Abstract
Golgi cells are important elements of the cerebellar cortex, controlling the flow of mossy fibre information to other cells via granule cells. Several anatomical reports suggest that climbing fibre afferents contact Golgi cells, and electrophysiological studies suggest that they depress Golgi cell firing. We reinvestigated this issue and, given that climbing fibres mediate synaptic plasticity in the cerebellar cortex, we have examined the effects of conjunctive stimulation of peripheral afferents and climbing fibres on Golgi cell responses. The results confirm that climbing fibre stimulation depresses Golgi cell firing at short latency. Golgi cells responded to stimulation of peripheral afferents with longer latency depressions of firing and after conjunctive stimulation with climbing fibres these were significantly reduced. The reductions developed progressively over 20 min of conjunctive stimulation and were persistent (up to 84 min). Temporal conjunction of the inputs was important because non-synchronous stimulation of climbing fibres and peripheral afferents failed to alter the peripheral afferent-evoked response in Golgi cells. In control experiments using either the same climbing fibre stimulation alone, or peripheral afferent stimulation paired with brainstem stimulation that did not activate climbing fibres, responses were not depressed. The results thus show that conjunctive stimulation of climbing fibres with other inputs to Golgi cells can induce long-term changes in Golgi cell responses in vivo. This raises the possibility that changes in Golgi cell peripheral responses mediated by climbing fibres can potentially contribute to cerebellar motor learning.
It is widely believed that climbing fibres carry important learning-related signals to the cerebellum. The connection from climbing fibre (CF) to Purkinje cell is one of the most powerful synapses known and is implicated in parallel fibre to Purkinje cell plasticity (Eccles et al. 1966; Llinas & Nicholson, 1976; Ito, 1989; Bloedel & Bracha, 1998). The CF influence on other cerebellar neurones is less well studied. Recent evidence shows that CFs can excite molecular layer interneurons directly via a spillover pathway (Szapiro & Barbour, 2007) and this may be the substrate for plastic changes in the responses of these interneurons when their activation is coupled with CF stimulation (Ekerot & Jorntell, 2003). Whether CFs also influence the Golgi cells directly is less well known. There is anatomical evidence that thin collaterals of olivocerebellar axons contact Golgi cells, but these are terminals with relatively few swellings or varicosities, unlike the multiple terminals climbing fibres make on Purkinje cells (Hámori & Szentágothai, 1965; Palay & Chan-Palay, 1974; Shinoda et al. 2000). The functional role of these connections has not been well studied electrophysiologically, the major study showing that stimulation in the inferior olive generated a depression of Golgi cell firing rather than excitation (Schulman & Bloom, 1981).
Golgi cells control the transmission of mossy fibre information to granule cells. Based on connectivity it has been suggested that they confer negative feedback on the mossy fibre–granule cell synapses in the cerebellar cortical glomeruli (Eccles et al. 1965b,a), since they are the only cells that can inhibit transmission through the mossy fibre–granule cell synapses. Subsequently Marr postulated that Golgi cells act as gain controllers to restrict the average number of active parallel fibre synapses (the ‘codon size’) for a given mossy fibre input pattern in order to enhance the number of possible discrete input patterns for a given Purkinje cell – thus increasing the number of input patterns that can be potentially learned by the cell (Marr, 1969). Until recently this role for Golgi cells was widely accepted. Several recent findings question this role: Dieudonne showed that parallel fibre–Golgi cell synapses are weak (Dieudonne, 1998). In addition, Golgi cells identified by juxtacellular labelling were inhibited by stimulation of wide peripheral receptive fields that transmitted via the lateral funiculus in the spinal cord (Holtzman et al. 2006a). These studies call into question whether Golgi cells confer a simple negative feedback onto Granule cells.
The objective of this study was to re-examine the effects of CF activation on Golgi cells. Furthermore, since they can mediate changes in molecular layer interneuron behaviour (Ekerot & Jorntell, 2001; Jorntell & Ekerot, 2002, 2003), we examined whether conjunctive activation of CF with peripheral afferents generates plastic changes in the responses of Golgi cells evoked by those inputs in the long term.
Methods
Experiments were performed on 13 adult Wistar rats (300–350 g) that were anaesthetized using urethane (1–1.2 g kg−1 i.p.). Depth of anaesthesia was deemed sufficient when there is no flexor withdrawal reflex to vigorous pinching of distal limbs. All procedures were approved by the UK Home Office regulations and have local ethical committee approval.
Surgery
Anaesthetized animals were fixed in a stereotaxic headholder. A heating blanket regulated by feedback from a rectal thermometer was used to maintain core body temperature at 37.9°C. The obex was exposed by removal of the overlying muscle and dura at the foramen magna, and a small craniotomy exposed the cerebellar cortex, either crus II or vermis.
Recording
Extracellular single-unit and field potential recordings were made using single parylene C insulated stainless steel electrodes with impedance ranging from 2 to 5 MΩ. Signals from the microelectrodes were amplified (gain, ×10 000), filtered (band-pass, 0.3–10 kHz) and digitized at 25 kHz.
