Abstract
The mechanisms by which proteases activate the epithelial sodium channel (ENaC) are not yet fully understood. We investigated the effect of extracellular proteases on rat ENaC heterologously expressed in Xenopus laevis oocytes. Application of trypsin increased ENaC whole-oocyte currents by about 8-fold without a concomitant increase in channel surface expression. The stimulatory effect of trypsin was preserved in oocytes expressing αγ-ENaC, but was abolished in oocytes expressing αβ-ENaC. Thus, the γ-subunit appears to be essential for channel activation by extracellular proteases. Site-directed mutagenesis of a putative prostasin cleavage site in the extracellular loop of the γ-subunit revealed that mutating the 181Lys residue to alanine (γK181A) increases ENaC baseline whole-oocyte currents, decreases channel surface expression, and largely reduces the stimulatory effect of extracellular proteases (trypsin, chymotrypsin and human neutrophil elastase). In single-channel recordings from outside-out patches we demonstrated that the γK181A mutation essentially abolishes the activation of near-silent channels by trypsin, while a stimulatory effect of trypsin on channel gating is preserved. This apparent dual effect of trypsin on channel gating and on the recruitment of near-silent channels was confirmed by experiments using the β518C mutant ENaC which can be converted to a channel with an open probability of nearly one by exposure to a sulfhydryl reagent. Interestingly, the γK181A mutation results in the spontaneous appearance of a 67 kDa fragment of the γ-subunit in the plasma membrane which can be prevented by a furin inhibitor and also occurs after channel activation by extracellular trypsin. This suggests that the mutation promotes channel cleavage and activation by endogenous proteases. This would lower the pool of near-silent channels and explain the constitutive activation and reduced responsiveness of the mutant channel to extracellular proteases. We conclude that the mutated site (K181A) affects a region in the γ-subunit of ENaC that is functionally important for the activation of near-silent channels by extracellular proteases.
The epithelial sodium channel (ENaC) is the rate-limiting step for sodium absorption in a number of epithelial tissues (Garty & Palmer, 1997; Kellenberger & Schild, 2002). The appropriate regulation of ENaC activity in the kidney is critically important for the maintenance of body sodium balance and hence for the long-term regulation of arterial blood pressure (Rossier et al. 2002). This is evidenced by gain-of-function mutations of ENaC which cause a severe form of arterial hypertension known as Liddle's syndrome (Rossier et al. 2002). Fluid absorption in the lung also critically depends on ENaC (Hummler et al. 1996), and airway-specific over-expression of the β-subunit of ENaC has recently been shown to cause airway surface liquid depletion and cystic fibrosis-like lung disease (Mall et al. 2004). These examples illustrate the importance of ENaC function and the need for an appropriate regulation of ENaC activity in a tissue-specific manner.
Recent evidence suggests that proteolytic processing of ENaC contributes to its regulation in a complex manner (Rossier, 2004; Kleyman et al. 2006). Enhanced activation by proteases may be one of the factors causing the hyperactivity of ENaC in Liddle's syndrome (Knight et al. 2006). Moreover, an inappropriate ENaC activation by endogenous proteases may be involved in sodium retention and the pathogenesis of arterial hypertension in the context of renal disease. Proteolytic activation of ENaC may also aggravate symptoms of cystic fibrosis during acute respiratory infections associated with the generation of local proteases, e.g. neutrophil elastase (Boucher, 2004; Caldwell et al. 2005; Harris et al. 2007).
Channel-activating proteases (CAPs) have been identified using the Xenopus laevis oocyte expression system and were shown to activate ENaC when co-expressed with the channel (Vallet et al. 1997, 2002; Vuagniaux et al. 2002). Membrane-bound and/or secreted proteases such as CAPs are likely to exist in ENaC-expressing epithelia and are thought to act on the extracellular domain of the channel. One possible candidate for an endogenous ENaC-activating protease is prostasin, the mammalian homologue of Xenopus CAP1 (Vuagniaux et al. 2000). Prostasin is an attractive candidate since it is expressed in the kidney and in cultured collecting duct cells (Adachi et al. 2001; Olivieri et al. 2005). Moreover, its expression seems to be regulated by aldosterone (Narikiyo et al. 2002), the main hormonal regulator of ENaC. However, prostasin has a pH optimum of about 9 and is practically inactive at physiological pH (Yu et al. 1994). Thus, it is likely that more than one protease is involved in ENaC regulation. Moreover, the recent discovery of endogenous CAP inhibitors adds to the complexity of this regulatory pathway (Wakida et al. 2006). Indeed, in analogy to known kinase/phosphatase networks, the channel-activating proteases may be part of a complex and highly regulated protease cascade with tissue-specific properties (Rossier, 2004).
Proteolytic cleavage by furin (Thomas, 2002) or related convertases is thought to be important for ENaC maturation in the biosynthetic pathway before the channel reaches the plasma membrane. Western blot analysis of a range of ENaC-expressing cells and tissues has revealed the presence of distinct ENaC cleavage products in particular of the α- and γ-subunit (Rossier, 2004; Kleyman et al. 2006). The channel is thought to be in its mature and active form in its cleaved state, but there is evidence for the presence of both cleaved and non-cleaved channels in the plasma membrane (Hughey et al. 2004b). The appearance of cleaved ENaC products is not an artifact of cultured cells or of heterologous expression systems, but has also been demonstrated in native renal tissue under quasi physiological conditions, i.e. in rats maintained on a low sodium diet to increase plasma aldosterone levels. Importantly, the appearance of cleaved products correlated with increasing ENaC currents (Ergonul et al. 2006; Frindt et al. 2008).
There is good evidence that at least two functionally distinct ENaC populations are present in the plasma membrane: active channels with a somewhat variable but rather high open probability (Po) of about 0.5 (Garty & Palmer, 1997) and near-silent channels with an exceedingly low Po of less than 0.05 (Firsov et al. 1996; Kellenberger & Schild, 2002; Harris et al. 2007). Patch-clamp experiments in cultured fibroblasts and respiratory cells have recently provided convincing evidence that near-silent channels can be activated by extracellularly applied trypsin or neutrophil elastase (Caldwell et al. 2004, 2005). However, the mechanism of this activation is still unclear. Proteolytic cleavage of the channel may cause a conformational change and lead to channel activation. In addition, indirect mechanisms involving the downstream activation of G-proteins have been reported to contribute to ENaC activation by trypsin (Bengrine et al. 2007). Recently, it has been reported that cleavage-induced release of inhibitory domains from the extracellular loops of the α- and γ-subunits of the channel mediates ENaC activation (Carattino et al. 2006; Bruns et al. 2007). Relieving Na+ self-inhibition may also contribute to ENaC activation by proteases (Sheng et al. 2006; Bize & Horisberger, 2007). Finally, recent experiments indicate that extracellular cleavage of the γ-subunit is particularly important for the proteolytic activation of ENaC (Adebamiro et al. 2007; Harris et al. 2007).
In the present study we used the Xenopus laevis oocyte expression system to further investigate the mechanisms of proteolytic ENaC activation. Trypsin was used as our main tool since it is known to have a large and rapid stimulatory effect on ENaC whole-oocyte currents in the oocyte expression system (Chraibi et al. 1998). Our aim was to investigate the effect of trypsin at the single-channel level using outside-out patch-clamp recordings and to determine a functionally relevant site for ENaC activation.
Part of this work has been published in abstract form (Diakov et al. 2007).
Methods
cDNA clones
The full-length cDNAs encoding the three subunits of wild-type (wt) rat ENaC (α, β, γ-ENaC) (Canessa et al. 1994) were in pGEM-HE. The FLAG-tagged rat β-ENaC (Firsov et al. 1996) and the MTSET-sensitive subunit βS518C (Kellenberger et al. 2002) were in pSD5. Clones were a kind gift of Drs Bernard C. Rossier and Laurent Schild (Lausanne, Switzerland). Linearized plasmids were used as templates for cRNA synthesis (mMessage mMachine, Ambion, Austin, TX, USA). Extension overlap PCR was used for site directed mutagenesis. Mutations were confirmed by sequence analysis (GATC Biotech, Konstanz, Germany). For the detection of γ-ENaC fragments by Western blot analysis we generated a γ-ENaC construct with a C-terminal V5 tag (GKPIPNPLLGLDST).
Two-electrode voltage-clamp experiments and surface expression assay
Defolliculated stage V–VI oocytes were obtained from ovarian lobes of adult female Xenopus laevis in accordance with the principles of German legislation, with approval by the animal welfare officer for the University of Erlangen-Nürnberg, and under the governance of the state veterinary health inspectorate. Animals were anaesthetized in 0.2% MS222 (Sigma, Taufkirchen, Germany). Isolation of oocytes and two-electrode voltage-clamp experiments were performed essentially as previously described (Konstas et al. 2000; Diakov & Korbmacher, 2004; Yang et al. 2006). Oocytes were injected with cRNA using 0.025, 0.1 or 0.2 ng of cRNA per ENaC subunit per oocyte. Injected oocytes were incubated in ND96 solution (mm: 96 NaCl, 2 KCl, 1.8 CaCl2, 1 MgSO4, 5 Hepes, adjusted to pH 7.4 with Tris) supplemented with 100 U ml−1 sodium penicillin and 100 μg ml−1 streptomycin sulphate. Unless stated otherwise, oocytes were studied 48 h after injection. The amiloride-sensitive current (ΔIami) was determined by subtracting the corresponding current value measured in the presence of 2 μm of amiloride from that measured in the absence of amiloride in ND96 solution. ENaC surface expression was determined using a chemiluminescence assay and a β-ENaC construct with a FLAG tag inserted into its extracellular loop essentially as previously described (Konstas et al. 2001, 2002a,b; Rauh et al. 2006).
Single-channel recordings in outside-out patches
Single-channel recordings in outside-out patches were essentially performed as previously described using oocytes kept in low sodium modified Barth's saline after cRNA injection to prevent Na+ overloading (Diakov & Korbmacher, 2004). Patch pipettes were pulled from borosilicate glass capillaries and had a tip diameter of about 1 μm after fire polishing. Pipettes were filled with potassium gluconate pipette solution (mm: 90 potassium gluconate, 5 NaCl, 2 Mg-ATP, 2 EGTA and 10 mm Hepes adjusted to pH 7.28 with Tris). Seals were routinely formed in a low sodium NMDG-Cl bath solution (mm: 95 NMDG (N-methyl-d-glucamine)-Cl, 1 NaCl, 4 KCl, 1 MgCl2, 1 CaCl2, 10 Hepes adjusted to pH 7.4 with Tris). In this bath solution the pipette resistance averaged about 11 MΩ. In NaCl bath solution the NMDG-Cl was replaced by 95 mm NaCl. Outside-out patches were routinely held at a holding pipette potential of −70 mV which was close to the calculated reversal potential of Cl− (ECl=−77.2) and K+ (EK=−79.4) under our experimental conditions with experiments performed at room temperature (∼23°C). Single-channel current data were initially filtered at 500 Hz and sampled at 2 kHz. In multi-channel patches current traces were re-filtered at 16 Hz to resolve the single-channel current amplitude (i) and channel activity. The latter was derived from binned amplitude histograms as the product NPo, where N is the number of channels and Po is open probability (Korbmacher et al. 1995; Diakov & Korbmacher, 2004). This method to estimate NPo does not require the knowledge of the number of channels present in the patch. The current level at which all channels are closed was determined in the presence of amiloride. Continuous current traces of 40–60 s were selected from different experimental periods to analyse changes in NPo. Data are presented as mean values ±s.e.m.; n indicates the number of individual oocytes; N indicates the number of different batches of oocytes used. Appropriate versions of Student's t test were used. P values less than 0.05 were required to reject the null hypothesis. *, ** and *** represent P values smaller than 0.05, 0.01 and 0.001, respectively.
