Abstract
Sympathetic vasoconstriction is blunted in the vascular beds of contracting skeletal muscle in humans, presumably due to the action of vasoactive metabolites (functional sympatholysis). Recently, we demonstrated that infusion of ATP into the arterial circulation of the resting human leg increases blood flow and concomitantly blunts α-adrenergic vasoconstriction in a similar manner to that during moderate exercise. Here we tested the hypothesis that ATP, rather than its dephosphorylated metabolites, induces vasodilatation and sympatholysis in resting skeletal muscle via activation of ATP/UTP-selective receptors. To this aim, we first measured leg blood flow (LBF), mean arterial pressure (MAP), cardiac output (Q̇), leg arterial–venous (a–v) O2 difference, plasma ATP and soluble nucleotidase activities during intrafemoral artery infusion of adenosine, AMP, ADP, ATP or UTP in nine healthy males. Comparison of the doses of nucleotides and adenosine required for a similar increase in LBF from ∼0.5 l min−1 at baseline to ∼3.5 l min−1 (without altering MAP but increasing Q̇ significantly) revealed the following rank order of vasoactive potency: ATP (100) = UTP (100) >> adenosine (5.8) > ADP (2.7) > AMP (1.7). The infusions did not cause any shifts in plasma ATP level or soluble serum nucleotidase activities. Combined infusion of the vasodilatory compounds and the sympathetic vasoconstrictor drug tyramine increased plasma noradrenaline in all hyperaemic conditions, but only caused leg and systemic vasoconstriction and augmented O2 extraction during adenosine, AMP and ADP infusion (LBF from 3.2 ± 0.3 to 1.8 ± 0.2 l min−1; 3.7 ± 0.4 to 1.7 ± 0.2 l min−1 and 3.3 ± 0.4 to 2.4 ± 0.3 l min−1, respectively, P < 0.05). These findings in humans suggest that the vasodilatory and sympatholytic effects of exogenous ATP in the skeletal muscle vasculature are largely mediated via ATP itself rather than its dephosphorylated metabolites, most likely via binding to endothelial ATP/UTP-selective P2Y2 receptors. These data are consistent with a role of ATP in skeletal muscle hyperaemia in conditions of increased sympathetic nerve drive such as exercise or hypoxia.
The potent and widespread vascular actions of purine nucleotides and nucleosides have long been recognized (Drury & Szent-Gyorgyi, 1929), and particularly ATP has been implicated in a number of physiological functions such as the hyperaemia seen during exercise and hypoxia (González-Alonso et al. 2002). Extracellular nucleotides mediate their effects via a series of cell surface P2 receptors composed of ligand-gated P2X receptors and G-protein-coupled P2Y receptors where the principal physiological agonists of the human P2Y receptors are ADP (P2Y1, P2Y12, P2Y13), UTP/ATP (P2Y2), UTP (P2Y4), UDP (P2Y6) and ATP (P2Y11) whereas adenosine binds to nucleoside-selective P1 receptors (Ralevic & Burnstock, 1998; Abbracchio et al. 2006). Activation of purinergic receptors in blood vessels induces vasodilatation or vasoconstriction depending upon receptor localization and subtype and therefore are thought to play important roles in the regulation of vascular tone and blood flow (Ralevic & Burnstock, 2003). To terminate signalling, ectonucleotidases are present in the circulation and on cell surfaces, rapidly degrading extracellular ATP into ADP, AMP and adenosine (Gordon, 1986; Zimmermann, 2006; Yegutkin, 2008). Thus, activation of the receptors represents a net balance between nucleotide release, inactivation and receptor affinity.
We have recently demonstrated that ATP, when infused intra-arterially into resting limb skeletal muscle at the levels released during moderate exercise (Rosenmeier et al. 2004), not only increases leg blood flow via purinergic receptor stimulation but also directly or indirectly overrides increases in muscle sympathetic vasoconstrictor activity, thereby simulating what has been demonstrated numerous times to occur during exercise (a phenomenon termed functional sympatholysis) (Remensnyder et al. 1962; Thomas & Victor, 1998; Buckwalter et al. 2001; Tschakovsky et al. 2002; Dinenno & Joyner, 2003; Rosenmeier et al. 2003a,b). Because extracellular ATP can be rapidly degraded to ADP, AMP and adenosine (Gordon, 1986; Zimmermann, 2006; Yegutkin, 2008), it remains unknown whether the vasodilatory and sympatholytic effects of ATP are partially or totally mediated via ATP itself or its dephosphorylated compounds and which particular receptor subtypes are involved.
We therefore used pharmacological dissection in this study to characterize the purinergic receptors responsible for endothelium-derived vasodilatation in human vessels by using adenosine and various purine and pyrimidine agonists and further comparing their vasoactive potency. To test which receptors are capable of producing sympatholysis, the different endothelial vasodilators were also investigated under conditions of tyramine-mediated sympathetic vasoconstriction. We hypothesize that ATP, rather than its dephosphorylated metabolites, induces vasodilatation and sympatholysis in resting skeletal muscle via activation of ATP-selective receptors. Lastly, since strenuous exercise and hypoxia are characterized by significantly increased blood flow and activation of intravascular nucleotide turnover (González-Alonso et al. 2002; Yegutkin et al. 2007), we also determined whether these vasoactive compounds when infused into the circulation of resting subjects would affect circulating ATP and soluble nucleotidase activities to the same extent as seen during exercise.
Methods
Subjects
This study was performed in nine healthy male subjects with a mean ±s.d. age of 27 ± 2 years, body weight of 75 ± 3 kg, and height of 178 ± 5 cm. The subjects were fully informed of any risks and discomforts associated with the experiments before giving their informed written consent to participate. The studies conformed to the code of Ethics of the World Medical Association (Declaration of Helsinki) and were approved by the Ethics Committee for Copenhagen and Frederiksberg communities.
