Abstract
Both complete knockout of the Igf2 gene (Igf2null+/−) and knockout of its placental specific transcript alone (Igf2P0+/−) lead to fetal growth restriction in mice. However, in the Igf2null+/− this growth restriction occurs concurrently in gestation with placental growth restriction, whereas, placental growth restriction precedes fetal growth restriction in the Igf2P0+/− mouse. Previous studies have shown that the Igf2P0+/− placenta has proportionate reductions in its cellular compartments and its diffusional exchange characteristics. Yet, nothing is known about the structural development or diffusional exchange characteristics of the Igf2null+/− mouse. Hence, this study compares the structural properties (using stereology) and diffusional exchange characteristics (using measurement of permeability–surface area product, P.S, of three inert hydrophilic tracers) of the Igf2null+/− and the Igf2P0+/− placenta to identify the role of Igf2 in the development of the labyrinthine exchange membrane and its functional consequences. Our data show disproportionate effects of complete Igf2 ablation on the compartments of the placenta, not seen when the placental-specific transcript alone is deleted. Furthermore, although the theoretical diffusing capacity (calculated from the stereological data) of the Igf2null+/− placenta was reduced relative to control, there was no effect of the complete knockout on permeability surface area available for small hydrophilic tracers. This is in contrast to the Igf2P0+/− placenta, where theoretical diffusion capacity and P.S values were reduced similarly. Total ablation of the Igf2 gene from the fetoplacental unit in the mouse therefore results in a disproportionate growth of placental compartments whereas, deleting the placental specific transcript of Igf2 alone results in proportional placental growth restriction. Thus, placental phenotype depends on the degree of Igf2 gene ablation and the interplay between placental and fetal Igf2 in the mouse.
Intrauterine growth restriction (IUGR) is a major complication of pregnancy. The perinatal mortality rate of the IUGR fetus is 4–10 times higher than that of normally grown babies (Chiswick, 1985) and approximately 5–10% of all pregnancies complicated by IUGR will result in stillbirth or neonatal death (McIntire et al. 1999; Thornton et al. 2004). Furthermore, there is a strong inverse correlation between size at birth and risk of adult-onset disease including hypertension and type 2 diabetes (Barker, 1994; Fowden et al. 2005; Hanson & Gluckman, 2008). In the Western world, where mothers generally have an adequate diet, the principal cause of IUGR is placental insufficiency, i.e. abnormal development of the placenta leading to inadequate structural and functional capacity to supply nutrients to the fetus (Fox, 1976; Fowden et al. 2006b). The causes of placental insufficiency are not known but there is evidence for both environmental and genetic effects
Genetically, the imprinted genes have been shown to have an important role in regulating placental development (Reik et al. 2003a; Coan et al. 2005a; Fowden et al. 2006a). These genes are expressed selectively from either the paternal or the maternal allele and are believed to have an important role in the allocation of maternal resources to fetal growth (Constancia et al. 2002; Reik et al. 2003a; Constancia et al. 2005). Consistent with this hypothesis, most of the known imprinted genes are expressed in the placenta, the main site of maternal–fetal nutrient transfer (Reik et al. 2003b; Fowden et al. 2006a). One of the first imprinted genes to be discovered, the insulin-like growth factor 2 gene, Igf2, is known to have a key role in regulating feto-placental development (Salmon & Daughaday, 1957; DeChiara et al. 1990; Ferguson-Smith et al. 1991; Baker et al. 1993; Ludwig et al. 1996; Louvi et al. 1997; Morrione et al. 1997; Murrell et al. 2001; Constancia et al. 2002). Deletion of this gene leads to placental and fetal growth restriction while, conversely, deletion of the type II insulin-like growth factor clearance receptor is associated with placentomegaly, putatively due to excess circulating IGF-II (Ludwig et al. 1996). Similarly, over expression of the Igf2 gene by imprint relaxation leads to placental and fetal overgrowth (Eggenschwiler et al. 1997). IGF-II enhances growth via paracrine and/or autocrine actions, which stimulate cell proliferation and survival (Morrione et al. 1997; Burns & Hassan, 2001; Carter et al. 2006).
The Igf2 gene has four fetal promoters that are expressed in a tissue specific manner in the fetus and placenta. In the labyrinthine zone of the mouse placenta, Igf2 is driven from two different promoters; 10% of Igf2 transcripts in the placenta derive from an upstream extra-embryonic specific promoter (P0), expressed exclusively in the labyrinthine trophoblast, whilst fetal promoters control further expression of Igf2 in the trophoblast and fetal endothelial cells (Redline et al. 1993; Constancia et al. 2000). In the junctional zone of the mouse placenta, Igf2 is expressed from fetal promoters; firstly from the spongiotrophoblasts and from E13 onwards from the glycogen cells (Redline et al. 1993).
Two mouse models have been generated using deletions of cell-type specific promoters to investigate the roles of Igf2 in the placenta and fetus. Mice lacking the labyrinthine trophoblast-specific Igf2P0 transcript (Igf2P0+/−) exhibit placental and fetal IUGR, with the former preceding the latter so that there is an increase in the fetal to placental weight ratio shortly after mid-gestation onwards (Constancia et al. 2002). These mutant placentas were also found to have perturbed diffusional exchange characteristics (as measured using inert hydrophilic tracers which can only cross the placenta by passive diffusion), which were related to alterations in placental morphology (Sibley et al. 2004). Furthermore, the placenta increases expression of Slc2a3 and Slc38a4 genes, enhancing glucose and amino acid transfer, respectively, and manages to maintain a normal growth trajectory until E16 (Sibley et al. 2004; Constancia et al. 2005). Despite these alterations in placental transport capacity, the Igf2P0+/− placenta is still limiting to fetal growth in late gestation as by term fetuses are smaller than their wild-type littermates (Constancia et al. 2005).