Stimulation
An insulated stainless steel stimulating electrode (impedance about 150 kΩ) was inserted into the midline at the obex at angles between 27 and 35 deg to the vertical. Initially with the recording electrode on the cerebellar surface the stimulating electrode was advanced whilst 0.2 ms duration 50 μA biphasic pulses were delivered: this allowed the position from which stimuli elicited the maximum field potential on the cerebellar surface of the vermis to be determined. Subsequent recordings of Purkinje cell complex spikes (Fig. 1) allowed the stimuli to be adjusted to a level at which CFs were recruited by the stimulus (range 10–130 μA).
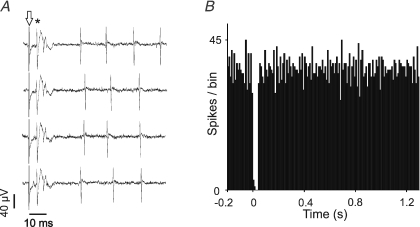
Figure 1. Complex spikes evoked in a Purkinje cell by CF stimulus.
A, extracellular recording of a Purkinje cell with arrow denoting stimulus artefact and asterisk denoting CF stimulus-driven complex spike. The complex spike is followed by a brief pause in simple spike firing. B, poststimulus time histogram (PSTH) of simple spike firing showing the characteristic post-complex spike pause.
Stimulation of peripheral afferents was achieved using percutaneous pins (as described by Holtzman et al. 2006a). Using the timing of the local field potential generated by both peripheral and climbing fibre (CF) stimulus, the two stimuli were timed to arrive at the cerebellar cortex simultaneously.
To assess whether plastic changes could be evoked in responses of Golgi cells by CF inputs, we used protocols for conjunctive stimulation of CF and peripheral afferent. In these we delivered paired stimuli at a frequency of 0.67 Hz with three different protocols: in one we delivered these stimuli simultaneously over 20 min (800 paired stimuli) and compared the peripheral evoked responses at the beginning and end of this period. In the second protocol we interrupted the conjunctive stimulation at 5 min intervals (after each successive 200 paired stimuli), to examine the development of changes in the peripheral evoked responses. In the third we assessed the importance of temporal synchrony of the stimuli by delivering CF stimuli 750 or 1125 ms after the peripheral stimulus. Note that the same stimulus frequency was maintained so a CF stimulus 1125 ms after a peripheral stimulus is also equivalent to a CF stimulus 375 ms before a peripheral stimulus. Control protocols involved CF stimulation alone or peripheral afferent stimulation alone. For the CF-only control stimulation protocol, CFs were stimulated alone for the same period and at the same frequency as in the conjunctive protocol and the response to peripheral afferents was examined before and after the stimulation period. For the peripheral afferents-only control stimulation protocol, the peripheral RF in question was stimulated with stimulation in the brainstem, but at strengths below the threshold to activate CFs and generate a depression of Golgi cell firing as described by Schulman & Bloom (1981).
Analysis
Raw signals were discriminated for spikes using a custom- made program (LabSpike – Bhumbra, http://www.pdn.cam.ac.uk/staff/dyball/labspike.html, Department of Physiology Development and Neuroscience, Cambridge University). Responses were evaluated as poststimulus time histograms (PSTH) made in MATLAB (The MathWorks Inc., Natick, MA, USA) and incorporated a cumulative sum (CUSUM) derivative analysis (Davey et al. 1986), which facilitates the detection of persistent trends in the histogram. A period of 200 ms immediately preceding the stimulus was used as a control spontaneous activity measure and to establish the CUSUM baseline. Responses were measured relative to this baseline.
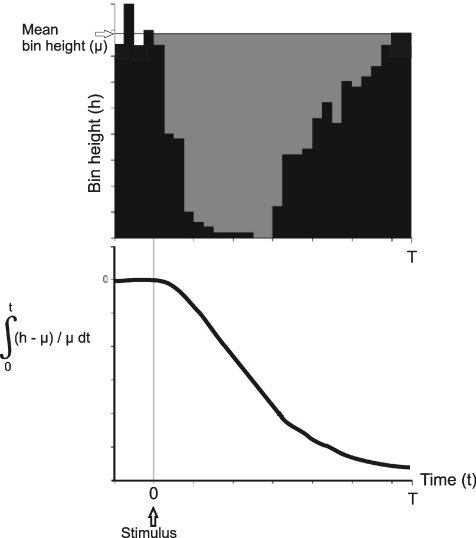
Response amplitudes were assessed from the PSTHs. From the 200 ms long prestimulus bin heights the mean background bin height was derived. Responses were quantified as the difference between this mean and the actual bin counts. The grey shaded area in the PSTH in Fig. 2 represents the difference between resting mean bin height and actual bin height from 0 s to 1.2 s. The lower graph plots the normalized (to background) size of the grey shaded area in the PSTH as a function of time. The modulus of the minimum value of this function is termed the cumulative response, and is an index of the size of the response.
