents
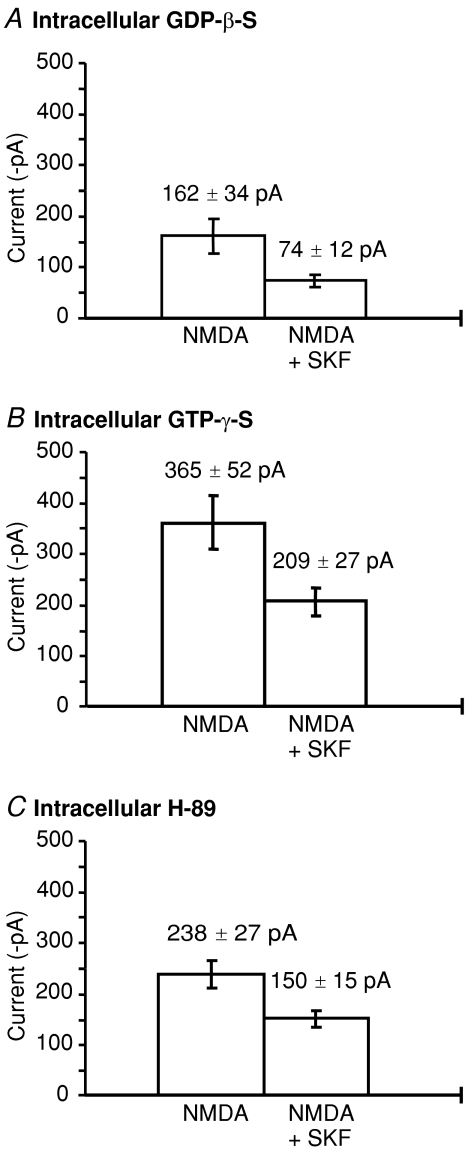
In order to test whether G proteins are required for D1 receptor inhibition of whole cell NMDA currents, intracellular GTP (1 mm) was replaced with a general G protein inhibitor, GDP-β-S (0.5 mm), in nine neurons. SKF-82958 still significantly decreased the NMDA whole-cell current from 162 ± 34 pA to 74 ± 12 pA (54 ± 12% inhibition, n= 9 cells) (Fig. 3A). Likewise, replacement of GTP with GTP-γ-S (0.5 mm), an irreversible activator of G proteins, did not occlude the SKF-82958 induced inhibition of NMDA responses (NMDA 365 ± 52 pA; SKF-82958 + NMDA 209 ± 27 pA; n= 10 cells, P < 0.05, 43 ± 6% inhibition) (Fig. 3B). The results suggest that G protein activation is not involved in D1 inhibition of NMDA responses. However, the mean NMDA response in the presence of G protein inhibition (GDP-β-S) is significantly smaller (162 ± 34 pA) than in the presence of an irreversible G protein activator, GTP-γ-S (365 ± 52 pA) although these were not significantly different to the control currents (ANOVA, P < 0.05). Therefore these results also indicate that G protein activation can potentiate NMDA responses in striatal neurons, although they give no information about the type of G protein involved.
Figure 3. G proteins and PKA are not involved in D1 inhibition of NMDA responses.
A, mean current (±s.e.m.) in the presence of intracellular GDP-β-S (0.5 mm) of the first NMDA response compared to the second response in the presence of SKF-82958 and spiperone (n= 9, P < 0.05). B, mean current (±s.e.m.) in the presence of intracellular GTP-γ-S (0.5 μm) of the first NMDA response compared to the second NMDA response (n= 10, P < 0.05). C, mean current (±s.e.m.) in the presence of intracellular H-89 (5 μm) of the first NMDA response compared to the second NMDA response in the presence of SKF-82958 and spiperone (n= 7, P < 0.05).
Activation of cAMP-dependent protein kinase (PKA) could be involved in D1 receptor modulation of NMDA receptors via the classical adenylate cyclase pathway (Greengard, 2001). To test for a role for PKA in D1 inhibition here, H-89 (5 μm), a PKA inhibitor, was added to the pipette solution which contained ATP (1 mm) and GTP (1 mm) (Fig. 3C). H-89 did not significantly affect the control NMDA response and did not block the significant attenuation of NMDA responses (33 ± 9%, n= 7, P < 0.05) induced by SKF-82958 (Fig. 3C). These results show that PKA activation is not required for D1 inhibition of NMDA receptor currents in medium spiny neurons. Since neither G proteins nor PKA were involved in D1 inhibition of NMDA responses in these experiments, we conclude an alternative mechanism may predominate here.
D1 inhibition is not evident in single-channel recordings
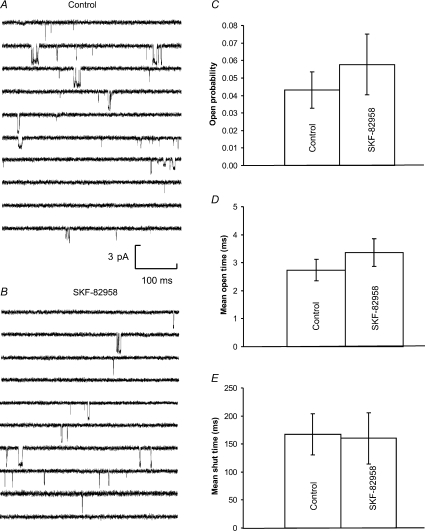
In order to investigate whether D1 inhibition of NMDA receptors could be observed in isolated membrane patches, we made outside-out patch-clamp recordings from medium spiny neurons in 21 different striatal slices from 7-day-old rats. In these experiments GDP-β-S (0.5 mm) and ATP (1 mm) were present in the pipette solution as whole-cell recordings demonstrated that active G proteins are not necessary to observe D1 inhibition and TTX (100 nm) was applied in the recording solution. Single channel openings were evoked by NMDA (1 μm) and glycine (10 μm) at −60 mV, and were recorded for 2 min both in the absence of SKF-82958 (20 nm) and spiperone (2 nm) (control) and in their presence. Examination of individual single channel recordings illustrates that SKF-82958 produced no obvious changes in NMDA channel behaviour (Fig. 4A and B).
Figure 4. Single channel activity mediated by NMDA receptors in control (A) and 20 nm SKF-82958 treated (B) outside-out patches.
Downward deflections in the baseline indicate single channel openings in the presence of 1.0 μm NMDA and 10 μm glycine. Both A and B show 5 s of continuous recording at a holding potential of −60 mV from the same patch. Currents were low-pass filtered at 2 kHz and digitized at 20 kHz. Mean channel open probability (C), open time (D) and shut time (E) were not altered significantly in the presence of SKF-82958.
Quantitative analysis of single channel distributions confirmed that D1 modulation of the NMDA receptor activity was not evident in outside-out patches (Supplemental Fig. 1S). Channel open probability was 0.043 ± 0.010 for control and 0.058 ± 0.017 in the presence of SKF-82958 (Fig. 4C). Neither the time constants nor the relative areas of the exponential components fitted to open time or closed time distributions were significantly changed in the presence of SKF-82958 (Supplemental Fig. 1S). The mean open time was 2.74 ± 0.39 ms for control and 3.36 ± 0.50 ms in the presence of SKF-82958 (Fig. 4D). The mean shut times were 167 ± 37 ms for control and 160 ± 46 ms in the presence of SKF-82958 (Fig. 4E). Therefore SKF-82958 did not significantly change the NMDA receptor single channel properties in outside out patches.