Complete ablation of Igf2 in the Igf2null+/− mouse results in both fetal and placental growth restriction, which occur concurrently and are more severe than seen in the Igf2P0+/− mutant that continues to express Igf2 in its fetal tissues (Constancia et al. 2005). The complete Igf2null+/− placenta supported less fetus per gram weight and, by the end of gestation, transferred less methyl-aminoisobutyric acid (MeAIB) per gram of placenta than its wild-type littermate in contrast to the Igf2P0+/− placenta (Constancia et al. 2005). Moreover, there is down-regulation of the gene expression of Slc38a2 and of certain other cationic and anionic amino acid transporters in the Igf2null+/− mouse, whereas in the Igf2P0+/− placenta there is an up-regulation of Slc38a4 in the presence of fetal Igf2 (Matthews et al. 1999; Constancia et al. 2005). Thus, fetal and placental Igf2 appear to play an important role in regulating the relationship between fetal and placental growth and placental capacity for transport of nutrients by facilitated and active transport. However, no published information on the morphology or diffusional exchange characteristics of the Igf2null+/− placenta is currently available. This is despite the fact that deficiency in placental-specific expression of Igf2 has been reported to proportionately affect both placental exchange barrier morphology and passive diffusion and this is likely to contribute to the growth restriction found close to term (Sibley et al. 2004). This study therefore investigates the interplay between fetal and placental Igf2 in controlling placental development by comparing the structure and diffusional exchange properties of the placenta in the Igf2null+/− and Igf2P0+/− mouse during late gestation when the effects of Igf2 are maximal.
Methods
Animals
All experiments were carried out in accordance with the UK Home Office Animals (Scientific Procedures) Act 1986. Fetuses and placentas on a C57BL/6J background were collected at E19 (embryonic day 1 (E1) = day of plug). Wet weights of placentas and whole fetuses were recorded. Igf2P0+/− mutant animals were distinguished from wild-types with a Southern blotting-based assay (Constancia et al. 2000). Paternal transmission of the LacZDMR2– genotype (Murrell et al. 2001) was used to produce Igf2null+/− placentas and fetuses. Animals were identified by the previously published method (Murrell et al. 2001). Details on the generation of the Igf2P0+/− model and Igf2null+/− (LacZDMR2–) mice have been previously described (Constancia et al. 2000; Murrell et al. 2001).
Measurement of permeability–surface area product (P.S) of inert, hydrophilic solutes
As described in detail in Sibley et al. (2004) the diffusional permeability of the placenta is usually described, based on Fick's law of diffusion, as the permeability–surface area product (P.S) for a series of inert hydrophilic tracers of increasing molecular size. The methodology for measuring P.S is also described in detail elsewhere (Sibley et al. 2004). Briefly, unidirectional materno-fetal transfer of non-metabolisable radioactive tracers was measured in pregnant mice at E19 (Igf2null+/−, 30 litters; Igf2P0+/−, 37 litters). The mice were anaesthetized with an intraperitoneal injection of 400 μl of fentanyl fluanisone and midazolam solutions in water (1 : 1 : 2 water, Janssen Animal Health). A neck incision was made, and the jugular vein was exposed. A 100 μl bolus of PBS either containing 1.295 × 104 Bq [14C]mannitol (NEN NEC314; specific activity 1.98 MBq mmol−1) or 2.59 × 105 Bq 51Cr-labelled EDTA (NEN NEZ147; specific activity 1201.4 MBq mg−1) or 2.59 × 105 Bq [14C]inulin-carboxyl (ICN; specific activity 8.88 MBq g−1) was then injected into the jugular vein via a short length of tubing attached to a 27-gauge needle. At times up to 6.5 min after injection of tracer, a maternal blood sample was taken and the animals were killed. Conceptuses were dissected out and killed by decapitation. Fetuses were minced and lysed overnight at 55°C in 4 ml of Biosol (National Diagnostics, Atlanta, GA, USA). Aliquots of the fetal lysates were then added to appropriate tubes containing scintillation liquid (Bioscint, National Diagnostics) for β counting (Packard Tri-Carb, 1900, GMI Inc. USA). Placental tissue was used for genotyping.
P.S for each tracer in μl min−1 (g of placenta)−1 was calculated (Sibley et al. 2004) as:
where Nx is counts in fetus taken at time x, when mother was killed; (AUC0−x) is area under curve, from time 0 to time of killing mother, derived from a graph of maternal arterial counts versus time, where each time point is given by the single sample from an individual mouse; W is the wet weight of the placenta. For assessing membrane porosity, P.S is normalized to the diffusion constant for each tracer in water at 37°C (Dw).
Stereological analysis of placental structure
Four litters of each genotype were collected at E19. Mice were killed and fetuses and placentas dissected out. Fetuses were decapitated and weighed. Placentas were weighed and cut in half; one half was fixed in 4% glutaraldehyde, the other was fixed in 4% paraformaldehyde and processed as previously described (Coan et al. 2004). Two wild-types and two mutant placentas from each litter were randomly selected. Briefly, the paraformadehyde fixed half was dehydrated, embedded in paraffin wax and completely sectioned at 10 μm. The corresponding glutaraldehyde fixed half was dehydrated and embedded in Spurr's epoxy resin and a 1 μm thick section cut near to the placental midline. The Computer Assisted Stereological Toolbox (CAST v2.0, Olympus) was employed to superimpose count grids on random fields of view within systematic random paraffin sections or resin sections. Volume and surface densities were determined using superimposed point grids, surface densities with cycloid arc grids, total capillary length using a counting frame, mean diameter from measured capillary cross sectional areas, and harmonic mean thickness with orthogonal intercept lengths. Volume and surface densities were converted to absolute values by multiplying with the absolute volume of the placenta and adjusted for shrinkage (Coan et al. 2004). Theoretical diffusion capacity was calculated from mean surface area and thickness and using in this instance Krogh's constant for oxygen diffusion (Coan et al. 2004).
Statistical analyses
Differences between fetal and placental weights and feto-placental weight ratios were assessed by ANOVA followed by Fisher's least significant difference post hoc test. Comparison of P.S and P.S/Dw between tracers within genotypes was assessed by ANOVA followed by Fisher's least significant difference post hoc test. Significant differences between mutants and wild-type littermates for individual tracer P.S and P.S/Dw values were assessed by paired t test. Stereological data were assessed for significant differences between mutants and wild-type siblings, as well as Igf2P0+/−versus Igf2null+/−, using ANOVA followed by Fisher's least significant difference post hoc test. Values are presented as means ±s.e.m.
Results
Fetal and placental weights of the Igf2 mutants at E19
Lack of Igf2 in the Igf2null+/− mice at E19 results in significant fetal and placental growth restriction compared to wild-type littermates (weights are 48% and 60% of wild-type, respectively) (Table 1). Compared to its wild-type counterpart, the Igf2null+/− placenta, at E19, supports significantly fewer grams of fetus per gram of placenta than its wild-type littermate (Table 1).
Table 1.