Figure 2. Derivation of cumulative response.
The upper panel shows a PSTH with the black bins representing the actual values. The grey shaded area represents the difference between the actual bins and the mean prestimulus bin height. The lower graph shows the integral of mean background bin height (μ) minus actual bin height (h) divided by firing (μ).
To assess the effects of conjunctive stimulation, pre- and poststimulation cumulative response values were quantified and plotted against each other. Linear regression lines were fitted to data points in order to determine the extent of correlation between peripheral responses before and after stimulus protocols. Student's two-tailed paired t test was performed on the values of responses before and after stimulus protocols.
Results
Identification of Golgi cells by their response to electrical stimulation of periphery
Golgi cells were identified according to the response patterns and spontaneous discharge patterns as described by Holtzman et al. (2006a). These cells responded with long-latency, long-lasting depressions, occasionally with short-latency excitations preceding them, to stimulation of peripheral afferents (Fig. 3). As previously described (Holtzman et al. 2006a) these responses could be evoked from wide receptive fields, including several limbs. In no case did we observe Purkinje cell complex spikes driven by stimulation of the peripheral afferents in the regions sampled.
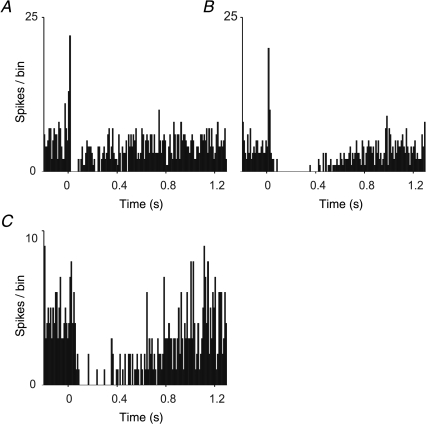
Figure 3. Representative Golgi cell responses to peripheral stimulation.
A–C, PSTH showing responses of a Golgi cell to stimulation of ipsilateral trigeminal afferents, ipsilateral forelimb and ipsilateral hindlimb afferents, respectively. Stimuli occurred at 0 ms. Each PSTH consists of 100 sweeps, bin width = 10 ms. In A and B weak short latency excitatory responses preceded the depression.
Responses of Golgi cells to climbing fibre stimulation
Effective CF stimulation was verified by the presence of driven complex spikes with latencies of about 5 ms in Purkinje cells nearby the recorded Golgi cells (Fig. 1). The short and invariant latency (∼5 ms) of the driven complex spike suggests that the stimuli activated olivocerebellar axons directly.
All Golgi cells tested responded to CF stimulation with a reduction in firing (Fig. 4) when the stimulus was confirmed to activate local CFs. The time to minimum bin in the PSTH had a mean of 7.7 ms (standard error of mean, SEM ± 3.1 ms, n= 13) and the duration of inhibition had a mean of 134.6 ms (SEM ± 21.9 ms, n= 13). These responses of Golgi cells are qualitatively similar to those described by Schulman & Bloom (1981). However, both times to peak inhibition and durations of inhibition were shorter than those described by Schulman & Bloom (138 SEM ± 48 ms and 439 SEM ± 47 ms, respectively).
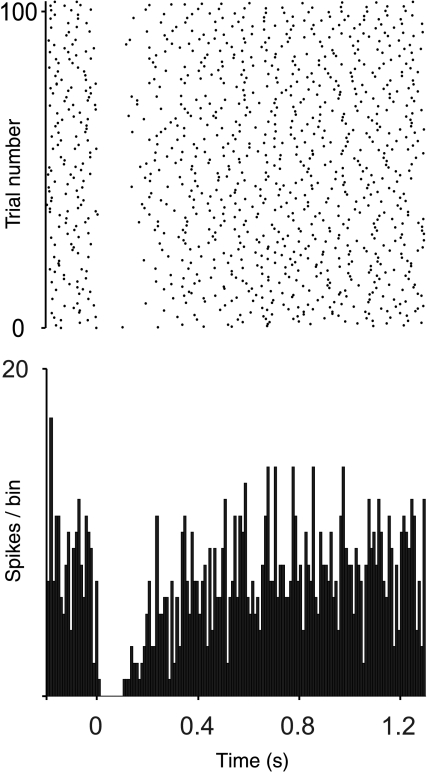
Figure 4. Spike raster and PSTH of a Golgi cell's response to CF stimulation.
Stimulus occurs at 0 ms. PSTH consists of 100 sweeps.
Changes in Golgi cell responses following conjunctive climbing fibre stimulation
To determine whether activation of CFs influenced Golgi cell responses, peripheral afferents and CFs were stimulated conjunctively. Golgi cells were first identified and their responses to stimulation of peripheral afferents characterized. A 20 min period of conjunctive stimulation was then applied (see Methods), and the response to the same peripheral afferent stimulation was assessed following the conjunctive period.