Experimental protocols
Subjects reported to the laboratory at 8 am or 1 pm, following the ingestion of a light breakfast or lunch. Upon arrival, they rested in a supine position while three catheters were placed under local anaesthesia (1% lidocaine (lignocaine)) into the femoral artery and vein of the right leg and in the femoral artery of the left leg using the Seldinger technique. The femoral artery and vein catheters were positioned 1–2 cm distal from the inguinal ligament. A thermistor (Edslab probe 94–0.3–2.5F) to measure venous blood temperature was inserted through the femoral venous catheter orientated in the anterograde direction for the determination of femoral venous blood flow. Adenosine (1.25 mg ml−1; Item Development AB, Stocksund, Sweden) and nucleotides AMP, ADP, ATP and UTP (all from Sigma) were dissolved in isotonic saline and infused at a rate sufficient to increase LBF to ∼3.5 l min−1. Next, the combined infusion of vasodilatory nucleotides and adenosine with tyramine (Sigma T-2879; infused at a rate of ∼13.2 μmol min−1) was performed to evoke a vasoconstrictor response in the resting leg of ∼50% with the adenosine infusion, without causing significant increases in arterial blood pressure. Following 30 min of rest, subjects first received separate infusions of adenosine, AMP, ADP, ATP or UTP for 4 min followed by combined infusions of vasodilator and tyramine for an additional 4 min period while they were resting seated in the upright position. Trials were separated by 30 min of rest and were counterbalanced across the subjects, except for the adenosine trial, which was always performed first and repeated at the end of the study. This comparison demonstrated a high reproducibility of the effect of adenosine + tyramine infusion on leg vasoconstriction. Separate tyramine infusion was performed once in each subject. The whole protocol lasted ∼4.5 h.
Blood flow and vascular conductance
Femoral venous blood flow (an index of leg blood flow; LBF) was determined by the constant infusion thermodilution technique as previously described (González-Alonso et al. 2000). Cardiac output was calculated by multiplying stroke volume by heart rate, using the Modelflow method to determine stroke volume from directly measured arterial blood pressure (Beat Scope 1.1a; Finapress Medical Systems BV, Amsterdam, the Netherlands) (Bogert & van Lieshout, 2005; González-Alonso et al. 2006). LBF represents the average of two measurements made after 1 min of the start of separate infusion of AMP, ADP, UTP, ATP or adenosine and after 1 min of subsequent combined infusion of these purines or pyrimidines and the vasoconstrictor drug tyramine. Arterial blood pressure was continuously monitored in all conditions by a pressure transducer (Pressure Monitoring Kit, Baxter) positioned at the level of the inguinal region and connected to a haemodynamic monitor (Dialogue, Danica Elektronic, Copenhagen, Denmark). Mean arterial pressure was calculated by integration of the pressure curve whereas heart rate was determined from an electrocardiogram. All data were continuously recorded using a Powerlab system (ADInstruments, Sydney, Australia). Leg vascular conductance was calculated as the quotient between LBF and mean arterial pressure.
Blood analysis and measurement of soluble nucleotidase activities
Blood samples (1.5 ml for blood gases, 2.7 ml for plasma ATP, 2 ml for noradrenaline and adrenaline analyses and 5 ml for serum nucleotidase assays) were drawn from the femoral vein of the right leg and femoral artery of the left leg at baseline and after 2 min of purine and pyrimidine infusion, as previously described (Rosenmeier et al. 2004). Plasma noradrenaline and adrenaline concentrations were determined with high performance liquid chromatography with electrochemical detection (Hallman et al. 1978). Arterial and femoral haemoglobin concentration, O2 saturation and PO2 were analysed with an automatic blood gas analyser (ABL720, Radiometer, Copenhagen, Denmark). ATP concentration in EDTA-containing plasma samples was determined by the luciferin–luciferase technique (Lundin, 2000) using an ATP kit (BioTherma AB, Sweden) and an ORION Microplate Luminometer with two automatic injectors (Berthold Detection System, Germany).
Soluble nucleotidase activities were measured by incubating serum at 37°C for 60 min in a final volume of 60 μl RPMI-1640 medium containing 5 mmol l−1β-glycerophosphate in the following ways: (1) for evaluation of total ATPase activity, 5 μl of serum was incubated with 100 μmol l−1 ATP with tracer [8-14C]ATP (specific activity 57 mCi mmol−1; Amersham), and (2) for ADPase/NTPDase activity serum (10 μl) was incubated with 50 μmol l−1 ADP and tracer [2,8-3H]ADP (27.5 Ci mmol−1; Perkin Elmer) in the presence of adenylate kinase inhibitor Ap5A (80 μmol l−1). Radiolabelled nucleotides and their dephosphorylated products were separated by thin layer chromatography (TLC) using Alugram SIL G/UV254 sheets (Macherey-Nagel, Germany), visualized in UV light and quantified by scintillation β-counting, as described previously (Yegutkin et al. 2003).
Statistical analysis
A one-way repeated measures analysis of variance (ANOVA) was performed to test significance between and within treatments. Following a significant F test, pair-wise differences were identified using Tukey's honestly significant difference (HSD) post hoc procedure. When appropriate, significant differences were also identified using Student's paired t tests. The significance level was set at P < 0.05. Data are presented as mean ±s.e.m.
Results
Haemodynamic responses, vasodilator potency and intravascular nucleotide turnover during separate infusions of purines and pyrimidines
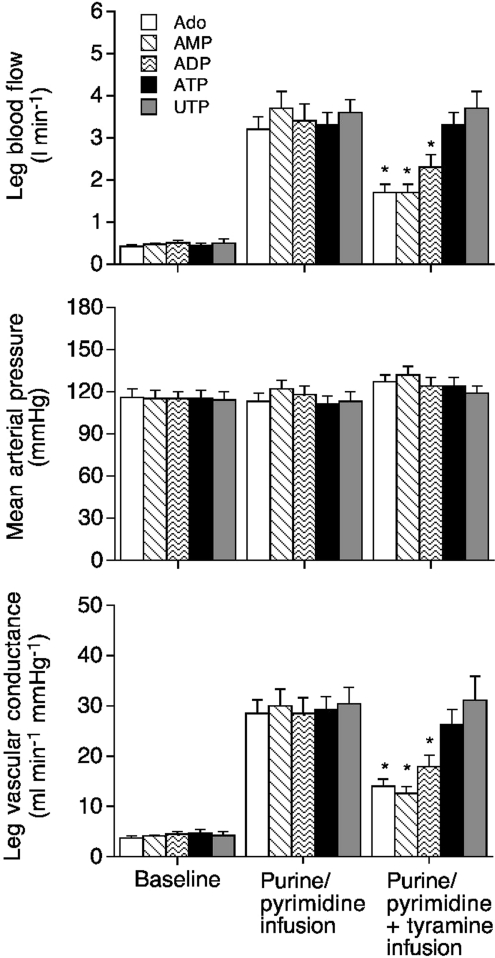
During pharmacologically induced vasodilatation, LBF increased from a baseline level of ∼0.5 l min−1 to 3.2 ± 0.3, 3.7 ± 0.4, 3.4 ± 0.4, 3.3 ± 0.3 and 3.6 ± 0.3 l min−1 for adenosine, AMP, ADP, ATP and UTP, respectively, while mean arterial pressure remained unchanged (Fig. 1). Consequently, leg vascular conductance increased from ∼4.2 ml min−1 mmHg−1 at baseline to 28.5 ± 2.7, 30.0 ± 3.3, 28.5 ± 3.1, 29.3 ± 2.5 and 30.4 ± 3.3 ml min−1 mmHg−1 for adenosine, AMP, ADP, ATP and UTP, respectively. During hyperaemia, heart rate increased to ∼68 beats min−1 in all conditions from similar resting levels of ∼59 beats min−1 accompanying an increase in stroke volume from ∼87 to ∼103 ml beat−1 (Fig. 2). Thus, cardiac output increased similarly in all conditions (Fig. 2).
