Abstract
The aim of this study was to identify the physiological mechanisms of exertional respiratory discomfort (breathlessness) in pregnancy by comparing ventilatory (breathing pattern, airway function, operating lung volumes, oesophageal pressure (Poes)-derived indices of respiratory mechanics) and perceptual (breathlessness intensity) responses to incremental cycle exercise in 15 young, healthy women in the third trimester (TM3; between 34 and 38 weeks gestation) and again 4–5 months postpartum (PP). During pregnancy, resting inspiratory capacity (IC) increased (P < 0.01) and end-expiratory lung volume decreased (P < 0.001), with no associated change in total lung capacity (TLC) or static respiratory muscle strength. This permitted greater tidal volume (VT) expansion throughout exercise in TM3, while preserving the relationship between contractile respiratory muscle effort (tidal Poes swing expressed as a percentage of maximum inspiratory pressure (PImax)) and thoracic volume displacement (VT expressed as a percentage of vital capacity) and between breathlessness and ventilation (V̇E). At the highest equivalent work rate (HEWR = 128 ± 5 W) in TM3 compared with PP: V̇E, tidal Poes/PImax and breathlessness intensity ratings increased by 10.2 l min−1 (P < 0.001), 8.8%PImax (P < 0.05) and 0.9 Borg units (P < 0.05), respectively. Pulmonary resistance was not increased at rest or during exercise at the HEWR in TM3, despite marked increases in mean tidal inspiratory and expiratory flow rates, suggesting increased bronchodilatation. Dynamic mechanical constraints on VT expansion (P < 0.05) with associated increased breathlessness intensity ratings (P < 0.05) were observed near peak exercise in TM3 compared with PP. In conclusion: (1) pregnancy-induced increases in exertional breathlessness reflected the normal awareness of increased V̇E and contractile respiratory muscle effort; (2) mechanical adaptations of the respiratory system, including recruitment of resting IC and increased bronchodilatation, accommodated the increased VT while preserving effort–displacement and breathlessness–V̇E relationships; and (3) dynamic mechanical ventilatory constraints contributed to respiratory discomfort near the limits of tolerance in late gestation.
Perceived respiratory discomfort (breathlessness) is reported during activities of daily living by as many as 75% of healthy pregnant women (Milne et al. 1978; Moore et al. 1987). The aetiology of gestational breathlessness, however, remains poorly understood and it is often difficult to differentiate physiological from patho-physiological breathlessness in this population. Purported mechanisms of gestational breathlessness include: (1) the normal awareness of increased ventilation (V̇E) (Moore et al. 1987; Field et al. 1991; Garcia-Rio et al. 1996); (2) an exaggerated central perception of discomfort at any given V̇E (Gilbert et al. 1962; Gilbert & Auchincloss, 1966); (3) increased O2 cost of breathing, secondary to progressive thoraco-abdominal distortion (Bader et al. 1959); or (4) any combination of the above.
A mechanistic study by Field et al. (1991) found that tidal volume (VT), inspiratory oesophageal pressure (Poes) swings and breathlessness intensity ratings were consistently higher during exercise in the third trimester (TM3) compared with the postpartum (PP) state, suggesting that pregnancy-induced increases in breathlessness reflect the normal awareness of increased V̇E and contractile inspiratory muscle effort. Thus, increased central respiratory motor output command and the attendant increase in central corollary discharge to the somatosensory cortex (Chen et al. 1991, 1992; Manning & Schwartzstein, 1995; O'Donnell et al. 2007) remains a plausible mechanistic explanation of gestational breathlessness. In the study of Field et al. (1991), however, comparisons were made at a standardized submaximal cycle work rate of only 48 W, corresponding to breathlessness intensity ratings of ‘very slight’ to ‘slight’ (i.e. 1–2 Borg units) in TM3 and PP, respectively. The concern remains that the exercise-testing protocol was too conservative to unmask potential mechanical ventilatory constraints relevant to the origin of exertional respiratory discomfort in this population. The present study is the first to examine detailed sensory–mechanical relationships at the limits of tolerance in human pregnancy.
Very little is known about the role of altered dynamic ventilatory mechanics in the perception of exertional breathlessness in pregnancy (Bader et al. 1959; Field et al. 1991). Conventional wisdom suggests that pregnancy-induced changes in the shape and configuration of the abdomen, diaphragm and chest wall may alter the normal mechanical response of the respiratory system to exercise, thereby constraining VT expansion especially when ventilatory requirements are high. Hypothetically, it is possible that an impaired ability to reduce end-expiratory lung volume during exercise in pregnancy (secondary to the mechanical effects of the gravid uterus) would compromise power-sharing between the inspiratory and expiratory muscles, and force dynamic end-inspiratory lung volume closer to total lung capacity. These restrictive changes in the setting of an increased central ventilatory drive (Jensen et al. 2007a, 2008) would be expected to uncouple the otherwise harmonious relationship between contractile respiratory muscle effort and thoracic volume displacement with attendant increases in perceived breathlessness. Accordingly, based on our previous work on the impact of mechanical restriction on exercise performance, we can predict that in the presence of significant restrictive ventilatory constraints, the ratio of contractile effort (tidal Poes swing expressed as a percentage of maximal inspiratory pressure (PImax)) to volume displacement (VT expressed as a percentage of vital capacity) will increase (O'Donnell et al. 1997, 2000, 2006). This increased effort–displacement ratio, an index of neuromechanical (un)coupling of the respiratory system, would in turn be associated with greater perceived respiratory discomfort at any given V̇E particularly during strenuous exercise in late gestation when the mechanical encumbrance of the gravid uterus is greatest. Contrary to our expectations, however, we recently found that neither pregnancy nor advancing gestation altered breathlessness–V̇E relationships during incremental cycle exercise (Jensen et al. 2007b). These results strongly suggested the existence of specific respiratory mechanical adaptations that accommodate the increased central ventilatory drive (i.e. VT expansion) of pregnancy while preserving neuromechanical coupling during exercise. The present study is the first to consider the potential relevance of resting inspiratory capacity recruitment and bronchodilatation (Rubin et al. 1956; Gee et al. 1967; Garrard et al. 1978; Gilroy et al. 1988; Berry et al. 1989; Contreras et al. 1991; Garcia-Rio et al. 1996, 1997) to respiratory sensation during exercise in pregnancy.
The aim of the present study therefore was to identify the physiological mechanisms of exertional breathlessness in pregnancy by comparing ventilatory (breathing pattern, airway function, operating lung volumes, Poes-derived indices of respiratory mechanics) and perceptual (breathlessness intensity) responses to incremental cycle exercise in 15 young, healthy women in TM3 (between 34 and 38 weeks gestation) and again 4–5 months PP. We hypothesized that: (1) breathlessness intensity would be consistently higher at any given work rate in TM3 compared with PP, reflecting the normal awareness of increased V̇E and contractile respiratory muscle effort; (2) effort–displacement and breathlessness–V̇E relationships would be preserved throughout exercise in TM3 compared with PP, indicating the existence of respiratory mechanical adaptations; and (3) mechanical ventilatory constraints would contribute importantly to perceived respiratory discomfort during exercise near the limits of tolerance in TM3.
Methods
Subjects
Subjects included 15 healthy women, 20–40 years, parity ≤2, who were experiencing uncomplicated singleton pregnancies; had no history of smoking or cardiovascular, respiratory, neuromuscular, musculoskeletal, metabolic or haematological (i.e. [haemoglobin]≥ 10 g dl−1) disease; and were not taking medications (other than prenatal vitamins) that could affect the ventilatory or perceptual response to exercise. Subjects were recruited via posted announcements, newspaper advertisements and contact with local obstetricians, midwives and health care providers. Prior to participation, subjects completed the Physical Activity Readiness Medical Examination for Pregnancy (available at http://www.csep.ca) and obtained medical clearance from their primary care giver. Approximately 1–2 weeks prior to TM3 tests, subjects underwent a fetal ultrasound and biophysical profile examination to ensure appropriate fetal growth, behaviour and amniotic fluid volume. As an incentive for study participation, subjects were given the opportunity to take part in a closely monitored prenatal muscle conditioning programme designed specifically to maintain (not improve) general muscular fitness, promote proper posture, and prevent urinary incontinence and diastasis recti, without causing improvement in the aerobic energy system. Fourteen women participated in 1.3 ± 0.1 classes week−1 (mean ±s.e.m.) for an average of 19.1 ± 1.1 weeks prior to TM3 testing; these classes were not continued in the postnatal period. The study protocol and consent form were approved by the Queen's University and Affiliated Teaching Hospitals Health Sciences Human Research Ethics Board in accordance with the Declaration of Helsinki; written informed consent was obtained from all participants.
Experimental design
This was a controlled, longitudinal study in which experimental tests were conducted at 36.6 ± 0.3 weeks gestation (i.e. when the mechanical encumbrance of the gravid uterus is greatest) and again 17.9 ± 0.9 weeks postpartum (i.e. long enough after delivery to ensure a complete return to the non-pregnant control state). The majority of available research suggests that menstrual cycle phase and oral contraceptive use has little or no effect on ventilatory control at rest and during exercise in healthy young women (Redman et al. 2003; Itoh et al. 2007; Nettlefold et al. 2007; Smekal et al. 2007). Therefore, no attempt was made to control for menstrual cycle status, lactation and/or oral contraceptive use in PP. Experimental visits included: evaluation of anthropometry (i.e. body height and mass) and persistent activity-related breathlessness using the modified baseline dyspnoea index (BDI) scale (Stoller et al. 1986); pulmonary function tests; and an incremental cycle exercise test. The BDI is a validated, reliable multidimensional discriminative instrument (Mahler et al. 1984): a focal score of ‘0’ indicates severe impairment and breathlessness at rest, while a score of ‘12’ indicates breathlessness only with intense physical activity. Subjects abstained completely from exercise, caffeine, heavy meals and alcohol for at least 12 h before TM3 and PP tests, which were conducted at the same time of day for each subject. Blood for the estimation of arterial PCO2 (PaCO2), [H+] and [HCO3−]; and serum progesterone ([P4]) and 17β-oestradiol ([E2]) concentrations was collected from each subject on a separate day within the same week, as previously described (Jensen et al. 2008).
