Abstract
In non-excitable cells, agonist-induced depletion of intracellular Ca2+ stores triggers Ca2+ influx via a process termed store-operated Ca2+ entry (SOCE). In T-lymphocytes, stromal interaction molecule 1 (STIM1) acts as the intra-store Ca2+ sensor and Orai1 functions as the Ca2+-permeable SOCE channel activated by STIM1 following store depletion. Two functionally distinct Ca2+ entry pathways exist in skeletal muscle; one activated by store depletion (SOCE) and a second by sustained/repetitive depolarization that does not require store depletion (excitation-coupled Ca2+ entry, ECCE). However, the role of STIM1 and Orai1 in coordinating SOCE and ECCE activity in skeletal muscle and whether these two Ca2+ entry pathways represent distinct molecular entities or two different activation mechanisms of the same channel complex is unknown. Here we address these issues using siRNA-mediated STIM1 knockdown, dominant-negative Orai1, and permeation-defective Orai1 to determine the role of STIM1 and Orai1 in store-operated and excitation-coupled Ca2+ entry in skeletal myotubes. SOCE and ECCE activity were quantified from both intracellular Ca2+ measurements and Mn2+ quench assays. We found that STIM1 siRNA reduced STIM1 protein by more than 90% and abolished SOCE activity, while expression of siRNA-resistant hSTIM1 fully restored SOCE. SOCE was also abolished by dominant-negative Orai1 (E106Q) and markedly reduced by expression of a permeation-defective Orai1 (E190Q). In contrast, ECCE was unaffected by STIM1 knockdown, E106Q expression or E190Q expression. These results are the first to demonstrate that SOCE in skeletal muscle requires both STIM1 and Orai1 and that SOCE and ECCE represent two distinct molecular entities.
Ca2+ influx through store-operated Ca2+ entry (SOCE) channels following intracellular store depletion provides a critical mechanism to replenish internal Ca2+ stores in non-excitable cells. In T lymphocytes, SOCE channels (termed Ca2+ release activated Ca2+ or CRAC channels) mediate Ca2+-dependent gene transcription by triggering calcineurin-dependent nuclear factor of activated T-cells (NFAT) dephosphorylation and nuclear translocation (Feske, 2007). However, the functional importance of SOCE in most cell types remains an open question. A major breakthrough in the field was the recent discovery of two molecular players in the SOCE pathway – STIM1 proteins located primarily in the membrane of the intracellular compartment (Liou et al. 2005; Roos et al. 2005) and Orai proteins located in the plasma membrane (Feske et al. 2006; Vig et al. 2006b). There are two mammalian STIM1 homologues (STIM1 and STIM2) and three mammalian Orai homologues (Orai1–3). STIM1 serves as the Ca2+ sensor for store depletion through Ca2+ binding to an N-terminal EF-hand motif situated in the store lumen (Liou et al. 2005), while Orai1 channels function as highly Ca2+-selective CRAC channels in the plasma membrane of T lymphocytes (Feske et al. 2006; Prakriya et al. 2006; Vig et al. 2006b). CRAC channel activation results from store depletion causing STIM1 aggregation and reorganization into discrete regions of the endoplasmic reticulum that subsequently interact and activate Orai1 channels in the adjacent plasma membrane (Feske, 2007). However, little information is available with regard to the roles of STIM1 and Orai1 in mediating SOCE in other cell types, particularly excitable cells, and SOCE in some cells involves STIM1 activation of C-type transient receptor potential (TRPC) channels or heteromultimers of Orai and TRPC proteins (Worley et al. 2007; Cheng et al. 2008; Liao et al. 2008).
SOCE is not restricted to non-excitable cells, but is also observed in excitable cells including neurones, cardiac myocytes and smooth muscle cells (Parekh & Putney, 2005). SOCE has also been documented in skeletal muscle fibres (Kurebayashi & Ogawa, 2001; Pan et al. 2002; Launikonis et al. 2003; Launikonis & Rios, 2007), although the electrophysiological correlate of SOCE in adult muscle remains elusive (Allard et al. 2006). While STIM1 and Orai1 coordinate SOCE in T lymphocytes, the importance of these proteins in mediating store-dependent Ca2+ influx in excitable cells remains obscure. For example, both TRPC family members (Albert et al. 2007) and Orai proteins (Peel et al. 2008) have been suggested to underlie SOCE in smooth muscle cells. Similarly, early work suggested that SOCE in skeletal muscle involves either IP3 (Launikonis et al. 2003) or ryanodine (Kiselyov et al. 2000; Pan et al. 2002) receptors as the sarcoplasmic reticulum (SR) Ca2+ sensor and sarcolemmal TRPC proteins as the SOCE channel (Kiselyov et al. 2000; Rosenberg et al. 2004; Sampieri et al. 2005). More recently, STIM1 and Orai1 were shown to be expressed at high levels in skeletal muscle and skeletal myopathy results from a deficiency in either protein (Vig et al. 2008; Stiber et al. 2008). Importantly, SOCE is absent in skeletal myotubes that lack functional STIM1 proteins and STIM1 haplo-insufficiency leads to defects in force generation and fatigue resistance (Stiber et al. 2008). Finally, an alternate store-independent Ca2+ entry mechanism triggered by sustained or repetitive depolarization (excitation-coupled Ca2+ entry or ECCE) is found in skeletal myotubes (Cherednichenko et al. 2004; Yang et al. 2007) and adult muscle fibres (Cherednichenko et al. 2008). While the precise mechanism for ECCE activation in muscle remains elusive, the skeletal muscle isoforms of the ryanodine (RyR1) and dihydropyridine (DHPR) receptors are required (Cherednichenko et al. 2004).
