Abstract
To maintain smooth and efficient gait the motor system must adjust for changes in the ground on a step-to-step basis. In the present study we investigated the role of sensory feedback as 19 able-bodied human subjects walked over a platform that mimicked an uneven supporting surface. Triceps surae muscle activation was assessed during stance as the platform was set to different inclinations (±3 deg, ±2 deg and 0 deg rotation in a parasagittal plane about the ankle). Normalized triceps surae muscle activity was significantly increased when the platform was inclined (2 deg: 0.153 ± 0.051; 3 deg: 0.156 ± 0.053) and significantly decreased when the platform was declined (−3 deg: 0.133 ± 0.048; −2 deg: 0.132 ± 0.049) compared with level walking (0.141 ± 0.048) for the able-bodied subjects. A similar experiment was performed with a subject who lacked proprioception and touch sensation from the neck down. In contrast with healthy subjects, no muscle activation changes were observed in the deafferented subject. Our results demonstrate that the ability to compensate for small irregularities in the ground surface relies on automatic within-step sensory feedback regulation rather than conscious predictive control.
To walk smoothly and efficiently over uneven ground the motor control system must compensate for changing mechanics on a step-to-step basis. It is commonly accepted that visual information is used to shape the locomotor drive (Patla et al. 2002). However, visual information in itself is not an essential requirement for the central nervous system's ability to adjust for changes in the supporting surface while walking on uneven ground – one can walk with closed eyes. As an extreme example, blind teenagers hiking in the Himalayas walked successfully in this challenging environment by relying on other sensory modalities (Puntillo, 2004). Though vestibular feedback is important for the maintenance of balance during walking, it is unlikely to provide an important contribution to the locomotor drive, nor to compensate for small irregularities in the ground, because small mechanical inputs to the ankle produced during walking would be damped to such an extent that any input to the vestibular apparatus would be negligible.
In contrast, sensory feedback from peripheral afferents is well suited to provide information used by the central nervous system to adapt to irregularities in the supporting surface (for review see: Zehr & Stein, 1999; Duysens et al. 2000; Donelan & Pearson, 2004). Rapid feedback from proprioceptive afferents may be particularly important for maintaining human walking. Stretch reflex responses mediated by muscle–tendon large-diameter afferents have been studied by applying rapid perturbations to the leg (Dietz et al. 1984) and to the ankle (Yang et al. 1991; Sinkjaer et al. 1996), and they probably contribute significantly to the production of corrective stumbling responses. Proprioceptive afferents may also transmit a peripheral feedback signal that modulates and contributes to the background locomotor activity (Sinkjaer et al. 2000; Grey et al. 2004, 2007; Mazzaro et al. 2005). Mazzaro et al. (2005, 2006) applied small-amplitude, slow-velocity ankle perturbations to the ankle that slightly increased or decreased the natural dorsiflexion during stance in treadmill walking and demonstrated that the soleus muscle activity increased when the dorsiflexion was slightly enhanced and decreased when dorsiflexion was reduced.
In the present study, we investigated the within-step modulation of plantar flexor locomotor activity during unrestrained over-ground walking. We hypothesized that sensory information from the leg modulates within-step locomotor activity in plantar flexor muscles during over-ground walking. To test this hypothesis, we designed an unrestrained over-ground walking protocol that modulated sensory input with very small changes in the slope of a stable supporting surface. As an extreme case of altered afferent feedback, we repeated the experiment with a deafferented subject.
Methods
Nineteen able-bodied volunteers (3 female and 16 male, mean age: 26, range: 20–50) with no known history of neuromuscular disorder and one deafferented subject (male, age: 54) participated in this study. The study conformed to the Declaration of Helsinki and was approved by the local ethics committee (VN 2004/24). All subjects provided informed written consent.