Pulmonary function testing
Pulmonary function measurements, including routine spirometry, constant volume body plethysmography, single breath diffusing capacity for carbon monoxide (DLCO), and maximum inspiratory (MIP) and expiratory (MEP) mouth pressures measured at functional residual capacity (FRC) (i.e. MIP was not corrected for the change in FRC during pregnancy) and total lung capacity (TLC), respectively, were collected according to recommended techniques (ATS/ERS, 2002; MacIntyre et al. 2005; Miller et al. 2005a,b; Wanger et al. 2005) using automated equipment (Vmax 229d with Autobox 6200 DL; SensorMedics, Yorba Linda, CA, USA) and expressed in absolute terms. Static lung recoil pressure (Pst) as well as both static (Cst) and dynamic (Cdyn) lung compliance was measured in a subgroup of seven volunteers using automated equipment (Vmax 229d with Autobox 6200 DL) according to recommended techniques (Gibson & Pride, 1976).
Cardiopulmonary exercise testing
Evidence-based guidelines for exercise during pregnancy (Davies et al. 2003) recommend that women participate in weight-supported exercise (e.g. stationary cycling) and avoid activities that increase the risk of loss of balance and fetal trauma (e.g. treadmill running). Therefore, incremental exercise tests were conducted on an electronically braked cycle ergometer (Ergometrics 800S; SensorMedics) by use of a cardiopulmonary exercise testing system (Vmax229d; SensorMedics). Prior to TM3 tests, the bicycle seat height was adjusted so that the subject's legs were almost completely extended when the pedals were at the lowest point; the seat was adjusted to the same height at PP. Exercise tests consisted of a steady-state resting period of at least 6 min followed by 25 W increases in cycle work rate every 2 min to the point of symptom-limitation. Pedalling cadence was maintained between 60 and 70 r.p.m. Subjects were verbally encouraged to cycle to the point of symptom-limitation.
Measurements were collected at rest and during exercise while subjects breathed through a mouthpiece and a low-resistance flow transducer with nasal passages occluded by a noseclip. Measurements included: standard cardiorespiratory and breathing pattern parameters (V̇E, oxygen uptake V̇O2, carbon dioxide production V̇CO2, end-tidal PO2 and PCO2 (PET,CO2), tidal volume (VT), breathing frequency (fR), inspiratory (TI) and expiratory time (TE), inspiratory duty cycle (TI/TTOT), and mean tidal inspiratory (VT/TI) and expiratory (VT/TE) flow) were collected on a breath-by-breath basis and compared with predicted normal values based on age and height (Jones, 1997); oxygen saturation by finger pulse oximetry; heart rate (HR) by 12-lead ECG; blood pressure by auscultation of the brachial artery using a sphygmomanometer with an arm cuff; intensity of perceived breathing and leg discomfort; dynamic operating lung volumes; and oesophageal pressure-derived indices of respiratory mechanics.
Symptom evaluation
Respiratory discomfort (breathlessness) was defined as the ‘sensation of laboured or difficult breathing’ and leg discomfort as ‘the level of leg discomfort experienced during pedalling.’ Before exercise testing, subjects were familiarized with Borg's 0–10 category ratio scale (Borg, 1982) and its endpoints were anchored such that ‘0’ represented ‘no respiratory (leg) discomfort’ and ‘10’ was ‘the most severe respiratory (leg) discomfort you have ever experienced or could ever imagine experiencing.’ By pointing to the Borg scale, subjects rated their intensity of perceived breathlessness and leg discomfort at rest, within the last 30 s of each 2 min interval during exercise, and at the symptom-limited peak of exercise. Symptom ratings preceded IC manoeuvres by at least 5 breaths to avoid interference with pre-IC breathing patterns, and to avoid the possible influence that the performance of an IC manoeuvre might have on perceived breathlessness. Upon exercise cessation, subjects were asked to verbalize their main reason for stopping exercise (i.e. respiratory discomfort, leg discomfort, combination of respiratory and leg discomfort or other) and this reason was documented. Finally, qualitative descriptors of respiratory discomfort at the symptom-limited peak of cycle exercise were collected by questionnaire (O'Donnell et al. 2000).
Operating lung volumes
Changes in end-expiratory lung volume (EELV) were estimated from IC measurements collected at rest, at the end of each 2 min interval during exercise and at end-exercise. Assuming that TLC does not change during exercise (Stubbing et al. 1980), changes in IC and inspiratory reserve volume (IRV) reflect changes in dynamic EELV (EELV = TLC – IC) and end-inspiratory lung volume (EILV = TLC – IRV), respectively. This has been found to be a reliable method of tracking acute changes in operating lung volumes (Yan et al. 1997; O'Donnell et al. 1998). Confirmation of satisfactory technique and reproducibility of IC manoeuvres for each subject was established during an initial practice session at rest by evaluating the consistency of volume and peak inspiratory Poes measurements, as described in detail previously (O'Donnell et al. 1997, 2001). Subjects were given a few breaths' warning before an IC manoeuvre, a prompt for the manoeuvre (i.e. ‘at the end of the next normal breath out, take a big, deep breath all the way IN’) and then strong verbal encouragement to make a maximal inspiratory effort (i.e. ‘IN…, IN…, IN…’). To verify that TLC was attained with each IC manoeuvre during exercise we confirmed that peak inspiratory Poes values matched those obtained at rest. When subjects indicated the desire to stop exercise, an end-exercise IC manoeuvre was performed within 15 s; or if an acceptable IC had been performed within the preceding 30 s and the breathing pattern had not restabilized, then the value from that IC was used as the end-exercise value. The resting IC was recorded as the mean of the two best reproducible efforts.
Oesophageal pressure-derived respiratory mechanics: measurement and analysis
Oesophageal pressure-derived measures of respiratory mechanics were collected on a breath-by-breath basis using an integrated data-acquisition setup. Briefly, an adult balloon-tipped oesophageal catheter (Ackrad Laboratories, Cranford, NJ, USA) containing 0.5 ml of air was positioned according to an accepted technique (Baydur et al. 1982). We have previously determined (authors' unpublished observations) that 0.5 ml of air represents the minimal balloon volume for zero pressure using our testing setup and this particular oesophageal catheter. The placement and position (i.e. right/left nostril, distance from the nare) of the catheter was identical at TM3 and PP for each subject. Oesophageal pressure was sampled continuously at a rate of 100 Hz using a differential pressure transducer (MP45; Validyne Engineering, Northridge, CA, USA), a signal conditioner (Carrier amplifier; Gould Electronics, Chandler, AZ, USA) and computer data-acquisition software (Advanced CODAS; Dataq Instruments, Akron, OH, USA). The continuous flow signal from the Vmax229d system was simultaneously input into this system for further analysis. Maximum inspiratory sniff manoeuvres against an occluded airway at EELV were performed at rest and within 30 s of exercise cessation to obtain maximal values for inspiratory Poes (PImax). In our experience, performance of PImax manoeuvres during exercise is not practical because it disrupts the pattern of breathing, thereby increasing breathlessness intensity ratings. Therefore, values for PImax at each submaximal work rate during exercise were calculated at iso-time (i.e. at the concurrent exercise time corresponding to minutes 2, 4, 6, etc.) by linear interpolation between simultaneous PImax and exercise time measurements taken at rest and at end-exercise. All tidal Poes swing measurements were subsequently compared with iso-time values of PImax.
Campbell diagrams were constructed to calculate: expiratory (WEres) and inspiratory (WIres) flow resistive work of breathing; inspiratory elastic work of breathing (WIel); and total inspiratory (WOB =WIel+WIres) and resistive (WRES=WEres+WIres) work of breathing (Otis, 1964). We assumed that pregnancy had no effect on static (or dynamic) chest wall compliance (Cw), which we estimated as 4% predicted vital capacity (VC; cmH2O−1) (Agostoni & Mead, 1964). The Cw curve was anchored using the predicted normal FRC (Crapo et al. 1982), i.e. resting relaxation volume of the respiratory system for each subject in PP and TM3. We must point out, however, that if our assumption is wrong and Cw decreased from PP to TM3 (Marx et al. 1970), then TM3 values for WIel and thus WOB at any given work rate and V̇E would tend to be overestimated. Work of breathing measurements were multiplied by fR and expressed as joules min−1. The tension–time index of the inspiratory muscles was calculated as the product of mean inspiratory Poes/PImax and TI/TTOT (Baydur et al. 1982). Pulmonary resistance was calculated as the average of 5 breaths at rest, at the highest-equivalent work rate and at end-exercise for each subject in PP and TM3 using the iso-volume method (Tobin, 1998). The ratio of contractile respiratory muscle effort (tidal Poes/PImax) to thoracic volume displacement (VT/VC) was calculated for each subject and used as an index of neuromechanical (un)coupling of the respiratory system (O'Donnell et al. 1997, 2000, 2006).
Analysis of exercise endpoints
All breath-by-breath measurements were averaged in 30 s intervals at rest and during exercise. Volume (integrated flow) and Poes signals were also averaged in 30 s intervals for reconstruction of Poes–volume loops. Cardiorespiratory and Poes-derived measurements collected over the first 30 s period of every second minute during exercise were linked with symptom ratings and IC measurements collected in the latter 30 s of the respective minute so as to avoid contamination of averaged breath-by-breath data by irregular breaths surrounding IC manoeuvres.