Fetal and placental weights (mg) and feto-placental weight ratio
| Fetus | Placenta | Feto-placental weight ratio | |
|---|---|---|---|
| WT (Igf2null) | 1224 ± 17 | 85 ± 2 | 14.9 ± 0.3 |
| Igf2null+/− | 595 ± 9* | 51 ± 2* | 12.2 ± 0.3* |
| WT (Igf2P0) | 1240 ± 13 | 91 ± 3 | 14.1 ± 0.3 |
| Igf2P0+/− | 949 ± 14* | 60 ± 2* | 15.9 ± 0.3* |
WT, wild-type. Mean values ±s.e.m.; significant differences between mutant and wild-type littermate assessed by ANOVA with Fisher's protected least significant difference (PLSD) post hoc test
P < 0.005 to < 0.0001.
At E19, the Igf2P0+/− fetuses and placentas are growth restricted compared to their wild-type littermates (76% and 65% of wild-type, respectively). However, growth restriction in Igf2P0+/− fetuses and placentas is less severe than in Igf2null+/− mice (Table 1). Furthermore, significantly more grams of fetus are produced per gram of placenta in Igf2P0+/− mutants compared to their wild-type littermates (Table 1). Fetal and placental weights of both mutants and their respective wild-type littermates are comparable to previously reported values (Constancia et al. 2005).
Diffusional exchange properties of the Igf2 mutant placentas
In order to evaluate the passive diffusion properties of the Igf2P0+/− and Igf2null+/− placentas against their respective wild-type littermates, P.S per gram of placenta for inert tracers of increasing molecular size ([14C]mannitol, 51Cr-EDTA, and [14C]inulin) was measured.
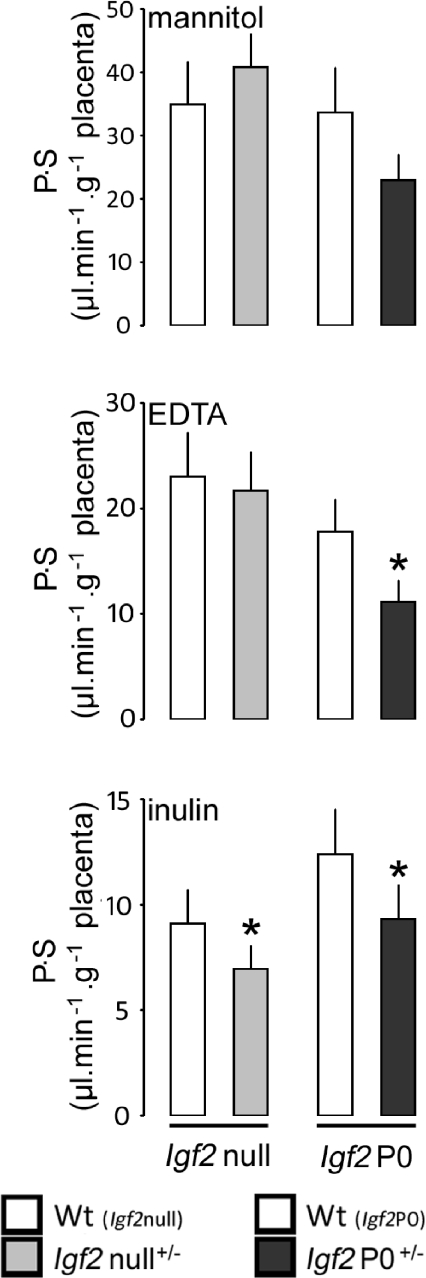
In the Igf2null+/− animals, there was no difference in P.S for [14C]mannitol or 51Cr-EDTA compared to wild-type littermates at E19 (Fig. 1). P.S for [14C]inulin was significantly lower at E19 in Igf2null+/− compared to wild-type siblings.
Figure 1. Permeability–surface area product (P.S) in relation to placental weight for three different sized radiolabelled solutes at E19 in Igf2 mutants.
Bars represent the mean +s.e.m. P.S Igf2null+/− litters: mannitol, 7; EDTA, 15, inulin, 8. Igf2P0+/− litters: mannitol, 6; EDTA, 23; inulin, 8. Significant differences between mutant and wild-type littermate were assessed by paired t test,*P < 0.05 to < 0.0001.
In the Igf2P0+/− animals, P.S for [14C]mannitol tended to be lower compared to their respective wild-type littermates at E19 but this did not reach significance (Fig. 1). P.S for both 51Cr-EDTA and [14C]inulin was significantly lower in Igf2P0+/−versus wild-type sibling animals at E19: these data for the P0 mice are similar to those we have reported previously (Sibley et al. 2004).
P.S was normalized to Dw for each tracer to assess potential differences in steric hindrance to diffusion of the tracers within each genotype through extracellular water filled channels (pores) (Stulc, 1989; Sibley et al. 2004). There were no significant differences in P.S/Dw between tracers for each genotype, for either mutants or wild-type littermates (Table 2).
Table 2.
P.S/Dw values (cm2 s−1× 106) for the trophoblast membrane in the Igf2 mutant placentas
| Dw | WT (Igf2null) | Igf2null+/− | WT (Igf2P0) | Igf2P0+/− | |
|---|---|---|---|---|---|
| [14C]Mannitol | 9.9 | 58.8 ± 11.4 | 68.7 ± 9.9 | 56.6 ± 11.9 | 38.7 ± 6.6 |
| [14C]EDTA | 7.0 | 54.8 ± 6.7 | 50.9 ± 7.1 | 51.3 ± 7.5 | 33.8 ± 5.4* |
| [14C]Inulin | 2.6 | 58.5 ± 10.2 | 44.6 ± 7.1* | 79.5 ± 13 | 59.6 ± 10* |
Mean values ±s.e.m., significant differences between tracers for each genotype were assessed by ANOVA with Fisher's PLSD post hoc test P > 0.05. Significant differences between mutant and respective wild-type sibling for each tracer were assessed by paired t test; *P < 0.02 to P < 0.0001.
Placenta structure
Labyrinthine and junctional zone volumes in the Igf2null+/− are reduced to 35% and 68% of wild-type, respectively, by E19 (Table 3). When expressed as a volume fraction, the labyrinthine zone is significantly reduced such that in the Igf2null+/− it occupies only 40% of the placenta compared to 52% in the wild-type littermate (Table 3). Furthermore, the junctional zone, which occupies 29% in the wild-type littermate, accounts for 38% in the Igf2null+/− placenta (Table 3).
Table 3.