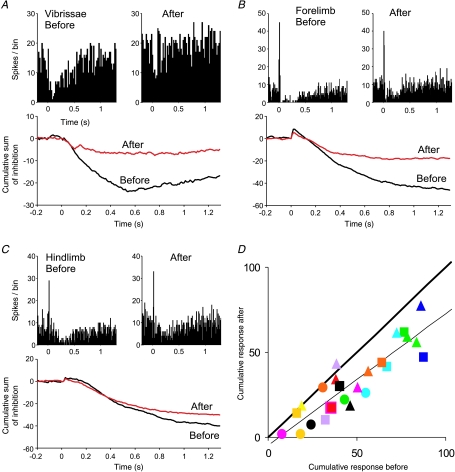
For all Golgi cells tested (n= 13), the response to peripheral stimulation was generally reduced after conjunctive stimulation. Figure 5A shows an example. The PSTHs compiled before and immediately after conjunctive stimulation show the same general form, but the depression is much weaker after conjunctive stimulation. This is clear in the direct comparisons of the superimposed CUSUMs. The baseline firing rate of the Golgi cells did not change significantly after conjunctive stimulation (P= 0.72).
Figure 5. Changes in a Golgi cell's response to peripheral stimulation after conjunctive CF stimulation.
A–C, PSTHs showing the responses to peripheral afferent stimulation before and after the conjunctive stimulation; below are CUSUMs comparing the responses. The source of the responses in A–C was ipsilateral vibrissal afferents, ipsilateral forelimb and ipsilateral hindlimb, respectively. Plot of cumulative response shows a universal reduction in response after conjunctive stimulation. Number of trials for each PSTH = 100, bin width = 10 ms. D, plot of the cumulative responses of the cells before and after CF conjunctive protocol. Each point represents a response to a particular group of peripheral afferents. Each colour represents a Golgi cell. Thick line represents the line of equality; thin line represents line of best fit of all data points (gradient = 0.77, R2= 0.81). Circles, triangles and squares represent ipsilateral vibrissae, forelimb and hindlimb, respectively.
Since Golgi cells have wide receptive fields, stimulation of afferents from different parts of the body could evoke responses in the same cell (see Holtzman et al. 2006a). For the same Golgi cells as above we were therefore able to examine the effects of conjunctive CF stimulation on responses to stimulation of different peripheral inputs that converge onto the same Golgi cell. In Fig. 5A the PSTHs show responses to stimulation of the trigeminal afferents that were stimulated conjunctively with CFs. Figure 5B and C shows responses of the same cell to stimulation of other groups of ipsilateral forelimb and ipsilateral hindlimb afferents, respectively. These responses were also reduced after conjunctive stimulation. Note that these responses in Golgi cells most likely involve convergence at a precerebellar level (Holtzman et al. 2006b), so changes evoked in the non-conjunctive receptive fields imply that these changes took place at the cerebellar cortical level.
In the cells tested there was no change in the timing to peak inhibition or the duration of inhibition to peripheral receptive field stimulation before and after conjunctive protocol (paired t tests, P > 0.5 and P > 0.8, respectively).
Quantification of plastic change
Since the time at which the CUSUM of a Golgi cell's PSTH reaches its minimum denotes the end of the response (firing reduction) in the PSTH (see Methods), we used this minimum to quantify the magnitude of Golgi cell response to peripheral stimulation. As described in Methods, we quantified the responses by calculating the relative difference between the number of spikes expected in a PSTH without stimulation (baseline) and the actual number of spikes in the PSTH following afferent stimulation, over the duration of the response as determined from the CUSUM: the modulus of this value is termed cumulative response (see Methods).
Taking this approach we were able to quantify and compare the responses pre- and post-conjunctive stimulation. Figure 5D plots the cumulative responses before and after conjunctive stimulation for all responses tested. The large majority of points lie below the line of equality (thick line), indicating a reduced response. A two-tailed paired t test comparing magnitudes of individual responses before and after the conjunctive stimulation protocol reveals that there is a highly significant reduction (P= 9.5 × 10−8). The linear regression line through these points is shown in Fig. 5D (thin line). It has a gradient of 0.77 and R2 value of 0.81. Where stability allowed we followed the peripherally evoked responses after conjunctive stimulation to assess the duration of the effects. The responses did not show recovery for as long as they could be held (up to 84 min).
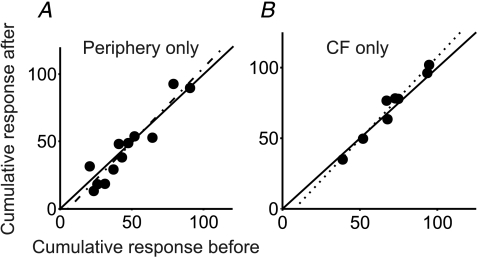
Control experiments were performed where stimuli were delivered to peripheral afferents using the same protocol but without conjunctive CF stimulation, or where CF stimuli were delivered using the same protocol but without pairing to the peripheral afferent stimuli. To test whether stimulation of peripheral afferents without conjunctive stimulation of CFs had any effect, for eight cells stimuli in the brainstem that were insufficiently strong to activate CFs were delivered conjunctively with peripheral afferent stimuli. In these experiments the brainstem stimulation did not activate complex spikes in local Purkinje cells and did not evoke depressions in Golgi cell firing as described by Schulman & Bloom (1981). To test whether CF stimulation alone evoked similar effects, we delivered CF stimulation alone in three cells and compared the peripheral evoked responses before and after. Cumulative responses (before and after) from these control experiments are plotted in Fig. 6A and B, respectively. In both cases the points lie close to the line of equality. Regression lines are shown and in neither case was there a statistically significant difference between cumulative responses before and after stimulation (P > 0.15, gradient = 1.1065, R2= 0.8892 for periphery-only stimulus and gradient = 1.17, R2= 0.9961 for CF-only stimulus).