Figure 1. Leg haemodynamics during purine or pyrimidine infusion alone and in combination with tyramine infusion.
Leg blood flow, mean arterial pressure and leg vascular conductance during separate intrafemoral artery infusion of adenosine, AMP, ADP, ATP or UTP or combined with tyramine infusion. Data are mean ±s.e.m. for 9 subjects. *Significantly different from control, P < 0.05.
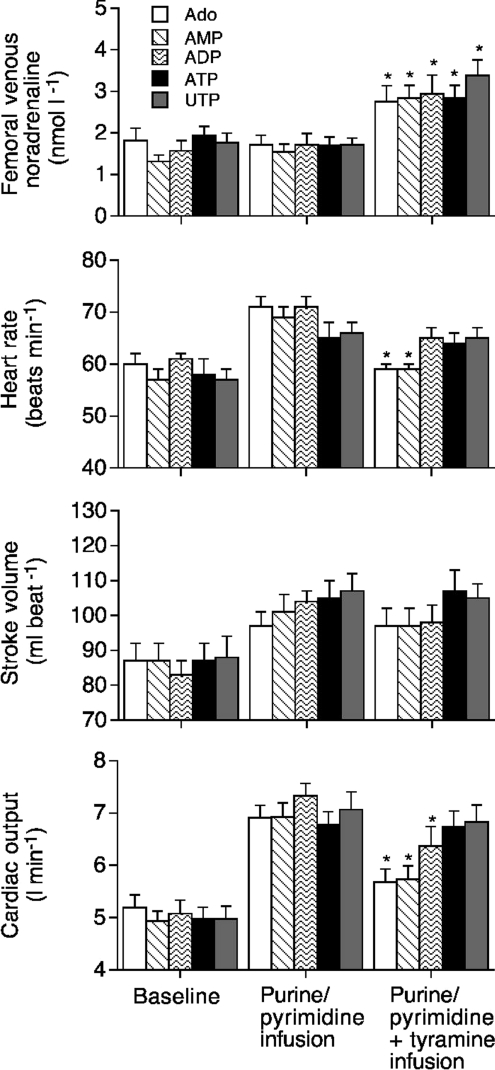
Figure 2. Plasma noradrenaline and systemic haemodynamics during purine or pyrimidine infusion alone and in combination with tyramine infusion.
Femoral venous noradrenaline concentration, heart rate, stroke volume and cardiac output during separate intrafemoral artery infusion of adenosine, AMP, ADP, ATP or UTP or combined with tyramine infusion. Data are mean ±s.e.m. for 9 subjects. *Significantly different from control, P < 0.05.
Comparable infusion rates of ∼1 μmol min−1 were required for both ATP and UTP to achieve increases in LBF from ∼0.5 to 3.6 l min−1. Compared to ATP/UTP, other infused compounds, adenosine, AMP and ADP, displayed much lower vasodilatory potency and caused similar increases in LBF only at rates of 20.9 ± 2.8, 71.3 ± 4.6 and 44.6 ± 2.6 μmol min−1, respectively. Thus, comparison of the relative vasoactive potencies of exogenous nucleotides and adenosine revealed the following rank order: ATP (100) = UTP (100) >> adenosine (5.8) > ADP (2.7) > AMP (1.7).
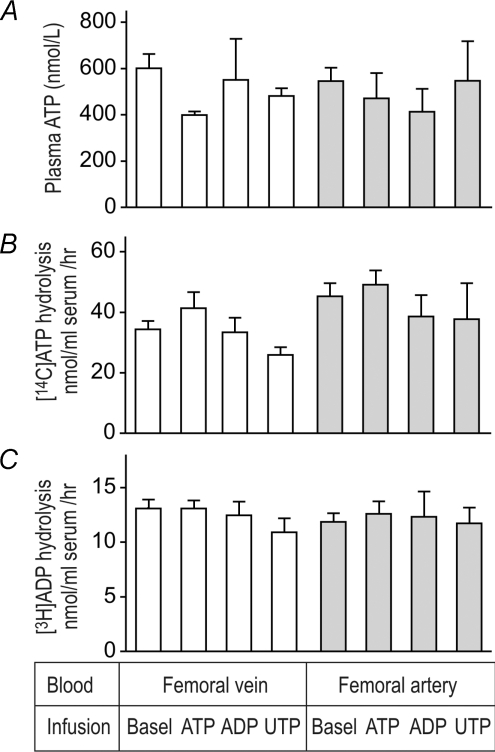
Blood samples were collected from the femoral vein of the right leg and femoral artery of the left leg at baseline and after intrafemoral artery infusion of ATP, UTP and ADP, at concentrations inducing an increase in blood flow above baseline of ∼3 l min−1. Use of the highly ATP-specific bioluminescent assay revealed that human plasma normally contains nanomolar concentrations of ATP and these circulating nucleotide levels remain unchanged in the arterial and femoral venous plasma under conditions of pharmacological nucleotide-induced vasodilatation (∼500 nmol l−1; Fig. 3A). Moreover, radio-TLC enzyme assay demonstrated that infusion of vasodilatory nucleotides also does not affect the rates of [14C]ATP (Fig. 3B) and [3H]ADP (Fig. 3C) hydrolyses by soluble nucleotidases circulating in human serum. Lastly, separate purine/pyrimidine infusion did not alter femoral venous or arterial noradrenaline concentration (Fig. 2).
Figure 3. Plasma ATP and soluble serum nucleotidase activities during infusion of exogenous nucleotides and adenosine in humans.
‘Basel’ stands for baseline (no infusion condition). ATP, ADP and UTP were infused into the femoral artery of the right leg at respective rates of ∼1, 40 and 1 μmol l−1. Blood was collected from the right femoral vein and left femoral artery before and after nucleotide infusion. Circulating ATP was measured in EDTA-plasma using a luciferin–luciferase assay (A), while soluble nucleotidases were assayed in human serum by TLC using 100 μmol l−1[14C]ATP (B) and 50 μmol l−1[3H]ADP (C) as substrates. Data are mean ±s.e.m. for 6 subjects.