Five main time points were used for the evaluation of exercise parameters: (1) pre-exercise rest; (2) the ventilatory threshold (Tvent); (3) the highest equivalent work rate (HEWR); (4) the VT/V̇E inflection; and (5) peak exercise. Pre-exercise rest was defined as the steady-state period after at least 3 min of breathing on the mouthpiece while seated on the cycle ergometer at rest before exercise was initiated: cardiorespiratory and Poes-derived measurements were averaged over the last 30 s of this period; and IC measurements for this period were collected during breathing on the same circuit immediately after completion of the quiet breathing period. Tvent was detected and verified individually using the V-slope and dual criterion methods (Beaver et al. 1986; Wasserman et al. 2005). HEWR was defined as the highest equivalent cycle work rate achieved during each of the symptom-limited incremental cycle exercise tests performed by a given subject (i.e. iso-work for each subject). The relationship between VT and V̇E (Hey et al. 1966) was examined and a point of inflection (i.e. VT/V̇E inflection) was determined for each subject at TM3 and PP. Finally, peak exercise was defined as the last 30 s of loaded pedalling: cardiorespiratory and Poes-derived measurements were averaged over this time period; and IC measurements and Borg ratings of breathlessness and leg discomfort were collected immediately at the end of this period. Peak work rate was defined as the highest cycle work rate that the subject was able to maintain for at least 30 s; endurance time was defined as the duration of loaded pedalling. Ventilation was compared with the maximal ventilatory capacity (MVC), which was estimated by multiplying the measured FEV1 by 35 (Gandevia & Hugh-Jones, 1957). Mean arterial blood pressure was estimated as the diastolic pressure plus one-third of the pulse pressure.
Statistical analysis
A one-way repeated measures analysis of variance (ANOVA) was used to examine the effects of human pregnancy on measured variables at each measurement time (SigmaStat for Windows Version 3.10, Systat Software, Inc., San Jose, CA, USA). Pregnancy- and exercise-induced changes in PImax and in peak inspiratory Poes recorded during sequential IC manoeuvres were compared using a two-way repeated measures ANOVA with Tukey's (HSD) post hoc test, respectively. Reasons for stopping exercise and qualitative descriptors of breathlessness at end-exercise were analysed using Fisher's exact test.
The effects of pregnancy on cardiorespiratory, perceptual and respiratory mechanical responses during exercise near the limits of tolerance were examined by comparing (1) measurements at the VT/V̇E inflection and at peak exercise both within and between conditions using a two-way repeated measures ANOVA with Tukey's (HSD) post hoc test and (2) the change in parameter values from the VT/V̇E inflection to end-exercise between PP and TM3 using a one-way repeated measures ANOVA. A P < 0.05 level of significance was used for all analyses. Results are reported as means ±s.e.m.
Results
Fifteen young, healthy, non-smoking, regularly active women with mild-to-moderate pregnancy-induced persistent activity-related breathlessness completed the study (Table 1). Nine women were nulliparous and six were primiparous. As expected, body mass and body mass index increased; [P4] and [E2] increased; and resting PaCO2, [H+] and [HCO3−] decreased from PP to TM3. Serum [P4] and [E2] values at PP were not significantly different (P > 0.05) than those previously reported from our laboratory in a group of 14 healthy eumenorrhoeic women in the follicular phase of their menstrual cycle (Slatkovska et al. 2006), suggesting that women had returned to their non-pregnant control state.
Table 1.
Subject characteristics and resting pulmonary function
| PP | TM3 | Δ(TM3– PP) | P value | |
|---|---|---|---|---|
| Age (years) | 30.6 ± 1.0 | — | — | — |
| Height (cm) | 166.4 ± 1.9 | — | — | — |
| Body mass (kg) | 69.6 ± 3.1 | 80.9 ± 3.0 | 11.3 ± 0.8 | <0.001 |
| Body mass index (kg m−2) | 25.0 ± 0.8 | 29.1 ± 0.7 | 4.1 ± 0.3 | <0.001 |
| Baseline dyspnoea index (focal score) | 11.9 ± 0.1 | 8.6 ± 0.4 | −3.3 ± 0.4 | <0.001 |
| Blood biochemistry | ||||
| PaCO2 (mmHg) | 39.5 ± 0.6 | 31.6 ± 0.4 | −7.9 ± 0.7 | <0.001 |
| [H+] (nequiv l−1) | 38.7 ± 0.4 | 35.8 ± 0.2 | −2.9 ± 0.4 | <0.001 |
| [HCO3−] (mequiv l−1) | 24.6 ± 0.3 | 21.3 ± 0.3 | −3.3 ± 0.4 | <0.001 |
| Progesterone (nmol l−1) | 1.1 ± 0.2 | 877 ± 73 | 876 ± 73 | <0.001 |
| 17β-Oestradiol (pmol l−1) | 106 ± 17 | 123 397 ± 47 068 | 123 291 ± 47 071 | 0.020 |
| Pulmonary function | ||||
| FEV1 (l) | 3.14 ± 0.09 | 3.28 ± 0.09 | 0.14 ± 0.04 | 0.002 |
| FEV1/FVC (%) | 79.7 ± 2.1 | 80.2 ± 1.5 | 0.6 ± 0.9 | 0.527 |
| FVC (l) | 3.98 ± 0.15 | 4.11 ± 0.15 | 0.13 ± 0.03 | 0.001 |
| PEF (l s−1) | 6.82 ± 0.23 | 6.80 ± 0.28 | −0.02 ± 0.14 | 0.898 |
| FEF25%-75% (l s−1) | 3.07 ± 0.20 | 3.26 ± 0.16 | 0.19 ± 0.09 | 0.061 |
| IC (l) | 2.92 ± 0.11 | 2.65 ± 0.11 | 0.28 ± 0.08 | 0.003 |
| FRC (l) | 2.36 ± 0.14 | 2.71 ± 0.14 | −0.36 ± 0.06 | <0.001 |
| sRaw (cmH2O s−1) | 6.59 ± 0.60 | 6.08 ± 0.54 | −0.52 ± 0.27 | 0.072 |
| DLCO (ml min−1 mmHg−1) | 21.3 ± 0.9 | 20.6 ± 0.8 | −0.7 ± 0.4 | 0.136 |
| MIP (cmH2O) | 76 ± 4 | 76 ± 5 | 0.1 ± 2.1 | 0.975 |
| MEP (cmH2O) | 113 ± 6 | 104 ± 6 | −9 ± 5 | 0.102 |
| Cst (cmH2O l−1) | 0.25 ± 0.03 | 0.30 ± 0.06 | 0.04 ± 0.07 | 0.561 |
| Cdyn (cmH2O l−1) | 0.17 ± 0.02 | 0.21 ± 0.04 | 0.04 ± 0.04 | 0.360 |
| Pst (cmH2O) | 30.6 ± 4.5 | 31.0 ± 2.3 | 0.4 ± 2.4 | 0.864 |
Values are means ±s.e.m. PP, postpartum; TM3, third trimester; Δ, pregnancy-induced change; PaCO2, arterialized venous partial pressure of CO2; [H+], arterialized venous hydrogen ion concentration; [HCO3−], plasma bicarbonate concentration; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; PEF, peak expiratory flow; FEF25%-75%, forced expiratory flow from 25% to 75% of the FVC manoeuvre; IC, inspiratory capacity; FRC, functional residual capacity; sRaw, specific airway resistance; DLCO, single breath diffusing capacity for carbon monoxide; MIP and MEP, maximal inspiratory and expiratory pressure, respectively; Cst and Cdyn, static and dynamic lung compliance, respectively (n= 7); Pst, static lung recoil pressure (n= 7).
Resting pulmonary function
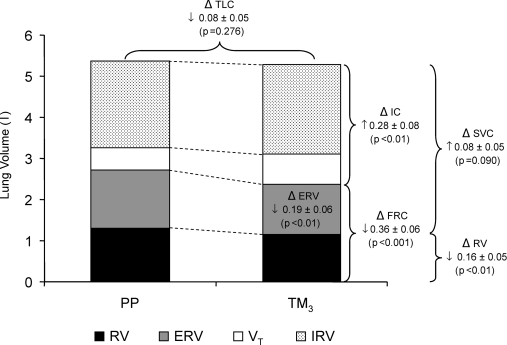
Pregnancy-induced changes in resting pulmonary function are shown in Table 1 and Fig. 1. In TM3 compared with PP: FEV1 and FVC increased, with no associated change in the FEV1/FVC ratio; specific airway resistance (sRaw) decreased by ∼9% (P= 0.072); IC increased; FRC, residual volume (RV) and expiratory reserve volume (ERV) decreased; and peak expiratory flow, TLC, slow vital capacity, DLCO, MIP, MEP, Cst and Cdyn did not change. The fact that TLC did not change with pregnancy is further supported by the observation that Pst was similar in TM3 and PP (Table 1).
Figure 1. Effects of pregnancy on static lung volumes and capacities.
In TM3 compared with PP: FRC, ERV and RV decreased, while IC increased with no associated change in TLC. PP, postpartum; TM3, third trimester; TLC, total lung capacity; ERV, expiratory reserve volume; IC, inspiratory capacity; FRC, functional residual capacity; SVC, slow vital capacity; RV, residual volume; VT, tidal volume, IRV, inspiratory reserve volume; Δ, pregnancy-induced change (i.e. mean difference ±s.e.m. between values at TM3 and PP); ↑, increase; ↓, decrease.
Physiological responses to symptom-limited cycle exercise
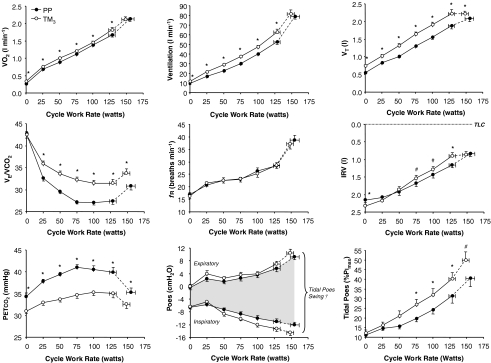
Ventilatory and metabolic responses to exercise are shown in Fig. 2. At rest and throughout exercise in TM3 compared with PP: V̇E increased significantly as a result of an increase in VT with no change in fR; and PETCO2 decreased significantly (Fig. 2). As expected, HR increased at rest (by 12 ± 3 beats min−1, P < 0001) and reached a peak value that was 6 ± 1 beats min−1 lower (P < 0.001) in TM3 compared with PP (Table 2). Measurements at peak exercise and at the HEWR are provided in Table 2. Peak work rate, V̇O2 and V̇E were similar, although the duration of loaded pedalling decreased by 0.6 ± 0.3 min (P= 0.046) in TM3 compared with PP. At the HEWR during exercise in TM3 compared with PP (Table 2): V̇O2 increased; V̇E and VT increased by 10.2 l min−1 (20%) and 0.35 l (19%), respectively, with no associated change in fR or breath timing (TI, TE, TI/TTOT). The V̇O2 at Tvent was not different despite a greater V̇E in TM3 compared with PP: V̇O2 averaged 1.10 ± 0.03 and 1.05 ± 0.04 l min−1 in TM3 and PP, respectively; and V̇E at Tvent averaged 32.4 ± 1.3 versus 27.1 ± 1.1 l min−1 in TM3versus PP, respectively (P= 0.005).