Stereological analysis of the Igf2 mutant placentas
| WT (Igf2null) | Igf2null+/− | % WT | WT (Igf2P0) | Igf2P0+/− | % WT | |
|---|---|---|---|---|---|---|
| Volume (mm3) | ||||||
| Placenta | 94 ± 8 | 43 ± 2*† | 46 | 116 ± 5 | 65 ± 4* | 56 |
| Labyrinthine zone | 49 ± 6 | 17 ± 1*† | 35 | 60 ± 2 | 32 ± 2* | 53 |
| Junctional zone | 25 ± 1 | 17 ± 3* | 68 | 34 ± 2 | 19 ± 3* | 56 |
| Decidua | 16 ± 1 | 11 ± 1* | 69 | 17 ± 2 | 13 ± 1 | 76 |
| FC | 11 ± 2 | 3 ± 0.3*† | 27 | 15 ± 2 | 7 ± 1* | 47 |
| MBS | 10 ± 1 | 3 ± 0.3*† | 30 | 10 ± 1 | 6 ± 1* | 60 |
| LT | 29 ± 3 | 10 ± 1*† | 34 | 35 ± 1 | 19 ± 1* | 54 |
| Volume fraction (%) | ||||||
| Labyrinthine zone | 52 ± 2 | 40 ± 2*† | — | 52 ± 3 | 49 ± 9 | — |
| Junctional zone | 29 ± 2 | 38 ± 6* | — | 27 ± 3 | 29 ± 3 | — |
| LT | 52 ± 1 | 52 ± 1 | — | 50 ± 1 | 51 ± 1 | — |
| MBS | 26 ± 1 | 26 ± 1 | — | 24 ± 1 | 25 ± 1 | — |
| FC | 27 ± 1 | 26 ± 2 | — | 29 ± 2 | 27 ± 1 | — |
| Surface area of the trophoblast membrane (cm2) | ||||||
| Fetal side | 21 ± 3 | 6 ± 0.6*† | 29 | 30 ± 2 | 15 ± 2* | 50 |
| Maternal side | 25 ± 4 | 7 ± 0.8*† | 28 | 30 ± 2 | 16 ± 2* | 53 |
| Total capillary length (m) | ||||||
| 85 ± 5 | 22 ± 3*† | 26 | 73 ± 10 | 37 ± 3* | 51 | |
| Mean capillary diameter (μm) | ||||||
| 14.6 ± 0.7 | 14.9 ± 0.7 | — | 13.6 ± 0.4 | 13.9 ± 0.8 | — | |
| Harmonic mean membrane thickness (μm) | ||||||
| 3.1 ± 0.16 | 3.73 ± 0.08*† | 120 | 3.31 ± 0.17 | 4.24 ± 0.13* | 128 | |
| Theoretical diffusion capacity (mm2 min−1 kPa−1) | ||||||
| 13.1 ± 2.1 | 3.08 ± 0.19*† | 24 | 15.9 ± 1.5 | 6.32 ± 0.51* | 40 | |
Abbreviations: FC, fetal capillaries; MBS, maternal blood spaces; LT, labyrinthine trophoblast; WT, wild-type littermate. N= 4 litters all groups, mean values ±s.e.m., significant differences assessed by paired t test between mutant and wild-type littermate
P < 0.05 to < 0.0001.
Significant differences assessed by unpaired t test between Igf2null+/− and Igf2P0+/−
P < 0.05 to < 0.001.
In the Igf2P0+/−, labyrinthine and junctional zone volumes are reduced to 53% and 56% of wild-type, respectively (Table 3). Comparison between the Igf2null+/− and Igf2P0+/− reveals that the Igf2null+/− has a significantly smaller labyrinthine zone, both in absolute terms and as a volume fraction (Table 3). Hence, the Igf2P0+/− placenta, unlike the Igf2null+/− placenta, is proportionately reduced with comparable volume fractions of labyrinthine and junctional zones to those of wild-type littermate placentas (Table 3).
Labyrinthine zone architecture
The labyrinthine zone can be divided into several components: trophoblast (syncytial and cellular), fetal capillaries and maternal blood spaces. Alterations in each of these components can have implications for the function of the placenta and consequently fetal development.
The Igf2null+/− labyrinthine zone at E19 contains significantly less trophoblast, maternal blood spaces and fetal capillary volumes compared to the labyrinthine zone of its wild-type littermate (34%, 30% and 27% of wild-type, respectively) (Table 3). Surface areas on both the apical (maternal) side and basal (fetal) side are also reduced compared to the wild-type sibling placenta (28%, 29% of wild-type, respectively) (Table 3). Furthermore, total capillary length is significantly diminished to 30% compared with the length of capillaries in the wild-type (Table 3). However, mean capillary diameter in the Igf2null+/− placenta is similar to the wild-type littermate placenta (Table 3). Despite reduction in most physiologically relevant parameters, the harmonic mean thickness of the interhemal membrane is increased to 120% compared the wild-type littermate placenta (Table 3). Consequently, with a reduction in surface area and an expansion in membrane thickness, the theoretical diffusion capacity is considerably reduced in the Igf2null+/− placenta to 24% of placentas expressing all transcripts Igf2 (Table 3).
Absence of the labyrinthine trophoblast-specific transcript Igf2P0 is also associated with a reduction in trophoblast, maternal blood spaces and fetal capillary volumes (54%, 60% and 47% of wild-type littermate placenta, respectively) (Table 3). Apical and basal surface areas of the interhemal membrane are also reduced (50% and 53% of wild-type, respectively) (Table 3). Total capillary length was reduced in the Igf2P0+/− placenta to 51% of wild-type, whereas there was no change in mean capillary diameter between genotypes (Table 3). In contrast to decreases in various components, the harmonic mean membrane thickness in the Igf2P0+/− placenta was increased by 128% of wild-type (Table 3). Hence, decreased surface area and increased membrane thickness results in a lower theoretical diffusion capacity in Igf2P0+/− placentas to 40% of that found in wild-type littermates (Table 3). Surface areas, thickness and the theoretical diffusion capacity are all comparable to the results we previously reported for the Igf2P0+/− placenta (Sibley et al. 2004).