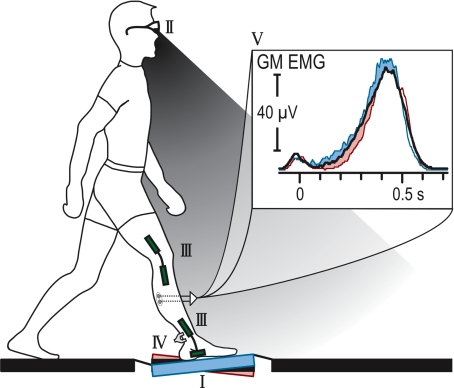
The set-up of the experiment is illustrated in Fig. 1. The able-bodied subjects walked barefoot at a self-selected speed on a 10 m path, such that one step with the right leg was made onto a robotic platform (van Doornik & Sinkjaer, 2004). On random trials the platform rotation was changed to one of five different inclinations (0 deg, ±2 deg, ±3 deg rotation in the parasagittal plane producing ankle increased dorsiflexion or plantar-flexion of the ankle) so that it was comparable to irregularities that are experienced during normal over-ground walking. Approximately 1 s before heel contact, the platform inclination was randomly adjusted for each trial and held throughout the step. Knowledge of the platform inclination was prevented with taped glasses that shielded the platform from their field of view and by masking the noise from the rotation.
Figure 1. Experimental set-up.
Subjects stepped on an inclined surface (I) in the middle of a 10 m walk-way. Knowledge of the platform's inclination was prevented by occluding part of the subject's view (grey area) with taped glasses (II). Ankle and knee rotation were recorded using goniometers (III) and Achilles' tendon force was estimated by an external buckle transducer (IV). Ensemble averaged (n > 20) electromyographic recordings from gastrocnemius medialis (GM) of a representative subject is presented for 0 deg (black), +3 deg and −3 deg (increase and decrease, respectively, in the EMG) degree inclination (V), showing modulation in muscle activity induced by the inclination of the platform.
Muscle activity was recorded from the three heads of the triceps surae with surface electromyography (EMG) (NeuroLine 720, AMBU A/S, Denmark, interelectrode distance 2 cm). The EMG signals were transmitted and band-pass filtered (10–1 kHz) using a wireless recording system (Telemyo 2400, Noraxon, USA). The right knee and ankle excursion was recorded using goniometry (models SG150 and SG110/A, Biometrics Ltd, Gwent, UK). In 18 able-bodied subjects, the Achilles' tendon force (ATF) was estimated for 10–15 trials of each platform inclination by clamping a custom-made E-buckle transducer to the tendon. The design of this device was based on the in vivo buckle transducer (Salmons, 1969; Komi et al. 1987) and an external tendon-clamp transducer (Berger et al. 1982). All signals were sampled at 2 kHz and stored for later processing. Subjects walked until 20–25 trials were recorded for each inclination.
Analysis and statistics
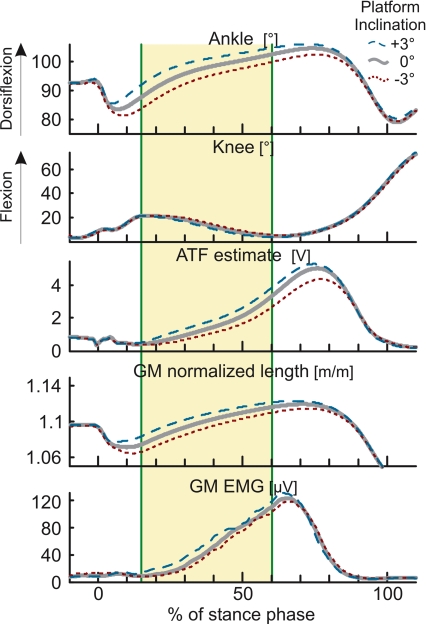
Data analysis was conducted off-line. The EMG records were rectified and low-pass filtered (20 Hz) to extract an amplitude envelope. The muscle–tendon lengths of the three muscles of the triceps surae, normalized to the length of the tibia, were estimated from the ankle and knee angular position records using the model of Hawkins & Hull (1990). The EMG, muscle–tendon force and length records were ensemble-averaged to create a single set of records for each subject and condition. To investigate the effect of the surface inclination on locomotor muscle activity, the area under the curve of the EMG records was calculated in a window placed 15–60% into stance (Fig. 2). For each muscle, the area measurement was normalized to the peak EMG activity during stance of the control condition (0 deg inclination) in order to compare the overall influence of the inclination in the triceps surae. Similarly, ATF was calculated by the area under the curve in the window of analysis and normalized to the peak force estimate in the control condition.
Figure 2. Analysis of the effect of stepping on an inclined surface.