Figure 2. Effects of pregnancy on oxygen uptake, ventilation, breathing pattern and respiratory mechanical/muscular responses to incremental cycle exercise.
Data points are mean ±s.e.m. values at rest, at standardized cycle work rates during exercise and at peak exercise. PP, postpartum; TM3, third trimester; V̇O2, metabolic rate of O2 uptake; V̇E/V̇O2, ventilatory equivalent for CO2; PETCO2, end-tidal partial pressure of CO2; VT, tidal volume; IRV, inspiratory reserve volume; fR, breathing frequency; Poes, oesophageal pressure; tidal Poes (%PImax), tidal oesophageal pressure expressed as a percentage of maximum inspiratory pressure (an index of contractile respiratory muscle effort). *P < 0.05; #P≤ 0.07 TM3versus PP; † tidal Poes swings increased from PP to TM3 (P < 0.05) during exercise at cycle work rates ≥ 75 W.
Table 2.
Effects of pregnancy on perceptual, cardio-metabolic, ventilatory and respiratory mechanical/muscular responses at the symptom-limited peak (i.e. last 30 s of loaded pedalling) and at the highest equivalent work rate (HEWR) of cycle exercise
| Peak exercise | HEWR | |||
|---|---|---|---|---|
| PP | TM3 | PP | TM3 | |
| Breathlessness (Borg scale) | 5.4 ± 0.6 | 6.5 ± 0.5* | 3.2 ± 0.4 | 4.1 ± 0.3* |
| Leg discomfort (Borg scale) | 6.6 ± 0.7 | 6.5 ± 0.6 | 4.1 ± 0.5 | 4.5 ± 0.5 |
| Work rate (W) | 155 ± 7 | 148 ± 5 | 128 ± 5 | 128 ± 5 |
| V̇O2 (l min−1) | 2.13 ± 0.08 | 2.12 ± 0.08 | 1.67 ± 0.06 | 1.82 ± 0.07† |
| V̇O2 (% predicted max) | 111 ± 4 | 110 ± 3 | 87 ± 2 | 94 ± 3† |
| V̇CO2 (l min−1) | 2.58 ± 0.08 | 2.40 ± 0.08* | 1.91 ± 0.07 | 1.99 ± 0.08 |
| HR (beats min−1) | 174 ± 3 | 168 ± 3‡ | 158 ± 4 | 157 ± 2 |
| O2 pulse (ml beat−1) | 12.3 ± 0.5 | 12.7 ± 0.5 | 10.7 ± 0.5 | 11.6 ± 0.5* |
| MAP (mmHg) | 104 ± 1 | 107 ± 1 | 102 ± 2 | 105 ± 5 |
| V̇E (l min−1) | 79.1 ± 2.6 | 81.5 ± 4.1 | 52.5 ± 2.6 | 62.7 ± 3.2‡ |
| V̇E/MVC (%) | 72.8 ± 3.2 | 71.5 ± 3.5 | 48.3 ± 2.7 | 54.7 ± 2.4† |
| VT (l) | 2.08 ± 0.08 | 2.22 ± 0.07† | 1.88 ± 0.09 | 2.22 ± 0.12‡ |
| VT/IC (%) | 71.8 ± 2.2 | 72.4 ± 2.3* | 61.8 ± 2.0 | 71.2 ± 2.8‡ |
| IC (l) | 2.92 ± 0.11 | 3.08 ± 0.08 | 3.04 ± 0.12 | 3.11 ± 0.08 |
| IC change from rest (l) | 0.21 ± 0.09 | −0.00 ± 0.10# | 0.33 ± 0.10 | 0.03 ± 0.09† |
| IRV (l) | 0.84 ± 0.08 | 0.86 ± 0.08 | 1.16 ± 0.08 | 0.89 ± 0.09† |
| fR (breaths min−1) | 38.7 ± 1.9 | 37.2 ± 2.0 | 28.4 ± 1.3 | 28.6 ± 1.3 |
| TI/TTOT (%) | 50.4 ± 0.9 | 48.7 ± 0.8 | 46.5 ± 0.7 | 47.3 ± 0.8 |
| VT/TI (l s−1) | 2.57 ± 0.11 | 2.77 ± 0.13 | 1.87 ± 0.07 | 2.20 ± 0.10† |
| VT/TE (l s−1) | 2.61 ± 0.09 | 2.65 ± 0.16 | 1.65 ± 0.10 | 1.98 ± 0.11‡ |
| SpO2 (%) | 94.5 ± 1.0 | 95.1 ± 0.6 | 95.5 ± 0.4 | 95.0 ± 0.6 |
| PETCO2 (mmHg) | 35.3 ± 0.9 | 32.6 ± 1.1* | 39.9 ± 1.1 | 35.1 ± 1.0‡ |
| V̇E/V̇O2 | 37.5 ± 1.4 | 38.6 ± 1.4 | 31.5 ± 1.2 | 34.5 ± 1.1† |
| V̇E/V̇CO2 | 30.8 ± 0.9 | 33.8 ± 1.0† | 27.4 ± 0.8 | 31.5 ± 0.8‡ |
| Peak inspiratory Poes (cmH2O) | −12.1 ± 0.9 | −14.4 ± 0.9 | −11.0 ± 0.9 | −13.3 ± 0.8# |
| Peak expiratory Poes (cmH2O) | 9.3 ± 0.9 | 10.5 ± 1.2 | 5.6 ± 1.0 | 7.0 ± 0.9 |
| Tidal Poes (cmH2O) | 21.4 ± 1.3 | 24.8 ± 1.7* | 16.9 ± 1.2 | 20.3 ± 1.2† |
| Tidal Poes/PImax (%) | 40.6 ± 4.3 | 49.8 ± 4.4# | 31.5 ± 3.9 | 40.2 ± 3.1* |
| Tidal Poes/PImax: VT/VC | 0.81 ± 0.11 | 0.94 ± 0.09 | 0.69 ± 0.09 | 0.76 ± 0.06 |
| Tension time index | 0.08 ± 0.01 | 0.08 ± 0.01 | 0.06 ± 0.01 | 0.07 ± 0.01 |
| WIres (joules min−1) | 45.1 ± 3.3 | 54.1 ± 8.2 | 23.0 ± 3.5 | 30.4 ± 5.4* |
| WEres (joules min−1) | 76.2 ± 6.6 | 79.2 ± 11.2 | 30.4 ± 5.8 | 41.8 ± 6.5‡ |
| WRES (joules min−1) | 121.3 ± 9.2 | 133.3 ± 8.8 | 53.5 ± 9.1 | 72.1 ± 11.5† |
| WIel (joules min−1) | 110.3 ± 6.5 | 132.2 ± 13.5# | 77.5 ± 4.1 | 109.1 ± 11.4† |
| WOB (joules min−1) | 155.5 ± 8.2 | 186.3 ± 18.6# | 110.6 ± 6.3 | 139.5 ± 14.2† |
| RL (cmH2O l−1 s−1) | 2.55 ± 0.15 | 2.65 ± 0.17 | 2.41 ± 0.16 | 2.55 ± 0.29 |
P < 0.05
P < 0.01
P < 0.001
P≤ 0.07 TM3versus PP. Values are means ±s.e.m. PP, postpartum; TM3, third trimester; O2, oxygen; CO2, carbon dioxide; V̇O2, metabolic rate of O2 uptake; V̇CO2, metabolic rate of CO2 production; HR, heart rate; MAP, mean arterial pressure; V̇E, minute ventilation; MVC, maximal ventilatory capacity estimated as FEV1× 35; VT, tidal volume; IC, inspiratory capacity; IRV, inspiratory reserve volume; fR, breathing frequency; TI/TTOT, inspiratory duty cycle; VT/TI and VT/TE, mean tidal inspiratory and expiratory flow, respectively; SpO2, arterial O2 saturation; PETCO2, end-tidal CO2 tension; V̇E/V̇O2 and V̇E/V̇CO2, ventilatory equivalent for O2 and CO2, respectively; Poes, oesophageal pressure; PImax, maximum inspiratory (negative) oesophageal pressure; VC, vital capacity; WIres, inspiratory flow resistive work of breathing; WEres, expiratory flow resistive work of breathing; WRES, total resistive work of breathing; WIel, inspiratory elastic work of breathing; WOB, total inspiratory work of breathing; RL, pulmonary resistance.
Respiratory mechanical responses to symptom-limited cycle exercise
The assumption that TLC did not change during exercise was supported by the fact that peak inspiratory Poes values did not change during sequential IC manoeuvres from rest (TM3, −26 ± 2 cmH2O; PP, −25 ± 2 cmH2O) and throughout exercise (e.g. TM3 at peak, −24 ± 2 cmH2O; PP at peak, −21 ± 2 cmH2O) both within and between conditions. These observations validate the use of serial IC measurements to track changes in operating lung volumes during exercise in healthy pregnant and non-pregnant women. We also found that PImax was not significantly different from its resting value at end-exercise within PP (–59 ± 4 versus−58 ± 5 cmH2O, respectively; P= 0.827) and TM3 (–57 ± 4 versus−53 ± 4 cmH2O, respectively; P= 0.316); in this regard, pregnancy had no effect on pre-exercise, end-exercise or the exercise-induced change in PImax.