Direct comparison between the Igf2null+/− and Igf2P0+/− labyrinthine zones reveals that the Igf2null+/− has significantly less volume of trophoblast, maternal blood spaces and fetal capillaries (Table 3). However, volume fractions of the same components are similar in both genotypes (Table 3). Surface areas are significantly reduced to 40% in the Igf2null+/− and capillary length is reduced to 60% compared to the Igf2P0+/− (Table 3). Moreover, harmonic mean thickness of the interhemal membrane is significantly less (88% of Igf2P0+/−) in the Igf2null+/− compared to the Igf2P0+/− (Table 3). However, a favourable reduction in membrane thickness, combined with an unfavourable smaller surface area of the Igf2null+/− interhemal membrane results in a significant reduction in theoretical diffusion capacity compared to the Igf2P0+/− (49% of Igf2P0+/−) (Table 3).
Discussion
The results show that the growth, morphology and diffusional characteristics of the mouse placenta depend on Igf2 and differ in mice lacking all Igf2 transcripts and those with deletion of the placental specific Igf2P0 transcript alone. In particular, it is development of the labyrinthine zone of the placenta that appears most sensitive to changes in Igf2 availability. The volume of this zone was reduced in proportion to the other regions in the placenta of the Igf2P0+/− mutant, whereas, it was disproportionately reduced in the complete Igf2null+/− placenta. The length of the capillaries, the thickness of the interhemal membrane and the absolute volume and surface area of trophoblast within the labyrinthine zone also differed between the two Igf2 mutants. Similarly, transplacental transfer of the three hydrophilic tracers was affected differently in these two mutants compared to their respective wild-type littermates. Placental phenotype therefore depends on the degree of Igf2 gene ablation in the mouse. These findings, together with our previous studies, show that interplay between placental and fetal Igf2 regulates placental morphology and transfer of substances by simple diffusion, facilitated diffusion and active transport (Sibley et al. 2004; Constancia et al. 2005).
In common with previous findings (Constancia et al. 2005), the current data on placental and fetal weights show that, by the end of gestation, growth restriction is less severe in the IgfP0+/− mutant than complete Igf2null+/− and that the Igf2null+/− placenta is less efficient whilst the Igf2P0+/− placenta is more efficient in terms of grams of fetus produced per gram of placenta, than their respective wild-type placentas. The data presented here indicate that the decreased efficiency of the complete null placenta may be partly due to the changes in placental morphology and, in particular, to the disproportionate reduction in the labyrinthine zone responsible for nutrient transfer to the fetus in late gestation. However, increased placental efficiency of the Igf2P0+/− placenta, at E19, cannot be explained morphologically and probably reflects, in part, the enhanced transfer of glucose observed previously in these placentas. (Constancia et al. 2005). Futhermore, the reduced passive permeability of the Igf2P0+/− placenta to all solutes will lower backflux of small molecules down their concentration gradient from the fetal to maternal circulation. This will thus increase the net flux of amino acids actively transported across the Igf2P0+/− placenta towards the fetus and consequently contribute to fetal tissue accretion and the increased fetal to placental weight ratio of these mutants.
The labyrinthine zone of both Igf2 mutant placentas is growth restricted. Igf2 deficiency may be leading to elongation of the labyrinthine trophoblast cell cycle and perturb the normal rate of proliferation, limiting growth of the labyrinthine zone (Baker et al. 1993). Another possibility may be that there is a higher turnover in the labyrinthine zone of the mutants. Igf2 is a known survival factor and ablation of this gene may lead to a tip in the balance between proliferation and apoptosis in the placenta (Burns & Hassan, 2001). However, structurally there are also striking differences between the Igf2 mutant placentas. The greater volume of labyrinthine zone in the Igf2P0+/− placenta may be associated with Igf2 expressed from the junctional zone. Furthermore, Igf2 from the fetus or from fetal endothelium may also influence labyrinthine trophoblast growth though there is currently no published evidence of these specific interactions. Interestingly, junctional zone volume in both mutants is similar suggesting that Igf2 expression, from spongiotrophoblasts and glycogen cells, may not be responsible for growth in this compartment. Thus in the complete absence of Igf2, junctional zone growth is likely to be modulated by various factors including Activin, Cdkn1c, Egfr, Follistatin, Grb10, Nodal and Peg3 (Li & Behringer, 1998; Rossant et al. 1998; Takahashi et al. 2000; Hiby et al. 2001; Ma et al. 2001; Charalambous et al. 2003; Candeloro & Zorn, 2007; Dackor et al. 2007). However, in the wild-type situation, Igf2 transcribed from the Igf2P0 transcript may be providing growth stimulation to the junctional zone. On the other hand, Igf2 could be acting as a survival factor in order to maintain the balance between apoptosis and proliferation in the junctional zone (Waddell et al. 2000).
Labyrinthine zone architecture as well as growth was affected by Igf2 deficiency and differently in the two Igf2 mutants. Total fetal capillary volume, surface area and length differ significantly between genotypes and may well indicate that Igf2 plays an important role in angiogenesis. Moreover it has been suggested that IGF-II exerts angiogenic effects through binding IGF2R (Herr et al. 2003). Igf2 deficiency also results in dramatically diminished volumes and surface areas concurrent with an increase in thickness of the interhemal membrane. Deficiency in Igf2P0 alone results in a less pronounced decrease in volumes and surface areas but a greater interhemal membrane thickness than in the total absence of Igf2. Therefore, Igf2 plays a critical role in the development and increased sophistication of the interhemal membrane's architecture. One explanation for the increased interhemal thickness in the Igf2P0+/− placenta may be a by-product of fetal (from the endothelium or from the fetus itself) or junctional zone Igf2 which is compensating Igf2P0 transcription by increasing labyrinthine zone growth and nutrient transporter gene expression. The increase in membrane thickness in the Igf2null+/− placenta may be connected to a membrane maturation problem, whereby Igf2 could promote the stretching and thinning of labyrinthine trophoblast syncytial layers, and in its absence, the syncytial layers are less stretched and so remain thicker. This could be compounded by the growth restricted Igf2null+/− with a potentially reduced fetal haematocrit which may exhibit less shear stress to promote capillary angiogenesis (Karimu & Burton, 1994; Resnick & Gimbrone, 1995).
In common with previous observations (Sibley et al. 2004), the passive permeability of the Igf2P0+/− placenta was significantly less for all three tracers than their respective wild-type placenta. The actual P.S values measured for the wild-type and mutant placentas of the Igf2P0+/− mouse in the present study were similar to those reported previously at E19 (Sibley et al. 2004). In contrast to the Igf2P0+/− mutants, the P.S values of the complete Igf2null+/− placenta, reported here for the first time, were only reduced compared to wild-type for inulin. The P.S values for mannitol and EDTA were similar in the wild-type and complete Igf2null+/− placentas, despite the even more severe reduction in labyrinthine zone growth than seen in the Igf2P0+/− placenta at E19. Indeed, the theoretical diffusion capacity of the two mutant placentas calculated from their surface areas and interhemal membrane thicknesses indicates that passive diffusion across the complete Igf2null+/− placenta should have been reduced to a greater extent than in the Igf2P0+/− mutant placenta.