Grand mean ensemble averages of 18 able-bodied subjects' ankle dorsiflexion, knee flexion, Achilles' tendon force (ATF) estimate, normalized gastrocnemius medialis (GM) muscle–tendon length and GM muscle activity are presented for level, +3 deg and −3 deg inclination of the platform (thick, dashed, and dotted, respectively). The analysis window for the kinematics, force and EMG recordings is represented by the shaded areas (15–60% of stance). Stepping on the inclined surface shifts the ankle rotation at foot touch down. This has an effect on the muscle–tendon length throughout the step, illustrated here for GM. ATF estimates and muscle activity also vary concomitantly with the inclination of the platform.
Repeated measures analysis of variance (rmANOVA) was used to test the effect of the inclination on the ATF estimates. Two-way rmANOVA was used to test the effect of muscle and inclination on the mean muscle–tendon lengths of SOL, GM and GL. A two-way rmANOVA was employed to test the effect of muscle (SOL, GM, GL) and inclination (−3 deg, −2 deg, 0 deg, 2 deg, 3 deg) on the normalized EMG measurement. In order to evaluate which muscle showed the greatest modulation in activity in relation to the inclinations of the stepping surface, the modulation was calculated for each subject's muscles by taking the difference between maximum and minimum area under the curve of the EMG trace across inclinations. A one-way rmANOVA was performed on these differences to test for significance. Scheffé's post hoc multiple comparison test was used to compare the significant effects at significance level P= 0.05.
Deafferented subject
We performed a similar experiment in a single subject with a rare neuropathy. At the age of 19 this person (IW) was struck by an autoimmune response to a viral infection causing deafferentation of the large-diameter sensory nerves below the neck, leaving him with neither touch nor proprioception (Cole & Sedgwick, 1992). With intense training, IW regained the ability to walk by relying on visual feedback to control his movements. The experiment was performed in the same manner as described for the able-bodied subjects with two exceptions. First, for safety reasons, we did not restrict IW's view of the platform, nor did we use the ATF transducer. Second, due to the physical demands required for IW to walk, we restricted the experiment to 0 deg and +3 deg inclination and limited the number of recorded trials to approximately 15. To determine if the muscle–tendon length increased with 3 deg inclination, three t tests were performed on the mean normalized muscle–tendon lengths of SOL, GM and GL during 15–60% of stance.
Results
As illustrated in Fig. 1 for a representative able-bodied subject, the triceps surae muscle activity increased when the subject stepped on the inclined platform and it decreased when the subject stepped on the declined platform. A similar result was observed across all subjects (Figs 2 and 3). Stepping onto a surface of greater inclination lengthened the muscle–tendon unit, while the negative inclinations shortened the muscle–tendon unit (Fig. 2). In all muscles the mean muscle–tendon length was significantly different for all levels of inclinations within the window of analysis (muscle F2,18= 635497.31, P < 0.001; inclination F4,18= 302.65; P < 0.001; SchefféP < 0.001 for all comparisons between inclination levels). These length changes were present throughout the window of analysis but were more pronounced closer to heel contact. ATF estimates also increased with the positive inclinations and decreased with the negative inclinations (F4,17= 68.35; P < 0.001; SchefféP < 0.001).
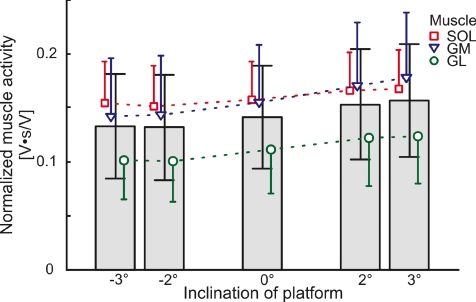
Figure 3. EMG modulations with respect to the inclination of the platform in able bodied subjects.
The bars represent the overall normalized EMG for all muscles. The individual muscle modulation is indicated for soleus (SOL, □), gastrocnemius medialis (GM, ∇) and gastrocnemius lateralis (GL, ◯). The normalized EMG is increased while walking over a positively inclined surface and decreased for negative inclinations. Error bars represent the standard deviation of the normalized muscle activation.