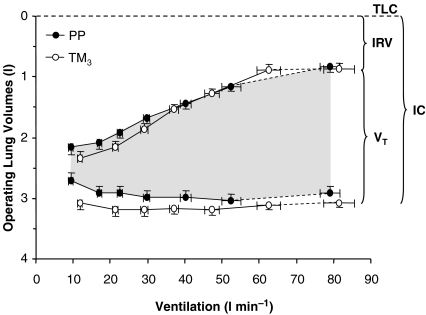
Dynamic operating lung volumes are shown in Fig. 2 (upper right panels) and Fig. 3. In contrast to PP where VT continued to increase throughout exercise, a plateau in the VT response was noted at the upper work rates in TM3 secondary to a corresponding plateau in dynamic IRV at its minimal level (Fig. 2). In TM3 compared with PP, IC increased by 0.37 l (P < 0.001) at rest (pre-exercise) and remained consistently larger during exercise up to 100 W, with no difference observed at the HEWR or at peak exercise (Table 2). Consequently, IC remained relatively constant throughout exercise in TM3 and did not progressively increase from its resting value during exercise as it did in PP. Pregnancy-induced increases in VT were accommodated from below (decreased EELV) at rest and throughout exercise (Fig. 3), and comprised a similar proportion (∼72%) of the available IC at end-exercise in TM3 compared with PP (Table 2).
Figure 3. Effects of pregnancy on the behaviour of dynamic operating lung volumes during incremental cycle exercise.
Data points are mean ±s.e.m. values at rest, at standardized cycle work rates during exercise and at peak exercise. PP, postpartum; TM3, third trimester; TLC, total lung capacity; IRV, inspiratory reserve volume; VT, tidal volume; IC, inspiratory capacity.
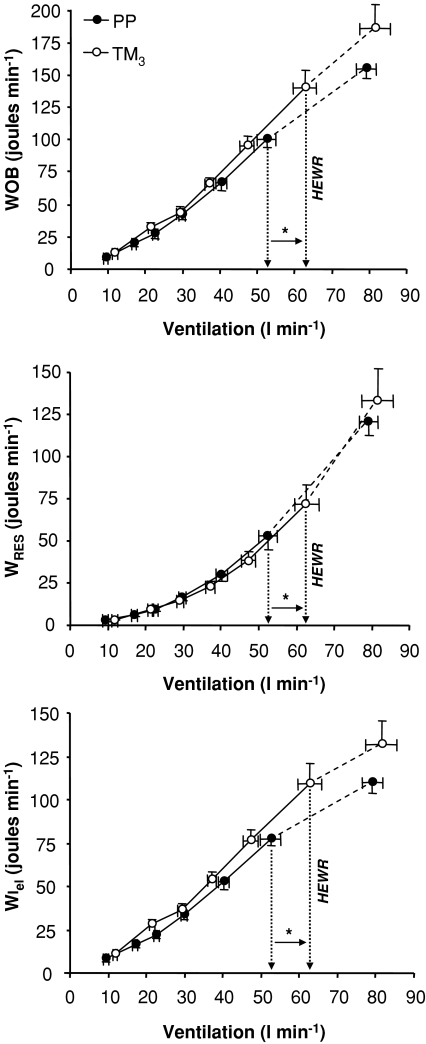
The effects of pregnancy on Poes-derived measures of respiratory mechanics during exercise are shown in Figs 2 and 4; measurements at the HEWR and at peak exercise are presented in Table 2. Tidal Poes, tidal Poes/PImax, WEres, WIres, WIel, WRES and WOB were consistently higher at any given submaximal cycle work rate in TM3 compared with PP, reflecting pregnancy-induced increases in exercise V̇E. The ratio of contractile respiratory muscle effort (tidal Poes/PImax) to thoracic volume displacement (VT/VC), an index of neuromechanical (un)coupling of the respiratory system, was not significantly different at any given work rate during incremental cycle exercise in TM3 compared with PP (Fig. 5). Pulmonary resistance was not significantly different at rest, at the HEWR or at end-exercise in TM3 compared with PP.
Figure 4. Effects of pregnancy on the relationship between ventilation and the total inspiratory work of breathing (WOB); total resistive work of breathing (WRES); and inspiratory elastic work of breathing (WIel) during incremental cycle exercise.
Data points are mean ±s.e.m. values at rest, at standardized cycle work rates during exercise and at peak exercise. Measurements of WOB, WRES and WIel were significantly higher in TM3 compared with PP during exercise at the highest equivalent work rate (HEWR = 128 ± 5 W); however, these differences were no longer significant when expressed against ventilation. PP, postpartum; TM3, third trimester. *P < 0.05 TM3versus PP during exercise at the HEWR.
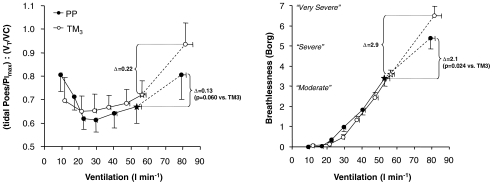
Figure 5. Effects of pregnancy on the relationship between contractile respiratory muscle effort (tidal Poes/PImax) and thoracic volume displacement (VT/VC), i.e. neuromechanical (un)coupling of the respiratory system; and Borg ratings of perceived breathlessness at the limits of exercise tolerance.
Data points are mean ±s.e.m. values at rest, at standardized cycle work rates and the V̇T/V̇E inflection point (⋆) during exercise and at peak exercise. Effort–displacement ratios were similar at any given work rate during incremental cycle exercise in TM3 compared with PP. The effort–displacement ratio and breathlessness intensity ratings were similar during exercise at the V̇T/V̇E inflection point in TM3 and PP. However, there was a progressively greater rise in the effort–displacement ratio and breathlessness intensity ratings after the V̇T/V̇E inflection point was exceeded in TM3 compared with PP, despite similar continued increases in ventilation. PP, postpartum; TM3, third trimester; tidal Poes/PImax, tidal oesophageal pressure expressed as a percentage of maximum inspiratory pressure; VT/VC, tidal volume expressed as a percentage of vital capacity.
A V̇T/V̇E inflection point was identified for all subjects in both TM3 and PP. This inflection occurred at a lower cycle work rate during exercise in TM3 compared with PP (120 ± 4 versus 133 ± 7 W; P= 0.013). Despite similar V̇E (56 ± 3 versus 53 ± 2 l min−1 at TM3versus PP, respectively) and Poes-derived mechanical measurements at this point, VT was larger (P < 0.05) by an average of 0.26 l or 14% and occupied a greater (P < 0.05) proportion of both the available IC and VC in TM3 than PP. Beyond the V̇T/V̇E inflection point in TM3, there was no further change (i.e. a plateau in the exercise response) in VT, IC and IRV, despite continued increases in work rate, V̇O2, V̇CO2, V̇E, tidal Poes, tidal Poes/PImax and work of breathing measurements. This is in contrast to PP, where VT continued to increase after this inflection point as IRV continued to decline until it reached a similarly reduced level at end-exercise. As a result, further increases in the tidal Poes/VT (by 2.64 and 1.41 cmH2O l−1, P= 0.041) and effort–displacement ratios (by 0.22 and 0.13 units, P= 0.060) were consistently greater during exercise beyond the V̇T/V̇E inflection point in TM3 compared with PP (Fig. 5). Interestingly, the V̇T/V̇E inflection point coincided with an inflection in the IRV/V̇E relationship, indicating that respiratory mechanical constraints probably contributed to the saturation of VT expansion near the limits of tolerance in TM3.
Perceptual responses to symptom-limited incremental cycle exercise
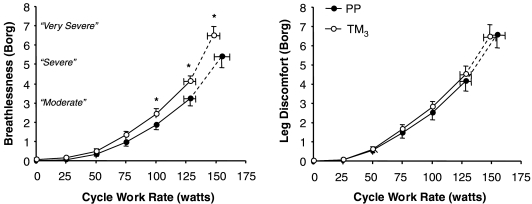
Pregnancy had no effect on the distribution of the reasons for stopping exercise. In TM3, two women stopped primarily due to breathing discomfort, eight due to leg discomfort and five due to a combination of both breathing and leg discomfort. In PP, one woman stopped due to breathing discomfort, 13 due to leg discomfort and one due to a combination of breathing and leg discomfort. The selection frequency of breathlessness descriptor phrases at end-exercise was similar in TM3 and PP. The majority (80%) of women in both TM3 and PP described their breathlessness at this point as an increased sense of respiratory ‘work/effort’. Borg ratings of perceived leg discomfort were not significantly different at any given work rate during exercise in TM3 compared with PP (Fig. 6). Breathlessness intensity ratings were significantly greater at 100 W (by 0.6 Borg units, P= 0.045), at the HEWR and at peak exercise in TM3 compared with PP (Fig. 6, Table 2). Breathlessness–V̇E relationships, however, were essentially superimposed throughout much of exercise in TM3 and PP (Fig. 5). Breathlessness intensity was similar at the V̇T/V̇E inflection point during exercise in TM3 and PP (Fig. 5). Similar to the response pattern seen with the tidal Poes/VT and effort–displacement ratios (discussed above), progressive increases in breathlessness intensity ratings (by 2.9 and 2.1 Borg units, P= 0.024) were significantly greater during exercise beyond the VT/V̇E inflection point in TM3 compared with PP (Fig. 5).
Figure 6. Effects of pregnancy on the intensity of perceived breathlessness and leg discomfort during incremental cycle exercise.
Data points are mean ±s.e.m. values at rest, at standardized cycle work rates during exercise and at peak exercise. Borg ratings of perceived breathlessness intensity were consistently higher at any given work rate during incremental cycle exercise in TM3 compared with PP. PP, postpartum; TM3, third trimester. *P < 0.05 TM3versus PP.