Subunit composition of neonatal striatal medium spiny neurone NMDA receptors
Our results showing D1 inhibition of NMDA currents are similar in some ways to those of Lee et al. (2002). However, they identified direct protein–protein interactions between the C-terminus of the D1 receptor (D1-t3) and the C-terminus of the NR2A subunit as involved in inhibition of NMDA currents in both transfected cell lines and in tissue cultured striatal and hippocampal neurones. In our outside-out patch experiments, stability plots (Colquhoun & Sigworth, 1995) of single channel amplitude measurements (Supplemental Fig. 2SA and B) showed that channel activity was stable during the recording period and that a variety of single channel current amplitudes are seen in the presence of NMDA, with the largest of these around 3 pA in size corresponding to 50 pS conductance at −60 mV. Single NMDA channel amplitude distributions were fitted with the sum of three Gaussian components both in control and in the presence of SKF-82958 and spiperone (Supplemental Fig. 2SC and D). Mean values (and relative areas) for these three components were 1.1 ± 0.1 pA (18 ± 4%), 1.9 ± 0.1 pA (48 ± 7%), 3.05 ± 0.2 pA (33 ± 7%) for control and 1.1 ± 0.1 pA (23 ± 4%), 1.9 ± 0.1 pA (45 ± 7%), 2.9 ± 0.1 pA (34 ± 7%) in the presence of SKF-82958, corresponding to average single channel conductances of 18 pS, 32 pS and 50 pS. Some evidence for receptor heterogeneity was apparent in these recordings with some patches, as illustrated in Fig. 4, exhibiting a majority of large (50 pS) openings, while in other patches intermediate and low (18 pS) conductance openings were more common. Large (50 pS) conductance single channel openings are characteristic of NR2A and/or NR2B subunit-containing NMDA receptors (Stern et al. 1992) while small conductance openings are characteristic of NR2C or NR2D subunit-containing receptors (Stern et al. 1992; Wyllie et al. 1996). We therefore tested in whole-cell recordings for the presence of functional NR2A-containing receptors (Paoletti et al. 1997) using ZnCl2 (100 nm) or for the presence of tonic zinc inhibition using the zinc chelating agent N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine (TPEN; 1 μm) (control 194 ± 20 pA; ZnCl2 198 ± 15 pA; TPEN 181 ± 11 pA; n= 5 cells). There was no significant difference among these three NMDA responses. In contrast, the NR2B selective antagonist ifenprodil (10 μm) produced a 68.1 ± 8.9% inhibition of the response to 200 μm NMDA and 10 μm glycine (control 862 ± 131 pA; ifenprodil 275 ± 76 pA; n= 11). A saturating concentration of NMDA was used in these experiments in order to avoid underestimating the effect of ifenprodil due to the increase in agonist affinity that accompanies ifenprodil binding to NR2B receptors (Kew et al. 1996). In addition, single channel recordings showed that ifenprodil (10 μm) decreased mean open time from 2.92 ± 0.2 ms in control to 0.77 ± 0.12 ms as expected if ifenprodil were acting at NR2B receptors to increase receptor proton sensitivity (Banke et al. 2005). These results suggested an absence of functional NR2A receptors and a predominance of NR2B subunit-containing receptors in 7-day-old rat striatum, while the presence of low-conductance (18 pS) single channel openings suggests the presence of NR2C or NR2D subunits. NR2D subunit-containing receptors display direct transitions between conductance levels that are asymmetric in their frequency when they involve the 18 pS conductance level (Wyllie et al. 1996) while NR2C subunit-containing receptors do not show this effect (Stern et al. 1992). In our experiments on neonatal striatal neurones direct transitions involving the 18 pS level were asymmetric with transitions from 50 pS to 18 pS occurring more frequently (58 ± 1.8% of transitions, n= 11 patches) than transitions from 18 pS to 50 pS (42 ± 1.8%) and transitions from 32 pS to 18 pS were also more frequent (57 ± 2.9%) than from 18 pS to 32 pS (43 ± 2.9%). In contrast, transitions between 50 pS and 32 pS levels were symmetrical in their transition frequency (49.1 ± 2.4% and 50.9 ± 2.4%). Application of SKF-82958 did not change the frequencies of direct transitions. Intermediate conductance receptors (32 pS) could be due to the presence of NR3A subunit-containing receptors (Pérez-Otaño et al. 2001; Wong et al. 2002) or due to triheteromeric receptors containing two types of NR2 subunit (Cheffings & Colquhoun, 2000). Similarly, protein immunohistochemistry (Portera-Cailliau et al. 1996; Wenzel et al. 1997; Wong et al. 2002) and in situ mRNA hybridization (Monyer et al. 1994; Wenzel et al. 1997) show a lack of NR2A and NR2C and the presence of NR2B (Portera-Cailliau et al. 1996; Wenzel et al. 1997) and low levels of NR2D (Dunah et al. 1996) and NR3A (Wong et al. 2002) subunit expression in P5–P7 rat striatum. Thus NR2A subunits may not be involved in D1 inhibition of striatal NMDA receptors at this stage of development whereas it is likely that NR2B and NR2D containing receptors are.
Since whole-cell currents are determined by the channel conductance, open probability and the number of channels, these results suggested that D1 inhibition of NMDA responses in whole-cell recordings could be due to a change in the number of surface NMDA receptors suggesting an effect on NMDA receptor trafficking.
Non-receptor tyrosine kinase inhibitors blocked D1 inhibition
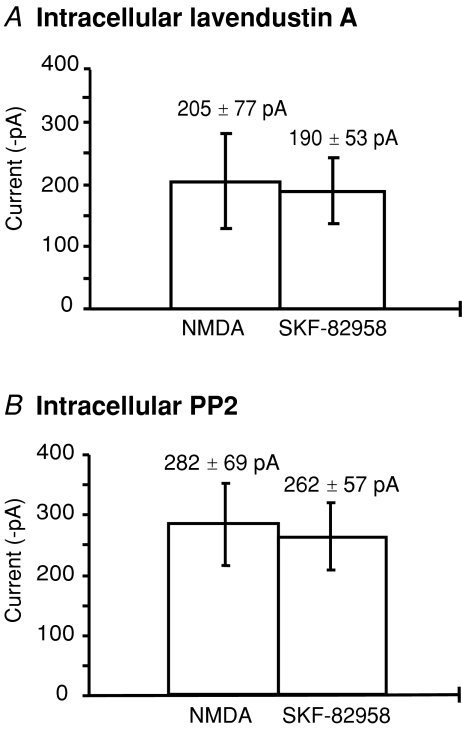
Non-receptor tyrosine kinases have been shown to modulate NMDA receptor trafficking in a range of preparations, including in the striatum where biochemical studies have shown clear evidence for a role of tyrosine kinases in regulating receptor trafficking (Dunah & Standaert, 2001; Dunah et al. 2004). In our initial experiments, lavendustin A (10 μm), a non-selective inhibitor of non-receptor tyrosine kinases (Onoda et al. 1989; Lu et al. 1999), was used to investigate the mechanism of D1 modulation of NMDA responses (Fig. 5A). In the presence of intracellular lavendustin A, D1 inhibition of the NMDA current was significantly reduced from 34.2 ± 12.3% (Fig. 5A) to 1 ± 8.7% (n= 14 cells, unpaired t test, P < 0.05), whereas its inactive analogue lavendustin B did not affect the D1 inhibition (40 ± 4% inhibition, n= 8). Intracellular lavendustin A did not significantly affect the size of control NMDA responses (control 249 ± 36 pA; lavendustin A 205 ± 77 pA). In a second series of experiments, the effects of SKF-82958 were tested during intracellular application of the selective Src tyrosine kinase inhibitor, PP2 (10 μm). In these experiments there was no significant difference between the NMDA response in control (282 ± 69 pA) compared to the NMDA response in the presence of SKF-82958 and spiperone (262 ± 57 pA, n= 9, P > 0.05) (Fig. 5B). These results suggest active tyrosine kinase is necessary for D1 receptor inhibition of NMDA responses.
Figure 5. D1 inhibition of NMDA responses is non-receptor tyrosine kinase dependent.
A, in the presence of intracellular lavendustin A (10 μm), the mean NMDA current (±s.e.m.), compared to the second NMDA response in the presence of SKF-82958 and spiperone (n= 14, P > 0.05). B, in the presence of intracellular PP2 (10 μm), the mean NMDA current (±s.e.m.) compared to the second response in the presence of SKF-82958 and spiperone (n= 9, P > 0.05).