In skeletal muscle, electrically evoked twitch contractions persist in the absence of extracellular Ca2+, demonstrating the exquisite fidelity and efficiency of Ca2+ release and reuptake in this tissue. Indeed, recent estimates indicate that Ca2+ release during a single twitch results in only ∼10 % reduction of releasable Ca2+ in the SR (Launikonis & Rios, 2007). Together, these results indicate that SOCE and ECCE are unlikely to contribute significantly to muscle contractility during a single twitch. However, recent work indicates that SOCE limits contractile decline during trains of high-frequency fatiguing stimulation (Pan et al. 2002) and that extracellular Ca2+ entry limits contractile fatigue in aged skeletal muscle fibres (Payne et al. 2007). More recently, Stiber et al. (2008) reported that EDL muscle from heterozygous STIM1 genetrap mice fatigue faster than wild-type muscle and that STIM1-deficient myotubes exhibit defects in refilling of internal Ca2+ stores. Together, these findings suggests that extracellular Ca2+ entry promotes store refilling and limits the development of high-frequency fatigue. Moreover, increased SOCE (Zhao et al. 2006) and ECCE (Yang et al. 2007; Cherednichenko et al. 2008) contribute to Ca2+ dysregulation in malignant hyperthermia (MH), a pharmacogenetic characterized by episodes of uncontrolled muscle contracture triggered by halogenated anaesthetics and depolarizing muscle relaxants. However, the relative contribution of SOCE and ECCE in protection from contractile decline during fatigue and uncontrolled muscle contractures during MH crises are unknown. While STIM1 is clearly required for SOCE in skeletal muscle (Stiber et al. 2008), the molecular identity of the STIM1-activated influx channel (e.g. Orai and/or TRPC channels) in muscle is unknown. Finally, the role of STIM1 and Orai1 in coordinating ECCE activity in skeletal muscle and whether SOCE and ECCE are mediated by single or distinct molecular entities has also not been determined. Here we show that both STIM1 and Orai1 are required for SOCE, but not ECCE, and demonstrate that these two Ca2+ influx pathways reflect two distinct molecular entities. Our results support the presence of two distinct molecular complexes in mediating Ca2+ influx across the transverse tubule (t-tubule) membrane that serve to limit contractile decline during high-frequency fatigue.
Methods
Ethical approval
All animals were housed in a pathogen-free area at the University of Rochester and all experiments were carried out in accordance with procedures reviewed and approved by the local University Committee on Animal Resources. Mice were killed by a CO2 overdose by regulated delivery of compressed CO2 followed by decapitation.
Primary culture, siRNA transfection and nuclear injection of Orai1 mutant cDNAs
Primary cultures of skeletal myotubes derived from newborn wild-type and RyR1-null (dyspedic) mice were prepared as previously described (Avila & Dirksen, 2001). Transfection of control and murine STIM1 siRNAs (Dahrmacon, Chicago, IL, USA; Supplemental Table 1) was conducted 2 days after initial plating of myoblasts using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) and did not produce a detectable change in myotube formation or differentiation. For STIM1 rescue experiments, a cDNA encoding hSTIM1 (0.3 μg μl−1) was injected into nuclei 1–2 days later. All Ca2+ measurements and Mn2+ quench experiments were conducted in myotubes on the fifth day after siRNA transfection. cDNAs encoding either dominant-negative (E106Q) and pore mutant (E190Q) Orai1 were injected 5 days after initial plating of myoblasts with myotube experiments conducted 2–3 days later. Nuclear injections also included a cDNA encoding CD8 (0.05 μg μl−1) to allow for subsequent identification with CD8 antibody-coated beads.
Western blot analysis
Cultured cells were harvested 7–8 day after initial plating. STIM1 protein was separated by SDS-PAGE on a 10% gel and then electrophoretically transferred to nitrocellulose membrane. Non-specific binding was blocked for 2 h at room temperature with 3% bovine serum albumin in a Tris-buffered saline containing 0.15% Tween 20 (TBST). Membranes were incubated with a mouse monoclonal GOK/STIM1 primary antibody (1: 350; BD Bioscience, Sparks, MD, USA) for 2 h in 3% BSA. Membranes were washed 3 times with TBST and then incubated with a secondary anti-mouse horseradish peroxidase-conjugated antibody (1: 1000; Bio-Rad, Hercules, CA, USA) for 1 h in TBST containing 5% milk. Enhanced chemiluminescence (Super Signal Substrate; Pierce, Rockford, IL, USA) was used to detect bound antibody. Western blot quantification of STIM1 knock-down was determined by densitometry using a UVP Bioimaging system and LabWorksTM software (UVP, Inc., Upland, CA, USA).