Haemodynamic responses during combined purine/pyrimidine and tyramine infusion
During separate tyramine infusion, LBF decreased from 0.5 ± 0.1 to 0.3 ± 0.1 l min−1 (44 ± 3%), without altering mean arterial pressure. During pharmacologically induced vasodilatation and combined tyramine infusion, LBF declined to 1.7 ± 0.2 l min−1 (56%), 1.7 ± 0.2 l min−1 (53%) and 2.3 ± 0.3 l min−1 (30%) during adenosine, AMP and ADP, respectively, but remained unchanged during ATP and tyramine infusion (3.3 ± 0.3 to 3.3 ± 0.3 l min−1; P= 0.97), and UTP and tyramine infusion (3.6 ± 0.4 to 3.7 ± 0.4 l min−1P= 0.79). The same pattern of response was observed in leg vascular conductance (Fig. 2). With superimposition of tyramine, heart rate and cardiac output declined during adenosine, AMP and ADP, but not during ATP and UTP conditions (Fig. 2). Femoral venous noradrenaline concentration increased during all the combined infusions of purine/pyrimidine and tyramine (from ∼2 to 3–4 nmol l−1) (Fig. 2).
Reflecting the LBF responses, leg a–v O2 difference increased from 5 to 8 ml l−1 during separate infusions of adenosine, AMP and ADP to 26.0 ± 2.2 ml l−1 with combined adenosine and tyramine infusion (P < 0.05), 15.3 ± 3.1 ml l−1 with combined AMP and tyramine infusion and 8.5 ± 0.8 ml l−1 (P= 0.07) with combined ADP and tyramine infusion. However, leg a–v O2 difference remained unchanged during combined ATP and tyramine infusion compared to separate infusion of ATP (7.9 ± 1.7 and 5.7 ± 1.6 ml l−1, respectively; P= 0.75) and combined UTP and tyramine infusion compared to separate UTP infusion (7.1 ± 0.9 and 7.1 ± 1.5 ml l−1, respectively; P= 0.98; Table 1).
Table 1.
Blood parameters during purine and pyrimidine infusion alone and in combination with tyramine infusion
| Baseline | Purine or pyrimidine infusion | Purine or pyrimidine + tyramine infusion | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ado | AMP | ADP | ATP | UTP | Ado | AMP | ADP | ATP | UTP | Ado | AMP | ADP | ATP | UTP | |
| Haemoglobin (g l−1) | |||||||||||||||
| a | 137 ± 4 | 134 ± 5 | 132 ± 5 | 132 ± 5 | 130 ± 5 | 131 ± 4 | 135 ± 5 | 134 ± 4 | 135 ± 5 | 131 ± 5 | 136 ± 5 | 134 ± 4 | 133 ± 5 | 131 ± 5 | 129 ± 4 |
| v | 137 ± 5 | 132 ± 4 | 130 ± 5 | 130 ± 5 | 128 ± 6 | 134 ± 5 | 134 ± 4 | 132 ± 5 | 132 ± 5 | 128 ± 4 | 131 ± 5 | 133 ± 5 | 131 ± 4 | 131 ± 5 | 128 ± 5 |
| O2 saturation (%) | |||||||||||||||
| a | 98.2 ± 0.2 | 98.7 ± 0.2 | 98.1 ± 0.1 | 98.4 ± 0.2 | 98.5 ± 0.2 | 98.8 ± 0.1 | 98.3 ± 0.2 | 98.4 ± 0.2 | 98.0 ± 0.1 | 98.0 ± 0.2 | 99.0 ± 0.2 | 98.3 ± 0.1 | 98.3 ± 0.2 | 98.6 ± 0.2 | 99.0 ± 0.2 |
| v | 63.1 ± 2.7 | 61.1 ± 2.9 | 64.8 ± 1.9 | 62.7 ± 2.8 | 66.3 ± 3.0 | 96.7 ± 0.4 | 95.4 ± 1.1 | 95.3 ± 0.7 | 95.9 ± 0.7 | 96.0 ± 0.6 | 85.9 ± 0.4*† | 83.4 ± 0.2*† | 88.2 ± 0.5*† | 94.6 ± 0.6 | 98.2 ± 0.5 |
| PO2 (mmHg) | |||||||||||||||
| a | 100 ± 4 | 101 ± 3 | 100 ± 4 | 101 ± 2 | 100 ± 2 | 105 ± 2 | 106 ± 2 | 104 ± 3 | 106 ± 3 | 108 ± 2 | 112 ± 5 | 110 ± 6 | 108 ± 2 | 109 ± 2 | 110 ± 2 |
| v | 35 ± 4 | 36 ± 3 | 35 ± 3 | 31 ± 3 | 32 ± 4 | 79 ± 1 | 76 ± 2 | 74 ± 2 | 76 ± 2 | 77 ± 3 | 54 ± 4*† | 56 ± 3*† | 60 ± 3*† | 70 ± 3 | 79 ± 3 |
Data are mean ±s.e.m. for 9 subjects. *Significantly different from separate purine or pyrimidine infusion, P < 0.05. †Significantly different from ATP and UTP, P < 0.05.
Discussion
There were three important findings in this study. First, intra-arterial infusion of ATP and UTP, but not of ADP, AMP or adenosine, fully blunts the effects of the tyramine-mediated elevation in sympathetic vasoconstrictor activity on leg muscle and systemic perfusion. Second, by comparing the relative vasoactive potencies of ATP, ADP, AMP and adenosine, when infused intra-arterially, we demonstrated that ATP exerts physiologically relevant vasodilatation at much lower doses than its dephosphorylated metabolites. Further, the infusion doses of ATP and UTP which have the P2Y2 receptor as the only common receptor were similar. Third, nucleotide-induced vasodilatation does not affect plasma ATP and serum nucleotidase activities. Collectively, these findings suggest that the vasodilatory and sympatholytic effects of exogenous ATP in the human skeletal muscle vasculature are largely mediated via ATP itself rather than its dephosphorylated metabolites, most likely via binding to endothelial ATP/UTP-selective P2Y2 receptors.