Figure 6. Comparison of responses from the control experiments.
Minimum value of cumulative response before and after periphery-only protocol (8 cells) (A) and CF-only control protocol (3 cells) (B). Each point represents a response to a particular group of peripheral afferents in one cell. Continuous line represents the line of equality; dotted line represents line of best fit of all data points. Dotted lines represent linear regression fits. R2 values are 0.8892 and 0.9964 for peripheral stimulus only and CF stimulus only protocols, respectively. t test comparing responses before and after the control protocols produced P values of 0.16 and 0.22 for periphery-only and CF-only stimulus protocols, respectively.
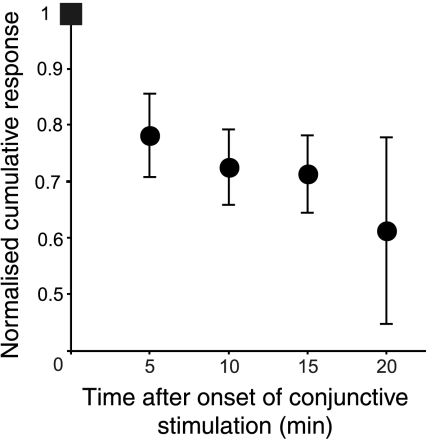
For five Golgi cells we examined the development of the changes in peripheral responses by interrupting the conjunctive stimulation briefly after 5, 10, 15 and 20 min to test the response to peripheral afferent stimulation alone. Cumulative response values were calculated from these PSTHs and these values were then normalized to the initial cumulative response value for that response just before the onset of the conjunctive protocol. This was plotted as a function of time (Fig. 7). The figure clearly shows a progressive decrease in cumulative response as time progresses, which reaches ∼70% of the initial value by around 15 min. The cumulative response values were already significantly reduced at 5 min (paired t test, P= 0.049), but became more pronounced with further conjunctive stimuli.
Figure 7. Progressive reduction of the response to peripheral afferent stimulation during conjunctive stimulation with CFs.
The conjunctive stimulation was stopped at 5, 10, 15 and 20 min to assess the responses. Each point is the mean response of 5 different Golgi cells not included in the preceding figures. Error bars represent standard errors of the mean.
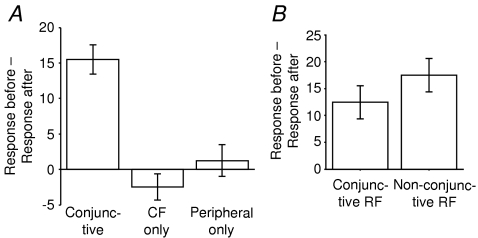
Grouping the data, the bar chart in Fig. 8A shows the mean change in response obtained by subtracting the cumulative response after conjunctive stimulation from the cumulative response at the outset. The changes in responses evoked by the afferents that were conjunctively stimulated and of those evoked by non-conjunctively stimulated afferents in the same cells are compared in Fig. 8B. The magnitude of the changes in responses to peripheral stimulation were similar in both the conjunctive and non-conjunctive groups, and were not significantly different (two-sample equal variance t test: P= 0.236).
Figure 8. Bar chart of the mean change in Golgi cell responses tested before and after conjunctive, CF-only and periphery-only stimulation protocols.
A, data from all receptive fields combined. Error bars represent standard error of the mean. B, no difference in magnitude of response alteration between the conjunctively stimulated afferents and the other afferents that evoked the same depression. The bar graph shows the mean decrease in response of RFs that were conjunctively stimulated with CF compared to RFs which were not.
Effect of varying the temporal juxtaposition of peripheral and climbing fibre stimuli
In the conjunctive stimulation protocol, peripheral and CF stimuli were delivered simultaneously. The effect of varying the relative timing between peripheral and CF during conjunctive stimulation was investigated by delaying the climbing fibre stimulus relative to the peripheral stimulus. For five Golgi cells the climbing fibre stimulus was delayed by 750 ms and for five other Golgi cells it was delayed by 1125 ms, whilst the total number of stimuli (800) and the stimulus interval (1500 ms) was kept constant We did not use shorter delays because the Golgi cell response to peripheral afferent stimulation can last for several hundred milliseconds (Holtzman et al. 2006a), and we did not want the responses to overlap.