The explanation for the difference in passive diffusion predicted by morphological and functional measurements in the complete Igf2null+/− is not readily apparent but three possibilities need to be considered. First, there may be alterations in the absolute or relative blood flow through either the maternal or fetal circulation of the labyrinthine zone which alter the effective delivery of the tracers to the fetus. This would be consistent with the greater reduction in capillary length in the Igf2null+/−. Furthermore, the reductions in the muscle volume and the ratio of radius4/length of umbilical arteries of Igf2null+/− fetuses suggest that blood volume flow may be reduced by more than half in these mutants (Ahmad et al. 2005). However, unidirectional transfer of hydrophilic tracers such as those used here is not normally expected to be altered by flow (Atkinson et al. 2006).
Secondly, a single measurement of interhemal membrane thickness may underestimate the complexity of the membrane as a diffusion barrier. There are three layers of trophoblast in the interhemal membrane of the mouse placenta. The outer layer is composed of mononucleate labyrinthine giant cells that line the maternal blood spaces, with two underlying layers of syncytiotrophoblast. The outer layer is not complete, however, and large perforations within the cells will allow maternal plasma to percolate into the narrow and irregular intercellular space between layers 1 and 2 (Coan et al. 2005b). The rate of diffusion will therefore be influenced by the frequency and dimensions of the perforations, and the width of the intercellular space, all of which may vary between the mutants. The two syncytial layers are linked by gap junctions so functionally act as a single layer for diffusion purposes. However, there is again a potentially variable subsyncytial fluid-filled space between it and the basement membrane, which could affect diffusion characteristics. Further studies at the ultrastructural level, preferably of tissues fixed by perfusion at physiological pressures, are required to test whether differences in the microscopic morphology of the interhemal membrane could account for the results obtained.
Finally at an ultrastructural level, transfer of hydrophilic tracers by diffusion across the syncytiotrophoblast is presumed to take place through extracellular water filled channels or pores (Stulc, 1989). These pores have never been morphologically identified unequivocally in the trophoblast. Therefore, it could be that whilst changes in trophoblast membrane surface area and interhemal barrier thickness normally approximate to pore surface area and length, this may not always be the case; complete Igf2 ablation might have differential effects on the trophoblast cell layer and paracellular diffusional pathway, which result in a leakier placenta than would be predicted from the morphological measurements alone. By considering all the morphological, P.S and P.S/Dw data, one could speculate that whilst the Igf2P0+/− interhemal membrane has a reduced number of pores, the Igf2null+/− interhemal membrane has a reduced number of larger pores, which offer less steric hindrance to the smaller tracer molecules. Consistent with this suggestion, our preliminary observations at E16 suggest that there is a small but significant increase in the inulin P.S in the complete Igf2null+/− placenta in contrast to the reduced inulin P.S values found previously in the Igf2P0+/− placenta (Sibley et al. 2004). These potential differences in pore size between mutants accord with the other differential effects of total versus partial ablation of the Igf2 gene on placental structure reported here.
Although there were no significant differences in placental structure or function between the wild-types of the two mutants, the current data hint at the possibility that development of the wild-type placenta is affected by the genotype of its neighbouring conceptus and/or the total uteroplacental availability of IGF-II. If placental Igf2 contributes to the pool of maternal circulating IGF-II, the mothers with Igf2null+/− placentas may have a lower IGF-II concentration than those with Igf2P0+/− placentas with consequences for resource allocation to growing feto-placental units.
Many of the features of both Igf2 mutants have also been reported in human cases of IUGR. An early study on small-for-gestational-age (SGA) placentas found a significant reduction in the total exchange surface area despite being in the normal weight range (Teasdale, 1984). Other, more recent, IUGR studies have reported fewer arterial vessels per unit of stem villous, reduction in fetoplacental perfusion, reduction in volume of intervillous space, villi and capillaries, a smaller exchange surface area and thicker trophoblast epithelium (Jackson et al. 1995; Mayhew et al. 2003). However, the extent to which changes in Igf2 gene expression are linked to IUGR in the human placenta remain unclear (Antonazzo et al. 2008).
In conclusion, total ablation of the Igf2 gene from the fetoplacental unit in the mouse results in a disproportionate growth of the structural compartments of the placenta. These effects contrast to those of solely deleting the placental-specific transcript of Igf2, where there are proportionate effects on the compartments and diffusional exchange characteristics, and an ability of the placenta to increase its transport efficiency, leading to a less severe growth restriction. Future studies are required to elucidate the molecular networks by which IGF-II operates to regulate composition of the placenta and development of its exchange membrane.
Acknowledgments
The authors would like to thank the Multi-Imaging Centre of the School of Biological Sciences and Mrs Olivera Spasic-Boscovic for their assistance in preparing the resin sections, Wolf Reik for discussion and Jenny Hughes for help with the mice. This work is supported by the ASGBI and the BBSRC.