ATP/UTP-selective receptor stimulation opposes pharmacologically induced vasoconstriction
A major finding of this study was that intra-arterial infusion of ATP and UTP, but not of ADP, AMP or adenosine, inhibited the effects of tyramine-mediated increases in sympathetic vasoconstrictor activity on reducing muscle and systemic perfusion in resting humans. To characterize the purinergic receptors responsible for endothelium-derived vasodilatation and pharmacologically induced sympatholysis, we used here a pharmacological dissection model that extended our previous approach using exercise and infusion of ATP and adenosine in combination with tyramine infusion (Rosenmeier et al. 2004). Because the adenine nucleosides and nucleotides infused in this study are known to stimulate different receptors in humans (i.e. adenosine (P1), AMP (P1 via degradation to adenosine), ADP (P2Y1, P2Y12, P2Y13), UTP (P2Y2, P2Y4), and ATP (P2Y2, P2Y4, P2Y11)) (Ralevic & Burnstock, 1998; Abbracchio et al. 2006), we can establish that the only known receptors in common amongst all these compounds are P2Y2 and P2Y4. Yet, ATP is known to be a competitive antagonist to P2Y4 receptors in humans (Kennedy et al. 2000), making it unlikely that this receptor plays a major role in the strikingly similar vasodilatation and sympatholysis found with ATP and UTP. In favour of a preferential action of the P2Y2 receptor are also the facts that: (i) human skeletal muscle expresses mainly P2Y2 purinergic receptors located in the vascular endothelium (S. P. Mortensen, J. González-Alonso, L. Bune, B. Saltin, H. Pilegaard & Y. Hellsten, unpublished observations), (ii) the expression of P2Y4 receptors on endothelial cells from the human umbilical vein is very scarce (Wang et al. 2002) and cannot be detected at the mRNA level in human skeletal muscle (S. P. Mortensen et al., unpublished observations), (iii) unlike adenosine, the breakdown product of UTP; i.e. uridine, is an inactive metabolite with limited additional pharmacological effects (Vassort, 2001), and (iv) UTP is only known be released from endothelial cells during pathological conditions such as cardiac ischaemia in humans (Wihlborg et al. 2006). Taken together, these observations in humans suggest that the P2Y2 receptor might be physiologically relevant for the regulation of vascular tone and blood flow in conditions of transient ATP or UTP release into the bloodstream combined with increases in noradrenaline-induced vasoconstriction.
LBF responses to separate purine/pyrimidine infusion and combined purine/pyrimidine + tyramine administration were met by parallel cardiac output responses. This raises the question of whether the leg infusions were having systemic effects that alter LBF indirectly. Arguing against this possibility, only ATP and UTP infusion prevented the decline of both LBF and cardiac output. It is reasoned that if the metabolites and tyramine acted via systemic effects, cardiac output would be the same across all the interventions given the equal initial hyperaemia and subsequent vasoconstrictor challenge. Further, blood flow to non-infused tissues (i.e. contralateral leg and upper body tissues) did not increase in any of the conditions, in agreement with the previous observations during intrafemoral artery ATP infusion that contralateral limb blood flow remains unchanged and that LBF and cardiac output increase proportionally (González-Alonso et al. 2002, 2008). Lastly, cardiac output and LBF do not increase when adding ATP into the venous blood returning to the heart during administration of ATP in the femoral vein (González-Alonso et al. 2008). Therefore, these findings jointly indicate that the infused metabolites and tyramine are having primarily a local effect.
Although the precise mechanism by which ATP or UTP overrides the vasoconstrictor influence of noradrenaline is not readily evident, the present difference in vasoconstrictor responses in the leg and systemic circulations between ATP/UTP and the other purines and pyrimidines does not appear to involve a blunting in presynaptic release of noradrenaline since tyramine infusion increased femoral venous noradrenaline concentration to the same level in all conditions (Fig. 2). Because neither ATP, ADP, AMP, nor adenosine can readily cross the endothelium (Mo & Ballard, 2001), it is unlikely that these compounds act via direct modulation of postjunctional α-adrenoreceptors located on vascular smooth muscle cells. Rather, the sympatho-inhibitory difference between these related compounds are probably due to either activation of different receptor subtypes, signal transduction pathways, and/or second metabolite release involved in the communication between the endothelial and smooth muscle cells in the skeletal muscle microvasculature (Burnstock & Kennedy, 1986).
Binding of ATP to endothelial P2Y receptors is known to stimulate the release of NO, adenosine, prostaglandins and endothelial-derived hyperpolarization factors (EDHF) leading to vascular smooth muscle relaxation (Collins et al. 1998; Olearczyk et al. 2004; van Ginneken et al. 2004; Winter & Dora, 2007). In the context of the present findings, these downstream signals could also lead to the modulation of direct noradrenaline-induced α-adrenoceptor stimulation. The findings by Kirby et al. (2008) demonstrating that exogenous ATP (but not adenosine) can abolish direct postjunctional α1- and α2-adrenergic vasoconstriction indicate that the postjunctional α-adrenoceptors are indeed involved in ATP/UTP-induced sympatholysis. However, evidence for the exact intracellular signals involved in this phenomenon is lacking. NO, adenosine or prostaglandins seem unlikely signals since they are not obligatory for functional sympatholysis (Thomas & Segal, 2004). EDHF is also an unlikely signal because it is not exclusively triggered by ATP or UTP (Wihlborg et al. 2003). EDHF can be released by acetylcholine, which does not have sympatholytic properties (Busse et al. 2002). Thus, elucidation of the precise mechanisms for the ATP/UTP-induced sympatholysis in human skeletal muscle is warranted.
Potency differences between adenine and uridine compounds
We have recently shown that intrafemoral artery ATP infusion, but not intrafemoral vein infusion, can elevate blood flow up to ∼80% of the maximal leg exercise hyperaemia established during incremental cycling to exhaustion (∼9 l min−1) (Rosenmeier et al. 2004; González-Alonso et al. 2008). Although ATP and UTP in this study were found to be equipotent with regard to leg blood flow at infusion rates of ∼1 μmol min−1, progressive infusion of UTP in three subjects demonstrated a plateau response in leg blood flow at ∼6 l min−1, a peak value which is lower than the ∼8 l min−1 peak leg blood flow observed with ATP infusion (Rosenmeier et al. 2004; González-Alonso et al. 2008). This poses the question of whether the degradation of ATP to its other derivatives such as ADP contributes to the higher peak ATP-induced vasodilatation. This is an attractive possibility in the light of the recent observation that strenuous exercise-induced hyperaemia is accompanied by concurrent up-regulation of soluble nucleotide-inactivating enzymes (Yegutkin et al. 2007). Yet the present observation that the rates of adenosine (21 μmol min−1), AMP (71 μmol min−1) and ADP (44 μmol min−1) infusion needed to achieve the same leg blood flow levels of about 3.5 l min−1 are very high in comparison with that of ATP and UTP (1 μmol min−1) suggests that ATP-dephosphorylated metabolites do not contribute importantly to ATP-induced vasodilatation and sympatholysis. Although a rapid uptake and metabolic breakdown mechanisms of adenosine might partially explain its lower vasodilator potency (Murrant & Sarelius, 2002), adenosine infusion is not capable of opposing direct sympathetic vasoconstriction in humans (Tschakovsky et al. 2002; Rosenmeier et al. 2003b; Kirby et al. 2008), rendering it an unlikely candidate for functional sympatholysis. Therefore, the present finding supports a smaller contribution of adenosine, AMP and ADP to limb muscle hyperaemia, because ATP, for instance, does not seem to be largely degraded to adenosine derivates in the circulation as previously demonstrated using P1 receptor blockade with theophylline during ATP infusion (Rongen et al. 1994) and the soluble purinergic enzyme concentration did not increase during infusion as discussed below.