Detection of ENaC cleavage products at the cell surface
Biotinylation experiments were performed as recently described by Harris et al. (2007) using 30 oocytes per group. All biotinylation steps were performed at 4°C. The biotinylation buffer (pH 9.5) contained 10 mm triethanolamine, 150 mm NaCl, 2 mm CaCl2 and 1 mg ml−1 EZ-link sulfo-NHS-SS-Biotin (Pierce, Rockford, IL, USA). Oocytes were incubated in this buffer for 15 min with gentle agitation. The biotinylation reaction was stopped by washing the oocytes with quench buffer containing 192 mm glycine, 25 mm Tris-Cl, pH 7.5. Oocytes were then washed with ND96 solution and lysed by passing them through a 27-gauge needle in lysis buffer containing 1% Triton X-100, 1% Igepal CA-630 (Sigma), 500 mm NaCl, 5 mm EDTA, 50 mm Tris-Cl, pH 7.4, and a protease inhibitor cocktail (‘Complete Mini EDTA-free’ protease inhibitor cocktail tablets, Roche Diagnostics, Mannheim, Germany). The lysates were centrifuged for 10 min at 10 000 g. Biotinylated proteins were precipitated with 100 μl of Immunopure immobilized Neutravidin beads (Pierce) on a rotating wheel at 4°C overnight. The beads were washed three times with lysis buffer by centrifugation for 1 min at 3000 g. One hundred microlitres of 2× SDS-PAGE sample buffer (Rotiload 1, Roth, Karlsruhe, Germany) was added to the beads. Samples were boiled for 5 min at 95°C before loading them on the 10% SDS-PAGE. Monoclonal anti-V5 antibody was obtained from Invitrogen (Karlsruhe, Germany) and used at a dilution of 1 : 5000. Horseradish peroxidase-labelled secondary sheep anti-mouse antibodies were purchased from Sigma (Taufkirchen, Germany) and used at a dilution of 1 : 10 000.
Solution and chemicals
Amiloride hydrochloride (Sigma-Aldrich, Taufkirchen, Germany) was added from an aqueous 10 mm stock solution. MTSET ([2-(trimethylammonium)ethyl] methanethiosulphonate bromide) was from Toronto Research Chemicals (Toronto, Canada). Trypsin type I from bovine pancreas, α-chymotrypsin (TLCK treated) type VII from bovine pancreas, and aprotinin were purchased from Sigma-Aldrich (Taufkirchen, Germany). Human neutrophil elastase (hNE) was obtained from Serva Electrophoresis (Heidelberg, Germany) and collagenase type 2 from Worthington Biochemical Corporation (Lakewood, NJ, USA). Furin inhibitor decanoyl-Arg-Val-Lys-Arg-chloromethyl ketone (dec-RVKR-cmk) was from Calbiochem/Merck Biosciences GmbH (Schwalbach, Germany). A 10 mm stock solution of dec-RVKR-cmk was prepared in DMSO and was diluted in ND96 to a final concentration of 40 μm.
Results
Trysin stimulates ENaC by increasing the activity of channels already present in the plasma membrane
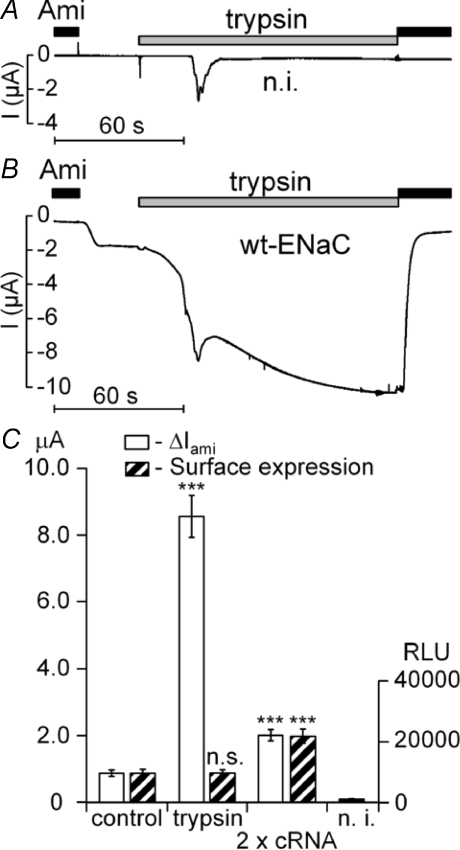
Extracellular application of trypsin has been reported to stimulate amiloride-sensitive whole-oocyte currents (ΔIami) in Xenopus laevis oocytes heterologously expressing ENaC (Chraibi et al. 1998). We confirmed this observation in oocytes expressing the three subunits (αβγ) of rat ENaC 2 days after cRNA injection. Figure 1B shows a representative whole-oocyte current trace illustrating the stimulatory effect of trypsin on ENaC. At the beginning of the experiment the bath solution contained 2 μm of amiloride. In non-injected control oocytes, washout of amiloride had no significant effect (Fig. 1A). In contrast, in ENaC-expressing oocytes washout of amiloride revealed the presence of an amiloride-sensitive inward current component (ΔIami). Application of trypsin (2 μg ml−1) caused an inward current increase with an initial peak response and a subsequent increase to a sustained plateau (Fig. 1B). Application of amiloride (2 μm) during the plateau phase returned the current level towards its initial baseline value. This demonstrates that the sustained component of the trypsin-stimulated inward current is caused by a stimulation of ENaC activity. In contrast, the initial peak response to trypsin (2 μg ml−1) was also observed in control oocytes (Fig. 1A) and in ENaC-expressing oocytes in the presence of amiloride (data now shown). Thus, it is not caused by an activation of ENaC but probably caused by an activation of an endogenous calcium-activated chloride conductance (Chraibi et al. 1998). At a holding potential of −60 mV, application of trypsin (2 μg ml−1) caused on average a 7.7 ± 0.3-fold increase in ΔIami (n= 115; N= 11) within 2 min.
Figure 1. Stimulation of ENaC currents by trypsin is not caused by an increase in channel surface expression.
The representative whole-oocyte current (I) traces shown in A and B were recorded at a holding potential (Vhold) of −60 mV. Trypsin (2 μg ml−1) and amiloride (Ami; 2 μm) were applied as indicated by the bars. In A, a control recording is shown from an oocyte injected with RNase-free water (n.i.) whereas the trace shown in B was recorded from an oocyte expressing αβγ-ENaC (wt-ENaC). Experiments shown in C were performed in oocytes expressing wild-type α- and γ-ENaC together with a FLAG-tagged β-subunit for the detection of ENaC surface expression using a chemiluminescence assay. Open columns represent the average amiloride (2 μm)-sensitive whole-oocyte currents (ΔIami) before the application of trypsin (control) and 2 min after the application of trypsin (trypsin) at a holding potential of −60 mV (n= 14). Hatched columns show the corresponding surface expression values measured in oocytes from the same batch that were either exposed for 2 min to a bath solution with trypsin (2 μg ml−1) or to a control solution without trypsin (n= 22 for each group). As an additional control, baseline ΔIami(n= 14) and surface expression (n= 22) were measured in oocytes injected with twice the normal amount of cRNA (2 × cRNA), i.e. 0.2 rather than 0.1 ng subunit−1 oocyte−1. Non-injected oocytes (n.i.) were used to determine the non-specific background chemiluminescence signal (n= 22).
The stimulation of ΔIami by trypsin may be caused by an increase in channel open probability (Po) or by an increase of the overall number of channels expressed at the cell surface. Therefore, we assessed in parallel the effect of trypsin on ΔIami and on channel surface expression by using a FLAG-tagged β-subunit of ENaC (FLAG-β-ENaC) and a chemiluminescence assay. The FLAG-β-ENaC was co-expressed with wild-type α- and γ-ENaC. As shown in Fig. 1C, exposure to trypsin for 2 min had no significant effect on channel surface expression while it increased ΔIami by about 9-fold. To confirm that the chemiluminescence assay can reliably detect an increase in channel surface expression under our experimental conditions, we performed control experiments in which the amount of cRNA injected per oocyte was doubled. As expected, this increased both ΔIami and surface expression by a factor of about two (Fig. 1C). Similar results as shown in Fig. 1C were obtained in four different batches of oocytes. We conclude that the large stimulatory effect of trypsin on ΔIami is not caused by an increase in channel surface expression. This confirms previous reports that neither trypsin nor CAP-1 increased ENaC surface expression (Chraibi et al. 1998; Vuagniaux et al. 2002). Thus, ENaC stimulation by trypsin is mainly caused by an activation of channels that are already present in the plasma membrane.
γ-ENaC is critical for the trypsin effect
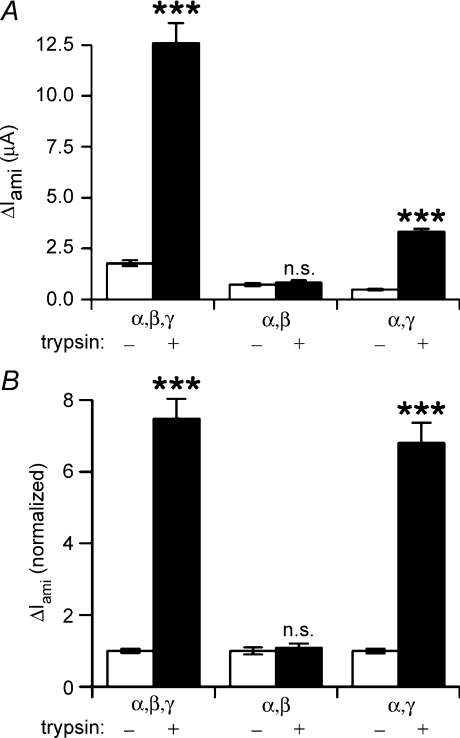
To investigate which ENaC subunit may be critical for mediating the stimulatory effect of extracellular trypsin, we performed experiments in which we compared the effect of trysin on ΔIami in oocytes expressing all three ENaC subunits (αβγ) to its effect on ΔIami in oocytes expressing either αγ-ENaC or αβ-ENaC. The latter two subunit combinations give rise to typical amiloride-sensitive ENaC currents but show reduced channel surface expression and slightly altered single-channel properties compared to wild-type αβγ-ENaC (Canessa et al. 1994; Firsov et al. 1996; McNicholas & Canessa, 1997; Konstas & Korbmacher, 2003). To compensate for the reduced surface expression and to achieve sizable currents in oocytes expressing αβ-ENaC or αγ-ENaC, 1 ng cRNA per subunit was injected in these oocytes instead of 0.1 ng per subunit used for the oocytes expressing all three subunits. Representative data obtained in one batch of oocytes are shown in Fig. 2A. Normalized data from similar experiments in three different batches of oocytes are summarized in Fig. 2B. Trypsin had the usual stimulatory effect in αβγ-ENaC-expressing oocytes causing a 7.5 ± 0.6-fold (n= 29; N= 3) increase in ΔIami. In contrast, in αβ-ENaC-expressing oocytes the stimulatory effect of trypsin was essentially abolished, while the stimulatory effect was preserved in the αγ-ENaC-expressing oocytes. The 6.8 ± 0.6-fold (n= 29; N= 3) increase in ΔIami in αγ-ENaC oocytes was similar to that observed in matched αβγ-ENaC oocytes. The absence of a significant trypsin effect in αβ-ENaC-expressing oocytes indicates that the γ-subunit is critically important for mediating trypsin's stimulatory effect on ENaC. It should be noted that our findings do not dismiss the well-documented role of α-ENaC in the proteolytic processing and activation of the heterotrimeric channel (Hughey et al. 2004a; Sheng et al. 2006). We also have to consider the possibility that αβ-ENaC has a constitutively high open probability which may prevent its further activation by trypsin. Indeed, it has been reported that in cell-attached patches from oocytes expressing αβ-ENaC, single-channel activity could only be resolved with 1 μm amiloride in the pipette solution. From this it was concluded that in the absence of amiloride the Po of αβ-channels is close to one (Fyfe & Canessa, 1998). However, previously published single-channel recordings do not support this hypothesis (McNicholas & Canessa, 1997).
Figure 2. The γ-subunit is critical for ENaC activation by trypsin.
Experiments were performed in oocytes expressing the following subunit combinations: αβγ-ENaC (α,β,γ; n= 29), αβ-ENaC (α,β; n= 29), or αγ-ENaC (α,γ; n= 29). To achieve sizeable currents in oocytes expressing αβ-ENaC or αγ-ENaC, these oocytes were injected with 1 ng cRNA per subunit while 0.1 ng cRNA per subunit was used in oocytes expressing all three subunits. ΔIami was determined before (open columns) and after (filled columns) exposing the oocytes to trypsin (2 μg ml−1) for 2 min. A, representative ΔIami values obtained in a single batch of oocytes (n= 10 per group). B, to summarize experiments from three different batches of oocytes (N= 3) and to compare the relative responses to trypsin in oocytes expressing different subunit combinations, the ΔIami values were normalized for each group to the corresponding mean ΔIami value before trypsin application (n= 29 per group).