Intracellular dynamin inhibitory peptide abolished D1 inhibition
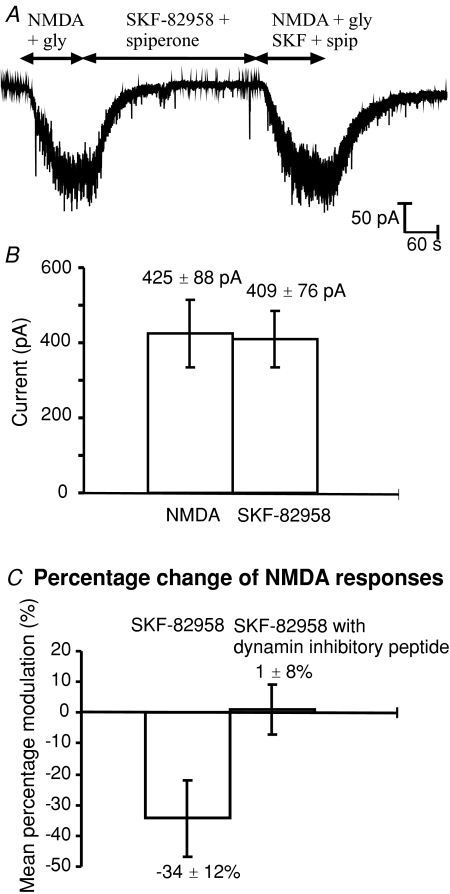
Dynamin plays an essential role in clathrin mediated endocytosis (Schmid, 1997), which has been shown to mediate endocytosis of GABAA receptors (Kittler et al. 2000), AMPA receptors (Carroll et al. 1999) and NMDA receptors (Nong et al. 2003; Newpher & Ehlers, 2008). Here we applied intracellular dynamin inhibitory peptide (QVPSRPNRAP; 50 μm) to prevent receptor endocytosis (Fig. 6A). In whole cell recordings, the response to bath application of NMDA reflects activation of both synaptic and extra-synaptic NMDA receptors and is thus likely to be unaffected by mechanisms influencing the surface movement of receptors between synaptic and extra-synaptic membranes (Groc et al. 2006; Newpher & Ehlers, 2008). In the presence of dynamin inhibitory peptide, NMDA responses were significantly increased compared to all other control NMDA responses (Fig. 6B). Control currents recorded with our standard pipette solution averaged 251 ± 27 pA (n= 32) whereas in the presence of intracellular dynamin inhibitory peptide, control currents averaged 497 ± 62 pA (n= 18). D1 inhibition of NMDA responses was abolished by intracellular dynamin inhibitory peptide; the average percentage inhibition decreased from 34.3 ± 12.3% (Fig. 1B) to −1.1 ± 8.2% (Fig. 6C) (n= 9 cells). In control experiments, intracellular application of a myristolated scrambled dynamin inhibitory peptide (Myr-QPPASNPRVR, 50 μm) was used to test the selectivity of the effect of the dynamin inhibitory peptide effect on D1 inhibition. In the presence of the scrambled dynamin inhibitory peptide, SKF-82958 significantly reduced the mean NMDA responses (from 168 ± 31 pA, to 127 ± 27 pA) by 25 ± 4% (n= 9, P < 0.05). These results suggest dynamin-dependent endocytosis is essential in order to observe D1 inhibition of NMDA responses.
Figure 6. Intracellular dynamin inhibitory peptide (50 μm) abolishes D1 inhibition of whole cell NMDA receptor currents.
A, response to NMDA (10 μm) and glycine (10 μm) applied for 2 min, followed by 5 min of SKF-82958 (20 nm) and spiperone (2 nm), and finally NMDA (10 μm), glycine (10 μm), SKF-82958 (20 nm) and spiperone (2 nm) for 2 min. B, mean current (±s.e.m.) of the first NMDA response compared to the second response in the presence of SKF-82958 and spiperone (n= 9, P > 0.05). C, mean percentage SKF-82958 inhibition (±s.e.m.) in experiments with only ATP and GTP in the pipette solution (Fig. 1A) compared to experiments in the presence of 50 μm intracellular dynamin inhibitory peptide (unpaired t test P < 0.05).
In nine control experiments where two successive NMDA responses were evoked in cells dialysed with 50 μm dynamin inhibitory peptide, without application of SKF-82958, there was no significant difference between first and second NMDA responses (first responses 569 ± 86pA; second responses 526 ± 93pA) suggesting dynamin-independent processes involving the NMDA receptors had reached a steady-state within the first few minutes of whole-cell recording. If we assume that dynamin-dependent receptor internalization is the dominant NMDA receptor internalization mechanism, these results suggest that approximately 50% of NMDA receptors are located on the cell surface at any one time (average current increased from 251 pA in control to 497 pA in the presence of the dynamin inhibitory peptide). Furthermore, since the NMDA current in the presence of dynamin inhibition was stable within 5 min of beginning whole-cell recording, these results suggest an estimate of the lifetime of a surface receptor (at room temperature) of around 1.0 min (given exponential processes normally require around five time constants for establishment of a new steady-state). Given that NPopen in outside-out patches was approximately 0.05 at 1 μm NMDA, then assuming an EC50 for NMDA of 20 μm and a maximal single channel open probability of 0.2 (Gibb & Colquhoun, 1992; Rycroft & Gibb, 2002), our average control response of 250 pA at −60 mV corresponds to a total of 1250 NMDA receptors on the cell surface and this increases to 2500 receptors in the presence of the dynamin inhibitory peptide.
Discussion
Dopamine modulation of NMDA receptors in the striatum is not only crucial to the efficacy of synaptic transmission in the cortico-striatal pathway (Calabresi et al. 2000; Centonze et al. 2003) but may also influence excitotoxicity in some neuronal pathologies. NMDA receptor-dependent long-term potentiation (LTP) and long-term depression (LTD) have been described in several brain areas including the striatum (Calabresi et al. 1992; Charpier & Deniau, 1997) and NMDA receptor mediated excitotoxicity contributes to the death of striatal neurons under some pathological conditions (Koroshetz et al. 1990; Cepeda et al. 1998b; Zeron et al. 2002; Li et al. 2003). In this study we have shown that dopamine D1 receptor activation attenuates NMDA receptor currents in neonatal striatal medium spiny neurons. Our experimental data suggest this is a G protein-independent, Src family tyrosine kinase-mediated mechanism involving NMDA receptor trafficking.
G protein-independent NMDA receptor inhibition
In whole cell recordings, D1 receptor activation by SKF-82958 significantly decreased NMDA receptor currents in striatal medium spiny neurons. This result is not consistent with the hypothesis of the classical adenylate cyclase pathway involving GS G protein stimulation, where D1 activation has been shown to enhance NMDA responses via the adenylate cyclase–protein kinase-A and DARPP-32 (dopamine and cyclic adenosine 3′,5′-monophosphate-Regulated PhosphoProtein, 32 kDa) cascade (Levine et al. 1996; Blank et al. 1997; Flores-Hernandez et al. 2002). In our experiments D1 inhibition of NMDA receptor currents could not be abolished by intracellular application of either GDP-β-S (an inhibitor of G protein function) or occluded by GTP-γ-S (an irreversible activator of G proteins), which strongly suggests that in these young medium spiny neurones D1 receptor activation attenuates NMDA receptor currents by a G protein-independent mechanism. In addition, the PKA inhibitor H-89 did not affect D1 inhibition.