Discussion
The main findings of this study were: (1) pregnancy-induced increases in exertional breathlessness reflected the normal awareness of increased V̇E and contractile respiratory muscle effort; (2) mechanical adaptations of the respiratory system, including recruitment of resting IC and increased bronchodilatation, accommodated the increased VT expansion during exercise in pregnancy, while preserving effort–displacement and breathlessness–V̇E relationships; and (3) dynamic mechanical ventilatory constraints (as evidenced by a plateau in the VT and IRV response during exercise beyond the VT/V̇E inflection point) contributed to perceived respiratory discomfort near the limits of tolerance in late gestation.
Normal awareness of an increased contractile respiratory muscle effort
The women in our study reported mild-to-moderate persistent activity-related breathlessness, as evidenced by a ∼3.5 unit decrease in the BDI focal score from PP to TM3. Consistent with the results of previous studies (Sady et al. 1989; Lotgering et al. 1995), however, aerobic working capacity was well preserved in late gestation: women exercised to at least 100% of their predicted maximum work rate and V̇O2 in TM3 and PP, respectively.
In keeping with the results of Field et al. (1991), we found that V̇E, tidal Poes/PImax and breathlessness intensity ratings were significantly higher at any given work rate during incremental cycle exercise in TM3 compared with PP (e.g. by 10.2 l min−1 (20%), 8.8%PImax (28%) and 0.9 Borg units (28%) at the HEWR). We also found that breathlessness–V̇E relationships were similar throughout exercise in TM3 compared with PP; and that pregnancy-induced increases in breathlessness at the HEWR correlated significantly with concomitant changes in indices of maternal hyperventilation, namely V̇E/V̇CO2 (Pearson r= 0.576, P= 0.025) and PETCO2 (Pearson r=−0.744, P= 0.002). Collectively, these findings support the hypothesis that pregnancy-induced increases in exertional breathlessness reflect the normal conscious awareness of increased V̇E and contractile respiratory muscle effort. The neurophysiological basis of breathlessness in this circumstance is thought to be increased central corollary discharge to the somatosensory cortex, secondary to increased brainstem (autonomic) and cortical respiratory motor drive (Chen et al. 1991, 1992; Manning & Schwartzstein, 1995; O'Donnell et al. 2007). Direct sensory afferent information arising from mechano- and metaboreceptors in the vigorously contracting inspiratory pump muscles may also contribute to the sense of increased respiratory ‘work/effort’ when ventilatory requirements are high (Manning & Schwartzstein, 1995).
Role of respiratory mechanical factors in gestational breathlessness
This is the first study to examine the potential contribution of respiratory mechanical factors to perceived respiratory discomfort during exercise in human pregnancy. It is reasonable to assume that the progressive thoraco-abdominal distortion of pregnancy may, in the setting of an increased central ventilatory drive, uncouple the otherwise harmonious relationship between contractile respiratory muscle effort and thoracic volume displacement, i.e. neuromechanical uncoupling. Thus, it is possible that restrictive mechanical constraints on exercise hyperpnoea may develop during exercise in TM3 with attendant amplification of breathlessness intensity ratings at any given V̇E. In support of this idea, previous studies have found that imposing a mechanical constraint to VT expansion (by chest wall strapping), in the face of an increased central ventilatory drive (by dead space loading), significantly increased (versus control) the intensity of perceived breathlessness at any given V̇E during incremental cycle exercise in healthy subjects (Harty et al. 1999; O'Donnell et al. 2000). Surprisingly, we found that breathlessness–V̇E and effort–displacement relationships (Fig. 5) were essentially superimposed throughout much of exercise in TM3 compared with PP. This suggests that respiratory mechanical factors did not contribute importantly to gestational breathlessness and/or that physiological adaptations were in place in TM3 to minimize the sensory consequences of increased respiratory mechanical loading.
In keeping with the results of several previous studies (Gilroy et al. 1988; Berry et al. 1989; Contreras et al. 1991; Garcia-Rio et al. 1996, 1997), we found that pre-exercise resting IC increased (by 0.37 l or ∼14%) and EELV decreased (by 0.45 l or ∼17%) from PP to TM3 (Fig. 3). Thus, IC increased because EELV decreased with no associated change in TLC. Static (and dynamic) lung compliance and inspiratory muscle strength were not affected by pregnancy. Preserved inspiratory muscle strength probably reflects the combined physiological effects of an increased area of apposition (Gilroy et al. 1988) and increased diaphragmatic mid-position (Thomson & Cohen, 1938) with attendant improvement in the length–tension relationship of the diaphragm, secondary to reductions in resting EELV. The reduced resting EELV in TM3 may be explained by reductions in chest wall and therefore total respiratory system compliance (Marx et al. 1970).
This is the first study to document the behaviour of dynamic operating lung volumes during exercise in pregnancy. In contrast to PP, dynamic EELV did not decrease from its resting value during incremental cycle exercise in TM3 (Fig. 3), despite similar progressive increases in expiratory effort in both conditions (Fig. 2). We postulate that further decreasing EELV into an already reduced ERV during exercise in TM3 would be mechanically disadvantageous (with negative sensory consequences), particularly in the setting of an increased central ventilatory drive. In this regard, reductions in dynamic EELV would position VT closer to RV where tidal expiratory flow generation may become compromised. Considered together, the recruitment of resting IC (Fig. 3) and the preserved relationship between contractile respiratory muscle effort and thoracic volume displacement throughout much of exercise in TM3 (Fig. 5) strongly suggests that the greater demand for VT expansion was accommodated within the most compliant portion of the respiratory system's pressure–volume relation. Interestingly, because of adequate IC recruitment at rest, the reduced ability to dynamically decrease EELV during exercise in TM3 did not result in further encroachment on dynamic IRV (Fig. 3). It follows that if pregnant women failed to recruit resting IC, then VT would expand to reach a critical minimal IRV even earlier in exercise. The attendant earlier onset of increased elastic loading of the inspiratory muscles would be expected to have important negative sensory consequences.
Reductions in EELV, alone or in combination with a reduced PaCO2, would be expected to increase airway resistance at rest and during exercise in pregnancy (Briscoe & Dubois, 1958; Newhouse et al. 1964; Sterling, 1968). Consistent with the results of previous studies (Rubin et al. 1956; Gee et al. 1967; Garrard et al. 1978), however, we found that resting and dynamic airway function was modestly, but consistently improved in late gestation: FEV1 and FEF25%-75% increased, while RV and sRaw decreased from PP to TM3. Moreover, pulmonary resistance was not significantly different at rest or during exercise in pregnancy, despite substantial increases in V̇E, VT/TI and VT/TE (e.g. by ∼20%, 18% and 21% at the HEWR, respectively). Even relatively minor reductions in airway diameter would be expected to have substantial deleterious effects on breathing mechanics (and therefore perceived respiratory discomfort) in the setting of high ventilatory requirements during exercise in healthy individuals. The corollary of this is that the above-mentioned improvements in resting and dynamic airway function probably contributed to the preservation of effort–displacement and breathlessness–V̇E relationships throughout much of exercise in TM3 compared with PP (Fig. 5).
The physiological mechanisms of bronchodilatation in pregnancy remain highly conjectural and were not explored in this study. Based on the collective results of several previous studies, we postulate that pregnancy-induced reductions in pulmonary vagal efferent activity (Severinghaus & Stupfel, 1955; Butler et al. 1960; Newhouse et al. 1964; Sterling, 1968; Avery et al. 2001; Ito et al. 2006), alone or in combination with progesterone-mediated alterations in airway smooth muscle tone (Foster et al. 1983; Perusquia et al. 1997) and/or genioglossus muscle activity (Popovic & White, 1998) may be responsible.
Mechanisms of breathlessness near the limits of tolerance in late gestation
The present study is the first to examine the physiological events at high exercise intensities when breathlessness intensity ratings became ‘severe’ to ‘very severe’. Recent studies from our laboratory have suggested that attainment of the VT/V̇E inflection point (at a critically reduced dynamic IRV) during exercise represents a critical mechanical event with important sensory consequences (Ofir et al. 2007, 2008). In the present study, V̇E, tidal Poes/PImax, the effort–displacement ratio and breathlessness intensity ratings were similar during exercise at the VT/V̇E inflection point in TM3 and PP (Fig. 5). However, clear pregnancy-related differences emerged after this point was exceeded: VT expansion was more constrained, and effort–displacement ratios and breathlessness intensity ratings were consistently higher (Fig. 5), despite similar levels of V̇E, tidal Poes/PImax and V̇O2 in TM3 compared with PP. We have previously argued that exertional breathlessness intensity ratings increase abruptly when dynamic IRV reaches a minimal value of ∼0.5–1.0 l below TLC and a widening disparity emerges between increased central ventilatory drive and the simultaneous mechanical/muscular response of the respiratory system, i.e. neuromechanical uncoupling (O'Donnell et al. 1997, 2000, 2006, 2007; Ofir et al. 2007, 2008).
Critique of methods
Weight-supported cycle exercise tests were employed to minimize the risk of falls as well as the potentially confounding effects of maternal weight gain on Poes-derived measures of dynamic ventilatory mechanics during weight-bearing (e.g. treadmill) exercise. Pregnancy-induced increases in body mass (by an average of ∼11.5 kg in the present study) would be expected to increase the metabolic cost of locomotion during weight-bearing activities (e.g. stair climbing) with attendant increases in V̇E, tidal Poes/PImax and therefore perceived breathlessness. In this regard, our results probably underestimate the effects of healthy human pregnancy on respiratory discomfort during activities of daily living. Furthermore, we acknowledge that the aetiology of breathlessness is multifactorial and we cannot rule out the possibility that during the course of human pregnancy psychological or affective factors (e.g. temporal sensitization) modulated the quality and intensity of exertional respiratory discomfort. Nevertheless, if these factors were contributory then one would expect both breathlessness–V̇E and leg discomfort–work rate relationships to be altered in TM3 compared with PP; however, this was not the case.