RNA extraction and reverse transcriptase PCR
Total RNA was isolated using TRIZOL reagent following the manufacturer's protocol. Prior to reverse transcription, total RNA was treated with DNAase I for 15 min at room temperature. RNA was then reverse-transcribed using Superscript III reverse transcriptase and oligo dT. PCR was performed using specific primers designed against STIM1 or Orai1–3 (Supplemental Table 2). In order to prevent amplification of genomic DNA, forward and reverse primers used spanned neighbouring exons. PCR products were visualized by ethidium bromide staining and confirmed by direct sequencing.
Intracellular Ca2+ measurements
Intracellular Ca2+ levels were measured in intact indo-1 AM-loaded myotubes as previously described (Avila & Dirksen, 2001). Briefly, myotubes grown on glass coverslips were incubated with 6 μm indo-1 AM for 1 h at 37°C in a Ca2+-free rodent Ringer solution consisting of (mm): 145 NaCl, 5 KCl, 1 MgCl2, 10 Hepes, 0.2 Cs-EGTA, pH 7.4. For SOCE measurements, SR Ca2+ stores were depleted by exposing normal or dyspedic myotubes to 1 μm thapsigargin in Ca2+-free Ringer solution for 15 min. SOCE was not significantly different following a longer exposure (60 min) with a higher concentration of thapsigargin (3 μm). For ECCE measurements, RyR-mediated Ca2+ release was blocked by prior exposure of myotubes to 500 μm ryanodine for 1 h. Myotubes were examined using an inverted microscope with indo-1 excited at 350 nm using a DeltaRam illumination system (Photon Technology Inc., Princeton, NJ, USA). Fluorescence emission at 405 and 485 nm was collected (100 Hz) from a small rectangular region of the myotube using a dual photomultiplier detection system. Results are presented as the ratio (R = F405/F485) of fluorescence emission at 405 nm (F405) divided by the emission at 485 nm (F485). Relative changes in intracellular Ca2+ were monitored following rapid perfusion of either 2 mm CaCl2 Ringer solution (SOCE) or 50 mm KCl (ECCE). Results were acquired/analysed using FeliX software (Photon Technology Inc., Princeton, NJ, USA).
Mn2+ quench measurements
Mn2+ quench of fura-2 emission was measured in myotubes loaded with 5 μm fura-2 AM for 1 h at 37 °C in a normal rodent Ringer solution consisting of (mm): 145 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 Hepes, pH 7.4. Prior to Mn2+ quench measurements, myotubes were treated with either 1 μm thapsigargin in Ca2+-free Ringer solution for 15 min to fully deplete SR Ca2+ stores (SOCE measurements) or 500 μm ryanodine for 1 h to block RyR-mediated Ca2+ release (ECCE experiments). Fura-2-loaded myotubes were excited at the experimentally determined fura-2 isosbestic point (366 nm) and emission monitored at 510 nm during perfusion of either 0.5 mm Mn2+ alone (SOCE) or 50 mm KCl in the presence of 0.5 mm Mn2+ (ECCE).
Data analysis
Maximum rates of change in Ca2+ entry and Mn2+ quench were obtained from the peak differential of the indo-1 ratio and fura-2 emission traces, respectively. Results are given as mean ±s.e.m. with statistical significance (**P < 0.001) determined using a one-way analysis of variance (ANOVA) and post hoc Fisher LSD test for multiple comparisons.
Results
STIM1 and Orai1–3 expression in primary skeletal myotubes
The purpose of this study was to determine the role of STIM1 and Orai1 in coordinating SOCE and ECCE in skeletal muscle cells. RT-PCR analysis confirmed expression of mRNA transcripts for STIM1 and all three Orai isoforms in primary mouse myotubes (Supplemental Fig. 1A, upper panel) and C2C12 myotubes (Supplemental Fig. 1A, lower panel). Western blot analysis confirmed STIM1 protein expression in normal and dyspedic myotubes (Supplemental Fig. 1B).