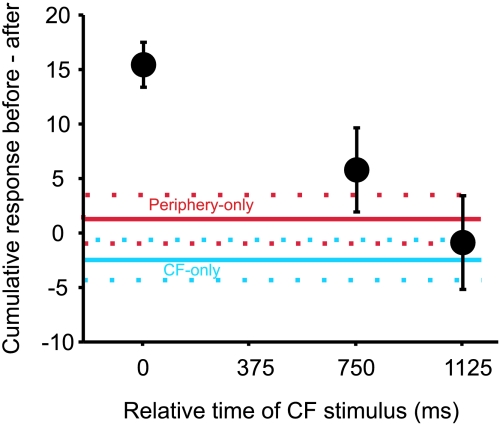
Figure 9 plots the differences in Golgi cell cumulative responses (response before minus response after the conjunctive stimulation) from experiments using temporally separated CF and peripheral stimulation. The data at 0 ms show the changes described for simultaneous CF and peripheral afferent stimulation (described in the preceding sections).
Figure 9. Peripheral responses are not changed if CF stimuli are not timed to coincide with the peripheral response.
The averaged differences in cumulative responses to peripheral stimuli before and after conjunctive stimulation with CF are plotted against the delay of the CF stimulus (with respect to the peripheral stimulus which occurred at time 0). Points show means and standard errors of the mean (n= 15 for time 0, n= 5 for 750 ms and 1125 ms). The points at 750 ms and 1125 ms were not significantly different from the peripheral-only and CF-only control values (shown, respectively, by the red and blue horizontal continuous lines; dotted lines represent their respective standard error values).
The changes in response sizes in both the 750 ms and 1225 ms groups were not significantly different from either the peripheral-only or CF-only control (P > 0.1 for both groups).
Discussion
The current study shows firstly that CFs confer a powerful and fast inhibition upon Golgi cell firing, and secondly that Golgi cell responses to peripheral stimulation can undergo long-lasting modification after conjunctive stimulation with climbing fibres in vivo.
Climbing fibre projections to cerebellar interneurons
It is widely believed that climbing fibres carry error signals to the cerebellum (Marr, 1969; Albus, 1971; Simpson et al. 1996; Wolpert et al. 1998), and it is therefore important to know how they affect cerebellar neurons other than Purkinje cells. Both electrophysiological and anatomical evidence suggests that CFs innervate other cerebellar neurons besides Purkinje cells, but not through synapses on the scale of those they make onto Purkinje cells (Palay & Chan-Palay, 1974; Schulman & Bloom, 1981; Sugihara et al. 1999; Jorntell & Ekerot, 2002, 2003). Jorntell & Ekerot (2002, 2003) showed that synapses between parallel fibres and molecular layer interneurons can undergo CF-specific LTP but their intracellular recordings showed the CF response to be weak. This suggests neurons other than the output Purkinje cell are subject to plasticity, so the actions they have on Purkinje cell firing are also subject to change. Coupled with the discovery that CFs signal to molecular layer interneurons exclusively via glutamate spillover (Szapiro & Barbour, 2007), it would seem that for a given CF-specific plastic change in a Purkinje cell, molecular interneurons in the local vicinity will undergo concomitant reciprocal plastic changes, albeit in a spatially imprecise fashion.
Anatomical studies suggest that CF collaterals confer some direct synaptic contacts onto Golgi cells. Shinoda et al. (2000) have observed that thin CF collaterals touch presumed Golgi cells (see also Palay & Chan-Palay, 1974). Apart from the results of the present study, the only published in vivo electrophysiological study of CF input onto Golgi cells was by Schulman & Bloom (1981). The present results regarding Golgi cells' CF responses qualitatively match those of Schulman & Bloom, but have two advantages over the latter: firstly, the stimulus strengths used to activate climbing fibres were on average an order of magnitude smaller and therefore less likely to recruit other brainstem structures. Secondly, Golgi cells can now be unequivocally identified in this type of experiment by their firing properties and unique in vivo responses to peripheral stimulation (Holtzman et al. 2006a).
There are several potential ways in which Golgi cells might be inhibited by CF stimulation. These include:
(1) spillover CF-activation of basket/stellate cells (Szapiro & Barbour, 2007), which in turn confer GABA-ergic inhibition onto Golgi cells (Dumoulin et al. 2001);
(2) CF-activation of Purkinje cells, which in turn confer GABA-ergic inhibition onto Golgi cells via collaterals (Palay & Chan-Palay, 1974);
(3) direct synaptic or spillover glutamate from CFs activating mGluR2 receptors on Golgi cells that generate hyperpolarizations (Watanabe & Nakanishi, 2003), parallel to the spillover pathway shown by Szapiro & Barbour (2007) for molecular layer interneurons;
(4) CF-activation of Lugaro cells, which in turn confer GABA/glycinergic inhibition onto Golgi cells (Dumoulin et al. 2001).
Whatever the mechanism, it is distinct from the pathway through which the peripheral afferents induced depressions, since climbing fibres were not activated by the peripheral afferent stimuli (Holtzman et al. 2006a), which was confirmed in the Purkinje cell recordings made in this study.