These results are similar in some ways to those of Lee et al. (2002). They identified direct protein – protein interactions between the C-terminus of the D1 receptor (D1-t3) and the C-terminus of the NR2A subunit which led to inhibition of NMDA receptor currents that was not dependent on G proteins or PKA. However, their results also showed that the carboxyl tail of NR2B could not form a complex with the D1 receptor directly. In our experiments we could not find evidence for the presence of functional NR2A subunits in the striatum of 7-day-old rats using either the zinc chelating agent TPEN to unmask any tonic zinc inhibition of NR2A receptors or addition of zinc to cause inhibition of NR2A receptors (Paoletti et al. 1997). These results are consistent with the absence or very weak expression of NR2A mRNA (Monyer et al. 1994; Wenzel et al. 1997) or protein (Portera-Cailliau et al. 1996; Dunah et al. 1996; Wenzel et al. 1997) from the striatum of P5–P7 rats. At postnatal days P5–P7 there is a strong expression of the NR2B mRNA (Monyer et al. 1994) and protein (Portera-Cailliau et al. 1996; Wenzel et al. 1997) in the striatum (Standaert et al. 1994; Wenzel et al. 1997) and only low levels of NR2D subunit protein are detected (Dunah et al. 1996), which is consistent with the large single channel conductance (50 pS) observed and lower conductance events (18 pS) in outside-out patch recordings in these experiments. These results suggest that NR2B receptors along with a proportion of NR2D-containing receptors are likely to be the most common receptor subtypes in 7-day-old rat striatum and that NR2A subunits are unlikely to be involved in the D1 inhibition of striatal NMDA receptors observed in this study.
D1 receptor activation does not change NMDA receptor single channel activity in isolated membrane patches
D1 receptor inhibition of NMDA receptors could not be observed in outside-out membrane patches from the cell body; application of SKF-82958 did not change the single channel conductance, mean open time, mean shut time or open probability in 21 outside-out patch-clamp recordings. These results are important because they show that SKF-82958 (20 nm) has no direct effect on NMDA receptor function although much higher concentrations have been shown to directly inhibit NMDA receptors (Castro et al. 1999; Cui et al. 2006). Whole-cell currents are determined by the channel conductance, the open probability and the channel number. Therefore we hypothesized that D1 inhibition of NMDA responses in whole-cell recordings may be mediated by a change in surface receptor number caused by NMDA receptor trafficking between surface and intracellular receptor pools, although there is also the possibility that D1 receptors are not colocalized with NMDA receptors in the somatic membrane patches used for these single channel recordings. Likewise, Lee et al. (2002) also found that D1 receptor activation decreased the number of NMDA receptors expressed on the cell surface.
Non-receptor tyrosine kinase is required for D1 inhibition of NMDA receptors
The Src family of protein tyrosine kinases, which consists of Src, Fyn, Lyn, Lck and Yes (Ali & Salter, 2001), has been shown to up-regulate the activity of NMDA receptors by phosphorylation (Kohr & Seeburg, 1996; Zheng et al. 1998; Ali & Salter, 2001). In recombinant NMDA receptors, Kohr & Seeburg (1996) showed that Src potentiated whole cell currents mediated by NR1/NR2A but not by NR1/NR2B. Zheng et al. (1998) demonstrated that Src potentiation was mediated by a decrease in tonic zinc inhibition. Salter (1998) suggested there might be an additional possibility that phosphorylation of NR2B subunits by tyrosine kinase does not affect channel gating but rather affects another function such as receptor trafficking, and subsequent biochemical experiments have uncovered a wide range of trafficking effects that are modulated by tyrosine kinases and by interaction with intracellular scaffolding proteins (reviewed by Wenthold et al. 2003; Lau & Zukin, 2007).
G protein-coupled receptors have been reported to potentiate NMDA receptor function (Lu et al. 1999) and trafficking (Dunah & Standaert, 2001; Hallett et al. 2006) by the activation of Src family kinases. Heuss et al. (1999) have proposed that a Src-family tyrosine kinase mediates the metabotropic glutamate receptor (mGluR) EPSC by associating with the receptor either directly or via an adaptor protein; furthermore Benquet et al. (2002) demonstrated that the activation of mGluR1 potentiates the NMDA current via a G protein-independent mechanism involving Src kinase activation. In terms of D1 receptor modulation, Dunah et al. (2004) showed that deletion of the gene for the protein tyrosine kinase Fyn, inhibits dopamine D1 receptor-induced enhancement of the abundance of NR1, NR2A and NR2B subunits in the synaptosomal membrane fraction and demonstrated that Fyn and tyrosine phosphorylation are required for D1-dependent redistribution of NMDA receptor protein (Dunah et al. 2004; Hallett et al. 2006).
In this study intracellular application of lavendustin A, an inhibitor of non-receptor protein tyrosine kinases, blocked D1 inhibition of NMDA receptor currents, while lavendustin B, an inactive analogue of lavendustin A, had no effect. In addition, intracellular application of PP2, a selective Src family tyrosine kinase inhibitor, also blocked D1 inhibition of NMDA currents. These results indicate D1 inhibition of NMDA currents requires tyrosine kinase activation.
Dynamin-dependent D1 inhibition
In this study, D1 inhibition of NMDA responses was blocked by intracellular application of a dynamin inhibitory peptide. The dynamin inhibitory peptide also produced an approximate doubling of the average whole-cell response to 10 μm NMDA. These results support the concept that normally there is constitutive turnover of NMDA receptors in the cell membrane (Roche et al. 2001; Wenthold et al. 2003; Lavezzari et al. 2004; Washbourne et al. 2004) and our results suggest this turnover is rapid (even at room temperature) with around 50% of the active pool of receptors spending on average 1 min or less on the cell surface. Such rapid receptor trafficking is not unexpected given that in neonatal hippocampus, subunit switching of NR2A for NR2B receptors occurs at CA1 synapses within seconds of a potentiating stimulus (Bellone & Nicoll, 2007). In tissue cultured cortical neurones, Washbourne et al. (2004) estimate an average time of 5 min for the full endo/exocytic cycle and using immuno-gold labelling that ∼15% of total NMDA receptors are on the cell surface at any given time while our experiments suggest that in neonatal striatal neurones ∼50% of receptors are on the cell surface. In our experiments inhibition of receptor internalization resulted in an accumulation of receptors on the cell surface and occlusion of D1 inhibition suggesting that dynamin-dependent internalization is essential in order to be able to observe D1-dependent inhibition.
Mechanism of D1 inhibition
NMDA receptor NR2B subunits have one PDZ-binding motif and one tyrosine phosphorylation motif (YEKL 1472–1475) at the C-terminal, which influence receptor internalization (Wenthold et al. 2003). The main site of tyrosine phosphorylation of NR2B is Y1472 (Nakazawa et al. 2001). Roche et al. (2001) demonstrated that the YEKL motif regulates a robust endocytosis of NMDA receptors in cultured neurons and there was a developmental decline in NMDA receptor endocytosis as neurons mature. This is consistent with a decrease of NR2B subunits together with an increase of NR2A subunits while neurons are maturing (Watanabe et al. 1993; Monyer et al. 1994; Wenzel et al. 1997; Laurie et al. 1997). PSD-95 is abundant in the postsynaptic density (PSD) and is a membrane associated guanylate kinase (MAGUKs), contributing to anchoring NMDA receptors at the synapse (Wenthold et al. 2003; Chung et al. 2004; Lin et al. 2004). PSD-95 associates with the last four amino acids (ESDV) of the NR2 subunit (Kornau et al. 1995; Niethammer et al. 1996; Cousins et al. 2008) and this binding site is very close to Y1472 (Nakazawa et al. 2001), the main site of tyrosine phosphorylation. Roche et al. (2001) suggest that phosphorylation of the NMDA receptor could inhibit the receptor's interaction with PSD-95. They concluded that the disruption of the NMDA receptor–PSD-95 complex destabilized the NMDA receptor, thereby allowing receptor internalization. In addition the tyrosine motif binds to AP-2 adaptor complexes allowing a rapid internalization of surface NMDA receptors (Bonifacino & Dell'Angelica, 1999; Lavezzari et al. 2003). Such a mechanism is consistent with the results we have observed here in neonatal rat striatum and with the results of Gu et al. 2007) showing that in HEK cells coexpressing D1, NR1, NR2B and PSD-95, in the presence of the PKA blocker, H89, dopamine caused an inhibition of the NMDA receptor response that is dependent on the expression of PSD-95. These observations are also consistent with the elegant work of Misale and coworkers (Fiorentini et al. 2003, 2006) who used BRET to show that D1 and NMDA receptors directly interact in transfected HEK cells and that in cells coexpressing D1, NR1, NR2B and PSD-95, coactivation of D1 and NMDA receptors is necessary to observe D1-evoked NMDA receptor internalization.