Summary
In conclusion: (1) pregnancy-induced increases in exertional breathlessness reflected the normal awareness of increased (humorally mediated) ventilation and contractile respiratory muscle effort; (2) mechanical adaptations of the respiratory system, including recruitment of resting inspiratory capacity and increased bronchodilatation, permitted the respiratory system to accommodate the demand for greater tidal volume expansion during exercise in pregnancy, while preserving effort–displacement (i.e. neuromechanical coupling) and breathlessness–ventilation relationships; and (3) dynamic mechanical ventilatory constraints became evident only at the limits of tolerance in late gestation with attendant negative sensory consequences. These novel findings have potentially important implications for the clinical assessment of pregnant women who seek medical attention for troublesome activity-related breathlessness. In this regard, variation in the severity of gestational breathlessness may reflect variation in the amplitude of central ventilatory drive and/or impaired respiratory mechanical adaptations, including inadequate inspiratory capacity recruitment and/or bronchodilatation.
Acknowledgments
The authors would like to acknowledge Dr Larry A. Wolfe (25 May 1950–29 July 2005) for his contribution to the original study design and grant application. Financial support was provided by the Ontario Thoracic Society (Grant-in-Aid); Ontario Thoracic Society Block Term Grant; and the William M. Spear Endowment Fund for Respiratory Research at Queen's University. D. Jensen was supported by an Ontario Graduate Scholarship and the John Alexander Stewart Fellowship (Department of Medicine, Queen's University and Kingston General Hospital).
References
- Agostoni E, Mead J. Statics of the respiratory system. In: Fehn WO, Rahn H, editors. Handbook of Physiology, section 3, vol. 1, Respiration. Washington, DC, USA: American Physiological Society; 1964. pp. 387–409. [Google Scholar]
- American Thoracic Society/European Respiratory Society. ATS/ERS statement on respiratory muscle testing. Am J Respir Crit Care Med. 2002;166:518–624. doi: 10.1164/rccm.166.4.518. [DOI] [PubMed] [Google Scholar]
- Avery ND, Wolfe LA, Amara CE, Davies GAL, McGrath MJ. Effects of human pregnancy on cardiac autonomic function above and below the ventilatory threshold. J Appl Physiol. 2001;90:321–328. doi: 10.1152/jappl.2001.90.1.321. [DOI] [PubMed] [Google Scholar]
- Bader RA, Bader ME, Rose DJ. The oxygen cost of breathing in dyspnoeic subjects as studied in normal pregnant women. Clin Sci. 1959;18:223–235. [PubMed] [Google Scholar]
- Baydur A, Behrakis PK, Zin WA, Jaeger M, Milic-Emili J. A simple method for assessing the validity of the esophageal balloon technique. Am Rev Respir Dis. 1982;126:788–791. doi: 10.1164/arrd.1982.126.5.788. [DOI] [PubMed] [Google Scholar]
- Beaver WL, Wasserman K, Whipp BJ. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol. 1986;60:2020–2027. doi: 10.1152/jappl.1986.60.6.2020. [DOI] [PubMed] [Google Scholar]
- Berry MJ, McMurray RG, Katz VL. Pulmonary and ventilatory responses to pregnancy, immersion, and exercise. J Appl Physiol. 1989;66:857–862. doi: 10.1152/jappl.1989.66.2.857. [DOI] [PubMed] [Google Scholar]
- Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–381. [PubMed] [Google Scholar]
- Briscoe WA, Dubois AG. The relationship between airway resistance, airway conductance, and lung volume in subjects of different age and body size. J Clin Invest. 1958;37:1279–1285. doi: 10.1172/JCI103715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler J, Caro CG, Alcala R, DuBois AB. Physiological factors affecting airway resistance in normal subjects and in patients with obstructive respiratory disease. J Clin Invest. 1960;39:584–591. doi: 10.1172/JCI104071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Eldridge FL, Wagner PG. Respiratory-associated rhythmic firing of midbrain neurons in cats: relation to level of respiratory drive. J Physiol. 1991;437:305–325. doi: 10.1113/jphysiol.1991.sp018597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Eldridge FL, Wagner PG. Respiratory-associated thalamic activity is related to the level of respiratory drive. Respir Physiol. 1992;90:99–113. doi: 10.1016/0034-5687(92)90137-l. [DOI] [PubMed] [Google Scholar]
- Contreras G, Gutierrez M, Beroiza T, Fantin A, Oddo H, Villarroel L, Cruz E, Lisboa C. Ventilatory drive and respiratory muscle function in pregnancy. Am Rev Respir Dis. 1991;144:837–841. doi: 10.1164/ajrccm/144.4.837. [DOI] [PubMed] [Google Scholar]
- Crapo RO, Morris AH, Clayton PD, Nixon CR. Lung volumes in healthy non-smoking adults. Bull Eur Physiopath Respir. 1982;18:419–425. [PubMed] [Google Scholar]
- Davies GAL, Wolfe LA, Mottola MF, MacKinnon C. Joint SOGC/CSEP clinical practice guideline: exercise in pregnancy and the post-partum period. Can J Appl Physiol. 2003;28:330–341. [PubMed] [Google Scholar]
- Field SK, Bell SG, Cenaiko DF, Whitelaw WA. Relationship between inspiratory effort and breathlessness in pregnancy. J Appl Physiol. 1991;71:1897–1902. doi: 10.1152/jappl.1991.71.5.1897. [DOI] [PubMed] [Google Scholar]
- Foster PS, Goldie RG, Paterson JW. Effects of steroids on β-adrenoreceptor-mediated relaxation of pig bronchus. Br J Pharmacol. 1983;78:441–445. doi: 10.1111/j.1476-5381.1983.tb09409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandevia B, Hugh-Jones P. Terminology for measurements of ventilatory capacity: a report to the thoracic society. Thorax. 1957;12:290–293. doi: 10.1136/thx.12.4.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rio F, Pino JM, Gomez L, Alvarez-Sala R, Villasante C, Villamor J. Regulation of breathing and perception of dyspnea in healthy pregnant women. Chest. 1996;110:446–453. doi: 10.1378/chest.110.2.446. [DOI] [PubMed] [Google Scholar]
- Garcia-Rio F, Pino-Garcia JM, Serrano S, Racionero MA, Terreros-Caro JG, Alvarez-Sala R, Villasante C, Villamor J. Comparison of helium dilution and plethysmographic lung volumes in pregnant women. Eur Respir J. 1997;10:2371–2375. doi: 10.1183/09031936.97.10102371. [DOI] [PubMed] [Google Scholar]
- Garrard GS, Littler WA, Redman CWG. Closing volume during normal pregnancy. Thorax. 1978;33:488–492. doi: 10.1136/thx.33.4.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee JBL, Packer BS, Millen JE, Robin ED. Pulmonary mechanics during pregnancy. J Clin Invest. 1967;46:945–952. doi: 10.1172/JCI105600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson GJ, Pride NB. Lung distensibility: the static pressure-volume curve of the lung and its use in clinical assessment. Br J Dis Chest. 1976;70:143–184. doi: 10.1016/0007-0971(76)90027-9. [DOI] [PubMed] [Google Scholar]
- Gilbert R, Auchincloss JH. Dyspnea of pregnancy: clinical and physiological observations. Am J Med Sci. 1966;52:270–276. doi: 10.1097/00000441-196609000-00004. [DOI] [PubMed] [Google Scholar]
- Gilbert R, Epifano L, Auchincloss JH. Dyspnea of pregnancy: a syndrome of altered respiratory control. J Am Med Assoc. 1962;182:1073–1077. [PubMed] [Google Scholar]
- Gilroy RJ, Mangura BT, Lavietes MH. Rib cage and abdominal volume displacements during breathing in pregnancy. Am Rev Respir Dis. 1988;137:668–672. doi: 10.1164/ajrccm/137.3.668. [DOI] [PubMed] [Google Scholar]
- Harty HR, Corfield DR, Schwartzstein RM, Adams L. External thoracic restriction, respiratory sensation, and ventilation during exercise in men. J Appl Physiol. 1999;86:1142–1150. doi: 10.1152/jappl.1999.86.4.1142. [DOI] [PubMed] [Google Scholar]
- Hey EN, Lloyd BB, Cunningham DJC, Jukes MGM, Bolton DPG. Effects of various respiratory stimuli on the depth and frequency of breathing in man. Respir Physiol. 1966;1:193–205. doi: 10.1016/0034-5687(66)90016-8. [DOI] [PubMed] [Google Scholar]
- Ito S, Sasano H, Sasano N, Hayano J, Fisher JA, Katsuya H. Vagal nerve activity contributes to improve the efficiency of pulmonary gas exchange in hypoxic humans. Exp Physiol. 2006;91:935–941. doi: 10.1113/expphysiol.2006.034421. [DOI] [PubMed] [Google Scholar]
- Itoh M, Ueoka H, Aoki T, Hotta N, Kaneko Y, Takita C, Fukuoka Y. Exercise hyperpnea and hypercapnic ventilatory responses in women. Respir Med. 2007;101:446–452. doi: 10.1016/j.rmed.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Jensen D, Duffin J, Lam YM, Webb KA, Simpson JA, Davies GAL, Wolfe LA, O'Donnell DE. Physiological mechanisms of hyperventilation during human pregnancy. Respir Physiol Neurobiol. 2008;161:76–86. doi: 10.1016/j.resp.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Jensen D, Webb KA, O'Donnell DE. Chemical and mechanical adaptations of the respiratory system at rest and during exercise in human pregnancy. Appl Physiol Nutr Metab. 2007a;32:1239–1250. doi: 10.1139/H07-120. [DOI] [PubMed] [Google Scholar]