STIM1 and Orai1 are required for SOCE
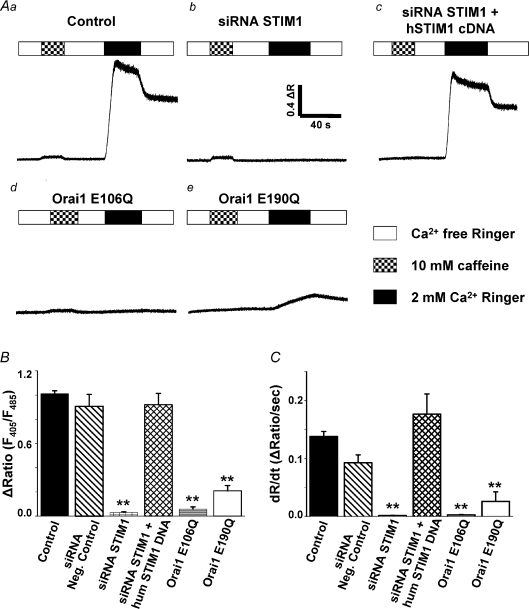
We determined SOCE activity from the magnitude and maximal rate of Ca2+ influx following reintroduction of extracellular Ca2+ to indo-1 AM-loaded myotubes in which SR Ca2+ stores had been depleted by prior exposure to 1 μm thapsigargin (Fig. 1). For all conditions, store depletion was confirmed by the absence of robust Ca2+ mobilization following the addition of 10 mm caffeine (Fig. 1A, checkered bars). Subsequent perfusion of naïve myotubes with a normal 2 mm Ca2+ Ringer solution (Fig. 1A, black bars) elicited robust and rapid Ca2+ entry (Fig. 1A). We quantified both the peak of the Ca2+ transient (Fig. 1B; ΔR=Rpeak–Rbaseline, where R=F405/F485) and the maximal rate of Ca2+ influx from the peak differential of the indo-1 ratio (Fig. 1C; dR/dt). Consistent with the results of Stiber et al. (2008), SOCE following reintroduction of Ca2+ was eliminated following siRNA-mediated STIM1 knockdown (Fig. 1Ab, B and C), but not following transfection with negative control siRNAs (Fig. 1B and C). In our experiments, siRNA transfection efficiency was nearly 100% (Supplementary Fig. 2) and transfection of a mixture of four different murine-specific STIM1 siRNAs resulted in > 90 % reduction in STIM1 protein (Supplementary Fig. 1C). Expression of human STIM1 (hSTIM1) fully restored SOCE in siRNA-treated myotubes (Fig. 1Ac, B and C), demonstrating the specificity of the murine STIM1 siRNAs. SOCE was also abolished by expression of a dominant-negative Orai1 construct (E106Q) that eliminates ion permeation (Vig et al. 2006a) (Fig. 1Ad, B and C). In addition, SOCE was markedly reduced following expression of E190Q, an Orai1 pore mutant that exhibits reduced Ca2+ selectivity/permeation (Prakriya et al. 2006) (Fig. 1Ae, B and C). Average values for the peak magnitude and maximal rate of change in indo-1 ratio in control, siRNA negative control, STIM1 knockdown, hSTIM1 rescue, E106Q-expressing, and E190Q-expressing myotubes are summarized in Fig. 1B and C, respectively.
Figure 1. STIM1 and Orai1 are required for store-operated Ca2+ entry (SOCE) in myotubes.
A, Ca2+ stores were depleted by incubating myotubes for 15 min with 1 μm thapsigargin in the absence of external Ca2+. SR Ca2+ depletion was confirmed by the absence of Ca2+ mobilization following addition of 10 mm caffeine (hatched bars). Re-introduction of 2 mm external Ca2+ (black bars) elicited robust Ca2+ influx through SOCE channels in naïve myotubes (a, n= 50). SOCE was eliminated in myotubes transfected with a siRNA mixture directed against murine STIM1 (b, n= 33) and entry was fully rescued by expression of human STIM1 (c, n= 6). SOCE was also eliminated following expression of dominant-negative E106Q Orai1 (d, n= 6) and markedly reduced following expression of permeation-defective E190Q Orai1 (e, n= 14). In the absence of store depletion, 10 mm caffeine triggers robust SR Ca2+ release (ΔR= 0.50 ± 0.02, n= 16). B and C, summary of maximum amplitude and Ca2+ entry rate, respectively, for the conditions in A and for siRNA negative control (n= 8). **P < 0.01.
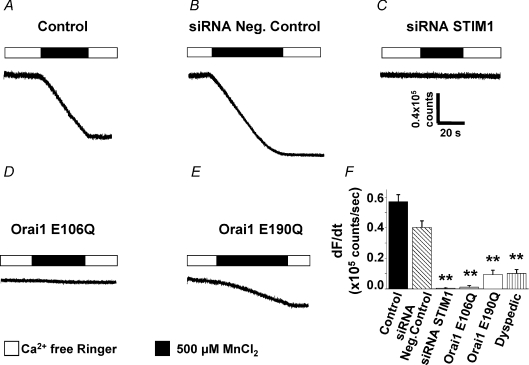
We also used Mn2+ quench of fura-2 fluorescence as an alternate assay to confirm the requirement of STIM1 and Orai1 for SOCE in myotubes (Fig. 2). The relative level of SOCE activity was determined from the maximum rate of fura-2 quench (decrease in fura-2 emission when excited at the isosbestic wavelength – dF/dt) following the rapid addition of 0.5 mm extracellular Mn2+. In SR Ca2+-depleted naïve myotubes, basal fura-2 emission is stable under control conditions and the addition of extracellular Mn2+ initiates rapid fura-2 quench (Fig. 2A and F). The maximum rate of Mn2+ quench was not significantly different for myotubes transfected with a negative control siRNA (Fig. 2B and F). However, transfection of murine STIM1 siRNAs (Fig. 2C and F) and expression of E106Q Orai1 (Fig. 2D and F) eliminated fura-2 quench during the addition of extracellular Mn2+. Moreover, the maximum rate of Mn2+ quench was markedly reduced in dyspedic myotubes and following expression of permeation-defective E190Q Orai1 (Fig. 2E and F). In Western blot experiments, we found that STIM1 protein levels are similar in normal and dyspedic myotubes (data not shown). Thus, the observed decrease in SOCE in dyspedic myotubes could reflect either a regulatory role of RyR1 on SOCE (Kiselyov et al. 2000; Pan et al. 2002) or a deficiency in Orai1 expression. Together, the Ca2+ measurements and Mn2+ quench results demonstrate that STIM1 and Orai1 are both required for SOCE in skeletal muscle cells.