Identification of a highly conserved putative prostasin site in γ-ENaC
Our data and recently published studies (Adebamiro et al. 2007; Bruns et al. 2007; Harris et al. 2007) indicate that the γ-subunit is critical for ENaC activation by extracellular proteases. Therefore, we asked the question whether a non-furin cleavage site in γ-ENaC is responsible for mediating the stimulatory effect of trypsin. Since prostasin has been shown to activate ENaC (Vuagniaux et al. 2000; Adachi et al. 2001) we searched for a putative prostasin cleavage site in rat γ-ENaC. The substrate recognition motif for prostasin is thought to correspond to the following amino acid sequence: [R,K]-[H,K,R]-X-[R,K] (Shipway et al. 2004). We identified four such sites within the three ENaC subunits. Three of those correspond to previously described putative furin cleavage sites (Hughey et al. 2004a; Sheng et al. 2006). However, the fourth site is not a predicted furin cleavage site and is localized in the extracellular loop of γ-ENaC. This putative prostasin cleavage site (178RKRK181 in rat γ-ENaC) is highly conserved in mammalian and Xenopusγ-ENaC sequences (Fig. 3A) and corresponds to the 183RKRK186 sequence in mouse γ-ENaC. Recently, this latter motif has been identified as a putative prostasin site by another research group (Bruns et al. 2007). To investigate the functional importance of this site, we mutated the critical Lys residue in position 181 to an alanine (γK181A).
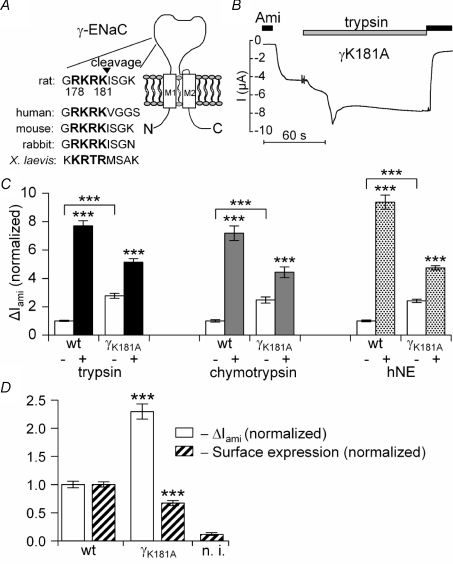
Figure 3. Mutating a putative prostasin site in the γ-subunit increases ENaC whole-oocyte currents, but decreases channel surface expression and reduces the stimulatory effect of extracellular proteases.
In A, the amino acid sequence alignment illustrates a highly conserved putative prostasin cleavage site (bold letters) in γ-ENaC of different species. In B and C, αβγ-ENaC (wt) or αβ-ENaC together with the mutant K181A γ-ENaC (γK181A) were expressed in Xenopus laevis oocytes to test the effect of the mutation on ΔIami baseline whole-oocyte currents and on the responsiveness to extracellular proteases. A representative whole-oocyte current (I) trace from an oocyte expressing γK181A is shown in B. In C, open columns represent the average ΔIami values before application of proteases. The black, grey and dotted columns represent the average ΔIami values 2 min after exposure to trypsin (2 μg ml−1; n= 82 for wt, n= 83 for γK181A; N= 8), chymotrypsin (2 μg ml−1; n= 43 for each group; N= 3) or human neutrophil elastase (hNE) (10 μg ml−1; n= 32 for each group; N= 3), respectively. ΔIami values were normalized to the average ΔIami measured in the αβγ-ENaC expressing oocytes before the application of proteases. In D, surface expression (hatched columns) and ΔIami (open columns) were measured in matched oocytes expressing either wild-type ENaC or the γK181A mutant channel (N= 4). Non-injected oocytes (n.i.) served as control.
The γK181A mutation increases baseline ENaC currents but reduces the stimulatory response to trypsin, chymotrypsin and human neutrophil elastase (hNE)
As shown in Fig. 3, average baseline ΔIami was significantly larger in oocytes expressing the γK181A channel compared to ΔIami measured in matched control oocytes expressing wild-type ENaC. On average, the γK181A mutation increased baseline ΔIami by 2.6 ± 0.1-fold (n= 158; N= 14; P < 0.001). However, in oocytes expressing the γK181A mutant channel the relative stimulatory response to trypsin was substantially reduced (Fig. 3B and C). On average, trypsin caused a 2.1 ± 0.1-fold (n= 83, N= 8) increase in ΔIami in oocytes expressing the γK181A mutant ENaC compared to a significantly larger 7.7 ± 0.4-fold (n= 82, N= 8; P < 0.001) increase in matched control oocytes expressing wild-type αβγ-ENaC. To investigate whether the γK181A mutation also affects the responsiveness of the channel to two other proteases known to stimulate ENaC, we also performed experiments with chymotrypsin and human neutrophil elastase (hNE). As shown in Fig. 3C, the stimulatory effects of chymotrypsin (2 μg ml−1) and neutrophil elastase (10 μg ml−1) on ΔIami were similar to that of trypsin. Importantly, the relative stimulatory effect of chymotrypsin and hNE on ΔIami was also reduced by the γK181A mutation.
The stimulatory effect of the γK181A mutation on baseline ΔIami is not caused by an increase in channel surface expression
The increased baseline ΔIami in oocytes expressing the γK181A mutant ENaC may be caused by an increase in surface expression of the mutant channel or by an increase in open probability (Po) of the channels expressed at the cell surface. To investigate this, we used FLAG-β-ENaC and the chemiluminescence assay to evaluate channel surface expression in parallel with ΔIami measurements. Surprisingly, channel surface expression of the mutant channel was significantly reduced compared to that of matched control oocytes, while ΔIami was significantly increased (Fig. 3D). In this set of experiments the γK181A mutation increased ΔIami by 2.3 ± 0.1-fold (n= 44; N= 4) while it reduced surface expression by about 30%. These findings suggest that fewer mutant channels than wild-type channels are expressed at the cell surface but that the mutant channels are more active.
Specificity of the γK181A mutation
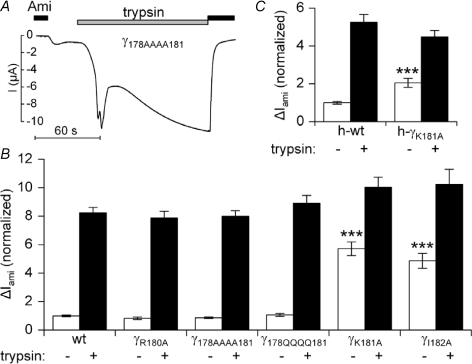
To investigate the specificity of the gain-of-function effect of the γK181A mutation, we generated a γ-ENaC construct in which we mutated the conserved arginine residue in position 180 to alanine. As shown in Fig. 4B the γR180A mutation did not significantly affect ENaC baseline currents nor did it reduce the stimulatory response to trypsin. Furthermore, mutating all four amino acids of the highly conserved RKRK prostasin consensus motif either to alanine (γ178AAAA181) or to glutamine (γ178QQQQ181) left the baseline ENaC currents remarkably unchanged and did not alter the responsiveness to extracellular trypsin (Fig. 4A and B). To further confirm the specificity of the effect of the γK181A mutation, we introduced a corresponding mutation in the γ-subunit of human ENaC (hγK181A). As shown in Fig. 4C, the hγK181A mutation had a similar gain-of-function effect as the corresponding mutation in rat γ-ENaC. Interestingly, mutating the isoleucine residue that follows the RKRK motif in rat γ-ENaC to alanine (γI182A), increased ENaC baseline currents almost to the same extent as the γK181A mutation and also reduced the relative stimulatory response to trypsin (Fig. 4B). Collectively, these findings indicate that mutating the last lysine residue (γK181) in the RKRK motif has a specific effect which cannot be mimicked by mutating the preceding arginine residue or by mutating the entire consensus motif. However, the gain-of-function effect of the γK181A can be mimicked by an adjacent mutation outside the RKRK motif.
Figure 4. Specificity of the γK181A mutation.
Rat αβγ-ENaC (wt) or αβ-ENaC together with different γ-ENaC mutants R180A (γR180A), 178AAAA181 (γ178AAAA181), 178QQQQ181 (γ178QQQQ181), K181A (γK181A) and I182A (γI182A) were expressed in Xenopus laevis oocytes to test the effect of the mutation on ΔIami baseline whole-cell currents and on the responsiveness to trypsin (n= 24; N= 3 for each group). A representative whole-oocyte current (I) trace from an oocyte expressing γ178AAAA181 is shown in A. Summary data for the various mutants are given in B. In C, human αβγ-ENaC (h-wt) or human αβ-ENaC together with the human mutant K181A γ-ENaC (h-γK181A) were expressed in Xenopus laevis oocytes (n= 39; N= 3 for each group). Open columns represent the average ΔIami values before application of trypsin. The filled columns represent the average ΔIami values 2 min after exposure to trypsin (2 μg ml−1). ΔIami values were normalized to the average ΔIami measured in the corresponding αβγ-ENaC expressing oocytes before the application of trypsin.
The γK181A mutation does not alter the single-channel conductance of ENaC
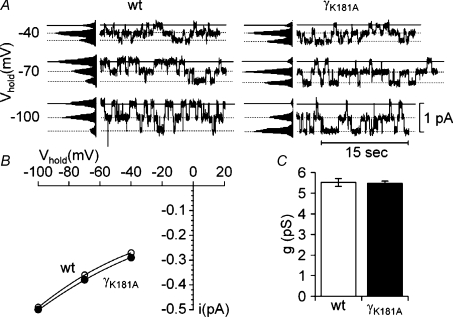
A gain-of-function phenotype of a channel mutation may be caused by an increase in the single-channel conductance of the mutant channel. Therefore, we investigated amiloride-sensitive single-channel currents in outside-out patches from αβγ-ENaC-expressing oocytes and from oocytes expressing the γK181A mutant channel 2 days after cRNA injection. Typical single-channel current traces and I–V plots are shown in Fig. 5 with no apparent differences between wild-type ENaC and the γK181A mutant channel. For wild-type ENaC, the single-channel current amplitude averaged −0.39 ± 0.01 pA (n= 8, N= 5) at a holding potential of −70 mV. This corresponds to a single-channel conductance of 5.52 ± 0.19 pS which is typical for rat ENaC (Canessa et al. 1994; Garty & Palmer, 1997; Kellenberger & Schild, 2002; Diakov & Korbmacher, 2004). The single-channel current amplitude of the γK181A mutant ENaC averaged −0.38 ± 0.01 pA (n= 6, N= 3), corresponding to a single-channel conductance of 5.48 ± 0.10 pS, not significantly different from the single-channel conductance of wild-type ENaC (Fig. 5C).
Figure 5. The γK181A mutation does not alter the single-channel conductance of ENaC.
The representative single-channel current traces shown in A were obtained at holding potentials (Vhold) of −40, −70 and −100 mV (as indicated) from outside-out patches of oocytes expressing wild-type αβγ-ENaC (wt) or wild-type αβ-ENaC together with the mutant K181A γ-ENaC (γK181A). Amplitude histograms (shown to the left of the traces) were used to determine the single-channel current amplitude (i) at each holding potential. The corresponding single-channel I–V plots are shown in B. Average single-channel conductance (g) derived from the single-channel current amplitude measured at a holding potential of −70 mV are shown in C for wild-type (wt; open column; n= 8; N= 5) and mutant ENaC (γK181A; filled column, n= 6; N= 3).