- Jensen D, Webb KA, Wolfe LA, O'Donnell DE. Effects of human pregnancy and advancing gestation on respiratory discomfort during exercise. Respir Physiol Neurobiol. 2007b;156:85–93. doi: 10.1016/j.resp.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Jones NL. Clinical Exercise Testing. 4th edn. Philadelphia, PA, USA: W.B. Saunders Co; 1997. Interpretation of stage 1 exercise test results; pp. 128–149. [Google Scholar]
- Lotgering FK, Struijk PC, Van Doorn MB, Spinnewijn WEM, Wallenburg HCS. Anaerobic threshold and respiratory compensation in pregnant women. J Appl Physiol. 1995;78:1772–1777. doi: 10.1152/jappl.1995.78.5.1772. [DOI] [PubMed] [Google Scholar]
- MacIntyre N, Crapo RO, Viegi G, Johnson DC, van der Grinten CPM, Brusasco V, Burgos F, Casaburi R, Coates A, Enright P, Gustafsson P, Hankinson J, Jensen R, McKay R, Miller MR, Navajas D, Pedersen OF, Pellegrino R, Wanger J. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005;26:720–735. doi: 10.1183/09031936.05.00034905. [DOI] [PubMed] [Google Scholar]
- Mahler DA, Weinberg DH, Wells CK, Feinstein AR. The measurement of dyspnea: contents, interobserver agreement, and physiologic correlates of two new clinical indexes. Chest. 1984;85:751–758. doi: 10.1378/chest.85.6.751. [DOI] [PubMed] [Google Scholar]
- Manning HL, Schwartzstein RM. Pathophysiology of dyspnea. N Eng J Med. 1995;333:1547–1553. doi: 10.1056/NEJM199512073332307. [DOI] [PubMed] [Google Scholar]
- Marx GF, Murthy PK, Orkin LR. Static compliance before and after vaginal delivery. Br J Anaesth. 1970;42:1100–1104. doi: 10.1093/bja/42.12.1100. [DOI] [PubMed] [Google Scholar]
- Miller MR, Crapo R, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Enright P, van der Grinten CPM, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J. General considerations for lung function testing. Eur Respir J. 2005a;26:153–161. doi: 10.1183/09031936.05.00034505. [DOI] [PubMed] [Google Scholar]
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coastes A, Crapo R, Enright P, van der Grinten CPM, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J. Standardisation of spirometry. Eur Respir J. 2005b;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- Milne JA, Howie AD, Pack AI. Dyspnea during normal pregnancy. Br J Obstet Gynaecol. 1978;85:260–263. doi: 10.1111/j.1471-0528.1978.tb10497.x. [DOI] [PubMed] [Google Scholar]
- Moore LG, McCullough RE, Weil JV. Increased HVR in pregnancy: relationship to hormonal and metabolic changes. J Appl Physiol. 1987;62:158–163. doi: 10.1152/jappl.1987.62.1.158. [DOI] [PubMed] [Google Scholar]
- Nettlefold L, Jensen D, Wolfe LA, O'Donnell DE. Ventilatory control and acid-base regulation across the menstrual cycle in oral contraceptive users. Respir Physiol Neurobiol. 2007;158:51–58. doi: 10.1016/j.resp.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Newhouse MT, Becklake MR, Macklem PT, McGregor M. Effect of alterations in end-tidal CO2 tension on flow resistance. J Appl Physiol. 1964;19:745–749. doi: 10.1152/jappl.1964.19.4.745. [DOI] [PubMed] [Google Scholar]
- O'Donnell DE, Banzett RB, Carrieri-Kohlman V, Casaburi R, Davenport PW, Gandevia SC, Gelb AG, Mahler DA, Webb KA. Pathophysiology of dyspnea in chronic obstructive pulmonary disease: a roundtable. Proc Am Thorac Soc. 2007;4:145–168. doi: 10.1513/pats.200611-159CC. [DOI] [PubMed] [Google Scholar]
- O'Donnell DE, Bertley JC, Chau LL, Webb KA. Qualitative aspects of exertional breathlessness in chronic airflow limitation: pathophysiologic mechanisms. Am J Respir Crit Care Med. 1997;155:109–115. doi: 10.1164/ajrccm.155.1.9001298. [DOI] [PubMed] [Google Scholar]
- O'Donnell DE, Hamilton AL, Webb KA. Sensory-mechanical relationships during high-intensity constant-work-rate exercise in COPD. J Appl Physiol. 2006;101:1025–1035. doi: 10.1152/japplphysiol.01470.2005. [DOI] [PubMed] [Google Scholar]
- O'Donnell DE, Hong HH, Webb KA. Respiratory sensation during chest wall restriction and dead space loading in exercising men. J Appl Physiol. 2000;88:1859–1869. doi: 10.1152/jappl.2000.88.5.1859. [DOI] [PubMed] [Google Scholar]
- O'Donnell DE, Lam M, Webb KA. Measurement of symptoms, lung hyperinflation and endurance during exercise in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;158:1557–1565. doi: 10.1164/ajrccm.158.5.9804004. [DOI] [PubMed] [Google Scholar]
- O'Donnell DE, Revill SM, Webb KA. Dynamic hyperinflation and exercise intolerance in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164:770–777. doi: 10.1164/ajrccm.164.5.2012122. [DOI] [PubMed] [Google Scholar]
- Ofir D, Laveneziana P, Webb KA, Lam YM, O'Donnell DE. Sex differences in the perceived intensity of breathlessness during exercise with advancing age. J Appl Physiol. 2008;104:1583–1593. doi: 10.1152/japplphysiol.00079.2008. [DOI] [PubMed] [Google Scholar]
- Ofir D, Laveneziana P, Webb KA, O'Donnell DE. Ventilatory and perceptual responses to cycle exercise in obese women. J Appl Physiol. 2007;102:2217–2226. doi: 10.1152/japplphysiol.00898.2006. [DOI] [PubMed] [Google Scholar]
- Otis AB. The work of breathing. In: Fehn WO, Rahn H, editors. Handbook of Physiology, section 3, vol. 1, Respiration. Washington, DC, USA: American Physiological Society; 1964. pp. 463–476. [Google Scholar]
- Perusquia M, Hernandez R, Montano LM, Villalon CM, Campos MG. Inhibitory effect of sex steroids on guinea-pig airway smooth muscle contractions. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1997;118:5–10. doi: 10.1016/s0742-8413(97)00029-7. [DOI] [PubMed] [Google Scholar]
- Popovic RM, White DP. Upper airway muscle activity in normal women: influence of hormonal status. J Appl Physiol. 1998;84:1055–1062. doi: 10.1152/jappl.1998.84.3.1055. [DOI] [PubMed] [Google Scholar]
- Redman LM, Scroop GC, Norman RJ. Impact of menstrual cycle phase on the exercise status of young, sedentary women. Eur J Appl Physiol. 2003;90:505–513. doi: 10.1007/s00421-003-0889-0. [DOI] [PubMed] [Google Scholar]
- Rubin A, Russo N, Goucher D. The effect of pregnancy upon pulmonary function in normal women. Am J Obstet Gynecol. 1956;72:963–969. doi: 10.1016/0002-9378(56)90058-8. [DOI] [PubMed] [Google Scholar]
- Sady SP, Carpenter MW, Thompson PD, Sady MA, Haydon B, Coustan DR. Cardiovascular response to cycle exercise during and after pregnancy. J Appl Physiol. 1989;66:336–341. doi: 10.1152/jappl.1989.66.1.336. [DOI] [PubMed] [Google Scholar]
- Severinghaus JW, Stupfel M. Respiratory dead space increase following atropine in man, and atropine, vagal or ganglionic blockade and hypothermia in dogs. J Appl Physiol. 1955;8:81–87. doi: 10.1152/jappl.1955.8.1.81. [DOI] [PubMed] [Google Scholar]
- Slatkovska L, Jensen D, Davies GAL, Wolfe LA. Phasic menstrual cycle effects on the control of breathing in healthy women. Respir Physiol Neurobiol. 2006;154:379–388. doi: 10.1016/j.resp.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Smekal G, Von Duvillard SP, Frigo P, Tegelhofer T, Pokan R, Hofmann P, Tschan H, Baron R, Wonisch M, Renezeder K, Bachl N. Menstrual cycle: no effect on exercise cardiorespiratory variables or blood lactate concentration. Med Sci Sports Exerc. 2007;39:1098–1106. doi: 10.1249/mss.0b013e31805371e7. [DOI] [PubMed] [Google Scholar]
- Sterling GM. The mechanism of bronchoconstriction due to hypocapnia in man. Clin Sci. 1968;34:277–285. [PubMed] [Google Scholar]
- Stoller JK, Ferranti R, Feinstein AR. Further specification and evaluation of a new clinical index for dyspnea. Am Rev Respir Dis. 1986;134:1129–1134. doi: 10.1164/arrd.1986.134.5.1129. [DOI] [PubMed] [Google Scholar]
- Stubbing DG, Pengelly LD, Morse JLC, Jones NL. Pulmonary mechanics during exercise in normal males. J Appl Physiol. 1980;49:506–510. doi: 10.1152/jappl.1980.49.3.506. [DOI] [PubMed] [Google Scholar]
- Thomson KJ, Cohen ME. Studies on the circulation in pregnancy. II. Vital capacity observations in normal pregnant women. Surg Gynecol Obstet. 1938;66:591–603. [Google Scholar]
- Tobin MJ. Monitoring respiratory mechanics in spontaneously breathing patients. In: Tobin MJ, editor. Principles and Practice of Intensive Care Monitoring. New York, NY, USA: McGraw-Hill, Inc.; 1998. pp. 617–654. [Google Scholar]
- Wanger J, Clausen JL, Coates A, Pedersen OF, Brusasco V, Burgos F, Casaburi R, Crapo R, Enright P, van der Grinten CPM, Gustafsson P, Hankinson J, Jensen R, Johnson D, MacIntyre N, McKay R, Miller MR, Navajas D, Pellegrino R, Viegi G. Standardisation of the measurement of lung volumes. Eur Respir J. 2005;26:511–522. doi: 10.1183/09031936.05.00035005. [DOI] [PubMed] [Google Scholar]
- Wasserman K, Hansen JE, Sue DY, Stringer WW, Whipp BJ. Principles of Exercise Testing and Interpretation. 4th edn. Baltimore, MD, USA: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- Yan S, Kaminski D, Sliwinski P. Reliability of inspiratory capacity for estimating end expiratory lung volume changes during exercise in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1997;156:55–59. doi: 10.1164/ajrccm.156.1.9608113. [DOI] [PubMed] [Google Scholar]