Figure 2. Rate of store-dependent Mn2+ quench in myotubes depends on STIM1 and Orai1.
A, following store depletion with 1 μm thapsigargin, perfusion of 0.5 mm Mn2+ (filled bars) caused rapid quench of fura-2 emission in control myotubes (n= 56). B, Mn2+ quench was not different in myotubes transfected with negative control siRNAs (n= 23). C–E, fura-2 quench during Mn2+ addition was eliminated in myotubes transfected with either STIM1 siRNA (C, n= 13) or following expression of E106Q (D, n= 13) and markedly reduced in myotubes expressing E190Q (E, n= 10). F, summary of maximum rate of store-dependent Mn2+ quench for the conditions shown in A–E and for RyR1-null (dyspedic) myotubes (n= 13). **P < 0.01.
Neither STIM1 nor Orai1 are required for ECCE
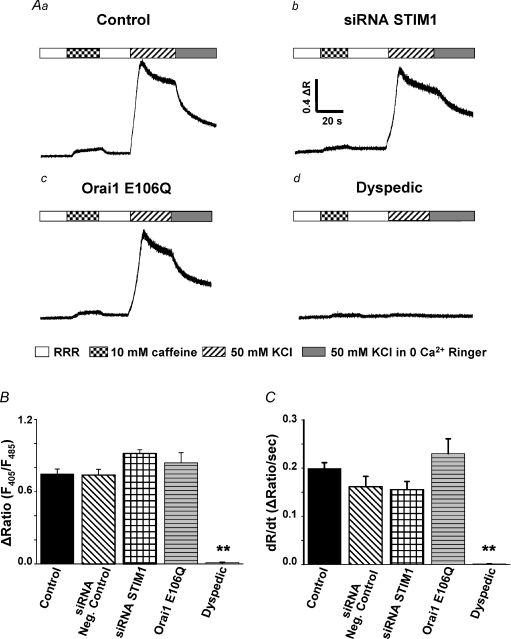
The potential role of STIM1 and Orai1 in ECCE activation and whether SOCE and ECCE reflect distinct molecular entities or result from different mechanisms for activation of the same channel is unknown. Therefore, we determined the STIM1 and Orai1 dependence of Ca2+ entry via the ECCE pathway in myotubes in which depolarization-induced Ca2+ release was eliminated by pre-incubation with 500 μm ryanodine (Fig. 3). Blockade of RyR channels was confirmed by the absence of Ca2+ release during 10 mm caffeine exposure (Fig. 3A, checkered bars). In naïve myotubes, robust Ca2+ influx through ECCE channels was elicited during sustained depolarization induced by 50 mm KCl (Fig. 3A, hatched bars). Figure 3Aa–d shows representative Ca2+ measurements of KCl-induced ECCE in control myotubes (Fig. 3Aa), STIM1 siRNA-treated myotubes (Fig. 3Ab), E106Q-expressing myotubes (Fig. 3Ac), and dyspedic myotubes (Fig. 3Ad). Average values for the peak magnitude and maximal rate of change in indo-1 ratio for each condition are summarized in Fig. 3B and C, respectively. The results indicate that Ca2+ entry through the ECCE pathway is ∼2× faster than via the SOCE pathway (compare Figs 1C and 3C). In addition, ECCE was unaltered by either STIM1 knockdown or E106Q Orai1, conditions that abolished SOCE (Figs 1 and 2). Consistent with prior findings (Cherednichenko et al. 2004), KCl-induced Ca2+ influx was absent in dyspedic myotubes.
Figure 3. STIM1 and Orai1 are not required for excitation-coupled Ca2+ entry (ECCE) in myotubes.
A, RyR-mediated Ca2+ release was blocked by pre-treating myotubes with 500 μm ryanodine for 1 h in Ringer solution. RyR1 blockade was confirmed by the absence of robust Ca2+ mobilization following the addition of 10 mm caffeine (checkered bars). Rapid perfusion of 50 mm KCl (hatched bars) elicited depolarization-induced Ca2+ entry in control myotubes (a, n= 46), STIM1 siRNA knock-down myotubes (b, n= 6), and Orai1 E106Q-expressing myotubes (c, n= 10). Dyspedic myotubes (d, n= 13) completely lacked KCl-induced Ca2+ entry. B and C, summary of maximum amplitude and Ca2+ entry rate, respectively, for the conditions shown in A and for siRNA negative control (n= 8). **P < 0.01.