Nucleotide-induced leg muscle vasodilatation does not affect plasma ATP and serum nucleotidase activities
As previously mentioned, exercise hyperaemia is accompanied by transient increases in circulating nucleotide levels (i.e. plasma ATP, ADP and AMP) as well as by up-regulation of soluble nucleotide pyrophosphatase/phosphodiesterase (NPP) and NTPDase activities (Yegutkin et al. 2007). With the current investigation we also wanted to determine whether regulation of leg haemodynamics by infusion of vasoactive nucleotides to the resting subjects would affect intravascular nucleotide turnover to the same extent as strenuous exercise. Infusion of vasodilatory nucleotides ATP, ADP and UTP was not accompanied by significant shifts in plasma ATP (Fig. 3A) as well as in other circulating nucleotides (data not shown), thus suggesting that, subsequent to signal transduction, nucleotides are immediately eliminated from the bloodstream.
In keeping with this rationale of rapid nucleotide breakdown, we believe that previously reported findings on exercise-mediated increases in circulating ATP, ADP and AMP levels (González-Alonso et al. 2002; Mortensen et al. 2007; Yegutkin et al. 2007) reasonably indicate that a net balance between nucleotide release and inactivation is markedly disturbed under exercising hyperaemic conditions and therefore, actual amounts of nucleotides liberated in the bloodstream of exercising humans should exceed their measured plasma concentrations of ∼1 μmol l−1. Interestingly, cultured endothelial cells have been shown to release E-NTPDases and ecto-5′-nucleotidase along with ATP in response to the application of shear force (Yegutkin et al. 2000). By analogy, it would be anticipated that the increased shear rate might also activate the release of membrane-bound nucleotidases in vivo, thus underlying the recently described phenomena of transient up-regulation of serum NTPDase and NPP under exercising hyperaemic conditions (Yegutkin et al. 2007). However, directional manipulation of LBF by injection of ATP and other vasoactive nucleotides into the femoral artery of the resting subjects did not affect the rates of [14C]ATP and [3H]ADP hydrolyses by human serum (Fig. 3), thus ruling out the possibility that the increased shear rate directly stimulates the release of soluble purine-converting activities.
Experimental limitations
There are limitations in manipulating vascular adenine nucleotides and adenosine with intravascular injections to investigate the role ATP and its dephosphorylated metabolites on the regulation of skeletal muscle blood flow during conditions of enhanced sympathetic vasoconstrictor drive such as moderate and intense exercise and/or hypoxia. Because nucleotides and adenosine in the bloodstream do not pass easily through the interstitium (Mo & Ballard, 2001), the intravascular injections employed here are likely to mimic the effects of purines when they are released from red blood cells, endothelial cells or generated extracellularly from other sources (González-Alonso et al. 2002; Rosenmeier et al. 2004; Erlinge & Burnstock, 2008). Their effects probably are largely mediated via stimulation of P1 and P2Y endothelial receptors. During exercise or hypoxia, however, nucleotides and/or adenosine might also increase in concentration in the interstitium (Hellsten et al. 1998; MacLean et al. 1998). Interstitial purines probably act mainly on the vascular smooth muscle receptors, possibly on abundant vasoconstrictor P2X receptors, and possibly on sympathetic prejunctional receptors (Erlinge & Burnstock, 2008).
Tyramine is a commonly used pharmacological tool to induce limb muscle vasoconstriction via stimulation of endogenous release of noradrenaline from sympathetic nerve endings (Rongen et al. 1994; Dinenno & Joyner, 2003). It must be emphasized that venous noradrenaline concentration does not always accurately reflect noradrenaline release from sympathetic neurons (Esler et al. 1990). Hence, an exact extrapolation cannot be made between the effects of tyramine and those of naturally evoked increases in sympathetic activity. Here we assumed that the equal increase in femoral venous noradrenaline with superimposition of tyramine infusion reflected an equal release of noradrenaline from the sympathetic nerve terminals and equivalent increase in vasoconstrictor drive in all conditions. However, the naturally evoked sympathetic activity occurs in bursts and groups of impulses and three different transmitters are released, namely noradrenaline, ATP and neuropeptide Y, where the relative importance of each of these compounds in contributing to vasoconstriction is different depending on the exact patterning of activity and the prevailing conditions. In future studies it will be important to test the ability of infused ATP/UTP to inhibit the vasoconstrictor influence of a naturally evoked increase in muscle sympathetic nerve activity (MSNA).
In conclusion, the present findings demonstrate that exogenous ATP and UTP possess the capacity to produce potent limb muscle vasodilatation and override vasoconstriction evoked by tyramine-induced release of noradrenaline at rest, possibly via their common P2Y2 receptor. These data are consistent with the additional observations in this study showing a greater vasodilatory potency of ATP than its dephosphorylated metabolites in the leg and the unchanged plasma nucleotide and soluble nucleotide concentrations in the blood. Because ATP and UTP are discharged in normal physiological or pathophysiological conditions where the sympathetic nervous drive or MSNA is also elevated, such as exercise (Yegutkin et al. 2007), hypoxia (González-Alonso et al. 2002; Hanada et al. 2003), anaemia (González-Alonso et al. 2006) and cardiac ischaemia (Erlinge et al. 2005), it seems possible that the present findings might have implications for the understanding of circulatory control during exercise and certain disease states.
Acknowledgments
Special thanks are given to Stefan Mortensen for his assistance with the study as well as to Paula Croft from Europe ADInstruments Ltd for her technical support. J. B. Rosenmeier was supported by the Danish Heart Foundation and Novo Nordisk Foundation. G. G. Yegutkin was supported by the Finnish Academy, Sigrid Juselius Foundation. J. González-Alonso was supported by grants from the Danish National Research Foundation.