Single-channel recordings demonstrate that trypsin activates near-silent channels
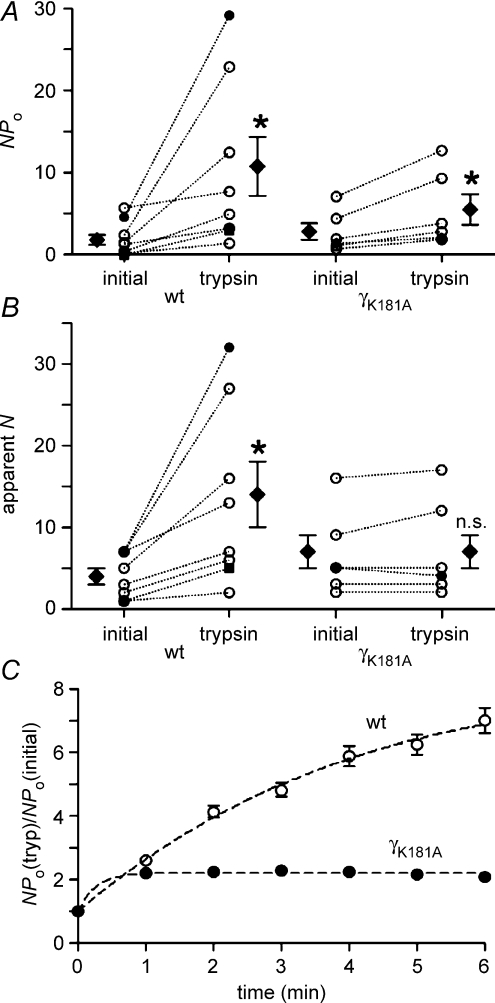
Our surface expression experiments demonstrated that insertion of new channels in the plasma membrane contributes very little if at all to ENaC stimulation by trypsin. Thus, trypsin is likely to stimulate ENaC by increasing the Po of channels already present in the plasma membrane. In this case, trypsin may either uniformly stimulate Po of all ENaC channels present in the plasma membrane or, alternatively, may activate near-silent channels (Caldwell et al. 2004, 2005). To investigate this, we performed single-channel recordings in outside-out patches and tested the effect of trypsin. In the recording shown in Fig. 6A the initial washout of amiloride revealed single-channel activity with up to seven apparent channel levels. About 2 min after trypsin application at least 19 channels were active in the patch (inset Fig. 6A). The single-channel amplitude remained unchanged at about – 0.4 pA. The trypsin effect reached its maximum after about 5–6 min. At this stage, NPo had increased to about 29 with at least 32 channels active in the patch. In some experiments with very low initial ENaC activity, the stimulatory effect of trypsin was particularly impressive. An example is shown in Fig. 6B. In this experiment only a few, brief openings of a single channel were observed prior to the application of trypsin (NPo= 0.01). However, trypsin caused the appearance of up to five additional channel levels and largely increased the duration of channel openings (NPo= 3.01). Results from similar experiments as shown in Fig. 6A and B are summarized in Fig. 7. On average, trypsin increased NPo by about 6-fold from 1.76 ± 0.60 to 10.74 ± 3.59 (n= 8; N= 5; P < 0.05) and increased the number of apparent channel levels from 4 ± 1 to 14 ± 4 (n= 8; N= 5; P < 0.05). In summary, our single-channel data indicate that recruitment of near-silent channels largely contributes to the stimulatory effect of trypsin on ENaC activity.
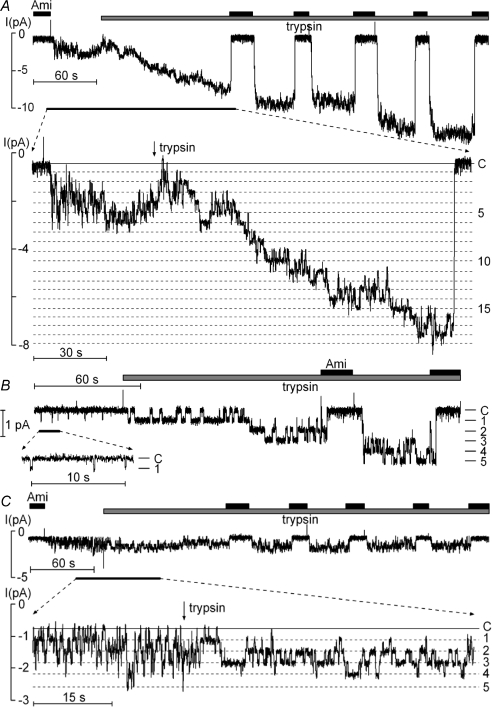
Figure 6. Trypsin activation of near-silent channels is absent in outside-out membrane patches from oocytes expressing the γK181A mutant ENaC.
The representative current recording shown in A was obtained at a holding potential of −70 mV from an outside-out patch of an oocyte expressing wild-type αβγ-ENaC. The inset shows a segment of the same current trace on expanded scales. The segment corresponds to the time interval indicated by the black bar below the trace. Amiloride (Ami; 2 μm) and trypsin (2 μg ml−1) were present in the bath solution as indicated above the trace by black and grey bars, respectively. The current level at which all channels are closed (C) was determined in the presence of amiloride. The different channel open levels are indicated by horizontal dotted lines. B, similar experiment as shown in A but with a lower initial ENaC activity. C, the experiment was performed as described in A but was obtained from an outside-out patch of an oocyte expressing the γK181A mutant ENaC.
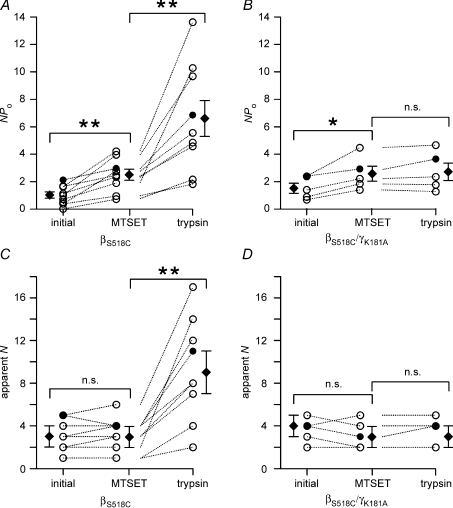
Figure 7. The γK181A mutation prevents the recruitment of near-silent channels but a stimulatory effect of trypsin on NPo is preserved.
Amplitude histograms were used to calculate NPo values (A) from outside-out patch-clamp recordings as shown in Fig. 6. The maximal number of apparent channel levels was determined (apparent N) by visual inspection of the traces (B). For each individual experiment, values are given before (initial) and after the application of trypsin (trypsin) and are connected by dotted lines. Data points represented by • and ▪ are from the experiments shown in Fig. 6. ⋄ mean values. C, time course of the ratio between NPo values after application of trypsin (NPo(tryp)) and the initial NPo value (NPo(initial)) for wild-type ENaC (wt; ◯;) and the γK181A mutant (γK181A; •).
No activation of near-silent channels by trypsin in outside-out patches from oocytes expressing γK181A mutant ENaC
To investigate the functional consequence of the γK181A mutation at the single-channel level, we tested the effect of trypsin on outside-out patches from oocytes expressing the γK181A mutant ENaC. A representative current trace is shown in Fig. 6C. Before trypsin application, up to five discrete channel levels were present in the trace. Before the application of trypsin NPo was about 1.4. While trypsin altered the single-channel kinetics of the γK181A mutant ENaC (inset in Fig. 6C), it did not cause additional channel levels to appear. After trypsin application, prolonged channel openings were regularly observed and the simultaneous closure of all channels became infrequent. These changes in channel gating caused an overall increase in NPo to about 2.1. In similar experiments, the average trypsin-induced increase in NPo was about 2-fold from 2.79 ± 1.02 to 5.48 ± 1.85 (n= 6; P < 0.05; Fig. 7). Thus, a doubling of the average single-channel Po of active channels in the patch can fully account for the trypsin-induced increase in NPo. In contrast, the absence of a trypsin-induced increase in the apparent number of active channels indicates that recruitment of near-silent channels does not contribute to the stimulatory effect of trypsin on NPo in patches with the γK181A mutant ENaC (Figs 6C and 7).
The altered responsiveness of the mutant channel to trypsin is also illustrated by the different time courses of current activation shown in Fig. 7C. While current activation of the mutant channel reaches a plateau within 1 min of trypsin application, the current continues to increase for up to 6 min in outside-out patches with wild-type ENaC. The rapid activation of the mutant channel reflects the rapid change in channel gating observed upon exposure to trypsin (Fig. 6C). In the mutant channel, this rapid stimulatory effect on channel gating is restricted to channels that are already active in the patch before the application of trypsin. In contrast, the slower time course of current activation in outside-out patches with wild-type ENaC can be attributed to the protracted and successive recruitment of near-silent channels after application of trypsin (Fig. 6A and B)which is not observed in patches with the mutant channel (Fig. 6C). The additional recruitment of near-silent channels also explains the larger overall effect of trypsin on wild-type ENaC compared to its effect on the mutant channel (Fig. 7C).
Separate effects of trypsin on channel gating and on the recruitment of near-silent channels
The stimulatory effect of trypsin on channel gating (inset in Fig. 6C) is reminiscent of the effect of the sulfhydryl reagent MTSET on rat ENaC with a S518C mutation in its β-subunit. The mutant channel can be converted to a channel with a Po of nearly one by exposing it to MTSET, which destabilizes the channel's closed state. This results in channel gating with long open dwell times and short channel closures. Open channels are readily modified by MTSET, with a mean time between channel opening and MTSET modification of about 170 ms. In contrast, brief channel openings of 10–20 ms appear not to be sufficient for the modification of the channel by MTSET (Kellenberger et al. 2002). Thus, near-silent channels with infrequent and brief channel openings are unlikely to be transformed by a short-term exposure to MTSET. We hypothesized that MTSET can mimic the effect of trypsin on the gating of channels that are already active in the patch. Consequently, the stimulatory effect of trypsin on channel gating should be largely reduced or even abolished after stimulating the βS518C mutant ENaC with MTSET. In contrast, trypsin after MTSET should still activate near-silent channels and this second effect of trypsin should be prevented by the K181A mutation in γ-ENaC. To test this hypothesis we performed co-expression experiments using wild-type α-ENaC and the S518C mutant β-subunit (βS518C) combined either with wild-type γ-ENaC or with the K181A mutant γ-subunit.
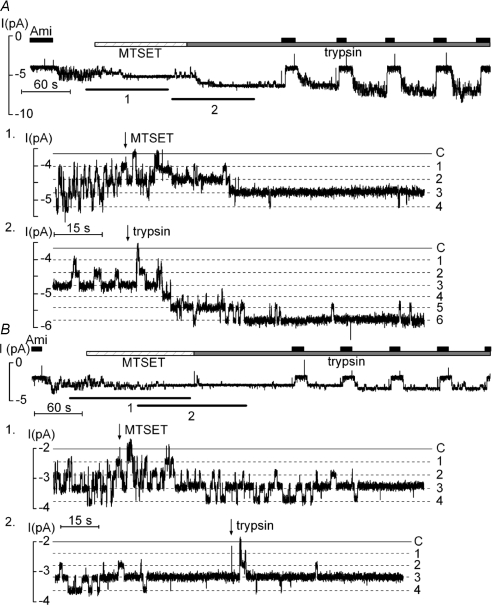
As shown in Fig. 8A, application of MTSET had the expected effect on the gating of the βS518C mutant channel and resulted in a channel activity characterized by long open dwell times and short channel closures. This effect of MTSET on channel gating is reflected by an overall increase in NPo. However, application of MTSET was not associated with an increase in the number of apparent channel levels. Thus, MTSET did not lead to the recruitment of near-silent channels (inset 1 in Fig. 8A). In contrast, subsequent application of trypsin was associated with the appearance of additional channel levels presumably by activating channels from the pool of near-silent channels (inset 2 in Fig. 8A). When similar experiments were performed with a βS518C/γK181A double mutant channel (Fig. 8B), the stimulatory effect of MTSET on gating was preserved (inset 1 in Fig. 8B). As expected, subsequent application of trypsin had no additional effect on channel gating and failed to recruit near-silent channels in patches with the βS518C/γK181A double mutant channel. Data from similar experiments as shown in Fig. 8 are summarized in Fig. 9. Taken together, these findings suggest that trypsin has two distinct effects on ENaC activity. Indeed, our experiments with the βS518C mutant channel and the combined βS518C/γK181A mutant channel demonstrate that the effect of trypsin on channel gating can be functionally separated from its effect on the recruitment of near-silent channels.
Figure 8. Dual effect of trypsin on channel gating and on the recruitment of near-silent channels is confirmed by experiments using the β518C mutant ENaC.
A, in outside-out patches trypsin activates additional near-silent channels after MTSET has altered the gating of active βS518C mutant ENaCs. The experiment was essentially performed as described in Fig. 6 but using an oocyte expressing the βS518C mutant ENaC. Moreover, MTSET (1 mm) was applied as indicated prior to the application of trypsin. The insets (1 and 2) show segments of the same current trace on expanded scales which correspond to the time intervals indicated by the black bars (1, 2) below the trace. B, the experiment was performed as in A but with an oocyte expressing the βS518C/γK181A double mutant ENaC.
Figure 9. After MTSET, the stimulatory effect of trypsin on near-silent channels is preserved in wild-type ENaC but lost in the γK181A mutant channel.