Relevance of neonatal D1 inhibition for synaptic NMDA receptors
The main excitatory input to striatal medium spiny neurones comes from cortical pyramidal neurones. At these glutamatergic corticostriatal synapses, NMDA receptor-dependent synaptic plasticity is modulated by dopamine D1 and D2 receptors (Calabresi et al. 1992; Kerr & Wickens, 2001; Surmeier et al. 2007; Shen et al. 2008). Striatal medium spiny neurones fall into two similar groups, expressing mainly D1 or D2 receptors corresponding to direct and indirect pathway neurones, respectively (reviewed by Surmeier et al. 2007). It follows that D1 modulation of NMDA receptors will be evident in direct pathway medium spiny neurones and not in indirect pathway cells. In neonatal rats this may cause a reduction in the synaptic strength at corticostriatal synapses in the direct pathway. However, the whole-cell responses studied in this paper will include current from both synaptic and extra-synaptic NMDA receptors and so it is not possible to predict from our data what the effect of D1 activation will be on synaptic NMDA receptors in P7 rats, although the mechanism of D1 modulation observed in this study is consistent with modulation of synaptic receptors in other systems (Newpher & Ehlers, 2008).
The idea that D1 activation evokes a tyrosine kinase-dependent NMDA receptor internalization appears at first sight to be inconsistent with biochemical experiments studying the effect of D1 receptor activation on NMDA receptor trafficking in the striatum (Dunah & Standaert, 2001; Dunah et al. 2004; Hallett et al. 2006). These show a tyrosine kinase-dependent increase in the density of synaptic NMDA receptors and stimulation of receptor trafficking to the dendrites (Hallett et al. 2006) following D1 receptor activation. In addition, in adult rats, Chen et al. (2004) and Seamans et al. (2001) have shown a potentiation of NMDA receptor-mediated synaptic currents in prefrontal cortex. One possible explanation for these apparently conflicting results may lie in the difference in NR2A subunit expression which is almost absent in P7 rat striatum (Monyer et al. 1994; Portera-Cailliau et al. 1996) but widely expressed in older rats or in long-term tissue culture. In younger animals NR2B subunits predominate and these can be rapidly internalized in response to tyrosine phosphorylation of the NR2B C-terminal domain (Lavezzari et al. 2003, 2004; Wenthold et al. 2003). In contrast, NR2A subunits are stabilized by PSD-95 binding at the synapse and NR2A endocytosis is regulated by a dileucine motif. These differences mean that the regulation of NMDA receptor trafficking and surface density is developmentally dependent on the presence of NR2A subunits (Newpher & Ehlers, 2008). Intriguingly, Hallett et al. (2006) show that in concert with a D1 receptor-dependent increase in NR2A subunit protein in the dendrites of striatal neurons in long-term tissue culture, there is an increase in surface NR2B. It could be that this reflects the movement of triheteromeric NR1/NR2A/NR2B receptors (Sheng et al. 1994; Chazot & Stephenson, 1997; Tovar & Westbrook, 1999) into the synapse. Whatever the underlying mechanisms, the results presented in this paper suggest that there is a developmental switch in D1 receptor modulation between early in development when NR2B receptors predominate and D1 receptor activation down-regulates NMDA receptors, to later in development when a mixed NR2A/NR2B receptor population is likely to be present and D1 receptor activation stabilizes NR2A-containing receptors at the synapse.
In summary, it is clear that the D1 receptor-dependent inhibition of NMDA receptors in our experiments in neonatal rats is not mediated by the classical G protein-dependent pathway but instead involves tyrosine kinase activation and dynamin-dependent receptor internalization. We suggest that in these experiments tyrosine kinase phosphorylation of the C-terminal of NR2B subunits may interfere with PSD-95 anchoring of NMDA receptors. As a result, fewer NMDA receptors remain on the cell surface producing smaller whole cell currents after D1 receptor activation.
Acknowledgments
A.J.G. is supported by the BBSRC and Wellcome Trust. H.X.T. received a UCL Graduate School Scholarship. We are grateful to Josef Kitler for advice on the use of dynamin inhibitory peptide
Supplemental material
Online supplemental material for this paper can be accessed at: http://jp.physoc.org/cgi/content/full/jphysiol.2008.158931/DC1
References
- Ali DW, Salter MW. NMDA receptor regulation by Src kinase signalling in excitatory synaptic transmission and plasticity. Curr Opin Neurobiol. 2001;11:336–342. doi: 10.1016/s0959-4388(00)00216-6. [DOI] [PubMed] [Google Scholar]
- Banke TG, Dravid SM, Traynelis SF. Protons trap NR1/NR2B NMDA receptors in a nonconducting state. J Neurosci. 2005;25:42–51. doi: 10.1523/JNEUROSCI.3154-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellone C, Nicoll RA. Rapid bidirectional switching of synaptic NMDA receptors. Neuron. 2007;55:779–785. doi: 10.1016/j.neuron.2007.07.035. [DOI] [PubMed] [Google Scholar]
- Benquet P, Gee CE, Gerber U. Two distinct signaling pathways upregulate NMDA receptor responses via two distinct metabotropic glutamate receptor subtypes. J Neurosci. 2002;22:9679–9686. doi: 10.1523/JNEUROSCI.22-22-09679.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank T, Nijholt I, Teichert U, Kugler H, Behrsing H, Fienberg A, Greengard P, Spiess J. The phosphoprotein DARPP-32 mediates cAMP-dependent potentiation of striatal N-methyl-D-aspartate responses. Proc Natl Acad Sci U S A. 1997;94:14859–14864. doi: 10.1073/pnas.94.26.14859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino JS, Dell'Angelica EC. Molecular bases for the recognition of tyrosine-based sorting signals. J Cell Biol. 1999;145:923–926. doi: 10.1083/jcb.145.5.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Gubellini P, Centonze D, Picconi B, Bernardi G, Chergui K, Svenningsson P, Fienberg AA, Greengard P. Dopamine and cAMP-regulated phosphoprotein 32 kDa controls both striatal long-term depression and long-term potentiation, opposing forms of synaptic plasticity. J Neurosci. 2000;20:8443–8451. doi: 10.1523/JNEUROSCI.20-22-08443.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Picconi B, Tozzi A, Di Filippo M. Dopamine-mediated regulation of corticostriatal synaptic plasticity. Trends Neurosci. 2007;30:211–219. doi: 10.1016/j.tins.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Pisani A, Mercuri NB, Bernardi G. Long-term potentiation in the striatum is unmasked by removing the voltage-dependent magnesium block of NMDA receptor channels. Eur J Neurosci. 1992;4:929–935. doi: 10.1111/j.1460-9568.1992.tb00119.x. [DOI] [PubMed] [Google Scholar]