References
- Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharm Rev. 2006;58:281–341. doi: 10.1124/pr.58.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogert LW, van Lieshout JJ. Non-invasive pulsatile arterial pressure and stroke volume changes from the human finger. Exp Physiol. 2005;90:437–446. doi: 10.1113/expphysiol.2005.030262. [DOI] [PubMed] [Google Scholar]
- Buckwalter JB, Naik JS, Valic Z, Clifford PS. Exercise attenuates α-adrenergic-receptor responsiveness in skeletal muscle vasculature. J Appl Physiol. 2001;90:172–178. doi: 10.1152/jappl.2001.90.1.172. [DOI] [PubMed] [Google Scholar]
- Burnstock G, Kennedy C. A dual function for adenosine 5′-triphosphate in the regulation of vascular tone. Excitatory cotransmitter with noradrenaline from perivascular nerves and locally released inhibitory intravascular agent. Circ Res. 1986;58:319–330. doi: 10.1161/01.res.58.3.319. [DOI] [PubMed] [Google Scholar]
- Busse R, Edwards G, Feletou M, Flemming I, Vanhoutte PM, Weston AH. EDHF: bringing the concepts together. Trends Pharmacol Sci. 2002;23:374–380. doi: 10.1016/s0165-6147(02)02050-3. [DOI] [PubMed] [Google Scholar]
- Collins DM, McCullough WT, Ellsworth ML. Conducted vascular responses: communication across the capillary bed. Microvasc Res. 1998;56:43–53. doi: 10.1006/mvre.1998.2076. [DOI] [PubMed] [Google Scholar]
- Dinenno FA, Joyner MJ. Blunted sympathetic vasoconstriction in contracting skeletal muscle of healthy humans: is nitric oxide obligatory? J Physiol. 2003;553:281–292. doi: 10.1113/jphysiol.2003.049940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury AN, Szent-Gyorgyi A. The physiological activity of adenine compounds with especial reference to their action upon the mammalian heart. J Physiol. 1929;68:213–237. doi: 10.1113/jphysiol.1929.sp002608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlinge D, Burnstock G. P2 receptors in cardiovascular regulation and disease. Purinergic Signal. 2008;4:1–20. doi: 10.1007/s11302-007-9078-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlinge D, Harnek J, van Heusden C, Olivecrona G, Jern S, Lazarowski E. Uridine triphosphate (UTP) is released during cardiac ischemia. Int J Cardiol. 2005;100:427–433. doi: 10.1016/j.ijcard.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Esler MD, Jennings G, Lambert G, Meredith I, Horne M, Eisenhofer G. Overflow of catecholamine neurotransmitters to the circulation: source, fate, and functions. Physiol Rev. 1990;70:963–985. doi: 10.1152/physrev.1990.70.4.963. [DOI] [PubMed] [Google Scholar]
- González-Alonso J, Mortensen SP, Dawson EA, Secher NH, Damsgaard R. Erythrocytes and the regulation of human skeletal muscle blood flow and oxygen delivery: role of erythrocyte count and oxygenation state of haemoglobin. J Physiol. 2006;572:295–305. doi: 10.1113/jphysiol.2005.101121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Alonso J, Mortensen SP, Jeppesen TD, Ali L, Barker H, Damsgaard R, Secher NH, Dawson EA, Dufour SP. Haemodynamic responses to exercise, ATP infusion and thigh compression in humans: insight into the role of muscle mechanisms on cardiovascular function. J Physiol. 2008;586:2405–2417. doi: 10.1113/jphysiol.2008.152058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Alonso J, Olsen DB, Saltin B. Erythrocyte and the regulation of human skeletal muscle blood flow and oxygen delivery: role of circulating ATP. Circ Res. 2002;91:1046–1055. doi: 10.1161/01.res.0000044939.73286.e2. [DOI] [PubMed] [Google Scholar]
- González-Alonso J, Quistorff B, Krustrup P, Bangsbo J, Saltin B. Heat production in human skeletal muscle at the onset of intense dynamic exercise. J Physiol. 2000;524:603–615. doi: 10.1111/j.1469-7793.2000.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JL. Extracellular ATP: effects, sources and fate. Biochem J. 1986;233:309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallman H, Farnebo LO, Hamberger B, Johnsson G. A sensitive method for the determination of plasma catecholamines using liquid chromatography with electrochemical detection. Life Sci. 1978;23:1049–1052. doi: 10.1016/0024-3205(78)90665-3. [DOI] [PubMed] [Google Scholar]
- Hanada A, Sander M, González-Alonso J. Human skeletal muscle sympathetic activity, heart rate and limb haemodynamics with reduced blood oxygenation and exercise. J Physiol. 2003;551:635–647. doi: 10.1113/jphysiol.2003.044024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellsten Y, Maclean D, Rådegran G, Saltin B, Bangsbo J. Adenosine concentrations in the interstitium of resting and contracting human skeletal muscle. Circulation. 1998;98:6–8. doi: 10.1161/01.cir.98.1.6. [DOI] [PubMed] [Google Scholar]
- Kennedy C, Qi AD, Herold CL, Harden TK, Nicholas RA. ATP, an agonist at the rat P2Y4 receptor, is an antagonist at the human P2Y4 receptor. Mol Pharmacol. 2000;57:926–931. [PubMed] [Google Scholar]
- Kirby BS, Voyles WF, Carlson RE, Dinenno FA. Graded sympatholytic effect of ATP on postjunctional a-adrenergic vasoconstriction in the human forearm: implications for vascular control in contracting muscle. J Physiol. 2008;586:4305–4316. doi: 10.1113/jphysiol.2008.154252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundin A. Use of firefly luciferase in ATP-related assays of biomass, enzymes, and metabolites. Methods Enzymol. 2000;305:346–370. doi: 10.1016/s0076-6879(00)05499-9. [DOI] [PubMed] [Google Scholar]
- MacLean DA, Sinoway LI, Leuenberger U. Systemic hypoxia elevates skeletal muscle interstitial adenosine levels in humans. Circulation. 1998;98:1990–1992. doi: 10.1161/01.cir.98.19.1990. [DOI] [PubMed] [Google Scholar]