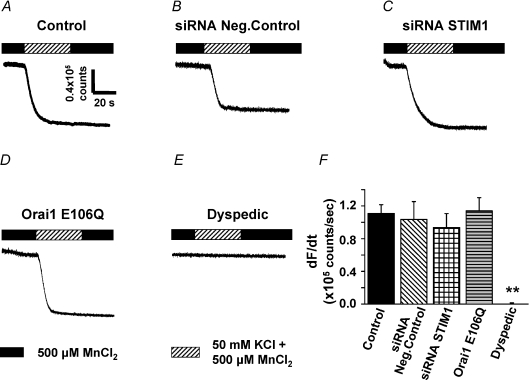
Mn2+ quench experiments were also conducted to confirm the independence of ECCE from STIM1 and Orai1 (Fig. 4). Consistent with the Ca2+ measurements, maximal rates of KCl-induced Mn2+ quench (i.e. ECCE activity) were not significantly different between control myotubes (Fig. 4A and F), myotubes transfected with negative control STIM1 siRNAs (Fig. 4B and F), myotubes transfected with murine STIM1 siRNAs (Fig. 4C and F), or E106Q-expressing myotubes (Fig. 4D and F). In addition, KCl depolarization failed to elicit Mn2+ quench in dyspedic myotubes (Fig. 4E and F). In addition, the maximum rate of ECCE-induced Mn2+ quench was also ∼2× greater than that mediated via the SOCE pathway (compare Figs 2F and 4F).
Figure 4. Rate of excitation-coupled Mn2+ quench in myotubes does not depend on STIM1or Orai1.
A–D, Mn2+ quench observed during perfusion of 50 mm KCl + 500 μm Mn2+ (hatched bar) in ryanodine-treated control myotubes (A, n= 23), siRNA negative control myotubes (B, n= 5), STIM1 siRNA myotubes (C, n= 5), and Orai1 E106Q-expressing myotubes (D, n= 12). E, dyspedic myotubes (n= 6) lacked KCl-induced Mn2+ quench. F, summary of maximum rate of Mn2+ quench for the conditions shown in A–E. **P < 0.01.
Discussion
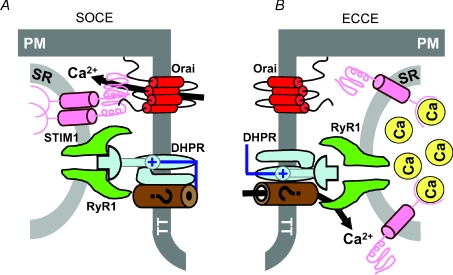
The fundamental new finding of this study is that a STIM1–Orai1 complex is required for SOCE in skeletal muscle cells, but is not involved in coordinating depolarization-dependent Ca2+ influx through the ECCE pathway. A critical extension of this observation is that the differential requirement of SOCE and ECCE on STIM1 and Orai1 indicates that these two functionally different Ca2+ influx pathways arise from distinct molecular entities. Proposed minimal molecular models for SOCE and ECCE complexes in skeletal muscle are depicted in Fig. 5. Our findings are consistent with the recent results of Stiber et al. (2008) who used STIM1 genetrap mice to demonstrate that STIM1 is required for SOCE in skeletal muscle. Our results extend these findings by being the first to demonstrate that SOCE in muscle reflects Ca2+ permeation through Orai1-containing channels (Fig. 5A). Additional support for this mechanism in muscle comes from the fact that both STIM1 (Stiber et al. 2008) and Orai1 (Vig et al. 2008) are expressed at high levels in skeletal muscle, STIM1 and Orai1 deficiency results in reduced muscle mass (Vig et al. 2008; Stiber et al. 2008), and one form of severe combined immunodeficiency results from an Orai1 loss-of-function mutation (R91W) that presents with a non-progressive myopathy (Feske et al. 2006). However, our results do not exclude potential direct and/or regulatory contributions to SOCE and/or ECCE function in skeletal muscle of other proteins including STIM2, Orai2/3, IP3 receptors (Launikonis et al. 2003), ryanodine receptors (Kiselyov et al. 2000; Pan et al. 2002), and TRPC proteins (Kiselyov et al. 2000; Rosenberg et al. 2004; Sampieri et al. 2005). It will also be important for future work to determine the impact of STIM1 knockdown and/or introduction of dominant-negative Orai1 constructs on skeletal muscle EC coupling, global and local Ca2+ release, and Ca2+ signalling triggered by activation of G protein-coupled receptors.
Figure 5. SOCE and ECCE are coordinated by distinct molecular complexes within the skeletal muscle triad.
A, proposed minimum molecular model for SOCE in skeletal muscle. SOCE activation requires an interaction between STIM1, which serves as the Ca2+ sensor for changes in SR Ca2+ content within the terminal cisternae, and Ca2+-permeable Orai1 channels in the t-tubule membrane. B, proposed minimum molecular model for ECCE in skeletal muscle. Functional ECCE requires the skeletal muscle DHPR and RyR1 isoforms, but neither STIM1 nor Orai1. The requirement for the DHPR (voltage sensor for activation) and RyR1 places this Ca2+ influx complex within the triad junction. The molecular identity of the Ca2+-permeation pathway for ECCE remains to be determined (see Discussion).