Summary of NPo (A and B) and apparent N (C and D) values derived from outside-out patch-clamp recordings as shown in Fig. 8. For each individual experiment, three NPo values and three apparent N values are given (initial, MTSET, trypsin) and are connected by dotted lines. ⋄ represent mean values. The values indicated by • were obtained from the experiments shown in Fig, 8.
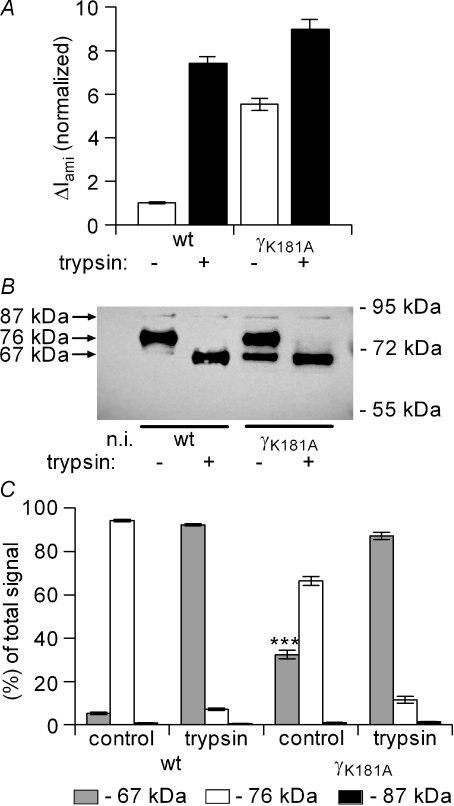
ENaC stimulation by trypsin and the γK181A mutation are associated with the appearance of a 67 kDa fragment of γ-ENaC
It has previously been shown that ENaC activation by exogenous hNE or by co-expression of prostasin is associated with the appearance of a 67 kDa fragment of γ-ENaC at the cell surface which is thought to correspond to the activated pool of channels (Bruns et al. 2007; Harris et al. 2007). To investigate whether trypsin has a similar effect and whether the cleavage pattern is altered by the γK181A mutation, we performed biotinylation experiments to detect trypsin cleavage products of V5-tagged γ-ENaC expressed at the cell surface using Western blot analysis. As shown in Fig. 10, the predominant γ-ENaC fragment detected in wild-type ENaC-expressing control oocytes had a molecular weight of about 76 kDa. This band is thought to correspond to the γ-subunit cleaved at its putative furin site by endogenous proteases when γ-ENaC is co-expressed with the α- and β-subunits (Hughey et al. 2004a; Bruns et al. 2007; Harris et al. 2007, 2008). The signal detected at about 87 kDa, the predicted size of the full-length rat γ-subunit, was usually very faint or even absent which is in agreement with previously reported data (Harris et al. 2007, 2008). In oocytes exposed to trypsin for 3 min, a time sufficient for maximal ENaC current activation, the 76 kDa band essentially disappeared. Instead, after exposure to trypsin the main γ-ENaC fragment detected at the cell surface had a molecular weight of about 67 kDa (Fig. 10B). Thus, trypsin activation of the channel was associated with cleavage of the γ-subunit at an additional site. The 67 kDa cleavage product observed after trypsin treatment is similar to that identified after channel activation by exogenous hNE (Harris et al. 2007) or by co-expression of prostasin (Bruns et al. 2007). This suggests that trypsin, hNE and prostasin can activate the channel by causing γ-ENaC cleavage at identical or closely adjacent sites.
Figure 10. The γK181A mutation is associated with the constitutive presence of a 67 kDa fragment which is similar to the cleavage product observed after trypsin application.
Constructs with a C-terminal V5 tag were used for wild-type γ-ENaC (wt) and the γK181A mutant subunit (γK181A) to detect biotinylated cell surface γ-ENaC fragments by Western blot analysis in parallel with ΔIami measurements. The V5 tagged γ-subunits were co-expressed in Xenopus laevis oocytes with wild-type α-ENaC and β-ENaC. A, average ΔIami values are shown for wt and for γK181A-expressing oocytes before (open columns; trypsin –) and after (filled columns, trypsin +) exposure to trypsin (2 μg ml−1) for 2 min. ΔIami values were normalized to the average ΔIami measured in wt-expressing oocytes from the same batch prior to the application of trypsin (for each column: n= 76; N= 8). B, the lanes of the representative Western blot correspond to the experimental conditions of the current data shown above the lanes in A. Non-injected oocytes (n.i.) were used to demonstrate the absence of a V5 signal in oocytes that do not express the tagged γ-ENaC. Numbers to the right of the gel represent the mobility of protein standards in kDa (PageRuler™ Prestained Protein Ladder, Fermentas). C, summary of Western blot results from similar experiments (n= 10; N= 8) as shown in B. For each lane the signals detected in the regions of about 67 kDa (grey columns), 76 kDa (open columns), and 87 kDa (black columns) were estimated and normalized to the sum of the signal detected. The resulting columns are grouped in triplets with the corresponding experimental conditions indicated below. The average signal for the 67 kDa fragment before exposure to trypsin was significantly larger in the γK181A-expressing oocytes compared to the 67 kDa signal detected in the wt-expressing oocytes (***P < 0.001).
Interestingly, a similar 67 kDa band was constitutively present at the cell surface of oocytes expressing the γK181A mutant channel even without application of trypsin (Fig. 10B). This suggests that the mutant channel is not dependent on exogenous proteases to appear at the cell surface in an activated cleaved form. If the appearance of the 67 kDa fragment indicates the transition of near-silent to active channels, this would mean that mutant channels expressed at the cell surface are usually more active than wild-type channels. This probably explains our finding that the γK181A mutation increases baseline ENaC currents while channel surface expression is reduced. A constitutive cleavage of the γK181A mutant may also explain the reduced stimulatory effect of exogenous proteases on the mutant channel and is consistent with our observation that trypsin fails to activate near-silent channels in oocytes expressing the mutant channel. Figure 10C summarizes results from similar experiments as shown in Fig. 10B. This summary confirms that the γK181A mutation results in the spontaneous appearance of a 67 kDa fragment which also appears upon exposure of the oocytes to exogenous trypsin.
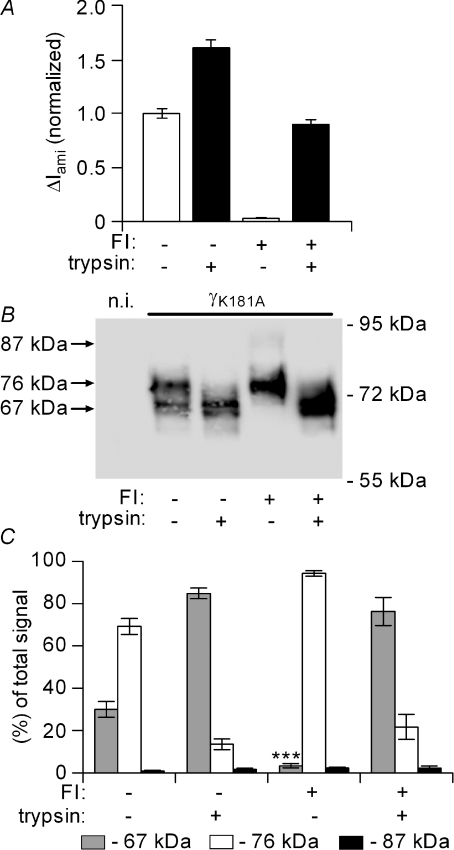
Furin inhibitor prevents the spontaneous appearance of the 67 kDa γ-ENaC fragment in oocytes expressing the γK181A mutant channel
We speculated that the γK181A mutation promotes channel cleavage by an endogenous protease. Therefore, we tested the effects of the serine protease inhibitor aprotinin (100 μg ml−1) and of the furin inhibitor dec-RVKR-cmk (40 μm). Exposure to aprotinin for 48 h did not alter the constitutive appearance of the 67 kDa cleavage product and did not change the stimulatory effect of the γK181A mutation on baseline ΔIami (data not shown). In contrast, a 48 h incubation of oocytes with furin inhibitor largely reduced ΔIami in oocytes expressing the γK181A mutant channel (Fig. 11A) or wild-type ENaC (data not shown). The inhibitory effect of the furin inhibitor on ΔIami is not surprising, since furin-dependent proteolysis is thought to be important for ENaC activation and since mutating putative furin sites in α- and γ-ENaC has previously been shown to reduce baseline ΔIami (Hughey et al. 2004a). Importantly, the furin inhibitor not only abolished the stimulatory effect of the γK181A mutant on ΔIami baseline currents, but also prevented the spontaneous appearance of the 67 kDa cleavage product (Fig. 11B). However, the furin inhibitor did not prevent the stimulatory effect of exogenous trypsin on ENaC currents. In oocytes pre-treated with furin inhibitor the current activation by trypsin was accompanied by the re-appearance of the 67 kDa band, supporting the view that this cleavage product represents the activated form of the γ-subunit. It should be noted that the furin inhibitor did not prevent the cleavage of full length γ-ENaC to the 76 kDa product, which would be expected if the 76 kDa fragment was the result of cleavage by endogenous furin. However, the putative furin cleavage site in γ-ENaC may be cleaved by other endogenous convertases which may explain the lack of effect of the furin inhibitor to favour the appearance of the 87 kDa full-length band. Figure 11C summarizes results from similar experiments as shown in Fig. 11B.
Figure 11. Furin inhibitor prevents the spontaneous appearance of the 67 kDa γ-ENaC fragment in oocytes expressing the γK181A mutant channel.
Experiments were essentially performed as described in Fig. 10 using oocytes expressing the γK181A mutant ENaC containing a C-terminal V5 tag. After cRNA injection, oocytes were divided into two groups. One group was exposed to 40 μm of the furin inhibitor dec-RVKR-cmk (FI +) for 48 h prior to the measurements, another group served as vehicle-treated control (FI –). In A, average ΔIami values are shown for FI + and for FI – incubated oocytes before (open columns; trypsin –) and after (filled columns, trypsin +) exposure to trypsin (2 μg ml−1) for 2 min. ΔIami values were normalized to the average ΔIami measured in FI – incubated oocytes from the same batch prior to the application of trypsin (for each column: n= 54; N= 7). B, the lanes of the representative Western blot correspond to the experimental conditions of the current data shown above the lanes in A. Non-injected oocytes (n.i.) were used to demonstrate the absence of a V5 signal in oocytes that do not express the tagged γ-ENaC. C, summary of Western blot results from similar experiments (n= 7; N= 7) as shown in B. The average signal for the 67 kDa fragment before exposure to trypsin was significantly smaller in the FI + incubated oocytes compared to the 67 kDa signal detected in the FI – incubated oocytes.
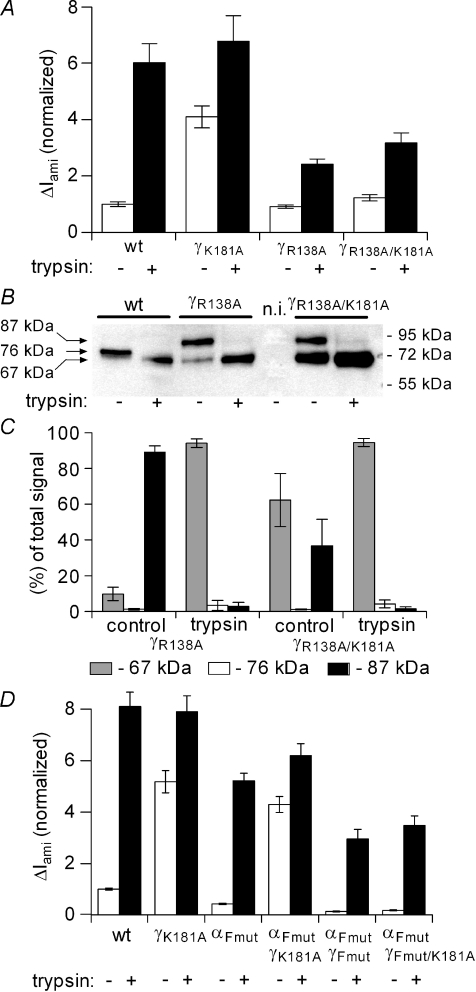
Mutating a putative furin site in γ-ENaC prevents the gain-of-function phenotype of the γK181A mutation
It has recently been shown that the γ-subunit of ENaC in αβγ-complexes appears mainly as a 76 kDa species at the cell surface (Harris et al. 2007, 2008), which is consistent with our findings shown in Figs 10 and 11. This 76 kDa fragment could not be detected when the P1 site in the furin consensus site of γ-ENaC was mutated (γR138A). Interestingly, the basal ENaC current was similar in oocytes expressing the mutant channel compared to oocytes expressing wild-type αβγ-ENaC (Harris et al. 2008). To investigate the possible importance of the furin cleavage site for the gain-of-function phenotype of the γK181A mutant channel, we produced a γ-ENaC construct with a double mutation γR138A/γK181A and expressed this in Xenopus laevis oocytes. Consistent with the work of Harris et al. (2008), oocytes expressing the γR138A mutant ENaC had similar currents as oocytes expressing wild-type ENaC. We also confirmed that in oocytes expressing the γR138A mutant channel only the 87 kDa full length γ-ENaC fragment was observed at the cell surface but not the 76 kDa fragment which is the predominant fragment in wild-type ENaC (Fig. 12A). This indicates that the γR138A mutant channel is no longer cleaved at the mutated furin consensus site. In addition, we demonstrated that the γR138A mutant channel remains responsive to trypsin and that channel activation is associated with the appearance of a 67 kDa fragment at the cell surface. The current data of oocytes expressing the double mutant γR138A/γK181A channel looked similar to those of oocytes expressing the γR138A mutant channel. However, in oocytes expressing the γR138A/γK181A double mutant we could detect a strong 67 kDa fragment at the cell surface even before channel activation by trypsin. Thus, the γK181A mutation also promotes spontaneous cleavage of the γ-subunit when it contains an additional γR138A mutation. Interestingly, this enhanced cleavage was not associated with a gain-of-function of the γR138A/γK181A double mutant which is in contrast to the finding with the γK181A single mutation. This suggests that cleavage at the putative furin site of γ-ENaC may be a prerequisite for the gain-of-function effect of the γK181A mutation.