- Carroll RC, Beattie EC, Xia H, Luscher C, Altschuler Y, Nicoll RA, Malenka RC, von Zastrow M. Dynamin-dependent endocytosis of ionotropic glutamate receptors. Proc Natl Acad Sci U S A. 1999;96:14112–14117. doi: 10.1073/pnas.96.24.14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro NG, de Mello MC, de Mello FG, Aracava Y. Direct inhibition of the N-methyl-D-aspartate receptor channel by dopamine and (+)- SKF38393. Br J Pharmacol. 1999;126:1847–1855. doi: 10.1038/sj.bjp.0702479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centonze D, Grande C, Saulle E, Martin AB, Gubellini P, Pavon N, Pisani A, Bernardi G, Moratalla R, Calabresi P. Distinct roles of D1 and D5 dopamine receptors in motor activity and striatal synaptic plasticity. J Neurosci. 2003;23:8506–8512. doi: 10.1523/JNEUROSCI.23-24-08506.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda C, Colwell CS, Itri JN, Chandler SH, Levine MS. Dopaminergic modulation of NMDA-induced whole cell currents in neostriatal neurons in slices: contribution of calcium conductances. J Neurophysiol. 1998a;79:82–94. doi: 10.1152/jn.1998.79.1.82. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Colwell CS, Itri JN, Gruen E, Levine MS. Dopaminergic modulation of early signs of excitotoxicity in visualized rat neostriatal neurons. Eur J Neurosci. 1998b;10:3491–3497. doi: 10.1046/j.1460-9568.1998.00357.x. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Levine MS. Where do you think you are going? The NMDA-D1 receptor trap. Science STKE. 2006;2006:pe20. doi: 10.1126/stke.3332006pe20. [DOI] [PubMed] [Google Scholar]
- Charpier S, Deniau JM. In vivo activity-dependent plasticity at cortico-striatal connections: evidence for physiological long-term potentiation. Proc Natl Acad Sci U S A. 1997;94:7036–7040. doi: 10.1073/pnas.94.13.7036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chazot PL, Stephenson FA. Molecular dissection of native mammalian forebrain NMDA receptors containing the NR1 C2 exon: direct demonstration of NMDA receptors comprising NR1, NR2A, and NR2B subunits within the same complex. J Neurochem. 1997;69:2138–2144. doi: 10.1046/j.1471-4159.1997.69052138.x. [DOI] [PubMed] [Google Scholar]
- Cheffings CM, Colquhoun D. Single channel analysis of a novel NMDA channel from Xenopus oocytes expressing recombinant NR1a, NR2A and NR2D subunits. J Physiol. 2000;526:481–491. [PubMed] [Google Scholar]
- Chen G, Greengard P, Yan Z. Potentiation of NMDA receptor currents by dopamine D1 receptors in prefrontal cortex. Proc Natl Acad Sci U S A. 2004;101:2596–2600. doi: 10.1073/pnas.0308618100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HJ, Huang YH, Lau LF, Huganir RL. Regulation of the NMDA receptor complex and trafficking by activity-dependent phosphorylation of the NR2B subunit PDZ ligand. J Neurosci. 2004;24:10248–10259. doi: 10.1523/JNEUROSCI.0546-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D, Sigworth FJ. Fitting and statistical analysis of single-channel records. In: Sakmann B, Neher E, editors. Single-Channel Recording. 2nd edn. New York: Plenum Press; 1995. pp. 483–587. [Google Scholar]
- Cousins SL, Papadakis M, Rutter AR, Stephenson FA. Differential interaction of NMDA receptor subtypes with the post-synaptic density-95 family of membrane associated guanylate kinase proteins. J Neurochem. 2008;104:903–913. doi: 10.1111/j.1471-4159.2007.05067.x. [DOI] [PubMed] [Google Scholar]
- Cui C, Xu M, Atzori M. Voltage-dependent block of N-methyl-D-aspartate receptors by dopamine D1 receptor ligands. Mol Pharmacol. 2006;70:1761–1770. doi: 10.1124/mol.106.028332. [DOI] [PubMed] [Google Scholar]
- Dunah AW, Sirianni AC, Fienberg AA, Bastia E, Schwarzschild MA, Standaert DG. Dopamine D1-dependent trafficking of striatal N-methyl-D-aspartate glutamate receptors requires Fyn protein tyrosine kinase but not DARPP-32. Mol Pharmacol. 2004;65:121–129. doi: 10.1124/mol.65.1.121. [DOI] [PubMed] [Google Scholar]
- Dunah AW, Standaert DG. Dopamine D1 receptor-dependent trafficking of striatal NMDA glutamate receptors to the postsynaptic membrane. J Neurosci. 2001;21:5546–5558. doi: 10.1523/JNEUROSCI.21-15-05546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunah AW, Yasuda RP, Wang YH, Luo J, Dávila-García M, Gbadegesin M, Vicini S, Wolfe BB. Regional and ontogenic expression of the NMDA receptor subunit NR2D protein in rat brain using a subunit-specific antibody. J Neurochem. 1996;67:2335–2345. doi: 10.1046/j.1471-4159.1996.67062335.x. [DOI] [PubMed] [Google Scholar]
- Edwards FA, Konnerth A, Sakmann B, Takahashi T. A thin slice preparation for patch clamp recordings from synaptically connected neurons of the mammalian central nervous system. Pflugers Arch. 1989;414:600–612. doi: 10.1007/BF00580998. [DOI] [PubMed] [Google Scholar]
- Fiorentini C, Gardoni F, Spano P, Di Luca M, Missale C. Regulation of dopamine D1 receptor trafficking and desensitization by oligomerization with glutamate N-methyl-D-aspartate receptors. J Biol Chem. 2003;278:20196–20202. doi: 10.1074/jbc.M213140200. [DOI] [PubMed] [Google Scholar]
- Fiorentini C, Rizzetti MC, Busi C, Bontempi S, Collo G, Spano P, Missale C. Loss of synaptic D1 dopamine/N-methyl-D-aspartate glutamate receptor complexes in L-DOPA-induced dyskinesia in the rat. Mol Pharmacol. 2006;69:805–812. doi: 10.1124/mol.105.016667. [DOI] [PubMed] [Google Scholar]
- Flores-Hernandez J, Cepeda C, Hernandez-Echeagaray E, Calvert CR, Jokel ES, Fienberg AA, Greengard P, Levine MS. Dopamine enhancement of NMDA currents in dissociated medium-sized striatal neurons: role of D1 receptors and DARPP-32. J Neurophysiol. 2002;88:3010–3020. doi: 10.1152/jn.00361.2002. [DOI] [PubMed] [Google Scholar]
- Gibb AJ, Colquhoun D. Activation of N-methyl-D-aspartate receptors by 1-glutamate in cells dissociated from adult rat hippocampus. J Physiol. 1992;456:143–179. doi: 10.1113/jphysiol.1992.sp019331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotz T, Kraushaar U, Geiger J, Lubke J, Berger T, Jonas P. Functional properties of AMPA and NMDA receptors expressed in identified types of basal ganglia neurons. J Neurosci. 1997;17:204–215. doi: 10.1523/JNEUROSCI.17-01-00204.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greengard P. The neurobiology of slow synaptic transmission. Science. 2001;294:1024–1030. doi: 10.1126/science.294.5544.1024. [DOI] [PubMed] [Google Scholar]
- Groc L, Heine M, Cousins SL, Stephenson FA, Lounis B, Cognet L, Choquet D. NMDA receptor surface mobility depends on NR2A-2B subunits. Proc Natl Acad Sci U S A. 2006;103:18769–18774. doi: 10.1073/pnas.0605238103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu WH, Yang S, Shi WX, Jin GZ, Zhen XC. Requirement of PSD-95 for dopamine D1 receptor modulating glutamate NR1a/NR2B receptor function. Acta Pharmacol Sin. 2007;28:756–762. doi: 10.1111/j.1745-7254.2007.00557.x. [DOI] [PubMed] [Google Scholar]