Climbing fibre-specific plasticity in peripherally evoked Golgi cell responses
Climbing fibres project only to the cerebellar cortex and the cerebellar output nuclei. Therefore the CF-evoked plastic changes in Golgi cell responses must have taken place at cerebellar cortical level. These plastic changes are non-specific for the RF stimulated during the conjunctive stimulation protocol, but this is explained by the convergence of inputs from wide RFs onto the pathway to Golgi cells at a site before the cerebellar cortex, such as the lateral reticular nucleus (Holtzman et al. 2006b).
Without knowing the exact mechanism of peripherally evoked Golgi cell responses, we can only speculate on the site of plasticity observed in the present study. However the two most likely mechanisms might be long-term potentiation (LTP) at the parallel fibre (PF)–Golgi cell excitatory synapse (which could be induced at the PF excitatory synapses in a CF-dependent manner similar to basket/stellate cells, as described by Jorntell & Ekerot, 2003), or long-term depression (LTD) at the inhibitory synapses from molecular layer interneurons to Golgi cells (as has been demonstrated in vitro at their synapses onto Purkinje cells (Mittmann & Hausser, 2007).
Functional implications of plasticity
Given that the negative feedback role of Golgi cells is called into question, the current plasticity results must be interpreted in the framework of a context-specific gating role of Golgi cells (Holtzman et al. 2006a).
In order to explore the functional implications of Golgi cell plasticity in this peripheral afferent pathway we must first propose an explanation for why Golgi cell firing is reduced by convergent sensory afferents signals from a widespread field in the first place. In terms of sensorimotor integration, widespread sensory afferent stimulation is a non-specific cue that would seem useful in general arousal or as a context cue rather than driving specific behaviour. Therefore the depressions of Golgi cell firing may lift the inhibition from the mossy fibre–granule cell pathway during generalized arousal, thus increasing the number of activated granule cells (e.g. increasing the average size of the codon as defined by Marr, 1969). On the one hand this would prevent any input patterns that are recognizable by Purkinje cells from coming through, because the large number of active sensory afferents would include very many patterns by chance, but if this general arousal signal is consistently associated with an error signal (carried by CFs) then it is possible that a real signal to which Purkinje cells have a learned response is contained within the whole set of inputs. The immediate response of Golgi cells to CF stimulation is of a strong reduction in firing (the response described by Schulman & Bloom, 1981, and confirmed here). During this period inhibition would be lifted from granule cells thus enabling the mossy fibre (MF) inputs that accompany the CF activation to strongly drive granule cell firing. The MF input that accompanies CF activation is likely to be the meaningful error-generating signal. Hence we postulate that the CF evoked response is an immediate gating mechanism that allows the real signal to pass to Purkinje cells. In the long term the CF-dependent reduction of Golgi cell response to peripheral stimulation would confer a relative increase in inhibition onto granule cells (a smaller decrease of Golgi cell firing). This increased inhibition could increase the signal to noise ratio for MF–granule cell transmission (Chadderton et al. 2004), thus enabling the real signal to pass through to the Purkinje cell. Therefore we hypothesize that Golgi cells can dynamically control the signal to noise ratio of transmission at the MF–granule cell synapse in a CF-dependent manner in order to adapt to different arousal contexts.
Acknowledgments
Wei Xu was supported by the Elmore Fund through the University of Cambridge Clinical School M.B. P.h.D programme.
References
- Albus JS. A theory of cerebellar function. Math Biosci. 1971;10:25–61. [Google Scholar]
- Bloedel JR, Bracha V. Current concepts of climbing fiber function. Anat Rec. 1998;253:118–126. doi: 10.1002/(SICI)1097-0185(199808)253:4<118::AID-AR7>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Chadderton P, Margrie TW, Hausser M. Integration of quanta in cerebellar granule cells during sensory processing. Nature. 2004;428:856–860. doi: 10.1038/nature02442. [DOI] [PubMed] [Google Scholar]
- Davey NJ, Ellaway PH, Stein RB. Statistical limits for detecting change in the cumulative sum derivative of the peristimulus time histogram. J Neurosci Methods. 1986;17:153–166. doi: 10.1016/0165-0270(86)90068-3. [DOI] [PubMed] [Google Scholar]