Figure 12. γR138A mutation prevents the gain-of-function phenotype of the γK181A mutation.
The experiments were essentially performed as described in Fig. 10 using oocytes expressing the C-terminal V5 tag constructs for wild-type γ-ENaC (wt), γK181A, γR138A or γR138R/K181A mutant subunits (γK181A, γR138A, γR138A/K181A, respectively). A, average ΔIami values are shown for wt, γK181A, γR138A and γR138A/K181A expressing oocytes before (open columns; trypsin –) and after (filled columns, trypsin +) exposure to trypsin (2 μg ml−1) for 2 min. ΔIami values were normalized to the average ΔIami measured in wt-expressing oocytes from the same batch prior to the application of trypsin (for each column: n= 24; N= 3). B, the lanes of the representative Western blot correspond to the experimental conditions of the current data shown in A. C, summary of Western blot results for γR138A- and γR138A/K181A-expressing oocytes from similar experiments (n= 3; N= 3) as shown in B. Average ΔIami values shown in D were obtained from oocytes expressing either wt-αβγ-ENaC (wt) or channels with one or two mutated subunits as indicated below the columns: γK181A, αFmut (αR205A/R231A), γFmut (γR138A) or γFmut/K181A (n= 18; N= 3 for each group).
Mutating the putative furin cleavage sites in α-ENaC does not prevent the gain-of-function phenotype of the γK181A mutation
To address the question whether the gain of function phenotype of the γK181A mutation depends on intact furin cleavage sites in α-ENaC, we mutated arginine residues to alanine in these putative sites in rat α-ENaC (αR205A/R231A) in analogy to similar mutations previously described for mouse α-ENaC (Hughey et al. 2004a). We confirmed by Western blot analysis that mutating these sites resulted in the expected loss (Hughey et al. 2004a; Harris et al. 2008) of an α-ENaC cleavage product in oocytes co-expressing all three ENaC subunits (data not shown). We also confirmed the findings of Hughey et al. (2004b) that αR205A/R231A reduced ENaC baseline currents but did not prevent ENaC activation by trypsin (Fig. 12D). Importantly, co-expression experiments with αR205A/R231A and γK181A demonstrated that mutating the putative furin consensus sites in α-ENaC does not prevent the gain-of-function effect of the γK181A mutation (Fig. 12D). As expected (Hughey et al. 2004a), ENaC baseline currents were reduced in oocytes expressing ENaC with all three putative furin consensus sites mutated (αR205A/R231A-γR138A) but the stimulatory effect of trypsin was preserved. However, mutating all three furin consensus sites essentially abolished the gain-of-function effect of the γK181A. These results are in good agreement with our observation that pre-treatment of the oocytes with furin inhibitor (Fig. 11) or mutating the putative furin cleavage site in γ-ENaC (Fig. 12A) prevents the stimulatory effect of the γK181A mutation on ENaC currents.
Discussion
In this study we present evidence that ENaC activation by trypsin involves a change in the gating of channels that are already active and a recruitment of additional near-silent channels. Moreover, we demonstrate that a single amino acid mutation (γK181A) within a putative prostasin cleavage site in the channel's γ-subunit causes a gain-of-function effect with increased basal ENaC currents but slightly reduced channel surface expression by about 30%. Surprisingly, this mutation enhances channel cleavage and activation by endogenous furin-like proteases, which probably explains the reduced responsiveness of the mutant channel to exogenous trypsin, chymotrypsin and hNE. The use of the β518C ENaC mutant allowed us to distinguish between the stimulatory effect of trypsin on channel gating and the trypsin-mediated recruitment of near-silent channels. While MTSET mimics the effect of trypsin on channel gating, it does not activate near-silent channels and does not prevent the subsequent recruitment of near-silent channels by trypsin. In the γK181A/β518C double mutant channel the effect of MTSET on channel gating is preserved but subsequent application of trypsin fails to recruit near-silent channels. This is consistent with the interpretation that the mutant channel is pre-activated by endogenous proteases, which reduces the population of near-silent channels. Collectively, our data indicate that trypsin has a dual effect on ENaC, and that the proteolytic activation of near-silent channels critically involves the region of the putative prostasin cleavage site in the channel's γ-subunit.
The γ-subunit of ENaC plays a major role in channel activation by extracellular proteases
In the present study we demonstrate the importance of the γ-subunit of ENaC for channel activation by extracellular proteases since the stimulatory effect of trypsin on ENaC currents was essentially abolished in oocytes expressing αβ-ENaC without the γ-subunit. In contrast, in oocytes expressing αγ-ENaC without the β-subunit the stimulatory effect of trypsin was preserved. Recent biotinylation experiments revealed that cell surface γ-ENaC (but not α- or β-ENaC) was cleaved by the application of neutrophil elastase and that proteolytic cleavage of γ-ENaC correlated with an increase in ENaC currents (Harris et al. 2007). These findings support our conclusion that γ-ENaC is critical for channel activation by extracellular proteases. Furthermore, our surface expression experiments demonstrated that ENaC activation by trypsin is mainly caused by an activation of channels that are already present in the plasma membrane which is in good agreement with previous reports (Chraibi et al. 1998; Vuagniaux et al. 2002). Thus, ENaC activation by extracellular proteases probably involves a modification of the γ-subunit within the heteromeric channel complex present at the cell surface. This could lead to a conformational change of the complex and to channel activation.
All three ENaC subunits (αβγ) have large extracellular domains that are likely targets for proteolytic cleavage. Indeed, three distinct furin cleavage sites have recently been identified, two in the channel's α-subunit and one in its γ-subunit (Hughey et al. 2004a). Interestingly, channel activation by trypsin application was preserved even when all three furin sites were mutated (Hughey et al. 2004a). This suggests that the stimulatory effect of trypsin involves additional cleavage sites. Recently, Bruns et al. (2007) described a putative prostasin cleavage site in the extracellular loop of mouse γ-ENaC which corresponds to the site in rat γ-ENaC identified in the present study. Deletion of the 43 amino acid tract between the known furin site and this novel putative cleavage site resulted in channels with a higher open probability than wild-type ENaC. Moreover, a 43-mer peptide corresponding to the cleaved sequence was shown to inhibit ENaC-mediated transepithelial sodium transport in cultured renal collecting duct cells and in airway epithelial cells in a concentration-dependent manner. It was concluded that excision of an inhibitory peptide from both the α- and γ-subunit of the channel is important for the proteolytic activation of ENaC (Carattino et al. 2006; Bruns et al. 2007). Interestingly, mutation of the furin site in γ-ENaC does not abolish channel activation by trypsin (Hughey et al. 2004a). This suggests that partial activation of ENaC by trypsin does not require simultaneous cleavage at the putative furin and prostasin sites of γ-ENaC, which on the other hand would be a prerequisite for the release of the postulated inhibitory peptide sequence between the two sites (Bruns et al. 2007). However, it is conceivable that trypsin may cleave at a basic residue in the vicinity of the mutated furin site thereby releasing an inhibitory peptide tract between this site and the putative prostasin site in γ-ENaC to activate the channel.
Functional importance of the γK181A mutation
In the present study we demonstrated that mutating a critical residue (K181A) in the putative prostasin cleavage site in γ-subunit of rat ENaC largely reduced the stimulatory effect of trypsin on ENaC currents. Importantly, our single-channel recordings in outside-out patches demonstrated that the residual activation of the K181A mutant ENaC by trypsin was caused by changing the gating of channels that were already active in the patch prior to the application of trypsin. However, trypsin failed to activate additional near-silent channels in patches with the K181A γ-ENaC mutant channel. In contrast, this recruitment of near-silent channels was a major effect of trypsin on wild-type ENaC. Thus, our findings demonstrate that the putative prostasin cleavage site in the γ-subunit is functionally important for the transition from a near-silent channel to an active channel in the membrane by application of extracellular trypsin.
The K181A mutation in γ-ENaC did not prevent the expression of active channels in the plasma membrane. Interestingly, compared to wild-type ENaC, fewer mutant channels are expressed at the cell surface but produce larger whole-oocyte currents. This indicates that the mutant channels that are present in the plasma membrane are more active than wild-type channels. Our biochemical experiments demonstrated that the γK181A mutation causes the spontaneous appearance of an additional 67 kDa fragment of the γ-subunit at the cell surface. A similar fragment appears when wild-type ENaC is stimulated in MDCK cells by the co-expression of prostasin (Bruns et al. 2007) or in oocytes by the application of hNE (Harris et al. 2007) or, as shown in Figs 10–12, by the application of trypsin. Thus, our findings suggest that the γK181A mutation favours the constitutive proteolytic cleavage of the γ-subunit. This conclusion is supported by our finding that a furin inhibitor prevents the spontaneous appearance of the 67 kDa fragment of the mutant γ-subunit. Enhanced constitutive channel cleavage and activation is likely to reduce the population of near-silent channels in the plasma membrane, which would explain the failure of trypsin to activate near-silent channels in oocytes expressing the mutant channel. It would also provide an explanation for the somewhat surprising finding that the mutation increases ENaC baseline currents but reduces channel surface expression.
The γK181A mutation may alter the protease preference of the putative prostasin cleavage site
The 67 kDa cleavage fragment indicates that the constitutive cleavage of the γK181A mutant subunit occurs at a site that is identical or close to the site that is cleaved by co-expression of prostasin (Bruns et al. 2007), by the application of hNE (Harris et al. 2007), or by exposure to trypsin (Figs 10–12). The large extracellular loop of γ-ENaC could be a direct substrate for these proteases, which all have different substrate specificities and predicted cleavage sites. Alternatively, different exogenous proteases with the ability to stimulate ENaC (e.g. trypsin, chymotrypsin and hNE) may converge to activate an endogenous protease cascade (Rossier, 2004), possibly involving a final cleavage step at a specific site resulting in the appearance of the 67 kDa band. Interestingly, the spontaneous cleavage of the γK181A subunit was prevented by a furin inhibitor. Thus, the endogenous protease responsible for the cleavage of the mutant subunit may be a furin-related protease expressed in Xenopus laevis oocytes (Korner et al. 1991; Nelsen et al. 2005).
This leads to the question of how the γK181A mutation may favour cleavage at this site. The ubiquitous proprotein convertase furin is concentrated in the trans-Golgi network (TGN) but may be able to cycle between the cell surface and the TGN (Molloy et al. 1994). It has been proposed that furin preferentially recognizes Arg-X-(Arg/Lys)-Arg as a cleavage site. The Arg in the P1 position seems to be essential and in the vast majority of biologically active furin substrates P2 is Lys or Arg. The minimal furin recognition site has been proposed to consist of Arg at P4 and P1. However, Lys and Val have also been reported to be suitable P4 residues for furin, and the preference for Arg at P4 is not as stringent as that at P1. Indeed, there may not be a strict requirement for a P4 residue in furin-related proprotein convertases. Importantly, efficiently cleaved furin substrates cannot contain bulky hydrophobic residues at both P′1 and P′2, nor can there be a Lys at either position (Rockwell et al. 2002). Thus, it is tempting to speculate that the γK181A mutation converts the highly conserved amino acid sequence 176GRKR180 into a cleavage site for furin or related proteases expressed in the Xenopus laevis oocytes with R180 being the P1 position (Fig. 3A). This would explain why cleavage of the γK181A mutant subunit occurs more readily than cleavage of the wild-type channel.