- Mo FM, Ballard HJ. The effect of systemic hypoxia on interstitial and blood adenosine, AMP, ADP and ATP in dog skeletal muscle. J Physiol. 2001;536:593–603. doi: 10.1111/j.1469-7793.2001.0593c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen SP, González-Alonso J, Damsgaard R, Saltin B, Hellsten Y. Inhibition of nitric oxide and prostaglandins, but not endothelial-derived hyperpolarizing factors, reduces blood flow and aerobic energy turnover in the exercising human leg. J Physiol. 2007;581:853–861. doi: 10.1113/jphysiol.2006.127423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrant CL, Sarelius IH. Multiple dilator pathways in skeletal muscle contraction-induced arteriolar vasodilation. Am J Physiol Regul Integr Comp Physiol. 2002;282:R969–R978. doi: 10.1152/ajpregu.00405.2001. [DOI] [PubMed] [Google Scholar]
- Olearczyk JJ, Stephenson AH, Lonigro AJ, Sprague RS. NO inhibits signal transduction pathway for ATP release from erythrocytes via its action on heterotrimeric G protein Gi. Am J Physiol Heart Circ Physiol. 2004;287:H748–H754. doi: 10.1152/ajpheart.00161.2004. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Involvement of purinergic signaling in cardiovascular diseases. Drug News Perspect. 2003;16:133–140. doi: 10.1358/dnp.2003.16.3.876886. [DOI] [PubMed] [Google Scholar]
- Remensnyder JP, Mitchell JH, Sarnoff SJ. Functional sympatholysis during muscular activity. Observations on influence of carotid sinus on oxygen uptake. Circ Res. 1962;11:370–380. doi: 10.1161/01.res.11.3.370. [DOI] [PubMed] [Google Scholar]
- Rongen GA, Smits P, Thien T. Characterization of ATP-induced vasodilation in the human forearm vascular bed. Circulation. 1994;90:1891–1898. doi: 10.1161/01.cir.90.4.1891. [DOI] [PubMed] [Google Scholar]
- Rosenmeier JB, Dinenno FA, Fritzlar SJ, Joyner MJ. α1- and α2-adrenergic vasoconstriction is blunted in contracting human muscle. J Physiol. 2003a;547:971–976. doi: 10.1113/jphysiol.2002.037937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenmeier JB, Dinenno FA, Joyner MJ. Exogenous NO administration and alpha adrenergic vasoconstriction in human limbs. J Appl Physiol. 2003b;95:2370–2374. doi: 10.1152/japplphysiol.00634.2003. [DOI] [PubMed] [Google Scholar]
- Rosenmeier JB, Hansen J, González-Alonso J. Circulating ATP-induced vasodilatation overrides sympathetic vasoconstrictor activity in human skeletal muscle. J Physiol. 2004;558:351–365. doi: 10.1113/jphysiol.2004.063107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas GD, Segal SS. Neural control of muscle blood flow during exercise. J Appl Physiol. 2004;15:893–901. doi: 10.1152/japplphysiol.00076.2004. [DOI] [PubMed] [Google Scholar]
- Thomas GD, Victor RG. Nitric oxide mediates contraction-induced attenuation of sympathetic vasoconstriction in rat skeletal muscle. J Physiol. 1998;506:817–826. doi: 10.1111/j.1469-7793.1998.817bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschakovsky ME, Sujirattanawimol K, Ruble SB, Valic Z, Joyner MJ. Is sympathetic neural vasoconstriction blunted in the vascular bed of exercising human muscle? J Physiol. 2002;541:623–635. doi: 10.1113/jphysiol.2001.014431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ginneken EE, Meijer P, Verkaik N, Smits P, Rongen GA. ATP-induced vasodilation in human skeletal muscle. Br J Pharmacol. 2004;141:842–850. doi: 10.1038/sj.bjp.0705589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassort G. Adenosine 5′-triphosphate: a P2-purinergic agonist in the myocardium. Physiol Rev. 2001;81:767–806. doi: 10.1152/physrev.2001.81.2.767. [DOI] [PubMed] [Google Scholar]
- Wang L, Karlsson L, Moses S, Hultgardh-Nilsson A, Andersson M, Borna C, Gudbjartsson T, Jern S, Erlinge D. P2 receptor expression profiles in human vascular smooth muscle and endothelial cells. J Cardiovasc Pharmacol. 2002;40:841–853. doi: 10.1097/00005344-200212000-00005. [DOI] [PubMed] [Google Scholar]
- Wihlborg AK, Balogh J, Wang L, Borna C, Dou Y, Joshi BV, Lazarowski E, Jacobson KA, Arner A, Erlinge D. Positive inotropic effects by uridine triphosphate (UTP) and uridine diphosphate (UDP) via P2Y2 and P2Y6 receptors on cardiomyocytes and release of UTP in man during myocardial infarction. Circ Res. 2006;98:970–976. doi: 10.1161/01.RES.0000217402.73402.cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wihlborg AK, Malmsjo M, Eyjolfsson A, Gustafsson R, Jacobson K, Erlinge D. Extracellular nucleotides induce vasodilatation in human arteries via prostaglandins, nitric oxide and endothelium-derived hyperpolarising factor. Br J Pharmacol. 2003;138:1451–1458. doi: 10.1038/sj.bjp.0705186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter P, Dora KA. Spreading dilatation to luminal perfusion of ATP and UTP in rat isolated small mesenteric arteries. J Physiol. 2007;582:335–347. doi: 10.1113/jphysiol.2007.135202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yegutkin GG. Nucleotide- and nucleoside-converting ectoenzymes: important modulators of purinergic signalling cascade. Biochim Biophys Acta. 2008;1783:673–694. doi: 10.1016/j.bbamcr.2008.01.024. [DOI] [PubMed] [Google Scholar]
- Yegutkin GG, Bodin P, Burnstock G. Effects of shear stress on the release of soluble ecto-enzymes ATPase and 5′-nucleotidase along with endogenous ATP from vascular endothelial cells. Br J Pharmacol. 2000;129:921–926. doi: 10.1038/sj.bjp.0703136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yegutkin GG, Samburski SS, Jalkanen S. Soluble purine-converting enzyime circulate in human blood and regulate extracellular ATP level via counteracting pyrophosphatase and phosphotransfer reactions. FASEB J. 2003;17:1328–1330. doi: 10.1096/fj.02-1136fje. [DOI] [PubMed] [Google Scholar]
- Yegutkin GG, Samburski SS, Mortensen SP, Jalkanen S, González-Alonso J. Intravascular ADP and soluble nucleotidases contribute to acute prothrombotic state during vigorous exercise in humans. J Physiol. 2007;579:553–564. doi: 10.1113/jphysiol.2006.119453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann H. Ectonucleotidases in the nervous system. Novartis Found Symp. 2006;276:113–128. Discussion 128–130, 233–237, 275–281. [PubMed] [Google Scholar]