While several studies have documented SOCE in neurones and muscle cells, ours is the first single study to unequivocally demonstrate that both STIM1 and Orai1 are required for SOCE in an excitable cell. Prior studies in skeletal muscle demonstrate that store depletion activates SOCE across the re-sealed t-tubule membrane of mechanically skinned skeletal muscle fibres (Launikonis et al. 2003; Zhao et al. 2005; Zhao et al. 2006; Launikonis & Rios, 2007). Moreover, activation of SOCE across the t-tubule membrane is remarkably rapid, occurring as fast as 1 s following initiation of SR Ca2+ release (Launikonis & Rios, 2007). Together with our findings, these results indicate that unlike T lymphocytes, STIM1 and Orai1 proteins required for SOCE activation in skeletal muscle must be co-localized within the t-tubule membrane even prior to store depletion. The pre-formation of STIM1–Orai1 complexes strategically within the triad junction (Fig. 5B) would provide efficient SR Ca2+ content sensing and responsiveness to store depletion required to promote SR store refilling and limit contractile decline during high-frequency fatigue.
Our results are also the first to demonstrate that ECCE in skeletal muscle requires neither STIM1 nor Orai1. While the complete molecular composition of the ECCE complex remains elusive, at a minimum it includes the DHPR, RyR1 and an as yet unidentified Ca2+-permeable channel. The molecular identity of the Ca2+-permeable ECCE channel will require further study, but may involve TRPC-related proteins. Nevertheless, since ECCE activity requires junctional DHPR and RyR1 proteins (Cherednichenko et al. 2004), the ECCE complex must also be located within the triad. Thus, the more rapid activation and store-independent properties of the ECCE pathway would permit junctional Ca2+ influx to limit contractile decline at early times during high-frequency skeletal muscle activation, even prior to significant store depletion or SOCE activation. The apparent redundant positioning of two functionally and molecularly distinct Ca2+ influx pathways within the triadic region underscores the potential physiological importance of Ca2+ influx in maintaining contractile function during strenuous muscle activity.
Acknowledgments
We would like to thank Drs T. Shuttleworth and A. Rao for providing us with cDNAs for hSTIM1, dominant-negative (E106Q), and permeation-defective (E190Q) Orai1 constructs. We would also like to thank Dr T. Shuttleworth for providing helpful comments on the manuscript and to Dr P. D. Allen for providing access to the dyspedic mice used in this study. This work was supported by National Institute of Health Grants AR044657 and 5P01AR052354 to R.T.D.
Supplemental material
Online supplemental material for this paper can be accessed at: http://jp.physoc.org/cgi/content/full/jphysiol.2008.160481/DC1
References
- Albert AP, Saleh SN, Peppiatt-Wildman CM, Large WA. Multiple activation mechanisms of store-operated TRPC channels in smooth muscle cells. J Physiol. 2007;583:25–36. doi: 10.1113/jphysiol.2007.137802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allard B, Couchoux H, Pouvreau S, Jacquemond V. Sarcoplasmic reticulum Ca2+ release and depletion fail to affect sarcolemmal ion channel activity in mouse skeletal muscle. J Physiol. 2006;575:69–81. doi: 10.1113/jphysiol.2006.112367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila G, Dirksen RT. Functional effects of central core disease mutations in the cytoplasmic region of the skeletal muscle ryanodine receptor. J Gen Physiol. 2001;118:277–290. doi: 10.1085/jgp.118.3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng KT, Liu X, Ong HL, Ambudkar IS. Functional requirement for Orai1 in storeoperated. TRPC1/STIM1 channels. J Biol Chem. 2008;283:12935–12940. doi: 10.1074/jbc.C800008200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherednichenko G, Hurne AM, Fessenden JD, Lee EH, Allen PD, Beam KG, Pessah IN. Conformational activation of Ca2+ entry by depolarization of skeletal myotubes. Proc Natl Acad Sci U S A. 2004;101:15793–15798. doi: 10.1073/pnas.0403485101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherednichenko G, Ward CW, Feng W, Cabrales E, Michaelson L, Samso M, Lopez JR, Allen PD, Pessah IN. Enhanced excitation-coupled calcium entry (ECCE) in myotubes expressing malignant hyperthermia mutation R163C is attenuated by dantrolene. Mol Pharmacol. 2008;73:1203–1212. doi: 10.1124/mol.107.043299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feske S. Calcium signalling in lymphocyte activation and disease. Nat Rev Immunol. 2007;7:690–702. doi: 10.1038/nri2152. [DOI] [PubMed] [Google Scholar]
- Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- Kiselyov KI, Shin DM, Wang Y, Pessah IN, Allen PD, Muallem S. Gating of store-operated channels by conformational coupling to ryanodine receptors. Mol Cell. 2000;6:421–431. doi: 10.1016/s1097-2765(00)00041-1. [DOI] [PubMed] [Google Scholar]