Near the end of the experiment, the subjects were asked whether or not they could detect the inclination. In all cases they were unable to detect the inclination of the platform until very late in stance despite the observation of a clear modulation of the triceps surae muscle activity with platform inclination. For the able-bodied subjects, platform inclination had a significant effect on the normalized EMG activity (F4,17= 8.12, P < 0.001). Across all muscles, EMG decreased for negative inclinations of the platform and increased for positive inclinations (−3 deg: 0.133 ± 0.048; −2 deg: 0.132 ± 0.049; 0 deg: 0.141 ± 0.048; 2 deg: 0.153 ± 0.051; 3 deg: 0.156 ± 0.053; Fig. 3). Scheffé's post hoc test revealed significant differences between the negative and positive inclinations (i.e. pairs (−3 deg, 2 deg) (−3 deg, 3 deg) (−2 deg, 2 deg) (−2 deg, 3 deg); P≤ 0.028), but there were no significant differences between all other paired comparisons (P > 0.05). The largest modulation of the muscle activity was seen in the gastrocnemius medialis (GM) muscle, for which the Scheffé's post hoc test showed a significantly greater modulation than the two other muscles (rmANOVA F2,17= 29.46; P < 0.001; SchefféP < 0.001 for all comparisons).
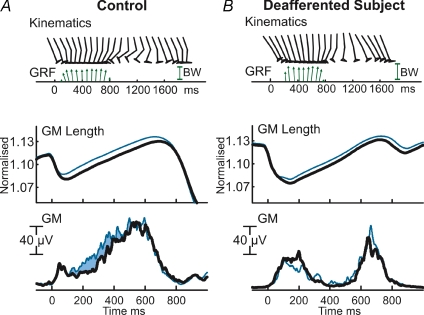
In Fig. 4, the kinematics, ground reaction forces (GRF) and ensemble averaged plots of GM normalized length and GM muscle activity are illustrated for subject IW and an age-matched control subject. While this control subject was asked to walk with a similar cadence as subject IW, his kinematic and electromyographic data are similar to the control group. As the largest difference in muscle activity in the pool of able-bodied subjects was observed for the GM muscle, only this muscle is illustrated in this figure; however, similar results were found in SOL and GL. All the muscle–tendon lengths were significantly increased with the 3 deg inclination of the platform (P= 0.0019, 0.0013 and 0.0014 for SOL, GM and GL, respectively). While it should be noted that the control step (0 deg inclination) muscle activity in IW did exhibit two EMG bursts, as compared to a single burst with the age matched subject, there was significant activity in mid-late stance. More interestingly, IW did not exhibit the increased EMG activity that was concomitant with the inclined slope observed in the age-matched subject or the group of able-bodied subjects. Furthermore, IW reported that he was unable to distinguish any of the platform inclinations.
Figure 4. Kinematics and ground reaction forces.
Kinematics and ground reaction forces (GRF; subjects' body weight (BW) is shown for comparison) of a typical step, and ensemble averaged plots of gastrocnemius medialis (GM) normalized muscle–tendon length and GM muscle activity in the age-matched control (A) and the deafferented subject IW (B) for 0 deg and +3 deg inclination (thick and thin line, respectively; 0 deg: n= 14, +3 deg: n= 16). The age-matched control shows a concomitant increase in EMG while stepping on a positively inclined surface (filled area). This increase is absent in the deafferented subject.
Discussion
In contrast to previous studies that have imposed perturbations during walking (e.g. Dietz et al. 1984; Yang et al. 1991; Sinkjaer et al. 1996; Grey et al. 2004, 2007; Mazzaro et al. 2005, 2006), this investigation was designed to mimic the natural variations in the ground surface encountered on an everyday basis and performed with unrestricted over-ground walking. Our results reveal within-step modulation of triceps surae EMG activity that is related to the inclination of the stepping surface in able-bodied subjects. While it might be possible that the mechanical properties of the muscle–tendon complex could provide some compensation for small irregularities in the surface, the EMG changes concomitant with the platform inclinations indicate that a distinct change in the sensory-driven neural drive to the muscle occurs in response to small changes in the supporting surface.