References
- Ahmad AM, Burns J, Gardner R, Graham C. Delayed and disturbed morphogenesis of the umbilical blood vessels in insulin-like growth factor-II deficient conceptuses (Igf2m+/p−) Dev Dyn. 2005;233:88–94. doi: 10.1002/dvdy.20320. [DOI] [PubMed] [Google Scholar]
- Antonazzo P, Alvino G, Cozzi V, Grati FR, Tabano S, Sirchia S, Miozzo M, Cetin I. Placental IGF2 expression in normal and intrauterine growth restricted (IUGR) pregnancies. Placenta. 2008;29:99–101. doi: 10.1016/j.placenta.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Atkinson DE, Boyd RD, Sibley CP. Placental transfer. In: Neill JD, editor. Knobil and Neill's Physiology of Reproduction. 3rd ed. London: Elsevier; 2006. pp. 2787–2846. [Google Scholar]
- Baker J, Liu J-P, Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993;75:73–82. [PubMed] [Google Scholar]
- Barker DJP. Mothers, Babies and Disease in Later Life. London: BMJ Publishing Group; 1994. [Google Scholar]
- Burns JL, Hassan AB. Cell survival and proliferation are modified by insulin-like growth factor 2 between days 9 and 10 of mouse gestation. Development. 2001;128:3819–3830. doi: 10.1242/dev.128.19.3819. [DOI] [PubMed] [Google Scholar]
- Candeloro L, Zorn TM. Distribution and spatiotemporal relationship of activin A and follistatin in mouse decidual and placental tissue. Am J Reprod Immunol. 2007;58:415–424. doi: 10.1111/j.1600-0897.2007.00525.x. [DOI] [PubMed] [Google Scholar]
- Carter AM, Nygard K, Mazzuca DM, Han VK. The expression of insulin-like growth factor and insulin-like growth factor binding protein mRNAs in mouse placenta. Placenta. 2006;27:278–290. doi: 10.1016/j.placenta.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Charalambous M, Smith FM, Bennett WR, Crew TE, Mackenzie F, Ward A. Disruption of the imprinted Grb10 gene leads to disproportionate overgrowth by an Igf2-independent mechanism. Proc Natl Acad Sci U S A. 2003;100:8292–8297. doi: 10.1073/pnas.1532175100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiswick ML. Intrauterine growth retardation. Br Med J (Clin Res Ed) 1985;291:845–848. doi: 10.1136/bmj.291.6499.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coan PM, Burton GJ, Ferguson-Smith AC. Imprinted genes in the placenta – a review. Placenta. 2005a;26(Suppl. A):S10–S20. doi: 10.1016/j.placenta.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Coan PM, Ferguson-Smith AC, Burton GJ. Developmental dynamics of the definitive mouse placenta assessed by stereology. Biol Reprod. 2004;70:1806–1813. doi: 10.1095/biolreprod.103.024166. [DOI] [PubMed] [Google Scholar]
- Coan PM, Ferguson-Smith AC, Burton GJ. Ultrastructural changes in the interhaemal membrane and junctional zone of the murine chorioallantoic placenta across gestation. J Anat. 2005b;207:783–796. doi: 10.1111/j.1469-7580.2005.00488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constancia M, Angiolini E, Sandovici I, Smith P, Smith R, Kelsey G, Dean W, Ferguson-Smith A, Sibley CP, Reik W, Fowden A. Adaptation of nutrient supply to fetal demand in the mouse involves interaction between the Igf2 gene and placental transporter systems. Proc Natl Acad Sci U S A. 2005;102:19219–19224. doi: 10.1073/pnas.0504468103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constancia M, Dean W, Lopes S, Moore T, Kelsey G, Reik W. Deletion of a silencer element in Igf2 results in loss of imprinting independent of H19. Nat Genet. 2000;26:203–206. doi: 10.1038/79930. [DOI] [PubMed] [Google Scholar]
- Constancia M, Hemberger M, Hughes J, Dean W, Ferguson-Smith A, Fundele R, Stewart F, Kelsey G, Fowden A, Sibley C, Reik W. Placental-specific IGF-II is a major modulator of placental and fetal growth. Nature. 2002;417:945–948. doi: 10.1038/nature00819. [DOI] [PubMed] [Google Scholar]
- Dackor J, Strunk KE, Wehmeyer MM, Threadgill DW. Altered trophoblast proliferation is insufficient to account for placental dysfunction in Egfr null embryos. Placenta. 2007;28:1211–1218. doi: 10.1016/j.placenta.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeChiara TM, Efstratiadis A, Robertson EJ. A growth-deficiency phenotype in heterozygous mice carrying an insulin-like growth factor II gene disrupted by targeting. Nature. 1990;345:78–80. doi: 10.1038/345078a0. [DOI] [PubMed] [Google Scholar]
- Eggenschwiler J, Ludwig T, Fisher P, Leighton PA, Tilghman SM, Efstratiadis A. Mouse mutant embryos overexpressing IGF-II exhibit phenotypic features of the Beckwith-Wiedemann and Simpson-Golabi-Behmel syndromes. Genes Dev. 1997;11:3128–3142. doi: 10.1101/gad.11.23.3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson-Smith AC, Cattanach BM, Barton SC, Beechey CV, Surani MA. Embryological and molecular investigations of parental imprinting on mouse chromosome 7. Nature. 1991;351:667–670. doi: 10.1038/351667a0. [DOI] [PubMed] [Google Scholar]
- Fowden AL, Giussani DA, Forhead AJ. Endocrine and metabolic programming during intrauterine development. Early Hum Dev. 2005;81:723–734. doi: 10.1016/j.earlhumdev.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Fowden AL, Sibley C, Reik W, Constancia M. Imprinted genes, placental development and fetal growth. Horm Res. 2006a;65(Suppl. 3):50–58. doi: 10.1159/000091506. [DOI] [PubMed] [Google Scholar]
- Fowden AL, Ward JW, Wooding FP, Forhead AJ, Constancia M. Programming placental nutrient transport capacity. J Physiol. 2006b;572:5–15. doi: 10.1113/jphysiol.2005.104141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox H. The histopathology of placental insufficiency. J Clin Pathol Suppl (R Coll Pathol) 1976;10:1–8. [PMC free article] [PubMed] [Google Scholar]
- Hanson MA, Gluckman PD. Developmental origins of health and disease: new insights. Basic Clin Pharmacol Toxicol. 2008;102:90–93. doi: 10.1111/j.1742-7843.2007.00186.x. [DOI] [PubMed] [Google Scholar]
- Herr F, Liang OD, Herrero J, Lang U, Preissner KT, Han VK, Zygmunt M. Possible angiogenic roles of insulin-like growth factor II and its receptors in uterine vascular adaptation to pregnancy. J Clin Endocrinol Metab. 2003;88:4811–4817. doi: 10.1210/jc.2003-030243. [DOI] [PubMed] [Google Scholar]