- Kurebayashi N, Ogawa Y. Depletion of Ca2+ in the sarcoplasmic reticulum stimulates. Ca2+ entry into mouse skeletal muscle fibres. J Physiol. 2001;533:185–199. doi: 10.1111/j.1469-7793.2001.0185b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launikonis BS, Barnes M, Stephenson DG. Identification of the coupling between skeletal muscle store-operated Ca2+ entry and the inositol trisphosphate receptor. Proc Natl Acad Sci U S A. 2003;100:2941–2944. doi: 10.1073/pnas.0536227100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launikonis BS, Rios E. Store-operated Ca2+ entry during intracellular Ca2+ release in mammalian skeletal muscle. J Physiol. 2007;583:81–97. doi: 10.1113/jphysiol.2007.135046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Erxleben C, Abramowitz J, Flockerzi V, Zhu MX, Armstrong DL, Birnbaumer L. Functional interactions among Orai1, TRPCs, and STIM1 suggest a STIM-regulated heteromeric Orai/TRPC model for SOCE/Icrac channels. Proc Natl Acad Sci U S A. 2008;105:2895–2900. doi: 10.1073/pnas.0712288105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr, Meyer T. STIM is a. Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Z, Yang D, Nagaraj RY, Nosek TA, Nishi M, Takeshima H, Cheng H, Ma J. Dysfunction of store-operated calcium channel in muscle cells lacking mg29. Nat Cell Biol. 2002;4:379–383. doi: 10.1038/ncb788. [DOI] [PubMed] [Google Scholar]
- Paraekh AB, Putney JW., Jr Store-operated calcium channels. Physiol Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- Payne AM, Messi ML, Zheng Z, Delbono O. Motor neuron targeting of IGF-1 attenuates age-related external Ca2+-dependent skeletal muscle contraction in senescent mice. Exp Gerontol. 2007;42:309–319. doi: 10.1016/j.exger.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peel SE, Liu B, Hall IP. ORAI and store-operated calcium influx in human airway smooth muscle cells. Am J Respir Cell Mol Biol. 2008;38:744–749. doi: 10.1165/rcmb.2007-0395OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443:230–233. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Velicelebi G, Stauderman KA. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg P, Hawkins A, Stiber J, Shelton JM, Hutcheson K, Bassel-Duby R, Shin DM, Yan Z, Williams RS. TRPC3 channels confer cellular memory of recent neuromuscular activity. Proc Natl Acad Sci U S A. 2004;101:9387–9392. doi: 10.1073/pnas.0308179101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampieri A, Díaz-Munoz M, Antaramian A, Vaca L. The foot structure from the type 1 ryanodine receptor is required for functional coupling to store-operated channels. J Biol Chem. 2005;280:24804–24815. doi: 10.1074/jbc.M501487200. [DOI] [PubMed] [Google Scholar]
- Stiber J, Hawkins A, Zhang ZS, Wang S, Burch J, Graham V, Ward CC, Seth M, Finch E, Malouf N, Williams RS, Eu JP, Rosenberg P. STIM1 signalling controls storeoperated calcium entry required for development and contractile function in skeletal muscle. Nat Cell Biol. 2008;10:688–697. doi: 10.1038/ncb1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vig M, Beck A, Billingsley JM, Lis A, Parvez S, Peinelt C, Koomoa DL, Soboloff J, Gill DL, Fleig A, Kinet JP, Penner R. CRACM1 multimers form the ion-selective pore of the CRAC channel. Curr Biol. 2006a;16:2073–2079. doi: 10.1016/j.cub.2006.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vig M, Dehaven WI, Bird GS, Billingsley JM, Wang H, Rao PE, Hutchings AB, Jouvin MH, Putney JW, Kinet JP. Defective mast cell effector functions in mice lacking the CRACM1 pore subunit of store-operated calcium release-activated calcium channels. Nat Immunol. 2008;9:89–96. doi: 10.1038/ni1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R, Kinet JP. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 2006b;312:1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worley PF, Zeng W, Huang GN, Yuan JP, Kim JY, Lee MG, Muallem S. TRPC channels as STIM1-regulated store-operated channels. Cell Calcium. 2007;42:205–211. doi: 10.1016/j.ceca.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Allen PD, Pessah IN, Lopez JR. Enhanced excitation-coupled calcium entry in myotubes is associated with expression of RyR1 malignant hyperthermia mutations. J Biol Chem. 2007;282:37471–37478. doi: 10.1074/jbc.M701379200. [DOI] [PubMed] [Google Scholar]
- Zhao X, Weisleder N, Han X, Pan Z, Parness J, Brotto M, Ma J. Azumolene inhibits a component of store-operated calcium entry coupled to the skeletal muscle ryanodine receptor. J Biol Chem. 2006;281:33477–33486. doi: 10.1074/jbc.M602306200. [DOI] [PubMed] [Google Scholar]
- Zhao X, Yoshida M, Brotto L, Takeshima H, Weisleder N, Hirata Y, Nosek TM, Ma J, Brotto M. Enhanced resistance to fatigue and altered calcium handling properties of sarcalumenin knockout mice. Physiol Genomics. 2005;23:72–78. doi: 10.1152/physiolgenomics.00020.2005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.