The effect of the platform inclination on the muscle–tendon lengths was the same for the deafferented subject as for the able-bodied subjects – i.e. positive inclinations of the platform lengthened the muscle–tendon complex during stance. However, the muscular response to stepping on the positively inclined surface differed in that the increased activation observed mid stance in the able-bodied subject was not present in IW. The combination of IW's rare disorder and his ability to walk is truly unique; consequently he is the only deafferented participant. It is unlikely that he used visual feed-forward control to prepare for stepping on the sloped surface because he reported that he was unable to differentiate if the platform was level or inclined. Similarly, anticipatory visual feed-forward mechanisms cannot explain the modulation in triceps surae muscle activity with the able-bodied subjects because they were unable to see the platform before stepping onto it. Though we cannot completely exclude vestibular feedback, this possibility is unlikely because the changes in surface inclination were so small that differences between slopes could not be discerned until several hundred milliseconds after heel contact. In pilot experiments, four subjects were asked to report the inclination as they walked over the platform. Even though, they were focusing on this task they were not able to report the inclination until late stance and then with only 55% correct guesses. The small input to the ankle would also have been damped by the body to such an extent that the input to the vestibular apparatus would be negligible. Any meaningful response through vestibular feedback would therefore require an unrealistically high gain. Furthermore, vestibular responses to galvanic stimulation in subject IW have been demonstrated to be normal but with an increased gain (Day & Cole, 2002). This suggests that if the vestibular influence were the cause of the modulation seen in the able-bodied subjects we should have expected an increased modulation in IW, and this was not the case. Therefore it can be concluded that vestibular feedback has little or no influence on modulating muscle activity for such small inclination changes of the ground.
Our results suggest that the modulation of plantar flexor EMG with platform inclination occurs via peripheral sensory feedback. Both proprioceptive and cutaneous afferents could provide this feedback because both types of receptors on the foot and leg are influenced by the inclination of the stepping surface. The muscle–tendon length was directly changed by the inclination of the platform and concomitant changes in ATF were recorded. While these estimates are subject to error, the general pattern is most likely present at muscle fibre level. Neural recordings in walking cats imply that proprioceptive information would change accordingly (Prochazka & Wand, 1980; Prochazka & Gorassini, 1998). Similarly, the pressure distribution on the sole would be altered due to the slope of the stepping surface. Grey et al. (2004) demonstrated that cutaneous afferents do not contribute to the afferent-mediated locomotor EMG in mid to late stance; however, they did not specifically address the contribution of cutaneous afferents to the modulation of the locomotor EMG. Further investigations are required to characterize the importance of the different pathways, whether proprioceptive or cutaneous.
Modulating within-step locomotor muscle activity by afferent related feedback might reduce the need for more complex neural processing. The benefit of such a system becomes apparent in subject IW, who required nearly two years to learn to walk following the onset of his neuropathy and who diverts nearly all of his attention to controlling his gait and predicting his footfall. Human walking may have complex and multiple systems for its control, with local spinal circuits likely to be controlling within-step adjustments while higher centres, both automatic and cognitive, are involved in more predictive and complex movements. Our results show that sensory information modulates the within-step locomotor activity in humans and could thus contribute to a smooth and efficient gait. However, whether or not this feedback is mediated at a spinal or higher level is left for future studies to discern.