More than one endogenous protease may cleave at or near the putative prostasin cleavage site under physiological conditions
Recently, it has been shown that mutating the putative RKRK prostasin motif in mouse γ-ENaC to QQQQ (R183Q/K184Q/R185Q/K186Q) essentially abolished the stimulatory effect of co-expressed prostasin on mouse ENaC currents in the Xenopus laevis oocyte expression system (Bruns et al. 2007). This led to the conclusion that prostasin is a likely candidate to contribute to ENaC activation under physiological conditions by cleaving γ-ENaC at this specific site. Interestingly, the homologous mutation in rat γ-ENaC (R178Q/K179Q/R180Q/K181Q9) used in our study did not prevent the stimulatory effect of trypsin on ENaC currents (Fig. 4). It is unlikely that this can be attributed to species differences since mouse and rat ENaC are highly homologous. Instead, it is likely that co-expression of prostasin affects ENaC in a different way than acute exogenous application of extracellular trypsin. Interestingly, in the same paper it was shown that co-expression of a prostasin mutated in its catalytic triad stimulated wild-type ENaC currents to a similar extent as wild-type prostasin but failed to stimulate ENaC with a mutated prostasin motif in γ-ENaC (Bruns et al. 2007). These findings suggest that the stimulatory effect of prostasin co-expression is dependent on the presence of an intact RKRK motif but does not require the proteolytic activity of prostasin which may act by altering the activity of a downstream protease cascade. This is in line with the recently reported finding that although the serine protease inhibitor aprotinin abolishes prostasin-induced activation of ENaC in Xenopus laevis oocytes (Adachi et al. 2001), mutations within the catalytic triad of prostasin do not prevent this activation (Andreasen et al. 2006).
Another possible explanation for the discrepancy between our findings and those reported by Bruns et al. (2007) may be a different cleavage specificity of prostasin and trypsin. While prostasin or dependent proteases may require the presence of the intact RKRK motif in the γ-subunit for ENaC activation, trypsin may still be able to cleave the channel at a closely adjacent site leading to channel activation even if the RKRK motif is mutated to QQQQ. This interpretation is consistent with the recently reported finding that several cleavage sites in this region may be targeted differentially by extracellular proteases, depending on their preferences for certain amino acid sequences (Adebamiro et al. 2007). Thus, in human ENaC, the valine residues in position 182 and 193 seem to be important for ENaC activation by neutrophil elastase but not for channel activation by trypsin. Importantly, inserting a novel cleavage site for thrombin in this region makes the channel responsive to proteolytic stimulation by thrombin which has no effect on wild-type ENaC. These elegant experiments and the mapping of putative elastase cleavage sites adjacent to the putative prostasin cleavage site in γ-ENaC (Adebamiro et al. 2007) suggest that similar stimulatory effects can be achieved by cleaving the channel at different but closely adjacent sites within this functionally important region of γ-ENaC.
This concept is also consistent with our interpretation that the γK181A mutation promotes channel cleavage and thereby causes a gain-of-function phenotype with a reduced responsiveness to trypsin. It should be pointed out that in vivo the predicted RKRK prostasin site in the channel's γ-subunit may not be an exclusive cleavage site for prostasin. While the amino acid sequence corresponds to a commonly accepted consensus sequence for prostasin cleavage, it is an interesting observation that the RKRK sequence is followed by a highly conserved isoleucine residue in all known mammalian ENaC sequences except the human sequence. In vitro studies have demonstrated that an isoleucine following the RKRK motif turns this motif into a rather poor cleavage site for prostasin (Shipway et al. 2004). While our mutagenesis experiments demonstrate that this region in γ-ENaC is functionally critical for the recruitment of near-silent channels by proteases, we cannot be certain that prostasin is the sole and preferred endogenous protease to cleave γ-ENaC at or near this site except perhaps in humans and in Xenopus where the isoleucine is missing. In this context, it is of interest that mutating the isoleucine residue in position 182 to alanine mimicked the effect of the γK181A mutation. This suggests that replacing the isoleucine by an alanine in position 182 may make the channel more accessible to prostasin or other endogenous proteases in analogy to the increased sensitivity to an endogenous furin-like protease as a result of the γK181A mutation. In contrast, mutating the arginine in position 180 or mutating the whole RKRK motif did not result in a similar gain-of-function phenotype as observed with the γK181A mutant channel. This demonstrates that the gain-of-function effect of the γK181A mutation is not a random effect of mutating any amino acid in the RKRK motif. This is further supported by our finding that the gain-of-function effect of the γK181A mutation is not restricted to rat ENaC but can also be observed in human ENaC with the corresponding hγK181A mutation. The human mutation also decreases the stimulatory effect of trypsin to a similar extent as the K181A mutation of rat γ-ENaC. Taken together, our findings confirm that this region of γ-ENaC is important for the proteolytic activation of ENaC by extracellular proteases. However, the relevant protease(s) that cleave in this region under physiological conditions remain elusive at present and may be different in different tissues.
Mutating the putative furin site in γ-ENaC prevents the gain-of-function phenotype of the γK181A mutation
As mentioned above, it has recently been reported that furin- and prostasin-dependent release of an inhibitory tract from the γ-subunit leads to full activation of ENaC (Bruns et al. 2007). If the release of this 43-mer tract is essential for proteolytic ENaC activation, a mutation that prevents cleavage at the furin site should abolish the gain-of-function effect of a mutation that promotes ENaC cleavage at or near the putative prostasin site. This is indeed what we found. We could demonstrate that mutating the furin site essentially prevents the appearance of a 76 kDa cleavage product. This confirms that this fragment is the result of ENaC cleavage at this furin site by endogenous proteases. Importantly, we found that mutating the furin site did not prevent the spontaneous appearance of a 67 kDa cleavage product when the γK181A site was mutated in addition. However, the channel with the double mutation γR138A/K181A did not show a gain-of-function phenotype. This suggests that cleavage at or near the putative prostasin site in γ-ENaC is not sufficient for channel activation which is likely to require additional cleavage at the furin site. Interestingly, trypsin had a residual stimulatory effect on both the γR138A mutant channel and the γR138A/γK181A double mutant channel. However, this residual stimulatory effect was much smaller than the stimulatory effect observed in control oocytes expressing wild-type ENaC and may not involve the recruitment of near-silent channels like the effect of trypsin we observed in outside-out patches from oocytes expressing the γK181A mutation.
Conclusion
In conclusion we have identified a lysine residue in the γ-subunit of ENaC (γK181) which is situated in a functionally important region for the activation of near-silent channels in the plasma membrane by extracellular trypsin, chymotrypsin or hNE. Mutating this lysine residue to an alanine promotes channel cleavage by endogenous proteases and thereby favours the conversion of near-silent channels into active channels. This lowers the pool of near-silent channels and explains the constitutive activation of the mutant channel and its reduced responsiveness to extracellular proteases. Furthermore, we demonstrate that trypsin enhances the gating of channels that are already active in the plasma membrane in addition to recruiting near-silent channels. This additional effect of trypsin is reminiscent of the effect of MTSET on the gating of the mutant β518C ENaC and may involve trypsin binding to the channel and/or channel cleavage. The dual effect of extracellular proteases on ENaC function may be a relevant mechanism to modify ENaC activity under physiological and pathophysiological conditions. In a situation in which most of the channels in the membrane are already active, the stimulatory effect of exogenous or endogenous proteases acting from the extracellular side may be limited to a factor of about two, i.e. an increase in average single-channel Po from about 0.5 to nearly one. In contrast, in a tissue with a sizeable pool of near-silent channels in the plasma membrane, the immediate stimulatory effect of extracellular proteases on ENaC activity may be substantially larger.
Acknowledgments
The expert technical assistance of Jessica Ott and Ralf Rinke is gratefully acknowledged. This work was supported by a grant of the Deutsche Forschungsgemeinschaft (SFB 423: Kidney injury: Pathogenesis and regenerative mechanism) and by a DAAD studentship award to M.M.
References
- Adachi M, Kitamura K, Miyoshi T, Narikiyo T, Iwashita K, Shiraishi N, Nonoguchi H, Tomita K. Activation of epithelial sodium channels by prostasin in Xenopus oocytes. J Am Soc Nephrol. 2001;12:1114–1121. doi: 10.1681/ASN.V1261114. [DOI] [PubMed] [Google Scholar]
- Adebamiro A, Cheng Y, Rao US, Danahay H, Bridges RJ. A segment of gENaC mediates elastase activation of Na+ transport. J Gen Physiol. 2007;130:611–629. doi: 10.1085/jgp.200709781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen D, Vuagniaux G, Fowler-Jaeger N, Hummler E, Rossier BC. Activation of epithelial sodium channels by mouse channel activating proteases (mCAP) expressed in Xenopus oocytes requires catalytic activity of mCAP3 and mCAP2 but not mCAP1. J Am Soc Nephrol. 2006;17:968–976. doi: 10.1681/ASN.2005060637. [DOI] [PubMed] [Google Scholar]
- Bengrine A, Li J, Hamm LL, Awayda MS. Indirect activation of the epithelial Na+ channel by trypsin. J Biol Chem. 2007;282:26884–26896. doi: 10.1074/jbc.M611829200. [DOI] [PubMed] [Google Scholar]
- Bize V, Horisberger JD. Sodium self-inhibition of human epithelial sodium channel: Selectivity and affinity of the extracellular sodium sensing site. Am J Physiol Renal Physiol. 2007;293:F1137–F1146. doi: 10.1152/ajprenal.00100.2007. [DOI] [PubMed] [Google Scholar]
- Boucher RC. New concepts of the pathogenesis of cystic fibrosis lung disease. Eur Respir J. 2004;23:146–158. doi: 10.1183/09031936.03.00057003. [DOI] [PubMed] [Google Scholar]
- Bruns JB, Carattino MD, Sheng S, Maarouf AB, Weisz OA, Pilewski JM, Hughey RP, Kleyman TR. Epithelial Na+ channels are fully activated by furin- and prostasindependent release of an inhibitory peptide from the gamma subunit. J Biol Chem. 2007;282:6153–6160. doi: 10.1074/jbc.M610636200. [DOI] [PubMed] [Google Scholar]
- Caldwell RA, Boucher RC, Stutts MJ. Serine protease activation of near-silent epithelial Na+ channels. Am J Physiol Cell Physiol. 2004;286:C190–C194. doi: 10.1152/ajpcell.00342.2003. [DOI] [PubMed] [Google Scholar]
- Caldwell RA, Boucher RC, Stutts MJ. Neutrophil elastase activates near-silent epithelial Na+ hannels and increases airway epithelial Na+ ransport. Am J Physiol Lung Cell Mol Physiol. 2005;288:L813–L819. doi: 10.1152/ajplung.00435.2004. [DOI] [PubMed] [Google Scholar]