- Dieudonne S. Submillisecond kinetics and low efficacy of parallel fibre–Golgi cell synaptic currents in the rat cerebellum. J Physiol. 1998;510:845–866. doi: 10.1111/j.1469-7793.1998.845bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumoulin A, Triller A, Dieudonne S. IPSC kinetics at identified GABAergic and mixed GABAergic and glycinergic synapses onto cerebellar Golgi cells. J Neurosci. 2001;21:6045–6057. doi: 10.1523/JNEUROSCI.21-16-06045.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Llinas R, Sasaki K. The inhibitory interneurones within the cerebellar cortex. Exp Brain Res. 1965a;1:1–16. doi: 10.1007/BF00235206. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Llinas R, Sasaki K. Inhibitory systems in the cerebellar cortex. Proc Aust Assoc Neurol. 1965b;3:7–14. [PubMed] [Google Scholar]
- Eccles JC, Llinas R, Sasaki K. The excitatory synaptic action of climbing fibres on the Purkinje cells of the cerebellum. J Physiol. 1966;182:268–296. doi: 10.1113/jphysiol.1966.sp007824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekerot CF, Jorntell H. Parallel fibre receptive fields of Purkinje cells and interneurons are climbing fibre-specific. Eur J Neurosci. 2001;13:1303–1310. doi: 10.1046/j.0953-816x.2001.01499.x. [DOI] [PubMed] [Google Scholar]
- Ekerot CF, Jorntell H. Parallel fiber receptive fields: a key to understanding cerebellar operation and learning. Cerebellum. 2003;2:101–109. doi: 10.1080/14734220309411. [DOI] [PubMed] [Google Scholar]
- Hámori J, Szentágothai J. Identification under the electron microscope of climbing fibers and their synaptic contacts. Exp Brain Res. 1965;1:65–81. doi: 10.1007/BF00235210. [DOI] [PubMed] [Google Scholar]
- Holtzman T, Mostofi A, Phuah CL, Edgley SA. Cerebellar Golgi cells in the rat receive multimodal convergent peripheral inputs via the lateral funiculus of the spinal cord. J Physiol. 2006b;577:69–80. doi: 10.1113/jphysiol.2006.117218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman T, Rajapaksa T, Mostofi A, Edgley SA. Different responses of rat cerebellar Purkinje cells and Golgi cells evoked by widespread convergent sensory inputs. J Physiol. 2006a;574:491–507. doi: 10.1113/jphysiol.2006.108282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M. Long-term depression. Annu Rev Neurosci. 1989;12:85–102. doi: 10.1146/annurev.ne.12.030189.000505. [DOI] [PubMed] [Google Scholar]
- Jorntell H, Ekerot CF. Reciprocal bidirectional plasticity of parallel fiber receptive fields in cerebellar Purkinje cells and their afferent interneurons. Neuron. 2002;34:797–806. doi: 10.1016/s0896-6273(02)00713-4. [DOI] [PubMed] [Google Scholar]
- Jorntell H, Ekerot CF. Receptive field plasticity profoundly alters the cutaneous parallel fiber synaptic input to cerebellar interneurons in vivo. J Neurosci. 2003;23:9620–9631. doi: 10.1523/JNEUROSCI.23-29-09620.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas R, Nicholson C. Reversal properties of climbing fiber potential in cat Purkinje cells: an example of a distributed synapse. J Neurophysiol. 1976;39:311–323. doi: 10.1152/jn.1976.39.2.311. [DOI] [PubMed] [Google Scholar]
- Marr D. A theory of cerebellar cortex. J Physiol. 1969;202:437–470. doi: 10.1113/jphysiol.1969.sp008820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittmann W, Hausser M. Linking synaptic plasticity and spike output at excitatory and inhibitory synapses onto cerebellar Purkinje cells. J Neurosci. 2007;27:5559–5570. doi: 10.1523/JNEUROSCI.5117-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palay SL, Chan-Palay V. Cerebellar Cortex: Cytology and Organization. Springer; 1974. [Google Scholar]
- Schulman JA, Bloom FE. Golgi cells of the cerebellum are inhibited by inferior olive activity. Brain Res. 1981;210:350–355. doi: 10.1016/0006-8993(81)90908-2. [DOI] [PubMed] [Google Scholar]
- Shinoda Y, Sugihara I, Wu HS, Sugiuchi Y. The entire trajectory of single climbing and mossy fibers in the cerebellar nuclei and cortex. Prog Brain Res. 2000;124:173–186. doi: 10.1016/S0079-6123(00)24015-6. [DOI] [PubMed] [Google Scholar]
- Simpson JI, Wylie DR, De Zeeuw CI. On climbing fiber signals and their consequence (s) Behav Brain Sci. 1996;19:368–388. [Google Scholar]
- Sugihara I, Wu HS, Shinoda Y. Morphology of single olivocerebellar axons labeled with biotinylated dextran amine in the rat. J Comp Neurol. 1999;414:131–148. [PubMed] [Google Scholar]
- Szapiro G, Barbour B. Multiple climbing fibers signal to molecular layer interneurons exclusively via glutamate spillover. Nat Neurosci. 2007;10:735–742. doi: 10.1038/nn1907. [DOI] [PubMed] [Google Scholar]
- Watanabe D, Nakanishi S. mGluR2 postsynaptically senses granule cell inputs at Golgi cell synapses. Neuron. 2003;39:821–829. doi: 10.1016/s0896-6273(03)00530-0. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Miall CR, Kawato M. Internal models in the cerebellum. Trends Cogn Sci. 1998;2:338. doi: 10.1016/s1364-6613(98)01221-2. [DOI] [PubMed] [Google Scholar]