- Hiby SE, Lough M, Keverne BE, Surani AM, Loke YW, King A. Paternal monoallelic expression of PEG3 in the human placenta. Human Mol Genet. 2001;10:1093–1100. doi: 10.1093/hmg/10.10.1093. [DOI] [PubMed] [Google Scholar]
- Jackson MR, Walsh AJ, Morrow RJ, Mullen JB, Lye SJ, Ritchie JW. Reduced placental villous tree elaboration in small-for-gestational-age pregnancies: relationship with umbilical artery doppler waveforms. Am J Obstet Gynecol. 1995;172:518–525. doi: 10.1016/0002-9378(95)90566-9. [DOI] [PubMed] [Google Scholar]
- Karimu AL, Burton GJ. Significance of changes in fetal perfusion pressure to factors controlling angiogenesis in the human term placenta. J Reprod Fertil. 1994;102:447–450. doi: 10.1530/jrf.0.1020447. [DOI] [PubMed] [Google Scholar]
- Li Y, Behringer RR. Esx-1 is an X-chromosome-imprinted regulator of placental development and fetal growth. Nat Genet. 1998;20:309–311. doi: 10.1038/3129. [DOI] [PubMed] [Google Scholar]
- Louvi A, Accili D, Efstratiadis A. Growth-promoting interaction of IGF-II with the insulin receptor during mouse embryonic development. Dev Biol. 1997;189:33–48. doi: 10.1006/dbio.1997.8666. [DOI] [PubMed] [Google Scholar]
- Ludwig T, Eggenschwiler J, Fisher P, D'Ercole A, Davenport M, Efstratiadis A. Mouse mutants lacking the type 2 IGF receptor (IGF2R) are rescued from perinatal lethality in Igf2 and Igf1r null backgrounds. Dev Biol. 1996;177:517–535. doi: 10.1006/dbio.1996.0182. [DOI] [PubMed] [Google Scholar]
- Ma GT, Solovena V, Tzeng S-J, Lowe LA, Pfendler KC, Iannaccone PM, Kuehn MR, Linzer DIH. Nodal regulates trophoblast differentiation and placental development. Dev Biol. 2001;236:124–135. doi: 10.1006/dbio.2001.0334. [DOI] [PubMed] [Google Scholar]
- Matthews JC, Beveridge MJ, Dialynas ET, Bartke A, Kilberg MS, Kaufman P. Placental anionic and cationic amino acid transporter expression in growth hormone overexpressing and null IGF-II or null IGF-I receptor mice. Placenta. 1999;20:639–650. doi: 10.1053/plac.1999.0421. [DOI] [PubMed] [Google Scholar]
- Mayhew T, Ohadike C, Baker P, Crocker I, Mitchell C, Ong S. Stereological investigation of placental morphology in pregnancies complicated by pre-eclampsia with and without intrauterine growth retardation. Placenta. 2003;24:219–226. doi: 10.1053/plac.2002.0900. [DOI] [PubMed] [Google Scholar]
- McIntire DD, Bloom SL, Casey BM, Leveno KJ. Birth weight in relation to morbidity and mortality among newborn infants. N Engl J Med. 1999;340:1234–1238. doi: 10.1056/NEJM199904223401603. [DOI] [PubMed] [Google Scholar]
- Morrione A, Valentinis B, Xu S-Q, Yumet G, Louvi A, Efstratiadis A, Baserga R. Insulin-like growth factor II stimulates cell proliferation through the insulin receptor. Proc Natl Acad Sci U S A. 1997;94:3777–3782. doi: 10.1073/pnas.94.8.3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrell A, Heeson S, Bowden L, Constância M, Dean W, Kelsey G, Reik W. An intragenic methylated region in the imprinted Igf2 gene augments transcription. EMBO Reports. 2001;21:1101–1106. doi: 10.1093/embo-reports/kve248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redline RW, Chernicky CL, Tan H-Q, Ilan J, Ilan J. Differential expression of insulin-like growth factor-II in specific regions of the late (post 9.5) murine placenta. Mol Reprod Dev. 1993;36:121–129. doi: 10.1002/mrd.1080360202. [DOI] [PubMed] [Google Scholar]
- Reik W, Constancia M, Fowlden A, Anderson N, Dean W, Ferguson-Smith A, Tycko B, Sibley C. Regulation of supply and demand for maternal nutrients in mammals by imprinted genes. J Physiol. 2003a;547:35–44. doi: 10.1113/jphysiol.2002.033274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reik W, Santos F, Dean W. Mammalian epigenomics: reprogramming the genome for development and therapy. Theriogenology. 2003b;59:21–32. doi: 10.1016/s0093-691x(02)01269-4. [DOI] [PubMed] [Google Scholar]
- Resnick N, Gimbrone MA., Jr Hemodynamic forces are complex regulators of endothelial gene expression. FASEB J. 1995;9:874–882. doi: 10.1096/fasebj.9.10.7615157. [DOI] [PubMed] [Google Scholar]
- Rossant J, Guillemot F, Tanaka M, Latham K, Gertsenstein M, AN Mash2 is expressed in oogenesis and preimplantation development but is not required for blastocyst formation. Mechanisms Dev. 1998;73:183–191. doi: 10.1016/s0925-4773(98)00051-3. [DOI] [PubMed] [Google Scholar]
- Salmon WD, Jr, Daughaday WH. A hormonally controlled serum factor which stimulates sulfate incorporation by cartilage in vitro. J Lab Clin Med. 1957;49:825–836. [PubMed] [Google Scholar]
- Sibley CP, Coan PM, Ferguson-Smith AC, Dean W, Hughes J, Smith P, Reik W, Burton GJ, Fowden AL, Constancia M. Placental-specific insulin-like growth factor 2 (Igf2) regulates the diffusional exchange characteristics of the mouse placenta. Proc Natl Acad Sci U S A. 2004;101:8204–8208. doi: 10.1073/pnas.0402508101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stulc J. Extracellular transport pathways in the haemochorial placenta. Placenta. 1989;10:113–119. doi: 10.1016/0143-4004(89)90012-x. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Kobayashi T, Kanayama N. p57Kip2 regulates the proper development of labyrinthine and spongiotrophoblasts. Mol Human Reprod. 2000;6:1019–1025. doi: 10.1093/molehr/6.11.1019. [DOI] [PubMed] [Google Scholar]
- Teasdale F. Idiopathic intrauterine growth retardation: histomorphometry of the human placenta. Placenta. 1984;5:83–92. doi: 10.1016/s0143-4004(84)80051-x. [DOI] [PubMed] [Google Scholar]
- Thornton JG, Hornbuckle J, Vail A, Spiegelhalter DJ, Levene M. Infant wellbeing at 2 years of age in the Growth Restriction Intervention Trial (GRIT): multicentred randomised controlled trial. Lancet. 2004;364:513–520. doi: 10.1016/S0140-6736(04)16809-8. [DOI] [PubMed] [Google Scholar]
- Waddell BJ, Hisheh S, Dharmarajam AM, Burton PJ. Apoptosis in rat placenta is zone-dependent and stimulated by glucocorticoids. Biol Reprod. 2000;63:1913–1917. doi: 10.1095/biolreprod63.6.1913. [DOI] [PubMed] [Google Scholar]