References
- Berger W, Quintern J, Dietz V. Pathophysiology of gait in children with cerebral palsy. Electroencephalogr Clin Neurophysiol. 1982;53:538–548. doi: 10.1016/0013-4694(82)90066-9. [DOI] [PubMed] [Google Scholar]
- Cole JD, Sedgwick EM. The perceptions of force and of movement in a man without large myelinated sensory afferents below the neck. J Physiol. 1992;449:503–515. doi: 10.1113/jphysiol.1992.sp019099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day BL, Cole JD. Vestibular-evoked postural responses in the absence of somatosensory information. Brain. 2002;125:2081–2088. doi: 10.1093/brain/awf212. [DOI] [PubMed] [Google Scholar]
- Dietz V, Quintern J, Berger W. Corrective reactions to stumbling in man: functional significance of spinal and transcortical reflexes. Neurosci Lett. 1984;44:131–135. doi: 10.1016/0304-3940(84)90070-3. [DOI] [PubMed] [Google Scholar]
- Donelan JM, Pearson KG. Contribution of sensory feedback to ongoing ankle extensor activity during the stance phase of walking. Can J Physiol Pharmacol. 2004;82:589–598. doi: 10.1139/y04-043. [DOI] [PubMed] [Google Scholar]
- Duysens J, Clarac F, Cruse H. Load-regulating mechanisms in gait and posture: comparative aspects. Physiol Rev. 2000;80:83–133. doi: 10.1152/physrev.2000.80.1.83. [DOI] [PubMed] [Google Scholar]
- Grey MJ, Mazzaro N, Nielsen JB, Sinkjaer T. Ankle extensor proprioceptors contribute to the enhancement of the soleus EMG during the stance phase of human walking. Can J Physiol Pharmacol. 2004;82:610–616. doi: 10.1139/y04-077. [DOI] [PubMed] [Google Scholar]
- Grey MJ, Nielsen JB, Mazzaro N, Sinkjaer T. Positive force feedback in human walking. J Physiol. 2007;581:99–105. doi: 10.1113/jphysiol.2007.130088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins D, Hull ML. A method for determining lower extremity muscle-tendon lengths during flexion/extension movements. J Biomech. 1990;23:487–494. doi: 10.1016/0021-9290(90)90304-l. [DOI] [PubMed] [Google Scholar]
- Komi PV, Salonen M, Jarvinen M, Kokko O. In vivo registration of Achilles tendon forces in man. I. Methodological development. Int J Sports Med. 1987;8(Suppl. 1):3–8. doi: 10.1055/s-2008-1025697. [DOI] [PubMed] [Google Scholar]
- Mazzaro N, Grey MJ, do Nascimento OF, Sinkjaer T. Afferent-mediated modulation of the soleus muscle activity during the stance phase of human walking. Exp Brain Res. 2006;173:713–723. doi: 10.1007/s00221-006-0451-5. [DOI] [PubMed] [Google Scholar]
- Mazzaro N, Grey MJ, Sinkjaer T. Contribution of afferent feedback to the soleus muscle activity during human locomotion. J Neurophysiol. 2005;93:167–177. doi: 10.1152/jn.00283.2004. [DOI] [PubMed] [Google Scholar]
- Patla AE, Niechwiej E, Racco V, Goodale MA. Understanding the contribution of binocular vision to the control of adaptive locomotion. Exp Brain Res. 2002;142:551–561. doi: 10.1007/s00221-001-0948-x. [DOI] [PubMed] [Google Scholar]
- Prochazka A, Gorassini M. Ensemble firing of muscle afferents recorded during normal locomotion in cats. J Physiol. 1998;507:293–304. doi: 10.1111/j.1469-7793.1998.293bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochazka A, Wand P. Tendon organ discharge during voluntary movements in cats. J Physiol. 1980;303:385–390. doi: 10.1113/jphysiol.1980.sp013293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puntillo K. Blind mountain climbers challenge prejudice, and reach for the sky. The New York Times. 2004 31 October 2004. [Google Scholar]
- Salmons S. The 8th International Conference on Medical and Biological Engineering – Meeting Report. Bio Med Eng. 1969:467–474. [Google Scholar]
- Sinkjaer T, Andersen JB, Ladouceur M, Christensen LOD, Nielsen JB. Major role for sensory feedback in soleus EMG activity in the stance phase of walking in man. J Physiol. 2000;523:817–827. doi: 10.1111/j.1469-7793.2000.00817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinkjaer T, Andersen JB, Larsen B. Soleus stretch reflex modulation during gait in humans. J Neurophysiol. 1996;76:1112–1120. doi: 10.1152/jn.1996.76.2.1112. [DOI] [PubMed] [Google Scholar]
- van Doornik J, Sinkjaer T. Hydraulically actuated platform for human gait and posture analysis. 2nd IASTED International Conference on Biomedical Engineering; Innsbruck, Austria. [Google Scholar]
- Yang JF, Stein RB, James KB. Contribution of peripheral afferents to the activation of the soleus muscle during walking in humans. Exp Brain Res. 1991;87:679–687. doi: 10.1007/BF00227094. [DOI] [PubMed] [Google Scholar]
- Zehr EP, Stein RB. What functions do reflexes serve during human locomotion? Prog Neurobiol. 1999;58:185–205. doi: 10.1016/s0301-0082(98)00081-1. [DOI] [PubMed] [Google Scholar]