- Hallett PJ, Spoelgen R, Hyman BT, Standaert DG, Dunah AW. Dopamine D1 activation potentiates striatal NMDA receptors by tyrosine phosphorylation-dependent subunit trafficking. J Neurosci. 2006;26:4690–4700. doi: 10.1523/JNEUROSCI.0792-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett PJ, Standaert DG. Rationale for and use of NMDA receptor antagonists in Parkinson's disease. Pharmacol Ther. 2004;102:155–174. doi: 10.1016/j.pharmthera.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Heuss C, Scanziani M, Gahwiler BH, Gerber U. G-protein-independent signaling mediated by metabotropic glutamate receptors. Nat Neurosci. 1999;2:1070–1077. doi: 10.1038/15996. [DOI] [PubMed] [Google Scholar]
- Jain M, Armstrong RJ, Barker RA, Rosser AE. Cellular and molecular aspects of striatal development. Brain Res Bull. 2001;55:533–540. doi: 10.1016/s0361-9230(01)00555-x. [DOI] [PubMed] [Google Scholar]
- Kebabian JW, Tarazi FI, Kula NS, Baldessarini RJ. Compounds selective for dopamine receptor subtypes. Drug Discovery Today. 1997;2:333–340. [Google Scholar]
- Kerr JN, Wickens JR. Dopamine D-1/D-5 receptor activation is required for long-term potentiation in the rat neostriatum in vitro. J Neurophysiol. 2001;85:117–124. doi: 10.1152/jn.2001.85.1.117. [DOI] [PubMed] [Google Scholar]
- Kew JN, Trube G, Kemp JA. A novel mechanism of activity-dependent NMDA receptor antagonism describes the effect of ifenprodil in rat cultured cortical neurones. J Physiol. 1996;497:761–772. doi: 10.1113/jphysiol.1996.sp021807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler JT, Delmas P, Jovanovic JN, Brown DA, Smart TG, Moss SJ. Constitutive endocytosis of GABAA receptors by an association with the adaptin AP2 complex modulates inhibitory synaptic currents in hippocampal neurons. J Neurosci. 2000;20:7972–7977. doi: 10.1523/JNEUROSCI.20-21-07972.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohr G, Seeburg PH. Subtype-specific regulation of recombinant NMDA receptor-channels by protein tyrosine kinases of the src family. J Physiol. 1996;492:445–452. doi: 10.1113/jphysiol.1996.sp021320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornau HC, Schenker LT, Kennedy MB, Seeburg PH. Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science. 1995;269:1737–1740. doi: 10.1126/science.7569905. [DOI] [PubMed] [Google Scholar]
- Koroshetz WJ, Freese A, DiFiglia M. The correlation between excitatory amino acid-induced current responses and excitotoxicity in striatal cultures. Brain Res. 1990;521:265–272. doi: 10.1016/0006-8993(90)91551-q. [DOI] [PubMed] [Google Scholar]
- Krupp JJ, Vissel B, Thomas CG, Heinemann SF, Westbrook GL. Calcineurin acts via the C-terminus of NR2A to modulate desensitization of NMDA receptors. Neuropharmacology. 2002;42:593–602. doi: 10.1016/s0028-3908(02)00031-x. [DOI] [PubMed] [Google Scholar]
- Lau CG, Zukin RS. NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat Rev Neurosci. 2007;8:413–426. doi: 10.1038/nrn2153. [DOI] [PubMed] [Google Scholar]
- Laurie DJ, Bartke I, Schoepfer R, Naujoks K, Seeburg PH. Regional, developmental and interspecies expression of the four NMDAR2 subunits, examined using monoclonal antibodies. Brain Res Mol Brain Res. 1997;51:23–32. doi: 10.1016/s0169-328x(97)00206-4. [DOI] [PubMed] [Google Scholar]
- Lavezzari G, McCallum J, Dewey CM, Roche KW. Subunit-specific regulation of NMDA receptor endocytosis. J Neurosci. 2004;24:6383–6391. doi: 10.1523/JNEUROSCI.1890-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavezzari G, McCallum J, Lee R, Roche KW. Differential binding of the AP-2 adaptor complex and PSD-95 to the C-terminus of the NMDA receptor subunit NR2B regulates surface expression. Neuropharmacology. 2003;45:729–737. doi: 10.1016/s0028-3908(03)00308-3. [DOI] [PubMed] [Google Scholar]
- Lee FJ, Xue S, Pei L, Vukusic B, Chery N, Wang Y, Wang YT, Niznik HB, Yu XM, Liu F. Dual regulation of NMDA receptor functions by direct protein–protein interactions with the dopamine D1 receptor. Cell. 2002;111:219–302. doi: 10.1016/s0092-8674(02)00962-5. [DOI] [PubMed] [Google Scholar]
- Lei G, Xue S, Chery N, Liu Q, Xu J, Kwan CL, Fu YP, Lu YM, Liu M, Harder KW, Yu XM. Gain control of N-methyl-D-aspartate receptor activity by receptor-like protein tyrosine phosphatase alpha. EMBO J. 2002;21:2977–2989. doi: 10.1093/emboj/cdf292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine MS, Altemus KL, Cepeda C, Cromwell HC, Crawford C, Ariano MA, Drago J, Sibley DR, Westphal H. Modulatory actions of dopamine on NMDA receptor-mediated responses are reduced in D1A-deficient mutant mice. J Neurosci. 1996;16:5870–5882. doi: 10.1523/JNEUROSCI.16-18-05870.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman DN, Mody I. Regulation of NMDA channel function by endogenous Ca2+-dependent phosphatase. Nature. 1994;369:235–239. doi: 10.1038/369235a0. [DOI] [PubMed] [Google Scholar]
- Lin JY, Dubey R, Funk GD, Lipski J. Receptor subtype-specific modulation by dopamine of glutamatergic responses in striatal medium spiny neurons. Brain Res. 2003;959:251–625. doi: 10.1016/s0006-8993(02)03757-5. [DOI] [PubMed] [Google Scholar]
- Lin Y, Skeberdis VA, Francesconi A, Bennett MV, Zukin RS. Postsynaptic density protein-95 regulates NMDA channel gating and surface expression. J Neurosci. 2004;24:10138–10148. doi: 10.1523/JNEUROSCI.3159-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu WY, Xiong ZG, Lei S, Orser BA, Dudek E, Browning MD, MacDonald JF. G-protein-coupled receptors act via protein kinase C and Src to regulate NMDA receptors. Nat Neurosci. 1999;2:331–338. doi: 10.1038/7243. [DOI] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Morishita W, Connor JH, Xia H, Quinlan EM, Shenolikar S, Malenka RC. Regulation of synaptic strength by protein phosphatease 1. Neuron. 2001;32:1133–1148. doi: 10.1016/s0896-6273(01)00554-2. [DOI] [PubMed] [Google Scholar]
- Mottola DM, Laiter S, Watts VJ, Tropsha A, Wyrick SD, Nichols DE, Mailman RB. Conformational analysis of D1 dopamine receptor agonists: pharmacophore assessment and receptor mapping. J Med Chem. 1996;39:285–296. doi: 10.1021/jm9502100. [DOI] [PubMed] [Google Scholar]
- Nakazawa T, Komai S, Tezuka T, Hisatsune C, Umemori H, Semba K, Mishina M, Manabe T, Yamamoto T. Characterization of Fyn-mediated tyrosine phosphorylation sites on GluR epsilon 2 (NR2B) subunit of the N-methyl-D-aspartate receptor. J Biol Chem. 2001;276:693–699. doi: 10.1074/jbc.M008085200. [DOI] [PubMed] [Google Scholar]
- Newpher TM, Ehlers MD. Glutamate receptor dynamics in dendritic microdomains. Neuron. 2008;58:472–497. doi: 10.1016/j.neuron.2008.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niethammer M, Kim E, Sheng M. Interaction between the C terminus of NMDA receptor subunits and multiple members of the PSD-95 family of membrane-associated guanylate kinases. J Neurosci. 1996;16:2157–2163. doi: 10.1523/JNEUROSCI.16-07-02157.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nong Y, Huang YQ, Ju W, Kalia LV, Ahmadian G, Wang YT, Salter MW. Glycine binding primes NMDA receptor internalization. Nature. 2003;422:302–307. doi: 10.1038/nature01497. [DOI] [PubMed] [Google Scholar]