- Canessa CM, Schild L, Buell G, Thorens B, Gautschi I, Horisberger JD, Rossier BC. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature. 1994;367:463–467. doi: 10.1038/367463a0. [DOI] [PubMed] [Google Scholar]
- Carattino MD, Sheng S, Bruns JB, Pilewski JM, Hughey RP, Kleyman TR. The epithelial Na+ channel is inhibited by a peptide derived from proteolytic processing of its a subunit. J Biol Chem. 2006;281:18901–18907. doi: 10.1074/jbc.M604109200. [DOI] [PubMed] [Google Scholar]
- Chraibi A, Vallet V, Firsov D, Hess SK, Horisberger JD. Protease modulation of the activity of the epithelial sodium channel expressed in Xenopus oocytes. J Gen Physiol. 1998;111:127–138. doi: 10.1085/jgp.111.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diakov A, Aspden K, Mokrushina M, Korbmacher C. A putative prostasin cleavage site in the g-subunit of the epithelial sodium channel (ENaC) is essential for the proteolytic activation of near-silent channels. Acta Physiol. 2007;189(Suppl 653):49. [Google Scholar]
- Diakov A, Korbmacher C. A novel pathway of ENaC activation involves an SGK1 consensus motif in the C-terminus of the channel's α-subunit. J Biol Chem. 2004;279:38134–38142. doi: 10.1074/jbc.M403260200. [DOI] [PubMed] [Google Scholar]
- Ergonul Z, Frindt G, Palmer LG. Regulation of maturation and processing of ENaC subunits in the rat kidney. Am J Physiol Renal Physiol. 2006;291:F683–F693. doi: 10.1152/ajprenal.00422.2005. [DOI] [PubMed] [Google Scholar]
- Firsov D, Schild L, Gautschi I, Merillat AM, Schneeberger E, Rossier BC. Cell surface expression of the epithelial Na+ channel and a mutant causing Liddle syndrome: a quantitative approach. Proc Natl Acad Sci U S A. 1996;93:15370–15375. doi: 10.1073/pnas.93.26.15370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frindt G, Ergonul Z, Palmer LG. Surface expression of epithelial Na channel protein in rat kidney. J Gen Physiol. 2008;131:617–627. doi: 10.1085/jgp.200809989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyfe GK, Canessa CM. Subunit composition determines single channel kinetics of the epithelial sodium channel. J Gen Physiol. 1998;112:423–243. doi: 10.1085/jgp.112.4.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garty H, Palmer LG. Epithelial sodium channels: Function, structure, and regulation. Physiol Rev. 1997;77:359–396. doi: 10.1152/physrev.1997.77.2.359. [DOI] [PubMed] [Google Scholar]
- Harris M, Firsov D, Vuagniaux G, Stutts MJ, Rossier BC. A novel neutrophil elastase inhibitor prevents elastase activation and surface cleavage of the epithelial sodium channel expressed in Xenopus laevis oocytes. J Biol Chem. 2007;282:58–64. doi: 10.1074/jbc.M605125200. [DOI] [PubMed] [Google Scholar]
- Harris M, Garcia-Caballero A, Stutts MJ, Firsov D, Rossier BC. Preferential assembly of ENaC subunits in Xenopus oocyte: Role of furin-mediated endogenous proteolysis. J Biol Chem. 2008;283:7455–7463. doi: 10.1074/jbc.M707399200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughey RP, Bruns JB, Kinlough CL, Harkleroad KL, Tong Q, Carattino MD, Johnson JP, Stockand JD, Kleyman TR. Epithelial sodium channels are activated by furin-dependent proteolysis. J Biol Chem. 2004a;279:18111–18114. doi: 10.1074/jbc.C400080200. [DOI] [PubMed] [Google Scholar]
- Hughey RP, Bruns JB, Kinlough CL, Kleyman TR. Distinct pools of epithelial sodium channels are expressed at the plasma membrane. J Biol Chem. 2004b;279:48491–48494. doi: 10.1074/jbc.C400460200. [DOI] [PubMed] [Google Scholar]
- Hummler E, Barker P, Gatzy J, Beermann F, Verdumo C, Schmidt A, Boucher R, Rossier BC. Early death due to defective neonatal lung liquid clearance in a-ENaC-deficient mice. Nat Genet. 1996;12:325–328. doi: 10.1038/ng0396-325. [DOI] [PubMed] [Google Scholar]
- Kellenberger S, Gautschi I, Schild L. An external site controls closing of the epithelial Na+ channel ENaC. J Physiol. 2002;543:413–424. doi: 10.1113/jphysiol.2002.022020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellenberger S, Schild L. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Rev. 2002;82:735–767. doi: 10.1152/physrev.00007.2002. [DOI] [PubMed] [Google Scholar]
- Kleyman TR, Myerburg MM, Hughey RP. Regulation of ENaCs by proteases: An increasingly complex story. Kidney Int. 2006;70:1391–1392. doi: 10.1038/sj.ki.5001860. [DOI] [PubMed] [Google Scholar]
- Knight KK, Olson DR, Zhou R, Snyder PM. Liddle's syndrome mutations increase Na+ transport through dual effects on epithelial Na+ channel surface expression and proteolytic cleavage. Proc Natl Acad Sci U S A. 2006;103:2805–2808. doi: 10.1073/pnas.0511184103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstas A-A, Bielfeld-Ackermann A, Korbmacher C. Sulfonylurea receptors inhibit the epithelial sodium channel (ENaC) by reducing surface expression. Pflugers Arch. 2001;442:752–761. doi: 10.1007/s004240100597. [DOI] [PubMed] [Google Scholar]
- Konstas AA, Koch JP, Tucker SJ, Korbmacher C. CFTR-dependent upregulation of Kir1.1 (ROMK) renal K+ channels by the epithelial sodium channel (ENaC) J Biol Chem. 2002a;277:25377–25384. doi: 10.1074/jbc.M201925200. [DOI] [PubMed] [Google Scholar]
- Konstas AA, Korbmacher C. The γ-subunit of ENaC is more important for channel surface expression than the β-subunit. Am J Physiol Cell Physiol. 2003;284:C447–C456. doi: 10.1152/ajpcell.00385.2002. [DOI] [PubMed] [Google Scholar]
- Konstas A-A, Mavrelos D, Korbmacher C. Conservation of pH sensitivity in the epithelial sodium channel (ENaC) with Liddle's syndrome mutation. Pflugers Arch. 2000;441:341–350. doi: 10.1007/s004240000430. [DOI] [PubMed] [Google Scholar]
- Konstas AA, Shearwin-Whyatt LM, Fotia AB, Degger B, Riccardi D, Cook DI, Korbmacher C, Kumar S. Regulation of the epithelial sodium channel by N4WBP5A, a novel Nedd4/Nedd4-2-interacting protein. J Biol Chem. 2002b;277:29406–29416. doi: 10.1074/jbc.M203018200. [DOI] [PubMed] [Google Scholar]
- Korbmacher C, Volk T, Segal AS, Boulpaep EL, Frömter E. A calcium-activated and nucleotide-sensitive nonselective cation channel in M-1 mouse cortical collecting duct cells. J Membr Biol. 1995;146:29–45. doi: 10.1007/BF00232678. [DOI] [PubMed] [Google Scholar]
- Korner J, Chun J, O'Bryan L, Axel R. Prohormone processing in Xenopus oocytes: characterization of cleavage signals and cleavage enzymes. Proc Natl Acad Sci U S A. 1991;88:11393–11397. doi: 10.1073/pnas.88.24.11393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNicholas CM, Canessa CM. Diversity of channels generated by different combinations of epithelial sodium channel subunits. J Gen Physiol. 1997;109:681–692. doi: 10.1085/jgp.109.6.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mall M, Grubb BR, Harkema JR, O'Neal WK, Boucher RC. Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nat Med. 2004;10:487–493. doi: 10.1038/nm1028. [DOI] [PubMed] [Google Scholar]
- Molloy SS, Thomas L, VanSlyke JK, Stenberg PE, Thomas G. Intracellular trafficking and activation of the furin proprotein convertase: localization to the TGN and recycling from the cell surface. EMBO J. 1994;13:18–33. doi: 10.1002/j.1460-2075.1994.tb06231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narikiyo T, Kitamura K, Adachi M, Miyoshi T, Iwashita K, Shiraishi N, Nonoguchi H, Chen LM, Chai KX, Chao J, Tomita K. Regulation of prostasin by aldosterone in the kidney. J Clin Invest. 2002;109:401–408. doi: 10.1172/JCI13229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelsen S, Berg L, Wong C, Christian JL. Proprotein convertase genes in Xenopus development. Dev Dyn. 2005;233:1038–1044. doi: 10.1002/dvdy.20378. [DOI] [PubMed] [Google Scholar]
- Olivieri O, Castagna A, Guarini P, Chiecchi L, Sabaini G, Pizzolo F, Corrocher R, Righetti PG. Urinary prostasin: a candidate marker of epithelial sodium channel activation in humans. Hypertension. 2005;46:683–688. doi: 10.1161/01.HYP.0000184108.12155.6b. [DOI] [PubMed] [Google Scholar]
- Rauh R, Dinudom A, Fotia AB, Paulides M, Kumar S, Korbmacher C, Cook DI. Stimulation of the epithelial sodium channel (ENaC) by the serum- and glucocorticoid-inducible kinase (Sgk) involves the PY motifs of the channel but is independent of sodium feedback inhibition. Pflugers Arch. 2006;452:290–299. doi: 10.1007/s00424-005-0026-5. [DOI] [PubMed] [Google Scholar]
- Rockwell NC, Krysan DJ, Komiyama T, Fuller RS. Precursor processing by kex2/furin proteases. Chem Rev. 2002;102:4525–4548. doi: 10.1021/cr010168i. [DOI] [PubMed] [Google Scholar]
- Rossier BC. The epithelial sodium channel: activation by membrane-bound serine proteases. Proc Am Thorac Soc. 2004;1:4–9. doi: 10.1513/pats.2306007. [DOI] [PubMed] [Google Scholar]
- Rossier BC, Pradervand S, Schild L, Hummler E. Epithelial sodium channel and the control of sodium balance: Interaction between genetic and environmental factors. Annu Rev Physiol. 2002;64:877–897. doi: 10.1146/annurev.physiol.64.082101.143243. [DOI] [PubMed] [Google Scholar]
- Sheng S, Carattino MD, Bruns JB, Hughey RP, Kleyman TR. Furin cleavage activates the epithelial Na+ channel by relieving Na+ self-inhibition. Am J Physiol Renal Physiol. 2006;290:F1488–F1496. doi: 10.1152/ajprenal.00439.2005. [DOI] [PubMed] [Google Scholar]
- Shipway A, Danahay H, Williams JA, Tully DC, Backes BJ, Harris JL. Biochemical characterization of prostasin, a channel activating protease. Biochem Biophys Res Commun. 2004;324:953–963. doi: 10.1016/j.bbrc.2004.09.123. [DOI] [PubMed] [Google Scholar]
- Thomas G. Furin at the cutting edge: from protein traffic to embryogenesis and disease. Nat Rev Mol Cell Biol. 2002;3:753–766. doi: 10.1038/nrm934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallet V, Chraibi A, Gaeggeler HP, Horisberger JD, Rossier BC. An epithelial serine protease activates the amiloride-sensitive sodium channel. Nature. 1997;389:607–610. doi: 10.1038/39329. [DOI] [PubMed] [Google Scholar]
- Vallet V, Pfister C, Loffing J, Rossier BC. Cell-surface expression of the channel activating protease xCAP-1 is required for activation of ENaC in the Xenopus oocyte. J Am Soc Nephrol. 2002;13:588–594. doi: 10.1681/ASN.V133588. [DOI] [PubMed] [Google Scholar]
- Vuagniaux G, Vallet V, Jaeger NF, Hummler E, Rossier BC. Synergistic activation of ENaC by three membrane-bound channel- activating serine proteases (mCAP1, mCAP2, and mCAP3) and serum- and glucocorticoid-regulated kinase (Sgk1) in Xenopus oocytes. J Gen Physiol. 2002;120:191–201. doi: 10.1085/jgp.20028598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuagniaux G, Vallet V, Jaeger NF, Pfister C, Bens M, Farman N, Courtois-Coutry N, Vandewalle A, Rossier BC, Hummler E. Activation of the amiloride-sensitive epithelial sodium channel by the serine protease mCAP1 expressed in a mouse cortical collecting duct cell line. J Am Soc Nephrol. 2000;11:828–834. doi: 10.1681/ASN.V115828. [DOI] [PubMed] [Google Scholar]
- Wakida N, Kitamura K, Tuyen DG, Maekawa A, Miyoshi T, Adachi M, Shiraishi N, Ko T, Ha V, Nonoguchi H, Tomita K. Inhibition of prostasin-induced ENaC activities by PN-1 and regulation of PN-1 expression by TGF-β1 and aldosterone. Kidney Int. 2006;70:1432–1438. doi: 10.1038/sj.ki.5001787. [DOI] [PubMed] [Google Scholar]
- Yang LM, Rinke R, Korbmacher C. Stimulation of the epithelial sodium channel (ENaC) by cAMP involves putative ERK phosphorylation sites in the C termini of the channel's β- and γ-subunit. J Biol Chem. 2006;281:9859–9868. doi: 10.1074/jbc.M512046200. [DOI] [PubMed] [Google Scholar]
- Yu JX, Chao L, Chao J. Prostasin is a novel human serine proteinase from seminal fluid. Purification, tissue distribution, and localization in prostate gland. J Biol Chem. 1994;269:18843–18848. [PubMed] [Google Scholar]