- Onoda T, Iinuma H, Sasaki Y, Hamada M, Isshiki K, Naganawa H, Takeuchi T, Tatsuta K, Umezawa K. Isolation of a novel tyrosine kinase inhibitor, lavendustin A, from Streptomyces griseolavendus. J Nat Prod. 1989;52:1252–1257. doi: 10.1021/np50066a009. [DOI] [PubMed] [Google Scholar]
- Paoletti P, Ascher P, Neyton J. High-affinity zinc inhibition of NMDA NR1-NR2A receptors. J Neurosci. 1997;17:5711–5725. doi: 10.1523/JNEUROSCI.17-15-05711.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Otaño I, Schulteis CT, Contractor A, Lipton SA, Trimmer JS, Sucher NJ, Heinemann SF. Assembly with the NR1 subunit is required for surface expression of NR3A-containing NMDA receptors. J Neurosci. 2001;21:1228–1237. doi: 10.1523/JNEUROSCI.21-04-01228.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portera-Cailliau C, Price DL, Martin LJ. N-methyl-D-aspartate receptor proteins NR2A and NR2B are differentially distributed in the developing rat central nervous system as revealed by subunit-specific antibodies. J Neurochem. 1996;66:692–700. doi: 10.1046/j.1471-4159.1996.66020692.x. [DOI] [PubMed] [Google Scholar]
- Roche KW, Standley S, McCallum J, Dune Ly C, Ehlers MD, Wenthold RJ. Molecular determinants of NMDA receptor internalization. Nat Neurosci. 2001;4:794–802. doi: 10.1038/90498. [DOI] [PubMed] [Google Scholar]
- Rycroft BK, Gibb AJ. Direct effects of calmodulin on NMDA receptor single channel gating in rat hippocampal granule cells. J Neurosci. 2002;20:8860–8868. doi: 10.1523/JNEUROSCI.22-20-08860.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rycroft BK, Gibb AJ. Inhibitory interactions of calcineurin (phosphatase 2B) and calmodulin on rat hippocampal NMDA receptors. Neuropharmacology. 2004;47:505–514. doi: 10.1016/j.neuropharm.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Rymar VV, Sasseville R, Luk KC, Sadikot AF. Neurogenesis and stereological morphometry of calretinin-immunoreactive GABAergic interneurons of the neostriatum. J Comp Neurol. 2004;469:325–339. doi: 10.1002/cne.11008. [DOI] [PubMed] [Google Scholar]
- Salter MW. Src, N-methyl-D-aspartate (NMDA) receptors, and synaptic plasticity. Biochem Pharmacol. 1998;56:789–798. doi: 10.1016/s0006-2952(98)00124-5. [DOI] [PubMed] [Google Scholar]
- Schmid SL. Clathrin-coated vesicle formation and protein sorting: an integrated process. Annu Rev Biochem. 1997;66:511–548. doi: 10.1146/annurev.biochem.66.1.511. [DOI] [PubMed] [Google Scholar]
- Scott L, Zelenin S, Malmersjö S, Kowalewski JM, Markus EZ, Nairn AC, Greengard P, Brismar H, Aperia A. Allosteric changes of the NMDA receptor trap diffusible dopamine 1 receptors in spines. Proc Natl Acad Sci U S A. 2006;103:762–767. doi: 10.1073/pnas.0505557103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans JK, Durstewitz D, Christie BR, Stevens CF, Sejnowski TJ. Dopamine D1/D5 receptor modulation of excitatory synaptic inputs to layer V prefrontal cortex neurons. Proc Natl Acad Sci U S A. 2001;98:301–306. doi: 10.1073/pnas.011518798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Flajolet M, Greengard P, Surmeier DJ. Dichotomous dopaminergic control of striatal synaptic plasticity. Science. 2008;321:848–851. doi: 10.1126/science.1160575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Cummings J, Roldan LA, Jan YN, Jan LY. Changing subunit composition of heteromeric NMDA receptors during development of rat cortex. Nature. 1994;368:144–147. doi: 10.1038/368144a0. [DOI] [PubMed] [Google Scholar]
- Standaert DG, Testa CM, Young AB, Penney JB., Jr Organization of N-methyl-D-aspartate glutamate receptor gene expression in the basal ganglia of the rat. J Comp Neurol. 1994;343:1–16. doi: 10.1002/cne.903430102. [DOI] [PubMed] [Google Scholar]
- Stern P, Béhé P, Schoepfer R, Colquhoun D. Single-channel conductances of NMDA receptors expressed from cloned cDNAs: comparison with native receptors. Proc Biol Sci. 1992;250:271–277. doi: 10.1098/rspb.1992.0159. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Ding J, Day M, Wang Z, Shen W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci. 2007;30:228–235. doi: 10.1016/j.tins.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Tovar KR, Westbrook GL. The incorporation of NMDA receptors with a distinct subunit composition at nascent hippocampal synapses in vitro. J Neurosci. 1999;19:4180–4188. doi: 10.1523/JNEUROSCI.19-10-04180.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washbourne P, Liu X-B, Jones EG, McAllister AK. Cycling of NMDA receptors during trafficking in neurons before synapse formation. J Neurosci. 2004;24:8253–8264. doi: 10.1523/JNEUROSCI.2555-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Inoue Y, Sakimura K, Mishina M. Distinct distributions of five N-methyl-D-aspartate receptor channel subunit mRNAs in the forebrain. J Comp Neurol. 1993;338:377–390. doi: 10.1002/cne.903380305. [DOI] [PubMed] [Google Scholar]
- Wenthold RJ, Prybylowski K, Standley S, Sans N, Petralia RS. Trafficking of NMDA receptors. Annu Rev Pharmacol Toxicol. 2003;43:335–358. doi: 10.1146/annurev.pharmtox.43.100901.135803. [DOI] [PubMed] [Google Scholar]
- Wenzel A, Fritschy JM, Mohler H, Benke D. NMDA receptor heterogeneity during postnatal development of the rat brain: differential expression of the NR2A, NR2B, and NR2C subunit proteins. J Neurochem. 1997;68:469–478. doi: 10.1046/j.1471-4159.1997.68020469.x. [DOI] [PubMed] [Google Scholar]
- Wong HK, Liu XB, Matos MF, Chan SF, Pérez-Otaño I, Boysen M, Cui J, Nakanishi N, Trimmer JS, Jones EG, Lipton SA, Sucher NJ. Temporal and regional expression of NMDA receptor subunit NR3A in the mammalian brain. J Comp Neurol. 2002;450:303–317. doi: 10.1002/cne.10314. [DOI] [PubMed] [Google Scholar]
- Wyllie DJ, Béhé P, Nassar M, Schoepfer R, Colquhoun D. Single-channel currents from recombinant NMDA NR1a/NR2D receptors expressed in Xenopus oocytes. Proc Biol Sci. 1996;263:1079–1086. doi: 10.1098/rspb.1996.0159. [DOI] [PubMed] [Google Scholar]
- Xiong ZG, Pelkey KA, Lu WY, Lu YM, Roder JC, MacDonald JF, Salter MW. Src potentiation of NMDA receptors in hippocampal and spinal neurons is not mediated by reducing zinc inhibition. J Neurosci. 1999;19:1–6. doi: 10.1523/JNEUROSCI.19-21-j0003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeron MM, Hansson O, Chen N, Wellington CL, Leavitt BR, Brundin P, Hayden MR, Raymond LA. Increased sensitivity to N-methyl-D-aspartate receptor-mediated excitotoxicity in a mouse model of Huntington's disease. Neuron. 2002;33:849–860. doi: 10.1016/s0896-6273(02)00615-3. [DOI] [PubMed] [Google Scholar]
- Zheng F, Gingrich MB, Traynelis SF, Conn PJ. Tyrosine kinase potentiates NMDA receptor currents by reducing tonic zinc inhibition. Nat Neurosci. 1998;1:185–191. doi: 10.1038/634. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.